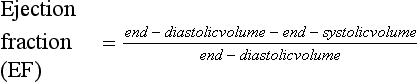Anaesthesia for Cardiac Surgery
TRENDS IN SURGICAL PRACTICE
Ischaemic Heart Disease
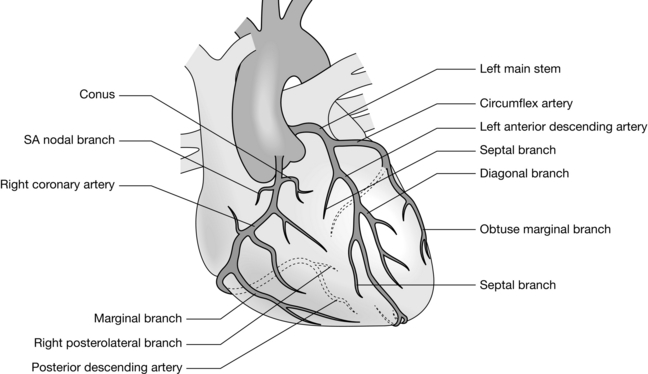
The concept of aorto-coronary bypass grafting for the relief of coronary ischaemia was conceived and performed in animals in the early 1900s. It was not until the 1960s, following development of the heart-lung machine and the chance discovery of coronary angiography, that direct revascularization of the ischaemic myocardium using the autologous saphenous vein (Fig. 34.1) replaced indirect therapies such as sympathectomy, thyroidectomy and pericardial poudrage.
Cardiopulmonary Bypass
The essential components of a cardiopulmonary bypasshe essential components of a cardiopulmonary bypass (CPB) circuit (Fig. 34.2) are:
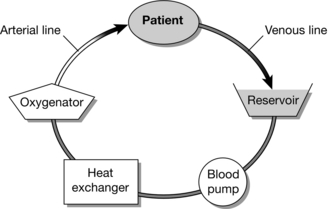
FIGURE 34.2 Components of a cardiopulmonary bypass circuit. (Adapted from JH Mackay and JE Arrowsmith (2012) Core Topics in Cardiac Anesthesia. Cambridge University Press.)
PREOPERATIVE ASSESSMENT
Cardiac Catheterization
Left heart catheterization typically comprises coronary angiography, aortography, left ventriculography and manometry. This provides the following information (Table 34.1):
TABLE 34.1
| Technique | Procedure | Parameter |
| Manometry | Pressure measurement with catheter in aortic root and LV | Aortic valve gradient LV end-diastolic pressure |
| Angiography | Coronary arteries selectively cannulated, contrast injected | Coronary anatomy |
| Ventriculogram | Catheter in LV, contrast injected very rapidly | LV size and function Ejection fraction Severity of mitral regurgitation |
| Aortogram | Catheter in aortic root, contrast injected | Severity of aortic regurgitation |
The efficiency of ventricular contraction (ejection fraction) can be estimated using the formula:
Right heart catheterization allows measurement of right heart and pulmonary artery pressures. When combined with measurements of cardiac output, these can be used to determine the pulmonary and systemic vascular resistances (Table 34.2).
TABLE 34.2
Measurements Obtained During Cardiac Catheterization
| Parameter | Normal Values | |
| Left heart | Systemic arterial/aortic pressure | < 140/90 (mean 105) mmHg |
| LV pressure | < 140/12 mmHg | |
| Right heart | RA pressure | < 6 (mean) mmHg |
| RV pressure | < 25/5 mmHg | |
| PA pressure | 25/12 (mean 22) mmHg | |
| PAWP | 12 mmHg | |
| Cardiac index | 2.5–4.2 l min– 1 m– 2 | |
| PVR | 100 dyne s cm– 5 | |
| SVR | 800–1200 dyne s cm– 5 |
Preoperative Drug Therapy
Nitrates should be continued to prevent rebound angina.
Diuretics should be continued until the day before surgery.
Potassium-channel activators may be continued up to the day of operation.
RISK ASSESSMENT
In the late 1980s, Parsonnet and colleagues identified 14 independent risk factors for death after cardiac surgery. The so-called Parsonnet score was adopted by many centres worldwide and is still in use today. However, most present day cardiac surgeons ‘out-perform’ Parsonnet, reducing the usefulness of the scoring system as a measure of both risk and surgical performance. The European System for Cardiac Operative Risk Evaluation (EuroSCORE), developed in the late 1990s, provides a more robust risk assessment, which, like its predecessor, can be calculated easily at the bedside (Table 34.3). The EuroSCORE has been validated in the UK, Europe and North America and has been shown to be predictive of major complications, duration of critical care stay and resource utilization.
TABLE 34.3
EuroSCORE—The European System for Cardiac Operative Risk Evaluation additive risk stratification model. http://www.euroscore.org.
| Factor | Points | |
| Age | Per 5 years or part thereof > 60 | 1 |
| Gender | Female | 1 |
| Chronic lung disease | Bronchodilators or steroids | 1 |
| Extra cardiac arteriopathy | Claudication, carotid stenosis > 50%, abdominal aortic, limb artery or carotid surgery planned or undertaken | 2 |
| Neurological dysfunction | Severe effect on ambulation or function | 2 |
| Previous cardiac surgery | Pericardium opened | 3 |
| Serum creatinine | > 200 μmol L– 1 before surgery | 2 |
| Active endocarditis | On antibiotics | 3 |
| Critical preoperative state | VT, VF, cardiac massage, invasive ventilation, inotropic support, IABP | 2 |
| Unstable angina | Angina at rest requiring intravenous nitrates | 2 |
| LV dysfunction | Moderate (LV EF 30–50%) Poor (LV EF < 30%) |
1 3 |
| Recent myocardial infarct | < 90 days | 2 |
| Pulmonary hypertension | PA systolic > 60 mmHg | 2 |
| Emergency operation | Carried out on referral, before next working day | 2 |
| Other than isolated CABG | 2 | |
| Surgery on thoracic aorta | 3 | |
| Post infarct septal rupture | 4 |
MONITORING
Electrocardiograph
PATHOPHYSIOLOGY
Preload and contractility determine the work performed by the heart (Fig. 34.3). In the failing heart, afterload determines the work expended in overcoming aortic pressure compared with that used to provide forward flow. Thus, cardiac output may be increased by increasing preload or contractility, or by reducing afterload. Any increases in heart rate, contractility, preload or afterload result in increased myocardial oxygen consumption. For this reason, augmentation of cardiac output by increasing preload or contractility may have a detrimental effect on oxygen balance. Reducing afterload may increase cardiac output while simultaneously reducing oxygen demand.
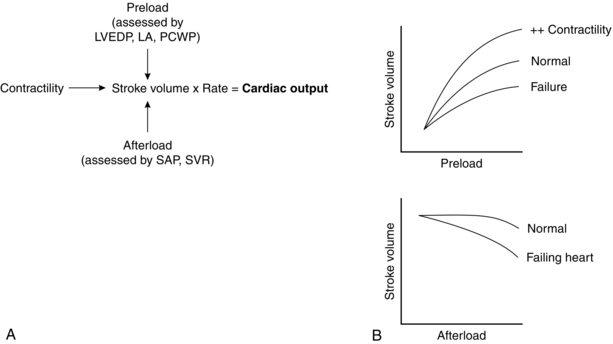
FIGURE 34.3 Important aspects of mechanical function. LA, left atrium; LVEDP, left ventricular end-diastolic pressure; PCWP, pulmonary capillary wedge pressure; SAP, systolic arterial pressure; SVR, systemic vascular resistance.
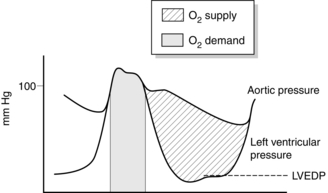
Adequate coronary perfusion demands the maintenance of an adequate diastolic aortic pressure. Oxygen supply to the myocardium occurs predominantly during diastole and is dependent on the gradient between diastolic aortic pressure and intraventricular pressure, and on the duration of diastole. The portion of myocardium most at risk of developing ischaemia is the left ventricular endocardium. Figure 34.4 illustrates how these variables affect oxygen supply and demand in the myocardium and how a satisfactory supply/demand ratio may be preserved.
ANAESTHETIC TECHNIQUE
Cardiopulmonary Bypass
Weaning from CPB
Low Cardiac Output State
Intra-Aortic Balloon Pump: This device is inserted through a femoral artery and positioned in the descending aorta, just distal to the left subclavian artery. The balloon is inflated during diastole immediately after closure of the aortic valve, and deflated prior to ventricular ejection. Inflation displaces blood in the aorta, simultaneously promoting distal flow and augmenting coronary perfusion. Following deflation of the balloon, proximal aortic pressure (afterload) is reduced, favouring improved ventricular ejection and lowering left ventricular end-diastolic pressure.
HAEMODYNAMICS AFTER CPB
Other Aspects
Cardiac Rhythm
Heart Block: This may follow aortic valve surgery and operations on the ventricular septum, or as a consequence of right coronary aeroembolism. Atrioventricular (sequential; D00, DDD) pacing using epicardial pacing wires ensures an adequate ventricular rate and maintains late diastolic ventricular filling, which is particularly important if ventricular compliance is poor.
Hensley, F.A., Martin, D.E., Gravlee, G.P. A practical approach to cardiac anesthesia, third ed. Williams & Wilkins, Philadelphia: Lippincott; 2002.
Kaplan H.A., Reich D.L., Konstadt S.N., eds. Cardiac anesthesia, fifth ed., Philadelphia: WB Saunders, 2006.
Klein A.A., Vuylsteke A., Nashef S.A.M., eds. Core topics in cardiothoracic critical care. Cambridge: Cambridge University Press, 2008.
Mackay J.H., Arrowsmith J.E., eds. Core topics in cardiac anaesthesia, second ed., Cambridge: Cambridge University Press, 2012.