49 Anaemia
Aetiology
The low haemoglobin level that defines anaemia results from two different mechanisms:
Reduced haemoglobin synthesis leads to either reduced proliferation of precursors or defective maturation of precursors or both (see Table 49.1). It is not unusual to find more than one cause in a single patient.
Table 49.1 Examples of conditions that cause reduced haemoglobin synthesis
| Reduced proliferation of precursors | Defective maturation of precursors |
|---|---|
| Iron deficiency | Vitamin B12 deficiency |
| Anaemia of chronic disease | Folate deficiency |
| Anaemia of renal failure | Iron deficiency |
| Aplastic anaemia (primary) | Disorders of |
| Aplastic anaemia (secondary to drugs, etc.) |
This chapter will cover some of the more common anaemias that involve drug therapy:
| Microcytic anaemias | iron deficiency anaemia |
| anaemia of chronic disease | |
| sideroblastic anaemia | |
| Megaloblastic anaemias | folate deficiency |
| vitamin B12 deficiency | |
| Haemolytic anaemias | autoimmune haemolytic anaemia |
| sickle cell disease | |
| thalassaemia | |
| glucose-6-phosphate dehydrogenase deficiency |
Normal erythropoiesis
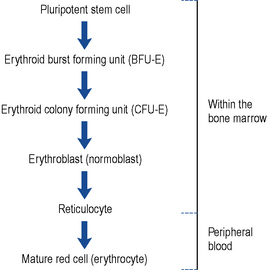
It is thought that white cells, red cells and platelets are all derived from a common cell known as the pluripotent stem cell found in the bone marrow. As these cells mature, they become committed to a specific cell line (Fig. 49.1). The red cells mature through the various stages, during which time they synthesise haemoglobin, DNA and RNA. Reticulocytes are found in the peripheral circulation for 24 h before maturing into erythrocytes. Reticulocytes are released into the peripheral circulation prematurely during times of increased erythropoiesis.
Clinical manifestations
In its mildest form anaemia results in tiredness and lethargy; at its most severe it results in death unless treated. There is some suggestion that even mild anaemia may inhibit physical exercise and result in reduced mental performance. The reduced oxygen-carrying capacity of the blood leads to reduced tissue oxygenation and widespread organ dysfunction (Box 49.1).
Investigations
The most important parameter to assess anaemia is the haemoglobin concentration of the blood. It is also usual to count the number of red cells. In addition the size, shape and colour all contribute to the investigation (Box 49.2). The mean corpuscular volume (MCV) is a useful parameter that helps determine the type of the anaemia. However, care must be taken since the MCV indicates the average size of the cells. If there are two pathologies, where one causes large cells and other causes small cells, the MCV may appear normal or be misleading. Following on from this baseline other investigations may be required. Bone marrow examination by either aspiration or trephine may be needed to make a diagnosis.
Iron deficiency anaemia
Aetiology
In Western societies, the commonest cause of iron deficiency is due to blood loss. In women of childbearing age, this is most commonly due to menstrual loss. Amongst adult males, the most likely cause is gastro-intestinal bleeding. Other causes of blood loss associated with iron deficiency anaemia include haemorrhoids, nosebleeds or postpartum haemorrhage. A loss of 100 mL of blood represents the amount of iron normally absorbed from a Western diet over 40 days. The major causes of iron deficiency are listed in Box 49.3.
Pathophysiology
Anaemia may result from a mismatch between the body’s iron requirements and iron absorption. The demand for iron varies with age (Table 49.2). Diets deficient in animal protein or ascorbic acid may not provide sufficient available iron to meet the demand. Poor nutrition in children in inner cities in the UK frequently leads to anaemia. Milk fortified with iron given to inner city infants up to the age of 18 months has been shown to increase haemoglobin levels and improve developmental performance compared to unmodified cow’s milk (Williams et al., 1999). A systematic review showed that iron supplementation in children over seven improved intelligence tests scores in those who were initially anaemic (Sachdev et al., 2005). However, universal iron supplementation may not be appropriate because there is a theoretic increased risk of susceptibility to infectious diseases, although this has only been demonstrated for diarrhoea (Gera and Sachdev, 2002) and malaria in endemic areas (Prentice et al., 2007). Targeting supplementation only to children with anaemia and withholding iron supplementation during malaria treatment is sensible as iron may inhibit treatment, and absorption of oral iron is blocked by the inflammatory response.
| Infant (0–4 months) | 0.5 mg |
| Adolescent male | 1.8 mg |
| Adolescent female | 2.4 mg |
| Adult male | 0.9 mg |
| Menstruating female | 2.0 mg |
| In pregnancy | 3–5 mg |
| Postmenopausal female | 0.9 mg |
Clinical manifestations
In addition to the general symptoms of anaemia, various other features may be present (Box 49.4). The colour of the skin is very subjective and often unreliable. Patients at risk of heart failure may present with breathlessness when anaemic. Koilonychia, dysphagia and pica are found only after chronic iron deficiency and are relatively rare.
Treatment
If gastro-intestinal investigation has been performed and any underlying cause treated, all patients should receive iron supplementation to correct their anaemia and replenish stores. Oral iron in the ferrous form is cheap, safe and effective in most patients. Depending on the state of the body’s iron stores, it may be necessary to continue treatment for up to 6 months to both correct the anaemia and replenish body stores. The standard treatment is ferrous sulphate 200 mg two to three times a day. It typically takes between 1 and 2 weeks for the haemoglobin level to rise 1 g/dL. An earlier indication of the patient’s response can be seen by looking at the reticulocyte count, which should start to rise 2–3 days after starting effective treatment. Nausea or abdominal pains trouble some patients and this tends to be related to the dose of elemental iron. Giving the iron with food makes it better tolerated but tends to reduce the amount absorbed. Alternative salts of iron are sometimes tried; these tend to have fewer side effects simply because they contain less elemental iron (Table 49.3). Taking fewer ferrous sulphate tablets each day would have the same effect. A change in bowel habit (either constipation or diarrhoea) is sometimes reported, and this is probably not dose related. During the early stages of treatment, the body absorbs oral doses of iron better. Absorption is commonly around 15% of intake for the first 2–3 weeks but falls off to an average of 5% thereafter. It has been shown that for some patients eradication of Helicobacter pylori aids recovery from iron deficiency anaemia (Annibale et al., 1999).
Table 49.3 Elemental iron content of common oral preparations
| Preparation | Approximate iron content (mg) |
|---|---|
| Tablets | |
| Ferrous sulphate 210 mg | 68 |
| Ferrous gluconate 300 mg | 35 |
| Ferrous fumarate 322 mg | 100 |
| Ferrous fumarate 210 mg | 68 |
| Oral liquids | |
| Ferrous fumarate 140 mg in 5 mL | 45 |
| Ferrous sulphate 125 mg in 1 mL | 25 |
| Sodium feredetate 190 mg in 5 mL | 27.5 |
There is a limited place for parenteral iron in iron deficiency anaemia; it should be reserved for patients who fail on oral therapy, usually because of poor adherence or intolerable gastro-intestinal side effects. For most patients when equivalent doses of oral and parenteral iron are used, there is no difference in the rate of at which the haemoglobin level rises. Patients who have lost blood acutely may require blood transfusions. The need for a rapid rise in haemoglobin is not an indication for parenteral iron. Intravenous iron has been shown to have some benefit during the perioperative management of anaemia in selected patients undergoing orthopaedic surgery, but not been observed in other types of surgery (Beris et al., 2008).
Anaemia of chronic disease
Investigation
Patients have a hypochromic microcytic anaemia similar to iron deficiency anaemia, but the two conditions can be differentiated by reviewing other serum factors (see Table 49.4).
Table 49.4 Differentiation between iron deficiency anaemia and anaemia of chronic disease
| Test | Iron deficiency anaemia | Anaemia of chronic disease |
|---|---|---|
| Serum iron | Low | Low |
| Serum ferritin | Low | Normal or high |
| Serum transferrin | High | Normal or low |
| Total iron binding capacity | High | Low |
Treatment
A number of patients with chronic renal failure appear to have a functional iron deficiency that responds to intravenous iron. These patients, despite receiving oral iron and erythropoietin analogues, do respond with a rise in haemoglobin when given regular intravenous iron together with an erythropoietin analogue (Silverberg et al., 1996). Intravenous iron in combination with erythropoietin analogues is widely used in chronic kidney disease. The patient’s serum ferritin is monitored to check for iron overload. Concerns have been expressed about the possible long-term complications of intravenous iron, for example, atherosclerosis or increased risk of infection (Cavill, 2003). There appears to be a slightly increased risk of infections, but the improvement in anaemia leads to an improved quality of life.
Patients with anaemia-associated inflammatory bowel disease or with rheumatoid arthritis respond to intravenous iron; however, the use of intravenous iron in chronic inflammatory conditions is not generally recommended because of an increased risk of infections and also possible increased risk of acute cardiovascular events. Some small studies have shown intravenous iron to be beneficial in patients with heart failure, but currently this should be reserved for patients with proven iron deficiency and failure on oral iron (Dec, 2009).
Some patients with chronic disorders respond to erythropoietin analogues, none are licensed for use in chronic disease states other than anaemia associated with chronic renal failure or cancer. Elevated endogenous erythropoietin levels in patients with heart failure are associated with adverse outcomes (Felker, 2010). Some clinical trial data show a higher mortality and increased risk of tumour progression in patients with anaemia associated with cancer who have been treated with erythropoietins. It is not recommended that erythropoietin analogues are routinely used in the management of cancer treatment-induced anaemia (NICE, 2008). However, they may be considered, in combination with intravenous iron, for:
Tocilizumab, the interleukin-6 antagonist monoclonal antibody licensed for use in rheumatoid arthritis, has been shown to improve haemoglobin levels (Raj, 2009). It has also been suggested that drugs which downregulate interleukin-6 may have some effect (Altschuler and Kast, 2005). Olanzepine and quetiapine (potent H1 antagonists) are known to be regulators of interleukin-6 but are not used clinically for this purpose. Furthermore, it is not known what the other effects of modifying interleukin-6 or hepcidin would have on the chronic inflammatory condition.
Sideroblastic anaemias
Aetiology
The acquired forms include idiopathic forms, forms associated with myeloproliferative disorders and forms secondary to the ingestion of drugs (Box 49.5). Regardless of the cause, there is impaired haem synthesis, where the body has adequate iron stores but is unable to incorporate it into haemoglobin.
Treatment
Although the peripheral blood cells are frequently hypochromic and microcytic, the condition is associated with increased iron stores; therefore, iron supplements should be avoided. The drugs and toxins (Box 49.5) tend to cause a reversible anaemia. Removing the offending agent usually resolves the anaemia.
Megaloblastic anaemias
Pathophysiology
Folate deficiency anaemia
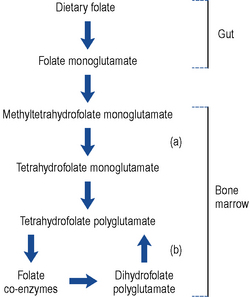
The folate found in food is mainly conjugated to polyglutamic acid. Enzymes found in the gut convert the polyglutamate form to monoglutamate, which is readily absorbed. During absorption, the folate is methylated and reduced to methyltetrahydrofolate monoglutamate. This travels through the plasma and is transported into cells via a carrier specific for the tetrahydrofolate form. Within the cell, the methyl group is removed (in a reaction requiring vitamin B12) and the folate is reconverted back to a polyglutamate form (Fig. 49.2). It has been suggested that the polyglutamate form prevents the folate leaking out of cells. The folate eventually acts as a coenzyme involved in a number of reactions including DNA and RNA synthesis. Defective DNA synthesis mainly affects cells with a rapid turnover, for example, gastro-intestinal cells and red blood cells, hence the sore tongue and anaemia seen in folate deficiency. During DNA synthesis, the folate coenzyme is oxidised to the dihydrofolate form, which is inactive and has to be reactivated by the enzyme dihydrofolate reductase. This is the enzyme inhibited by methotrexate and to a lesser extent by trimethoprim and pyrimethamine. Co-trimoxazole has been shown to increase the severity of megaloblastic anaemia.
Vitamin B12 deficiency
Since intrinsic factor is only produced by the gastric parietal cells, a total gastrectomy always leads to vitamin B12 deficiency. Approximately 10–15% of patients who have had a partial gastrectomy also develop deficiency. The onset of anaemia is usually delayed because the body typically has stores of 2–3 mg, which is sufficient for 2–3 years. Vitamin B12 is a coenzyme for the removal of a methyl group from methyltetrahydrofolate. Lack of vitamin B12 traps the folate as methyltetrahydrofolate and prevents DNA synthesis (Fig. 49.2). The exact mechanism by which vitamin B12 deficiency causes neuropathy is not clear but may be due to a defect in the methylation reactions needed for myelin formation.
Clinical manifestations
In addition to the general features of anaemia, megaloblastic anaemia, of which folic acid deficiency and B12 deficiency anaemia are the two most common examples, has certain characteristics described in Box 49.6.
Folate deficiency anaemia
Alcoholics and the elderly are particularly prone to nutritional deficiency. Elderly people living alone on tea and toast are typical examples of patients at risk. Many alcoholics develop deficiency due to their poor diet; although some beers contain small amounts of folate, spirits contain none. A number of drugs have been implicated in causing folate deficiency (Box 49.7). Actual megaloblastic anaemia from drug therapy is uncommon, and the exact mechanism(s) has not always been established. Serious malabsorption can occur in tropical sprue and coeliac disease. Reduced absorption is seen in Crohn’s disease and following partial gastrectomy or jejunal resection.
There is a physiological increase in folate utilisation during pregnancy. There may be an increased utilisation in various pathological conditions, in association with inflammation or in a number of chronic haemolytic anaemias, particularly thalassaemia major, sickle cell disease and autoimmune haemolytic anaemia. Chronic folate deficiency probably predisposes patient to thrombosis, depression and neoplasia (Green and Miller, 1999).
Vitamin B12 deficiency anaemia
The megaloblastic anaemia caused by vitamin B12 deficiency has similar features to folate deficiency (Box 49.6). In addition to the macrocytosis, anisocytosis and poikilocytosis, there is often mild thrombocytopenia. The spleen may be slightly enlarged and there may be a slight fever. Mild jaundice may be present due to the increased breakdown of haemoglobin found in the abnormal red cells. The onset is slow and insidious, so patients often present late or are diagnosed during other investigations. The feature that separates vitamin B12 deficiency from the other megaloblastic anaemias is progressive neuropathy. It is symmetrical and affects the legs rather than the arms. Patients notice a tingling in their feet and a loss of vibration sense. Occasionally, patients have muscle weakness, difficulty in walking or experience frequent falls.
Investigations
Vitamin B12 deficiency anaemia
The detection of autoantibodies is helpful to distinguish pernicious anaemia from other forms. The presence of intrinsic factor antibodies is virtually diagnostic of pernicious anaemia being rarely found in any other condition. However, it is not a very sensitive test since only half the patients with pernicious anaemia have these antibodies. Gastric parietal cell antibodies are found in 85% of patients with pernicious anaemia (a more sensitive test) but unfortunately also found in up to 10% of people without pernicious anaemia (less specific) (see Table 49.5).
| Intrinsic factor antibodies | Parietal cell antibodies | |
|---|---|---|
| Patients with pernicious anaemia | Detected in 50% of patients | Detected in 85% of patients |
| People who do not have pernicious anaemia | Rarely detected | Detected in up to 10% |
Treatment
Folate deficiency anaemia
Pregnancy
The folate requirement increases in pregnancy and is higher in twin pregnancies. Folate deficiency regularly occurs in patients with a poor diet who do not take supplements. Prophylaxis with folate (350–500 μcg daily) is now frequently given in pregnancy, often in combination with iron, starting before conception and during the first 12 weeks of pregnancy. It is important that these products with low doses of folate are not used to treat megaloblastic anaemia. Although this low-dose folate should be started before conception to prevent a first occurrence of neural tube defect, higher doses (5 mg daily) are required in women with a history of neural tube defects and again continued until week 12 (DH, 1992).
Haemolytic anaemias
In the haemolytic anaemias, there is a reduced life span of the erythrocytes. Anaemia occurs when the rate of destruction of the erythrocytes exceeds their rate of production. There are a wide range of haemolytic anaemias with both genetic and acquired disorders (Table 49.6). Only autoimmune haemolytic anaemia, sickle cell anaemia, thalassaemia and glucose-6-phosphate dehydrogenase deficiency will be briefly discussed. Haemolytic anaemias account for approximately 5% of all anaemias.
| Genetic disorders of | Examples |
|---|---|
| Haemoglobin | Sickle cell anaemias |
| Thalassaemias | |
| Energy pathways | Glucose-6-phosphate deficiency |
| Membrane | Hereditary spherocytosis |
| Hereditary ovalcytosis | |
| Acquired disorders | |
| Immune | Autoimmune |
| Rh or ABO incompatibility | |
| Non-immune | Infections (parasitic, bacterial) |
| Drugs and chemicals | |
| Hypersplenism | |
Autoimmune haemolytic anaemia
Aetiology
WAIHA is frequently associated with other diseases with an immunological component, for example, chronic lymphocytic leukaemia, systemic lupus erythematosus and hepatitis B. Acquired CAD is sometimes associated with viral or bacterial infections: cold agglutinins develop in more than 60% of patients following infectious mononucleosis but haemolytic anaemia is rare. A number of drugs have been implicated as leading to immune haemolysis (Box 49.8).
Treatment
Treatment is dependent on the specific cause (Pruss et al., 2003). In WAIHA, the standard treatment is high-dose corticosteroids. Patients who do not respond can be managed with azathioprine or cyclophosphamide. Blood transfusions are necessary in severe cases, although providing blood free from underlying alloantibodies is difficult. Rituximab, the anti CD-20 monoclonal antibody, has been shown to be beneficial in some patients who fail to respond to the forementioned conventional anti-inflammatory therapy. However, this is an unlicensed indication. (D’Arena et al., 2007) Patients with CAD need to be kept warm with supportive measures as well as treated for any underlying disorder.
Sickle cell anaemia
Aetiology
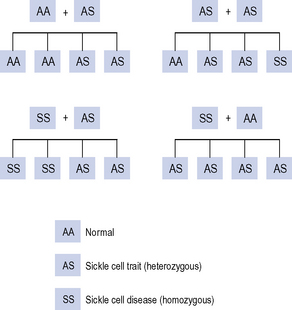
Sickle cell trait is where a person is a carrier of the gene (heterozygous for the sickle cell gene). These people are usually asymptomatic. Sickle cell trait provides some protection from malaria and is more common in those ethnic groups originating from geographical areas that are endemic for malaria. The offspring from a father with the trait and a mother with the trait has a one in four chance of having sickle cell disease (Fig. 49.3).
Treatment
Attempts have been made to increase the proportion of haemoglobin F and reduce the proportion of haemoglobin S in the circulation. Several drugs (Box 49.9) have been shown (some only in animal models) to stimulate fetal haemoglobin production (Charache et al., 1995).
Thalassaemias
Treatment
There is currently no effective treatment. Patients with thalassaemia minor (those who carry the thalassaemia gene, but still make enough normal haemoglobin) usually do not require treatment. Many patients with severe forms are dependent on blood transfusions from an early age. This inevitably leads to iron overload. Desferrioxamine, deferiprone and deferasirox are routinely needed for such patients (see sideroblastic anaemia for additional information). Splenectomy helps some patients, and allogeneic stem cell transplant is used in severe cases. Prevention is actively explored with genetic counselling programmes in Italy and Greece, and antenatal screening in a number of countries.
Glucose-6-phosphate dehydrogenase deficiency
Clinical manifestation
Clinically, the two most important types of G6PD deficiency occur in the black population and in people originating from the Mediterranean. The black population has a milder form that results in an acute self-limiting haemolytic anaemia following exposure to an oxidising agent, for example, infection, acute illness, broad beans (fava) beans or drugs (Box 49.10). The haemolytic anaemia is self-limiting because the young cells produced by the bone marrow have higher levels of G6PD activity than old cells. Following exposure to the oxidising agent, the old cells are haemolysed but the new cells produced in response are more capable of tolerating the insult until they grow old. In the Mediterranean form of the disorder, the enzyme activity is very low, haemolysis is not usually self-limiting and, indeed, some patients have a chronic haemolytic anaemia despite the absence of an obvious causative factor.
Patient care
Answers
Answers
Answers
Answers
Answers
Altschuler E.L., Kast R.E. Using histamine (H1) antagonists in particular atypical antipsychotics to treat anaemia of chronic disease via interleukin-6 suppression. Med. Hypotheses. 2005;65:65-67.
Annibale B., Marignani M., Monarca B., et al. Reversal of iron deficiency anaemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann. Intern. Med.. 1999;131:668-672.
Beris I., Munoz M., Garcia-Erce J.A., et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br. J. Anaesth.. 2008;100:599-609.
Cavill I. Intravenous iron as adjuvant therapy: a two edged sword? Nephrol. Dial. Transplant.. 2003;18(Suppl. 8):viii24-viii28.
Charache S., Terrin M.L., Moore R.D., et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anaemia. N. Engl. J. Med.. 1995;332:1317-1322.
D’Arena G., Califano C., Annunziata M., et al. Rituximab for warm-type idiopathic autoimmune hemolytic anemia: a retrospective study of 11 adult patients. Eur. J. Haematol.. 2007;79:53-58.
Dec W.G. Anemia and iron deficiency – new therapeutic targets in heart failure? N. Engl. J. Med.. 2009;361:2475-2477.
DH. Folic Acid and the Prevention of Neural Tube Defects: Report from an Expert Advisory Group. London: Department of Health; 1992.
Felker M. Too much, too little, or just right? Untangling endogenous erythropoietin in heart failure. Circulation. 2010;121:191-193.
Gera T., Sachdev H.P.S. Effect of iron supplementation on incidence of infectious illness in children: systematic review. Br. Med. J.. 2002;325:1142. Available at http://www.bmj.com/content/325/7373/1142.full.pdf
Green R., Miller J.W. Folate deficiency beyond megaloblastic anaemia: hyperhomocysteinemia and other manifestations of dysfunctional folate status. Semin. Haematol.. 1999;36:47-64.
National Institute for Health and Clinical Excellence. Epoetin Alfa, Epoetin Beta and Darbepoetin Alfa for Cancer Treatment-Induced Anaemia, Technology Appraisal 142. London: NICE. 2008. Available at http://www.nice.org.uk/nicemedia/pdf/TA142Guidance.pdf
Prentice A.M., Ghattas H., Doherty C., et al. Iron metabolism and malaria. Food Nutr. Bull.. 2007;28(4 Suppl.):S524-S539.
Pruss A., Salama N., Ahrens A., et al. Immune hemolysis-serological and clinical aspects. Clin. Exp. Med.. 2003;3:55-64.
Raj D.S. Role of interleukin in the anaemia of chronic disease. Semin. Arthritis Rheum.. 2009;38:382-388.
Sachdev H.P.S., Gera T., Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr.. 2005;8:117-132.
Silverberg D.S., Blum M., Peer G., et al. Intravenous ferric saccharate as an iron supplement in dialysis patients. Nephron. 1996;72:413-417.
Williams J., Wolff A., Daly A., et al. Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. Br. Med. J.. 1999;318:693-698.