Chapter 98 An Approach for Treatment of Complex Adult Spinal Deformity
The range of normal for cervical lordosis, thoracic kyphosis, and lumbar lordosis is quite variable.1–3 Varying degrees of scoliosis can be tolerated depending on many other factors. As a result, spinal balance apparently is more important in terms of symptoms and progression than the magnitude of scoliosis or kyphosis. A review by Kuntz et al.1 showed that there is only a narrow range of spinal balance and that this is highly conserved.
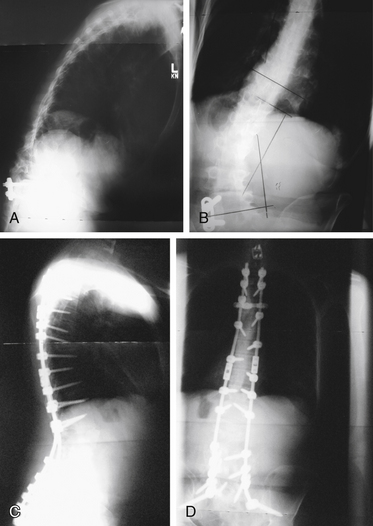
Clinically, spinal balance can be assessed by examining the head position of a standing patient in relation to the pelvis. In the lateral view, a plumb line from the ear canal should pass through or behind the greater trochanter. In the anteroposterior view, a plumb line from the inion should pass between the posterior superior iliac spines. Radiographically on a long cassette film, one can use either the C7 vertebral body or the odontoid as the starting point for a plumb line. Use of the odontoid as a marker allows assessment of cervical deformity in overall spinal balance. A plumb line from the odontoid should pass dorsal to the center of rotation of the hip in the lateral plane and should fall between the medial borders of the S1 pedicle in the anteroposterior plane. A plumb line from the C7 vertebral body should pass through the L5-S1 disc space. Figure 98-1 shows preoperative anteroposterior (see Fig. 98-1A) and lateral (see Fig. 98-1B) views of a patient with decompensated kyphoscoliosis with loss of both sagittal and coronal balance. Postoperative views of the same patient show restoration of balance (see Figs. 98-1C and D).
Define the Problem
Although it may seem simplistic, it is important to begin the process by defining the problem. In contrast to idiopathic adolescent scoliosis, in which the predominant focus is on the magnitude and progression of the deformity, there are more factors to consider in adult deformity. One key clinical difference in adult deformity is that adults generally seek treatment for the symptoms of the deformity rather than the deformity itself.4 As a result, the deformity is viewed within the context of the symptoms it produces. In addition, comorbidities need to be considered. In many cases, the patient will already have had other spine procedures.
The physical examination should include a detailed neurologic examination. Examination of spinal alignment and balance is important. Loss of sagittal and coronal balance is associated with increased symptoms and seems to have a higher risk of progression.5–7
In patients in whom surgery is being considered and comorbidities are present, general and specialty medical consultation for preoperative optimization should be used.8 Nutritional and bone health status are often overlooked in the workup and can have significant effects on outcome.9–12 Bone mineral density testing can help to assess bone health, although the presence of degenerative changes in the spine may artificially increase bone mineral density of the spine.13,14 Vitamin D testing and supplementation in the preoperative period should be considered, especially in regions or cultures where there is little direct sun exposure.15,16 In large-magnitude thoracic deformities, pulmonary function testing should be done for risk assessment.17
Goals
Prevention of progression is a common goal in treatment of deformity. In adult deformity, progression is unpredictable for many conditions, and progression of symptoms may or may not correlate with progression of deformity.18–20 As a result, prevention of progression is an uncommon indication for treatment after skeletal maturity.21–23
Generally, the goals of surgical treatment are to relieve compression of neural elements, stabilize instabilities, and correct and maintain the correction of the deformity. These goals need to be accomplished while minimizing risk in the short term and the long term. A primary end result of deformity treatment should be the restoration of sagittal and coronal balance. Outcome studies have shown weak, if any, correlation between correction of the Cobb angle and outcome but have shown clear correlation with spinal balance and outcome.6,7,24–27
Osteoporosis
Osteoporosis is common in patients with spinal deformity. It may be associated with vertebral fractures leading to increased deformity.21,28 It also may have an effect on outcome of surgery.11,29 Although osteoporosis does not affect bone healing, it does affect the holding power of spine instrumentation.30–32 For this reason, assessment of osteoporosis is an important step when considering surgery. Although there is no quoted level of bone density beyond which surgery is not an option, the risks of failure increase with higher degrees of bone loss. Preoperative optimization of bone health with vitamin D testing and supplementation as required and pretreatment with teriparatide have been advocated,16 but no studies have looked at outcomes of these interventions. Animal studies have suggested that teriparatide may improve healing of fusions.33,34
Options
Decompression Alone
In patients with a stable balanced spine with isolated radiculopathy, one option may be to consider an isolated decompression. Generally, compressive pathology occurs on the concavity of the deformity.35 If a single level can be identified either on the basis of clinical symptoms or with nerve root blocks, an isolated decompression may be a reasonable option. There is a risk that decompression may exacerbate deformity in these patients. Previous studies showed that the results of decompression alone in the presence of scoliosis may not be as good as decompression in a normally aligned spine.36–38 Many of these studies were done with more extensive decompression than would be done at the present time. Decompression alone is not an option in the presence of a rotatory subluxation or spondylolisthesis at the apex of the deformity. Anecdotally, decompressions of a keyhole or laminotomy type are associated with a lower risk of progression of deformity.39,40 This option may be particularly good in elderly patients with an isolated radiculopathy and relatively minimal axial back pain.
Limited Fusion
In many patients, symptoms can be isolated to a single level of pathology. An example would be a degenerative spondylolisthesis and a degenerative scoliosis. In these patients, it may be reasonable to treat only the symptomatic level. This is a controversial treatment. In a more recent study, reasonably good results were obtained with single-level fusion for degenerative spondylolisthesis in degenerative scoliosis. A few patients needed further surgery, and few if any had progression of deformity.41 Some authors have criticized this technique as having an unacceptably high rate of failure.42 However, there are no controlled trials comparing it with more extensive fusion; the literature contains few articles.43,44
One more recent trial45 looked at surgeons whose practice contained more than 50% deformity cases and showed that these surgeons were more likely to perform fusion of more levels than surgeons whose practice contained less than 50% deformity cases. The authors implied that the surgeons with more deformity cases were more likely to select a correct course; however, there was no clinical correlation in this study. It is perhaps equally valid to suggest that the surgeons with more deformity cases were more likely to perform fusion of excess levels.
Instrumented Correction and Fusion
In most cases, pedicle screw instrumentation is the mainstay of instrumented fusion. Pedicle screws allow better correction of most deformities.46–50 Pedicle screws are extremely versatile and have excellent holding power. They can exert or resist forces in multiple planes. Pedicle screws tend to be weakest in pull-out.51 As a result of their versatility, pedicle screws have become the main type of instrumentation used. Hooks and wires are less commonly used because they are more technically demanding and less versatile. Hooks and wires are relatively strong in pull-out but need intact posterior elements.
Obtaining solid biologic fusion is of utmost importance in the long-term. Fusion can be achieved through interbody, dorsal, or dorsolateral fusion. Interbody techniques generally have a higher fusion rate.52–54 In the lumbar spine, dorsolateral fusion is biomechanically superior and more effective than laminar onlay fusion.55 In the thoracic spine, dorsal fusion is more typically performed. The biology of fusion, choice of bone graft or bone graft substitute, and use of extenders are discussed elsewhere. In complex surgery with the high risk of fusion failure, the choice of bone graft and bone graft substitutes is of great importance. The use of bone morphogenetic protein in deformity seems to lead to significantly higher fusion rates. Limited evidence suggests that it is cost-effective in this indication.56–58
Selection of rostral and caudal levels is the first step in determining an operative plan. Generally, the construct should begin and end at a neutral vertebra in both the sagittal and the coronal planes. In complex or degenerative deformities, it is often more difficult to determine these levels than in an idiopathic scoliosis. The presence significant disc degeneration or instability below a neutral vertebra would generally necessitate extension of the fusion beyond this.59,60 Perhaps the most controversial question is whether or not to end a fusion at the L5 vertebra. Numerous studies have been performed and reached conflicting results.61–65
A series of studies by Lenke et al.61,62 looked at this question and concluded that if the L5-S1 disc is relatively normal on MRI and the L5 vertebral body does not have an oblique takeoff, preserving the L5-S1 motion segment is a reasonable option. In these patients, the incidence of repeat surgery to fuse the 5/1 level was lower than the incidence of repeat surgery for pseudarthrosis. In the presence of significant L5-S1 disc degeneration or oblique takeoff or instability at L5-S1, the incidence of repeat surgery to fuse the 5/1 level was higher than the incidence of surgery for pseudarthrosis.
Numerous factors must be looked at in considering the upper stop point of the construct. The thoracolumbar junction represents a transition from the mobile lumbar spine to the stiffer thoracic spine. Constructs extending up from the sacrum to the lumbosacral junction can create a stress riser if stopped at the junction. Typically, it has been considered acceptable to stop such a construct at L2, but constructs longer than this should extend to T1059,60,66,67; however, a more recent study has called this into question. In this study, there seemed to be no clearly defined level at which the risk of subsequent surgery was lessened.66 In deciding to stop in the lower thoracic spine, one must also consider whether this stop point is at the apex of the thoracic kyphosis. In patients in whom a fusion stops at the apex of the thoracic kyphosis, there is significant risk of proximal junctional kyphosis. It may be preferable in these patients to extend the construct up into the upper thoracic spine, typically T4 or T5.59,60
Long fusion constructs to the sacrum have a high incidence of failure because of pseudarthrosis at L5-S1. This pseudarthrosis is due to numerous biomechanical and anatomic factors. The S1 pedicle is more cancellous and has a short anteroposterior diameter, and the holding power of S1 pedicle screws is less than at other levels. In addition, forces at this level are magnified because of the relatively large lever arm exerted by the pelvis.65,69 Many strategies have been suggested to increase the fusion right at L5-S1. Primary among these strategies is the use of interbody fusion through either a ventral or a dorsal approach.65 This strategy has been shown to decrease pseudarthrosis.
More recent studies have assessed anterior lumbar interbody fusion and compared it with posterior lumbar interbody fusion or transforaminal lumbar interbody fusion. None of these techniques showed clear superiority in these studies.68–71 McCord et al.72 analyzed alternative fixation techniques at the lumbosacral junction; this study led to the concept of the pivot point, which is the region of the dorsal aspect of the anulus fibrosus at L5-S1. Fixation at the lumbosacral junction should extend ventral to this pivot point to provide increased stability. Sacral alar screws, S2 screws, iliac bars and screws, and iliosacral screws have been suggested for this procedure. Biomechanical studies showed increased rigidity with the use of iliac or sacroiliac screws, and clinical studies suggested that these two fixation types are superior to sacral alar or S2 screws.70–77 In a longitudinal series by Kostuik and Musha,65 pseudarthrosis rates were decreased from 83% to 3% by the use of interbody fusion and iliac fixation.
Many authors have advocated increasing deformity correction through the use of anterior releases and fusions.78,79 It is believed that this approach increases correction and increases the fusion rate. However, more recent studies have called this into question.80–82 With the use of segmental pedicle screw fixation and alternative release techniques, equivalent deformity correction can be obtained through purely dorsal procedures without the morbidity83 of an anterior release. These studies compared more traditional open anterior release techniques. With the advent of new or less invasive procedures and the use of interbody fusions through a direct lateral approach, the morbidity of anterior releases may be significantly less. Such minimal access lateral approaches and fusion techniques have been shown to give good correction, high fusion rates, and reasonably good clinical results.84–86 In the author’s practice, these techniques have replaced traditional open releases and fusions. The use of these techniques at the L4-5 level should be considered cautiously. The anatomic corridor is small,87,88 and there is a relatively high rate of L3 neurapraxia.86 The author no longer uses minimal access lateral techniques for the L4-5 level.
Instrumentation in Osteoporosis
The presence of osteoporosis increases the failure rate of instrumented constructs in deformity surgery. Osteoporosis compromises the holding power of the implants leading to this increased failure rate. Numerous strategies have been mediated to lessen this failure risk, and Hu29 summarized them well in a review article. Essentially, these strategies all are methods of dispersing or decreasing forces across the construct. Increasing the number of fixation points decreases the stress on each element of the construct. Cement augmentation of pedicle screws has been shown to increase their pull-out resistance. Generally, it is unnecessary to perform cement augmentation of all fixation points; only the points at the ends of the construct need to be reinforced with cement. There is relatively more loss of cancellous than cortical bone in osteoporosis, and fixation that uses cortical bone is relatively stronger. As a result, laminar hooks may be a good option in a kyphosis construct, which is likely to fail in pull-out. If correction can be obtained through osteotomies or releases, loads on the hardware are more likely to be neutral, and the construct is less susceptible to hardware failure.12,29,89,90
Osteoporosis has been considered a relative contraindication to the use of interbody fusions. Biomechanical studies by Cunningham and Polly91 showed that use of interbody fusion increases the strength and rigidity of constructs. Interbody grafts or cages placed asymmetrically can be used to obtain correction, allowing the hardware to be in neutral and decreasing the risk of hardware failure.92
Interspinous Spacers
Interspinous spacers such as the X-Stop (Medtronic, Memphis, TN) are indicated for treatment of spinal stenosis in the absence of deformity. In the U.S. Food and Drug Administration (FDA) studies, scoliosis was an exclusion criterion. It has been suggested that these spacers may be used in an off-label manner for the treatment of stenotic symptoms in the presence of deformity.93 The author has used interspinous spacers in rare cases of patients with severe medical comorbidities and significant deformity who would not tolerate traditional surgery. The results have been mixed, but there have been few complications. Further studies are warranted.
Osteotomies
The simplest osteotomies to perform are facet resection osteotomies as described by Ponte or Smith-Petersen. There is confusion as to nomenclature of these osteotomies. Smith-Petersen et al.94 described a procedure where the facet was resected and the disc released leading to a pivot at the dorsal corner of the vertebral body, causing closure of the osteotomy dorsally and extension through the disc space ventrally. This procedure was originally described in ankylosing spondylitis. The more common facet resection and closure through a mobile disc was first described by Wilson and Turkell95 but has been widely attributed to Ponte.96 For clarity, the author uses facet resection osteotomy.
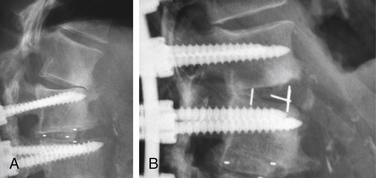
These osteotomies can be used anywhere there is a mobile disc. Correction of 5 to 10 degrees of kyphosis can readily be obtained, and multiple levels can be used.97,98 Some coronal correction can be obtained as well. Facet resections can also be used to increase the correction of the coronal deformity. A facet resection osteotomy augmented by an interbody fusion can increase the amount of correction obtained through this technique. An example is shown in Figure 98-2.
If larger degrees of correction are required, a pedicle subtraction osteotomy can be considered. This is a closing wedge osteotomy performed by removing the posterior elements of the pedicle and a portion of the vertebral body. First described by Scudese and Colabro,99 this is a very powerful technique that allows routine correction of 30 degrees or more.97,98 It has typically been performed at lumbar levels100–102 but can be performed safely in the thoracic spine as well.103,104 These procedures are technically demanding and associated with significant complications.105–107 Clinical results are very good. Biplanar correction can also be achieved allowing correction of deformity in more than one plane.100–102
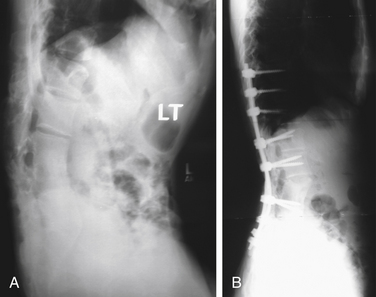
Two basic types of pedicle subtraction osteotomy have been described. In the first type, osteotomes are used to create a wedge, which is then removed. The alternative procedure is a decancellation osteotomy. In this procedure, the vertebra is decancellized, the dorsal wall is reduced into the cavity, the lateral wall is osteotomized, and the osteotomy is closed.108 No comparison studies of these two techniques exist. Figure 98-3 shows the preoperative and postoperative radiographs of a patient with a posttraumatic kyphosis treated with a pedicle subtraction osteotomy to restore lumbar lordosis.
Vertebral Column Resection
In some very complex high-magnitude deformities, complete resection of one or more vertebral segments may be required to correct deformity. This procedure is called a vertebal column resection. It can be done through a combined anteroposterior or a dorsal-only approach.97,102,109,110 This technique may be used in an apical kyphectomy for spina bifida.111 This procedure can be used for both kyphosis and scoliosis and may be used to obtain biplanar correction. In some cases, the anterior column is reconstructed with a graft or cage implant; in other cases, the spinal column is shortened. These procedures are also associated with significant risks. Reasonably good clinical results have been reported.97,101,109,112–115
Plans
In the preoperative period, steps should be taken to ensure the patient is optimally prepared for surgery. The author considers smoking cessation to be mandatory for all such procedures. Preoperative consultation with a hematologist for blood management may help optimize hemoglobin before surgery.116 Studies have suggested an increased risk of thrombotic complications with the use of erythropoietin analogues, so the risks and benefits must be balanced.117,118 Preoperative medical cardiology and pulmonology consultations should be obtained as indicated.8,17
If a combined anteroposterior approach is being considered, one must decide whether to use a single-day or staged multiday approach. Single-day procedures have the advantage of only a single anesthetic and recovery period but can result in very long procedures with excessive blood loss. Single-day procedures may also be more demanding on the surgeon. Staged procedures may be less physically demanding for the patient and the surgeon. Studies that have compared single-day and multiday approaches showed no clear benefit of one over the other.119–124 It is the author’s practice to do most of these procedures as a single-day surgery but to stage them if the procedure is particularly complex or complications arise.
Neurologic monitoring should be considered for all of these procedures. At the author’s institution, somatosensory-evoked and motor-evoked potentials are used for all spinal deformity cases. Stimulated electromyographic monitoring is used for minimal access lateral lumbar approaches. Neurologic monitoring has been shown to decrease the risk of neurologic injury.125,126 If motor-evoked potentials are to be used, this should be communicated to the anesthesiologist to ensure that neuromuscular blockade is not used during the procedure.
Intraoperative red blood cell salvage is routinely used in complex surgery to reduce the use of autologous blood donation.127–129
Intraoperative imaging is facilitated by the use of a radiolucent table. Radiographs obtained intraoperatively in both sagittal and coronal planes allow an estimate of correction obtained and implant placement. Intraoperative fluoroscopy may be used to guide implant placement. CT-based navigation systems have been shown to increase accuracy of screw placement, particularly in significant deformities.130,131 Clinically, however, freehand placement of pedicle screws has been shown to be safe and effective.132–134 The author prefers to use freehand techniques for placement of pedicle screws in most primary cases and to use fluoroscopy or navigation in complex or revision instrumentation.
Numerous techniques have been described for determining the magnitude of angle needed to be corrected in the sagittal plane to restore balance. Perhaps the simplest way to do this is to cut a 3-foot film at the level of the planned osteotomy, balance the head over the pelvis, and measure the subtended angle. With the advent of digital radiography, printed 3-foot films are becoming rare, and this is no longer as good an option. A second option is to measure the angle subtended between a vertical line at the pivot point of the planned osteotomy and either the C7 or the C1 vertebral body. An osteotomy higher in the lumbar spine requires a greater angle of correction for a given amount of linear translation of the head.135 Ondra et al.135–137 described two mathematical models for determining osteotomy correction. Although these models are effective, the author finds them cumbersome to use in clinical practice.
Outcomes of Complex Surgery for Adult Deformity
Yadla et al.138 performed a systematic review of outcomes of surgery for lumbar scoliosis. They showed that at a minimum 2-year follow-up there was consistent improvement in radiographic and clinical outcomes. The Oswestry Disability Index (ODI) showed an average 15.7 decrease. The Scoliosis Research Society (SRS)-30 showed a mean postoperative decrease of 23.1. These authors showed a relatively high (40%) complication rate.
Using a prospectively collected database, Daubs et al.139 analyzed 46 patients older than 60 years who underwent a procedure in the thoracic or lumbar spine with more than five levels. Average ODI scores improved from 49 to 25 for a 49% improvement. The overall complication rate was 37%, with 20% of complications being defined as major.
In a prospective cohort study, Alpert et al.140 used the 36-item Short Form Health Survey (SF-36) to assess 68 adults undergoing surgery for spinal deformity. These authors showed significant increases in physical function, social function, bodily pain, and perceived health change. They did not show a difference comparing patients older than 40 years with younger patients, and there was no difference in outcome observed in patients with complications.
In a matched cohort analysis, Glassman et al.141 compared patients with major complications, minor complications, and no complications. They noted no difference in scores on the SRS, SF-36, ODI, or visual analogue scale. There was a decrease in general health (12-item Short Form Health Survey [SF-12]) at 1 year for the group with major complications.
Li et al.142 performed a retrospective case-control study of 83 patients older than 65 years with scoliosis. Of these patients, 34 underwent surgery, and 49 were managed nonoperatively. The patients managed operatively were noted to have significantly less pain, better health-related quality of life, and better self-image and were more satisfied with treatment compared with conservatively treated patients. There was no difference in ODI or the physical and mental components of SF-12. The magnitude of preoperative deformity was not predictive of whether operative or nonoperative treatment was performed.
Two further studies have looked at operative versus nonoperative care. In a retrospective analysis of 55 patients, Kluba and Dikmenli143 showed that 24 patients who underwent operation had more significant pathology and symptoms preoperatively. At an average of 4 years postoperatively, surgical patients had better activity levels and less analgesic use but no difference in axial back pain. Two articles from the Spinal Deformity Study Group used prospectively collected data on nonmatched cohorts to look at leg pain and disability144 and quality of life145 and showed that at 2 years the operative patients were better off than the nonoperative patients despite having more disability, leg pain, and lower quality of life preoperatively.
Smith et al.146 performed a risk benefit analysis based on patient age for surgical treatment of adult scoliosis. They showed that although the risks were higher for older patients, these patients had a disproportionately greater improvement in pain and function.
Bess S., Boachie-Adjei O., Burton D., et al. Pain and disability determine treatment modality for older patients with adult scoliosis, while deformity guides treatment for younger patients. Spine (Phila Pa 1976). 2009;34:2186-2190.
Bridwell K.H. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine (Phila Pa 1976). 2006;31(Suppl 19):S171-S178.
Bridwell K.H., Glassman S., Horton W., et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa 1976). 2009;34:2171-2178.
Glassman S.D., Bridwell K., Dimar J.R., et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30:2024-2029.
Hu S.S. Internal fixation in the osteoporotic spine. Spine (Phila Pa 1976). 1997;22(Suppl 24):43S-48S.
Mok J.M., Hu S.S. Surgical strategies and choosing levels for spinal deformity: how high, how low, front and back. Neurosurg Clin N Am. 2007;18:329-337.
Pekmezci M., Berven S.H., Hu S.S., et al. The factors that play a role in the decision-making process of adult deformity patients. Spine (Phila Pa 1976). 2009;34:813-817.
Yadla S., Maltenford M.G., Ratliff J.K., et al. Adult scoliosis surgery: a systematic review. Neurosurg Focus. 2010;28:E3.
1. Kuntz C.4th, Levin L.S., Ondra S.L., et al. Neutral upright sagittal spinal alignment from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104-112.
2. Vialle R., Levassor N., Rillardon L., et al. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg [Am]. 2005;87:260-267.
3. Roussouly P., Gollogly S. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976). 2005;30:346-353.
4. Bess S., Boachie-Adjei O., Burton D., et al. Pain and disability determine treatment modality for older patients with adult scoliosis, while deformity guides treatment for younger patients. Spine (Phila Pa 1976). 2009;34:2186-2190.
5. Weinstein S.L. Idiopathic scoliosis natural history. Spine (Phila Pa 1976). 1986;11:780-783.
6. Ploumis A., Liu H., Mehbod A.A., et al. A correlation of radiographic and functional measurements in adult degenerative scoliosis. Spine (Phila Pa 1976). 2009;34:1581-1584.
7. Glassman S.D., Bridwell K., Dimar J.R., et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30:2024-2029.
8. Hu S.S., Berven S. Preparing the adult deformity patient for spinal surgery. Spine (Phila Pa 1976). 2006;31(Suppl 19):S126-S131.
9. Stambough J.L., Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992;5:277-285.
10. Klein J.D., Hey L.A., Yu C.S., et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine (Phila Pa 1976). 1996;21:2676-2682.
11. DeWald C.J., Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976). 2006;31(Suppl 19):S144-S151.
12. Glassman S.D., Alegre G.M. Adult spinal deformity in the osteoporotic spine: options and pitfalls. AAOS Instr Course Lect. 2003;52:579-588.
13. Hayirlioglu A., Gokaslan H., Cimsit C., et al. The importance of severity of arthrosis for the reliability of bone mineral density measurement in women. Rheumatol Int. 2009;29:371-375.
14. Pappou I.P., Girardi F.P., Sandhu H.S., et al. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa 1976). 2006;31:1614-1620.
15. Plehwe W.E., Carey R.P. Spinal surgery and severe vitamin D deficiency. Med J Aust. 2002;176:438-439.
16. Dipaola C.P., Bible J.E., Biswas D., et al. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J. 2009;9:537-544.
17. Hall J.E. Preoperative assessment of the patient with a spinal deformity. AAOS Instr Course Lect. 1985;34:127-139.
18. Marty-Poumarat C., Scattin L., Marpeau M., et al. Natural history of progressive adult scoliosis. Spine (Phila Pa 1976). 2007;32:1227-1234.
19. Edgar M.A. The natural history of unfused scoliosis. Orthopedics. 1987;10:931-939.
20. Djurasovic M., Glassman S.D. Correlation of radiographic and clinical findings in spinal deformities. Neurosurg Clin N Am. 2007;18:223-227.
21. Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925-948.
22. Pekmezci M., Berven S.H., Hu S.S., et al. The factors that play a role in the decision-making process of adult deformity patients. Spine (Phila Pa 1976). 2009;34:813-817.
23. Heary R.F., Kumar S. Decision making in adult deformity. Neurosurgery. 2008;63(Suppl 3):69-77.
24. Schwab F.J., Lafage V. Predicting outcome and complications in the surgical treatment of adult scoliosis. Spine (Phila Pa 1976). 2008;33:2243-2247.
25. Glassman S.D., Berven S., Bridwell K., et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976). 2005;30:682-688.
26. Wilson P.L., Newton P.O., Wenger D.R., et al. A multicenter study analyzing the relationship of a standardized radiographic scoring system of adolescent idiopathic scoliosis and the Scoliosis Research Society outcomes instrument. Spine (Phila Pa 1976). 2002;27:2036-2040.
27. Gilad R., Gandhi C.D., Arginteanu M.S., et al. Uncorrected sagittal plane imbalance predisposes to symptomatic instrumentation failure. Spine J. 2008;8:911-917.
28. Daffner S.D., Vaccaro A.R. Adult degenerative lumbar scoliosis. Am J Orthop. 2003;32:77-82.
29. Hu S.S. Internal fixation in the osteoporotic spine. Spine (Phila Pa 1976). 1997;22(Suppl 24):43S-48S.
30. Burval D.J., McLain R.F. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine (Phila Pa 1976). 2007;32:1077-1083.
31. Soshi S., Shiba R., Kondo H., et al. An experimental study on transpedicular screw fixation in relation to osteoporosis of the lumbar spine. Spine (Phila Pa 1976). 1991;16:1335-1341.
32. Kumano K., Hirabayashi S., Ogawa Y., et al. Pedicle screws and bone mineral density. Spine (Phila Pa 1976). 1994;19:1157-1161.
33. Lehman R.A.Jr., Dmitriev A.E., Cardoso M.J., et al. Effect of teriparatide [rhPTH(1,34)] and calcitonin on intertransverse process fusion in a rabbit model. Spine (Phila Pa 1976). 2010;35:146-152.
34. Abe Y., Takahata M. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1-34) in a rat spinal arthrodesis model. Bone. 2007;41:775-785.
35. Liu H., Ishihara H., Kanamori M., et al. Characteristics of nerve root compression caused by degenerative lumbar spinal stenosis with scoliosis. Spine J. 2003;3:524-529.
36. Frazier D.D., Lipson S.J., Fossel A.H., et al. Associations between spinal deformity and outcomes after decompression for spinal stenosis. Spine (Phila Pa 1976). 1997;22:2025-2029.
37. San Martino A., D’Andria F.M., San Martino C., et al. The surgical treatment of nerve root compression caused by scoliosis of the lumbar spine. Spine (Phila Pa 1976). 1983;8:261-265.
38. Hansraj K.K., Cammisa F.P.Jr., O’Leary P.F., et al. Decompressive surgery for typical lumbar spinal stenosis. Clin Orthop Relat Res. 2001;384:10-17.
39. Toyoda H., Nakamura H., Konishi S., et al. Clinical outcome of microsurgical bilateral decompression via unilateral approach for lumbar canal stenosis: minimum five-year follow-up. Spine (Phila Pa 1976). 2011;36:410-415.
40. Kelleher M.O., Timlin M., Persaud O., et al. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine (Phila Pa 1976). 2010;35:E981-E987.
41. Lavelle W, Evanchick K, Orr RD: Clinical outcome for limited transforaminal lumbar interbody fusion. Proceedings of the Lumbar Spine Research Society Meeting, Chicago, April 2009.
42. Hwang D.W., Jeon S.H., Kim J.W., et al. Radiographic progression of degenerative lumbar scoliosis after short segment decompression and fusion. Asian Spine J. 2009;3:58-65.
43. Cho K.J., Suk S.I., Park S.R., et al. Short fusion versus long fusion for degenerative lumbar scoliosis. Eur Spine J. 2008;17:650-656.
44. Weidenbaum M. Considerations for focused surgical intervention in the presence of adult spinal deformity. Spine (Phila Pa 1976). 2006;31(Suppl 19):S139-S143.
45. Protopsaltis T.S., Patel A., Lonner B., Berdo S.A., et al. Fellowship and practice composition impact surgical decision making in patients with degenerative scoliosis: spinal deformity versus degenerative spine surgeons. Spine J. 2010;10(Suppl 9):S5-S6.
46. Watanabe K., Lenke L.G., Bridwell K.H., et al. Comparison of radiographic outcomes for the treatment of scoliotic curves greater than 100 degrees: wires versus hooks versus screws. Spine (Phila Pa 1976). 2008;33:1084-1092.
47. Koptan W.M., Elmiligui Y.H., Elsebaie H.B. All pedicle screw instrumentation for Scheuermann’s kyphosis correction: is it worth it? Spine J. 2009;9:296-302.
48. Kim Y.J., Lenke L.G., Cho S.K., et al. Comparative analysis of pedicle screw versus hook instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2004;29:2040-2048.
49. Liljenqvist U., Lepsien U., Hackenberg L., et al. Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur Spine J. 2002;11:336-343.
50. Suk S.I., Lee C.K., Min H.J., et al. Comparison of Cotrel-Dubousset pedicle screws and hooks in the treatment of idiopathic scoliosis. Int Orthop. 1994;18:341-346.
51. Cho W., Cho S.K., Wu C. The biomechanics of pedicle screw-based instrumentation. J Bone Joint Surg [Br]. 2010;92:1061-1065.
52. Tay B.B., Berven S. Indications, techniques, and complications of lumbar interbody fusion. Semin Neurol. 2002;22:221-230.
53. Cunningham B.W., Polly D.W.Jr. The use of interbody cage devices for spinal deformity: a biomechanical perspective. Clin Orthop Relat Res. 2002;394:73-83.
54. Watters W.C.3rd, Bono C.M. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9:609-614.
55. Lee C.K., Langrana N.A. Lumbosacral spinal fusion: a biomechanical study. Spine (Phila Pa 1976). 1984;9:574-581.
56. Mulconrey D.S., Bridwell K.H., Flynn J., et al. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine (Phila Pa 1976). 2008;33:2153-2159.
57. Luhmann S.J., Bridwell K.H., Cheng I., et al. Use of bone morphogenetic protein-2 for adult spinal deformity. Spine (Phila Pa 1976). 2005;30(Suppl 17):S110-S117.
58. Maeda T., Buchowski J.M., Kim Y.J., et al. Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine (Phila Pa 1976). 2009;34:2205-2212.
59. Mok J.M., Hu S.S. Surgical strategies and choosing levels for spinal deformity: how high, how low, front and back. Neurosurg Clin N Am. 2007;18:329-337.
60. Kuklo T.R. Principles for selecting fusion levels in adult spinal deformity with particular attention to lumbar curves and double major curves. Spine (Phila Pa 1976). 2006;31(Suppl 19):S132-S138.
61. Kuhns C.A., Bridwell K.H., Lenke L.G., et al. Thoracolumbar deformity arthrodesis stopping at L5: fate of the L5-S1 disc, minimum 5-year follow-up. Spine (Phila Pa 1976). 2007;32:2771-2776.
62. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976). 2006;31:2329-2336.
63. Edwards C.C.2nd, Bridwell K.H., Patel A., et al. Long adult deformity fusions to L5 and the sacrum: a matched cohort analysis. Spine (Phila Pa 1976). 2004;29:1996-2005.
64. Swamy G., Berven S.H., Bradford D.S. The selection of L5 versus S1 in long fusions for adult idiopathic scoliosis. Neurosurg Clin N Am. 2007;18:281-288.
65. Kostuik J.P., Musha Y. Extension to the sacrum of previous adolescent scoliosis fusions in adult life. Clin Orthop Relat Res. 1999;364:53-60.
66. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Is the T9, T11, or L1 the more reliable proximal level after adult lumbar or lumbosacral instrumented fusion to L5 or S1? Spine (Phila Pa 1976). 2007;32:2653-2661.
67. Kim J.H., Kim S.S., Suk S.I. Incidence of proximal adjacent failure in adult lumbar deformity correction based on proximal fusion level. Asian Spine J. 2007;1:19-26.
68. Emami A., Deviren V., Berven S., et al. Outcome and complications of long fusions to the sacrum in adult spine deformity: Luque-Galveston, combined iliac and sacral screws, and sacral fixation. Spine (Phila Pa 1976). 2002;27:776-786.
69. Santos E.R., Rosner M.K., Perra J.H., et al. Spinopelvic fixation in deformity: a review. Neurosurg Clin N Am. 2007;18:373-384.
70. Crandall D.G., Revella J. Transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion as an adjunct to posterior instrumented correction of degenerative lumbar scoliosis: three year clinical and radiographic outcomes. Spine (Phila Pa 1976). 2009;34:2126-2133.
71. Lippman C.R., Spence C.A., Youssef A.S., et al. Correction of adult scoliosis via a posterior-only approach. Neurosurg Focus. 2003;14:e5.
72. McCord D.H., Cunningham B.W., Shono Y., et al. Biomechanical analysis of lumbosacral fixation. Spine (Phila Pa 1976). 1992;17(Suppl 8):S235-S243.
73. Tsuchiya K., Bridwell K.H., Kuklo T.R., et al. Minimum 5-year analysis of L5-S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine (Phila Pa 1976). 2006;31:303-308.
74. Allen B.L.Jr., Ferguson R.L. The Galveston technique of pelvic fixation with L-rod instrumentation of the spine. Spine (Phila Pa 1976). 1984;9:388-394.
75. Kasten M.D., Rao L.A., Priest B. Long-term results of iliac wing fixation below extensive fusions in ambulatory adult patients with spinal disorders. J Spinal Disord Tech. 2010;23:e37-e42.
76. Chang T.L., Sponseller P.D., Kebaish K.M., et al. Low profile pelvic fixation: anatomic parameters for sacral alar-iliac fixation versus traditional iliac fixation. Spine (Phila Pa 1976). 2009;34:436-440.
77. Glazer P.A., Colliou O., Lotz J.C., et al. Biomechanical analysis of lumbosacral fixation. Spine (Phila Pa 1976). 1996;21:1211-1222.
78. Berven S.H., Deviren V., Smith J.A., et al. Management of fixed sagittal deformity: outcome of combined anterior and posterior surgery. Spine (Phila Pa 1976). 2003;28:1710-1715.
79. Khan S.N., Hofer M.A., Gupta M.C. Lumbar degenerative scoliosis: outcomes of combined anterior and posterior pelvis surgery with minimum 2-year follow-up. Orthopedics. 32(4), 2009.
80. Kim Y.B., Lenke L.G., Kim Y.J., et al. Surgical treatment of adult scoliosis: is anterior apical release and fusion necessary for the lumbar curve? Spine (Phila Pa 1976). 2008;33:1125-1132.
81. Suk S.I., Kim J.H., Cho K.J., et al. Is anterior release necessary in severe scoliosis treated by posterior segmental pedicle screw fixation? Eur Spine J. 2007;16:1359-1365.
82. Burton D.C., Sama A.A., Asher M.A., et al. The treatment of large (>70 degrees) thoracic idiopathic scoliosis curves with posterior instrumentation and arthrodesis: when is anterior release indicated? Spine (Phila Pa 1976). 2005;30:1979-1984.
83. Kim Y.B., Lenke L.G. The morbidity of an anterior thoracolumbar approach: adult spinal deformity patients with greater than five-year follow-up. Spine (Phila Pa 1976). 2009;34:822-826.
84. Dakwar E., Cardona R.F., Smith D.A., et al. Early outcomes and safety of the minimally invasive lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28:E8.
85. Anand N., Baron E.M., Thaiyananthan G., et al. Minimally invasive multilevel percutaneous correction and fusion for adult degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21:459-467.
86. Tormenti M.J., Maserati M.B., Bonfield C.M., et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7.
87. Benglis D.M., Vanni S., Levi A.D. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139-144.
88. Regev G.J., Chen L., Dhawan M., et al. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976). 2009;34(12):1330-1335.
89. Kostuik J.P., Shapiro M.B. Open surgical treatment of osteoporotic fractures and deformity of the spine. AAOS Instr Course Lect. 2003;52:569-578.
90. Hasegawa K., Takahashi H.E. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine (Phila Pa 1976). 1997;22:958-962.
91. Cunningham B.W., Polly D.W.Jr. The use of interbody cage devices for spinal deformity: a biomechanical perspective. Clin Orthop Relat Res. 2002;394:73-83.
92. Heary R.F., Karimi R.J. Correction of lumbar coronal plane deformity using unilateral cage placement. Neurosurg Focus. 2010;28:E10.
93. Rolfe K.W., Zucherman J.F., Kondrashov D.G., et al. Scoliosis and interspinous decompression with the X-STOP: prospective minimum 1-year outcomes in lumbar spinal stenosis. Spine J. 2010;10:972-978.
94. Smith-Petersen M.N., Larson C.B., Aufranc O.E. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. J Bone Joint Surg [Br]. 1945. 271–11
95. Wilson M.J., Turkell J.H. Multiple wedge spinal osteotomy: its use in Marie Strumpel spondylitis. Am J Surg. 1949;77:777-782.
96. Geck M.J., Macagno A., Ponte A., et al. The Ponte procedure: posterior only treatment of Scheuermann’s kyphosis using segmental posterior shortening and pedicle screw instrumentation. J Spinal Disord Tech. 2007;20:586-593.
97. Bridwell K.H. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine (Phila Pa 1976). 2006;31(Suppl 19):S171-S178.
98. Cho K.J., Bridwell K.H., Lenke L.G., et al. Comparison of Smith-Petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine (Phila Pa 1976). 2005;30:2030-2037.
99. Scudese V.A., Colabro J.J. Vertebral wedge osteotomy: correction of rheumatoid (ankylosing) spondylitis. JAMA. 1963;186:627-631.
100. Dorward I.G., Lenke L.G. Osteotomies in the posterior-only treatment of complex adult spinal deformity: a comparative review. Neurosurg Focus. 2010;28:E4.
101. Gill J.B., Levin A., Burd T., et al. Corrective osteotomies in spine surgery. J Bone Joint Surg [Am]. 2008;90:2509-2520.
102. Boachie-Adjei O. Role and technique of eggshell osteotomies and vertebral column resections in the treatment of fixed sagittal imbalance. AAOS Instr Course Lect. 2006;55:583-589.
103. O’Shaughnessy B.A., Kuklo T.R., Hsieh P.C., et al. Thoracic pedicle subtraction osteotomy for fixed sagittal spinal deformity. Spine (Phila Pa 1976). 2009;34:2893-2899.
104. Chang K.W., Chen Y.Y., Lin C.C., et al. Apical lordosating osteotomy and minimal segment fixation for the treatment of thoracic or thoracolumbar osteoporotic kyphosis. Spine (Phila Pa 1976). 2005;30:1674-1681.
105. Buchowski J.M., Bridwell K.H., Lenke L.G., et al. Neurologic complications of lumbar pedicle subtraction osteotomy: a 10-year assessment. Spine (Phila Pa 1976). 2007;32:2245-2252.
106. Murrey D.B., Brigham C.D., Kiebzak G.M., et al. Transpedicular decompression and pedicle subtraction osteotomy (eggshell procedure): a retrospective review of 59 patients. Spine (Phila Pa 1976). 2002;27:2338-2345.
107. Willems K.F., Slot G.H., Anderson P.G., et al. Spinal osteotomy in patients with ankylosing spondylitis: complications during first postoperative year. Spine (Phila Pa 1976). 2005;30:101-107.
108. Bridwell K.H., Lewis S.J. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance: surgical technique. J Bone Joint Surg [Am]. 2004;86(Suppl 1):44-50.
109. Lenke L.G., Sides B.A., Koester L.A., et al. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468:687-699.
110. Pappou I.P., Papadopoulos E.C., Swanson A.N., et al. Pott disease in the thoracolumbar spine with marked kyphosis and progressive paraplegia necessitating posterior vertebral column resection and anterior reconstruction with a cage. Spine (Phila Pa 1976). 2006;31:E123-E127.
111. Karlin L.I. Kyphectomy for myelodysplasia. Neurosurg Clin N Am. 2007;18:357-364.
112. Wang Y., Zhang Y. Posterior-only multilevel modified vertebral column resection for extremely severe Pott’s kyphotic deformity. Eur Spine J. 2009;18:1436-1441.
113. Smith J.S., Wang V.Y., Ames C.P. Vertebral column resection for rigid spinal deformity. Neurosurgery. 2008;63(Suppl 3):177-182.
114. Wang Y., Zhang Y., Zhang X., et al. A single posterior approach for multilevel modified vertebral column resection in adults with severe rigid congenital kyphoscoliosis: a retrospective study of 13 cases. Eur Spine J. 2008;17:361-372.
115. Suk S.I., Chung E.R., Lee S.M., et al. Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976). 2005;30:E703-E710.
116. Tate D.E.Jr., Friedman R.J. Blood conservation in spinal surgery: review of current techniques. Spine (Phila Pa 1976). 1992;17:1450-1456.
117. Colomina M.J., Bago J., Pellise F., et al. Preoperative erythropoietin in spine surgery. Eur Spine J. 2004;13(Suppl 1):S40-S49.
118. Stowell C.P., Jones S.C., Enny C., et al. An open-label, randomized, parallel-group study of perioperative epoetin alfa versus standard of care for blood conservation in major elective spinal surgery: safety analysis. Spine (Phila Pa 1976). 2009;34:2479-2485.
119. Shufflebarger H.L., Grimm J.O., Bui V., et al. Anterior and posterior spinal fusion: staged versus same-day surgery. Spine (Phila Pa 1976). 1991;16:930-933.
120. Wright N. Single-surgeon simultaneous versus staged anterior and posterior spinal reconstruction: a comparative study. J Spinal Disord Tech. 2005;18(Suppl):S48-S57.
121. Rhee J.M., Bridwell K.H., Lenke L.G., et al. Staged posterior surgery for severe adult spinal deformity. Spine (Phila Pa 1976). 2003;28:2116-2121.
122. Dick J., Boachie-Adjei O., Wilson M. One-stage versus two-stage anterior and posterior spinal reconstruction in adults: comparison of outcomes including nutritional status, complication rates, hospital costs, and other factors. Spine (Phila Pa 1976). 1992;17(Suppl 8):S310-S316.
123. Powell E.T.4th, Krengel W.F.3rd, King H.A., et al. Comparison of same-day sequential anterior and posterior spinal fusion with delayed two-stage anterior and posterior spinal fusion. Spine (Phila Pa 1976). 1994;19:1256-1259.
124. Johnson J.R., Holt R.T. Combined use of anterior and posterior surgery for adult scoliosis. Orthop Clin North Am. 1988;19:361-370.
125. Fehlings M.G., Brodke D.S., Norvell D.C., et al. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976). 2010;35(Suppl 9):S37-S46.
126. Gonzalez A.A., Jeyanandarajan D., Hansen C., et al. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 27, 2009. E6
127. Elgafy H., Bransford R.J., McGuire R.A., et al. Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine (Phila Pa 1976). 2010;35(Suppl 9):S47-S56.
128. Gause P.R., Siska P.A., Westrick E.R., et al. Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine (Phila Pa 1976). 2008;33:571-575.
129. Reitman C.A., Watters W.C. The Cell Saver in adult lumbar fusion surgery: a cost-benefit outcomes study. Spine (Phila Pa 1976). 2004;29:1580-1583.
130. Tian N.F., Huang Q.S., Zhou P., et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J. 2011;20:846-859.
131. Tormenti M.J., Kostov D.B., Gardner P.A., et al. Intraoperative computed tomography image-guided navigation for posterior thoracolumbar spinal instrumentation in spinal deformity surgery. Neurosurg Focus. 2010;28:E11.
132. Modi H.N., Suh S.W., Hong J.Y., et al. Accuracy of thoracic pedicle screw using ideal pedicle entry point in severe scoliosis. Clin Orthop Relat Res. 2010;468:1830-1837.
133. Bergeson R.K., Schwend R.M., DeLucia T., et al. How accurately do novice surgeons place thoracic pedicle screws with the free hand technique? Spine (Phila Pa 1976). 2008;33:E501-E507.
134. Kim Y.W., Lenke L.G., Kim Y.J., et al. Free-hand pedicle screw placement during revision spinal surgery: analysis of 552 screws. Spine (Phila Pa 1976). 2008;33:1141-1148.
135. Van Royen B.J., De Gast A., Smit T.H. Deformity planning for sagittal plane corrective osteotomies of the spine in ankylosing spondylitis. Eur Spine J. 2000;9:492-498.
136. Yang B.P., Chen L.A., Ondra S.L. A novel mathematical model of the sagittal spine: application to pedicle subtraction osteotomy for correction of fixed sagittal deformity. Spine J. 2008;8:359-366.
137. Ondra S.L., Marzouk S., Koski T., et al. Mathematical calculation of pedicle subtraction osteotomy size to allow precision correction of fixed sagittal deformity. Spine (Phila Pa 1976). 2006;31:E973-E979.
138. Yadla S., Maltenford M.G., Ratliff J.K., et al. Adult scoliosis surgery: a systematic review. Neurosurg Focus. 2010;28:E3.
139. Daubs M.D., Lenke L.G., Cheh G., et al. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976). 2007;32:2238-2244.
140. Alpert T.J., Purtill J., Mesa J., et al. Health outcome assessment before and after adult deformity surgery: a prospective study. Spine (Phila Pa 1976). 1995;20:2002-2004.
141. Glassman S.D., Hamill C.L., Bridwell K.H., et al. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976). 2007;32:2764-2770.
142. Li G., Passias P., Kozanek M., et al. Adult scoliosis in patients over sixty-five years of age: outcomes of operative versus nonoperative treatment at minimum 2 years follow-up. Spine (Phila Pa 1976). 2009;34:2165-2170.
143. Kluba T., Dikmenli G. Comparison of surgical and conservative treatment for degenerative lumbar scoliosis. Arch Orthop Trauma Surg. 2009;129:1-5.
144. Smith J.S., Shaffrey C.I., Berven S., et al. Operative versus nonoperative treatment of leg pain in adults with scoliosis: a retrospective review of a prospective multicenter database with two-year follow-up. Spine (Phila Pa 1976). 2009;34:1693-1698.
145. Bridwell K.H., Glassman S., Horton W., et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa 1976). 2009;34:2171-2178.
146. Smith J.S., Polly D.W.Jr., Sansur C.A., et al. Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine (Phila Pa 1976). 2010;35:2140-2149.