Jang, KM, et al. Added value of diffusion-weighted MR imaging in the diagnosis of ampullary carcinoma. Radiology. 2013; 266(2):491–501.
Westgaard, A, et al. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol. 2013; 20(2):430–439.
Chung, YE, et al. Differentiation of benign and malignant ampullary obstructions on MR imaging. Eur J Radiol. 2011; 80(2):198–203.
Chung, YE, et al. Differential features of pancreatobiliary- and intestinal-type ampullary carcinomas at MR imaging. Radiology. 2010; 257(2):384–393.
Barauskas, G, et al. Tumor-related factors and patient’s age influence survival after resection for ampullary adenocarcinoma. J Hepatobiliary Pancreat Surg. 2008; 15(4):423–428.
Carter, JT, et al. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008; 207(2):210–218.
Chen, WX, et al. Multiple imaging techniques in the diagnosis of ampullary carcinoma. Hepatobiliary Pancreat Dis Int. 2008; 7(6):649–653.
O’Connell, JB, et al. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008; 15(7):1820–1827.
Schirmacher, P, et al. Ampullary adenocarcinoma – differentiation matters. BMC Cancer. 2008; 8:251.
Singh, S, et al. Palliative surgical bypass for unresectable periampullary carcinoma. Hepatobiliary Pancreat Dis Int. 2008; 7(3):308–312.
Smith, RA, et al. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008; 12(8):1422–1428.
Ahualli, J. The double duct sign. Radiology. 2007; 244(1):314–315.
Sugita, R, et al. Periampullary tumors: high-spatial-resolution MR imaging and histopathologic findings in ampullary region specimens. Radiology. 2004; 231(3):767–774.
Clements, WM, et al. Ampullary carcinoid tumors: rationale for an aggressive surgical approach. J Gastrointest Surg. 2003; 7(6):773–776.
Duffy, JP, et al. Improved survival for adenocarcinoma of the ampulla of Vater: fifty-five consecutive resections. Arch Surg. 2003; 138(9):941–948. [discussion 948-50].
Handra-Luca, A, et al. Adenomyoma and adenomyomatous hyperplasia of the Vaterian system: clinical, pathological, and new immunohistochemical features of 13 cases. Mod Pathol. 2003; 16(6):530–536.
Lindell, G, et al. Management of cancer of the ampulla of Vater: does local resection play a role? Dig Surg. 2003; 20(6):511–515.
Martin, JA, et al. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003; 13(4):649–669.
Nakano, K, et al. Combination therapy of resection and intraoperative radiation for patients with carcinomas of extrahepatic bile duct and ampulla of Vater: prognostic advantage over resection alone? Hepatogastroenterology. 2003; 50(52):928–933.
Smith, TR, et al. Prolapse of the common bile duct with small ampullary villous adenocarcinoma into third part of the duodenum. AJR Am J Roentgenol. 2003; 181(2):599–600.
Trimbath, JD, et al. Attenuated familial adenomatous polyposis presenting as ampullary adenocarcinoma. Gut. 2003; 52(6):903–904.
Irie, H, et al. MR imaging of ampullary carcinomas. J Comput Assist Tomogr. 2002; 26(5):711–717.
Jordan, PH, Jr., et al. Treatment of ampullary villous adenomas that may harbor carcinoma. J Gastrointest Surg. 2002; 6(5):770–775.
Kaiser, A, et al. The adenoma-carcinoma sequence applies to epithelial tumours of the papilla of Vater. Z Gastroenterol. 2002; 40(11):913–920.
Kim, JH, et al. Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics. 2002; 22(6):1335–1352.
Rodriguez, C, et al. How accurate is preoperative diagnosis by endoscopic biopsies in ampullary tumours? Rev Esp Enferm Dig. 2002; 94(10):585–592.
Skordilis, P, et al. Is endosonography an effective method for detection and local staging of the ampullary carcinoma? A prospective study. BMC Surg. 2002; 2(1):1.
Yoshida, T, et al. Hepatectomy for liver metastasis from ampullary cancer after pancreatoduodenectomy. Hepatogastroenterology. 2002; 49(43):247–248.
Eriguchi, N, et al. Carcinoma of the ampulla of Vater associated with other organ malignancies. Kurume Med J. 2001; 48(4):255–259.
Hirata, S, et al. Periampullary choledochoduodenal fistula in ampullary carcinoma. J Hepatobiliary Pancreat Surg. 2001; 8(2):179–181.
Nikfarjam, M, et al. Local resection of ampullary adenocarcinomas of the duodenum. ANZ J Surg. 2001; 71(9):529–533.
Wagle, PK, et al. Pancreaticoduodenectomy for periampullary carcinoma. Indian J Gastroenterol. 2001; 20(2):53–55.
Wittekind, C, et al. Adenoma of the papilla and ampulla—premalignant lesions? Langenbecks Arch Surg. 2001; 386(3):172–175.
Yeo, CJ, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998; 227(6):821–831.
Talamini, MA, et al. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg. 1997; 225(5):590–599. [discussion 599-600].
 Pancreatic duct (PD) obstructed in only ∼ 50%, and usually does not cause upstream pancreatic atrophy
Pancreatic duct (PD) obstructed in only ∼ 50%, and usually does not cause upstream pancreatic atrophy Tumors arising from duodenal epithelium (intestinal type): Prognosis comparable to duodenal carcinoma
Tumors arising from duodenal epithelium (intestinal type): Prognosis comparable to duodenal carcinoma
 Tumors arising from duodenal epithelium (intestinal type): Prognosis comparable to duodenal carcinoma
Tumors arising from duodenal epithelium (intestinal type): Prognosis comparable to duodenal carcinoma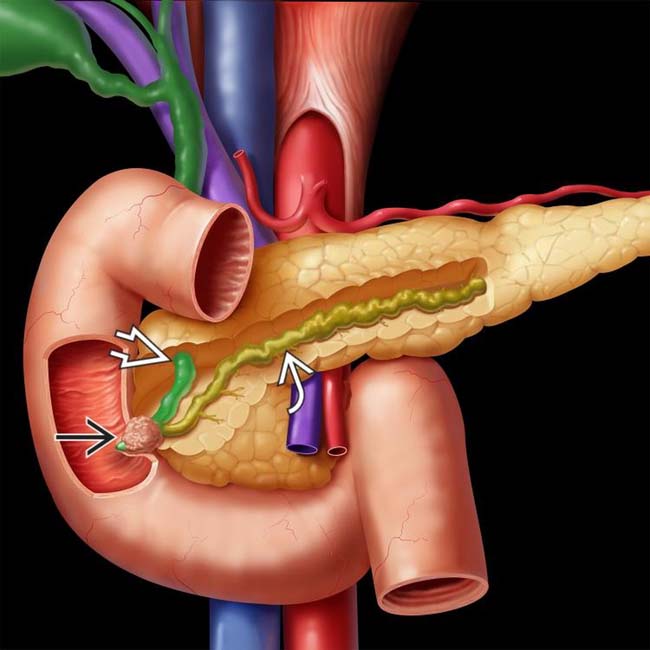
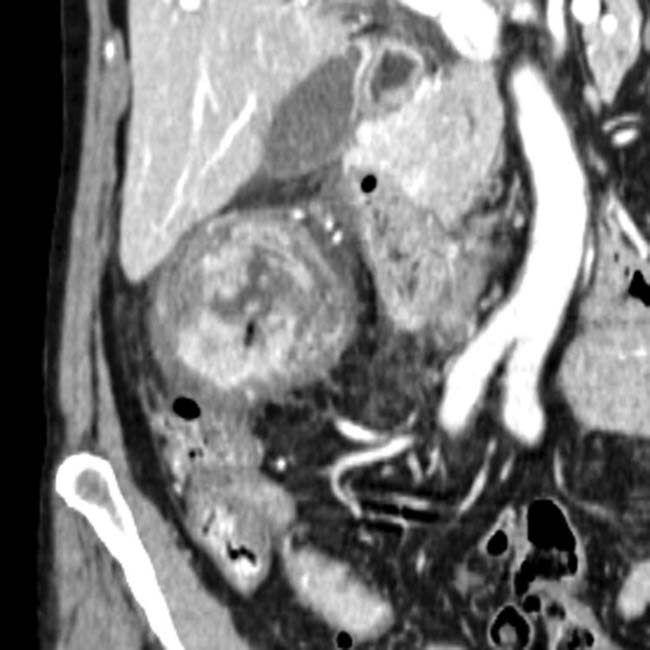
 causing obstruction of both the common bile duct (CBD)
causing obstruction of both the common bile duct (CBD)  and the pancreatic duct (PD)
and the pancreatic duct (PD) 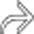 .
.
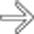 causing obstruction of the distal common duct at the ampulla. Patients with familial polyposis have a substantially increased risk of developing ampullary (and other) carcinomas. Lifelong surveillance is required to detect bowel, stomach, and biliary tumors.
causing obstruction of the distal common duct at the ampulla. Patients with familial polyposis have a substantially increased risk of developing ampullary (and other) carcinomas. Lifelong surveillance is required to detect bowel, stomach, and biliary tumors.
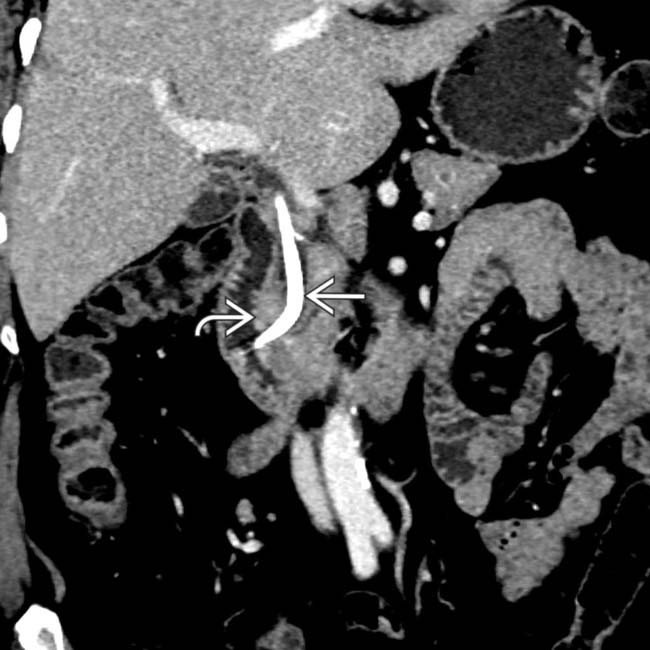
 , with obstruction of both the common bile duct
, with obstruction of both the common bile duct  and pancreatic duct
and pancreatic duct  . Note the abrupt occlusion of both ducts by the mass.
. Note the abrupt occlusion of both ducts by the mass.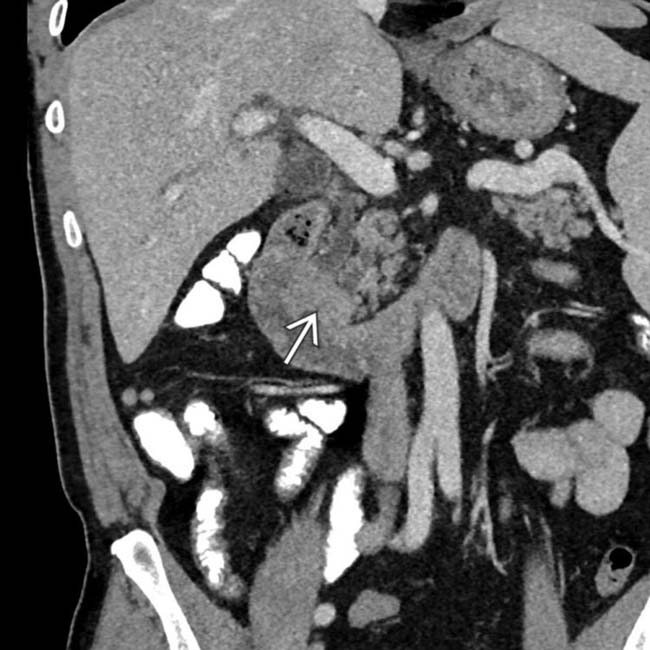
 centered at the ampulla obstructing the pancreatic and common bile ducts. While this was a pancreaticobiliary type lesion at histology, individual subtypes of ampullary carcinoma cannot be distinguished on imaging.
centered at the ampulla obstructing the pancreatic and common bile ducts. While this was a pancreaticobiliary type lesion at histology, individual subtypes of ampullary carcinoma cannot be distinguished on imaging.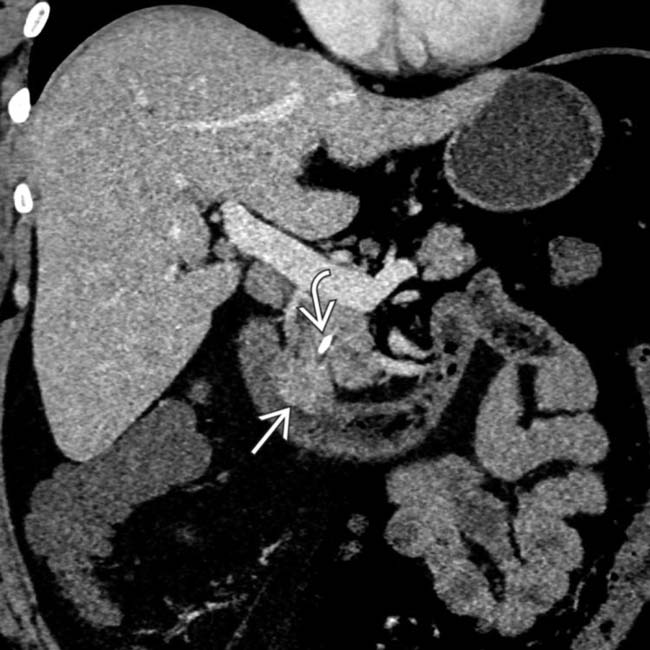
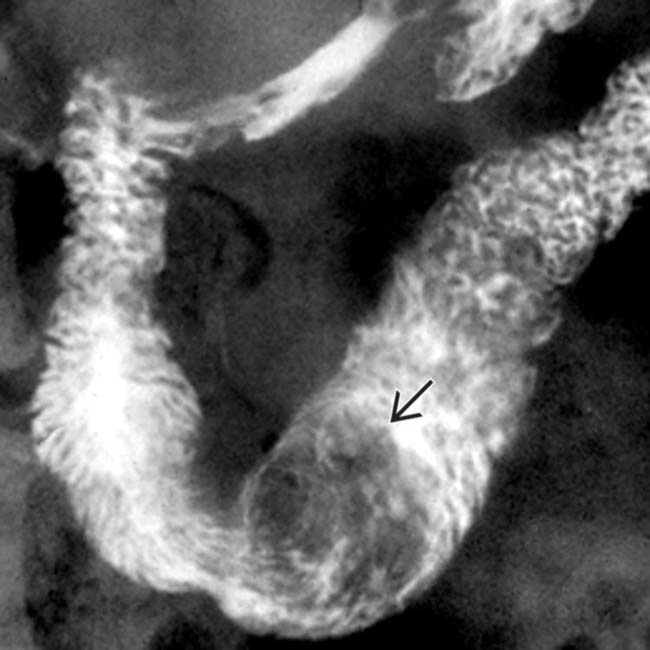
 centered at the ampulla, representing an ampullary carcinoma. A biliary stent
centered at the ampulla, representing an ampullary carcinoma. A biliary stent 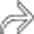 is partially visualized. The coronal plane is usually the best way of visualizing the ampulla and assessing a potential mass.
is partially visualized. The coronal plane is usually the best way of visualizing the ampulla and assessing a potential mass.
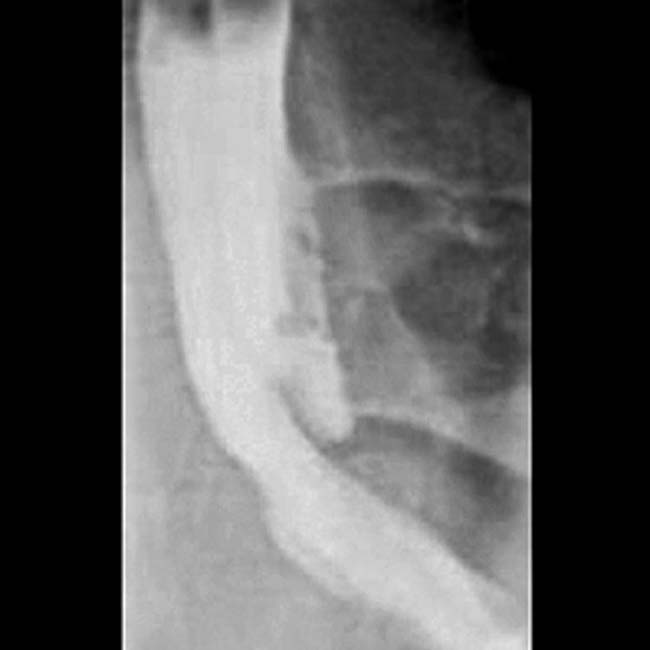
 obstructing the common bile duct
obstructing the common bile duct  , with only mild dilatation of the pancreatic duct
, with only mild dilatation of the pancreatic duct  . This was found to represent an ampullary carcinoma at surgery.
. This was found to represent an ampullary carcinoma at surgery.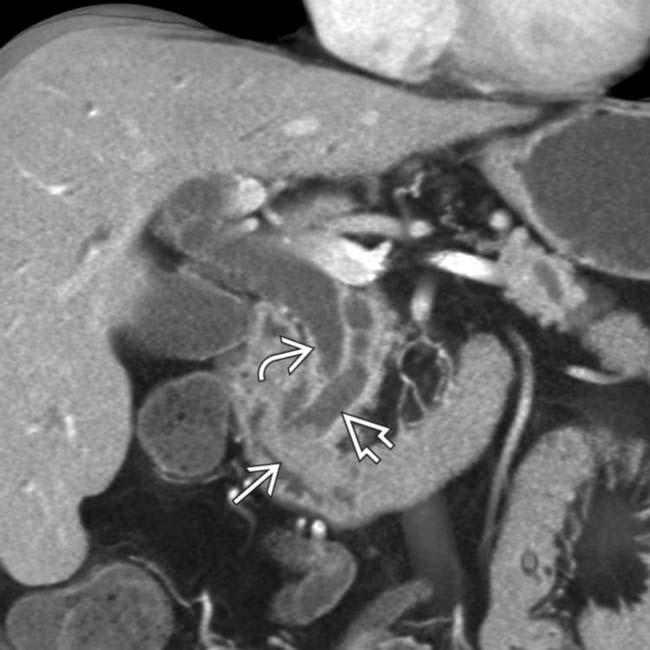
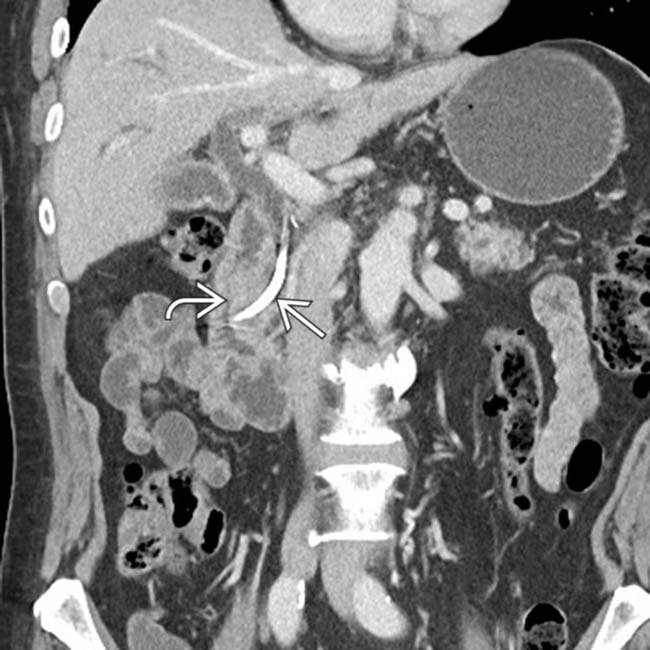
 and pancreatic duct
and pancreatic duct  by a polypoid ampullary carcinoma
by a polypoid ampullary carcinoma  .
.
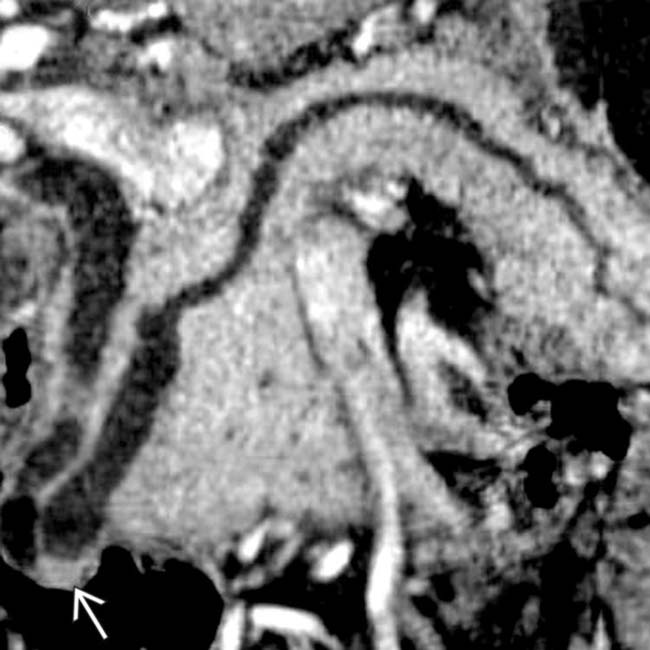
 centered around the ampulla with a biliary stent
centered around the ampulla with a biliary stent  . Ampullary carcinoma cannot be easily distinguished from a periampullary duodenal carcinoma at imaging, although surgical treatment for both lesions is the same (Whipple procedure).
. Ampullary carcinoma cannot be easily distinguished from a periampullary duodenal carcinoma at imaging, although surgical treatment for both lesions is the same (Whipple procedure).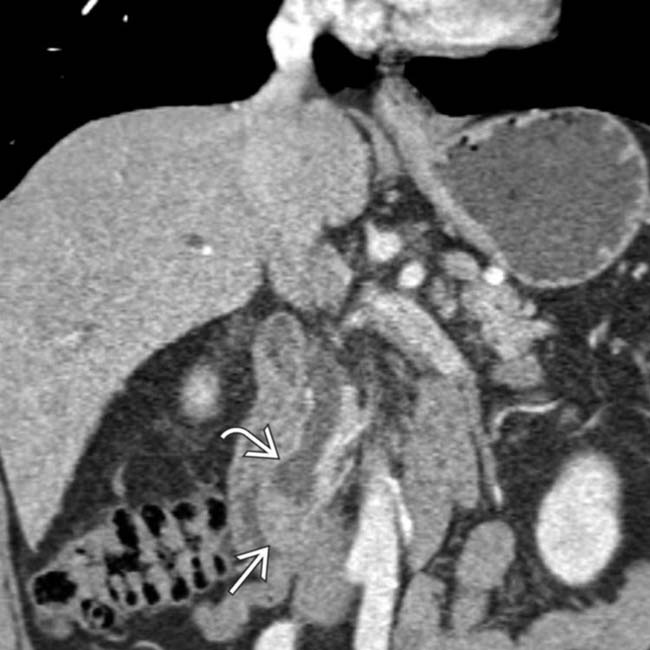
 obstructing the CBD
obstructing the CBD 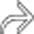 . The PD (not shown) was not obstructed. Ampullary carcinomas almost always obstruct the CBD, but obstruct the PD in only 50% of cases.
. The PD (not shown) was not obstructed. Ampullary carcinomas almost always obstruct the CBD, but obstruct the PD in only 50% of cases.
 at the junction of the 2nd and 3rd portions of the duodenum.
at the junction of the 2nd and 3rd portions of the duodenum.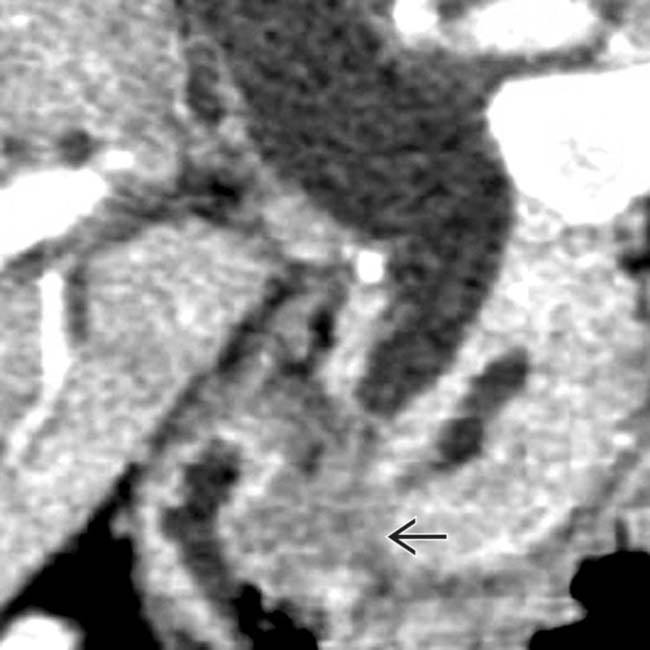
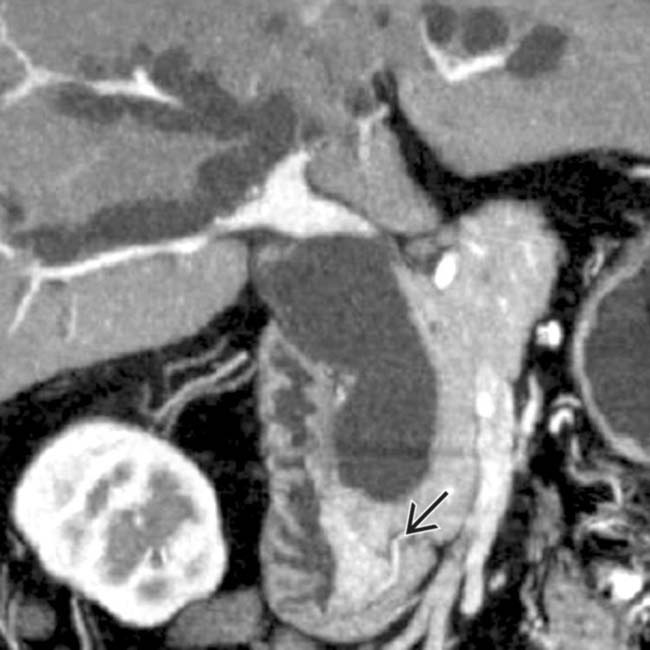
 obstructing the pancreatic and common bile ducts.
obstructing the pancreatic and common bile ducts.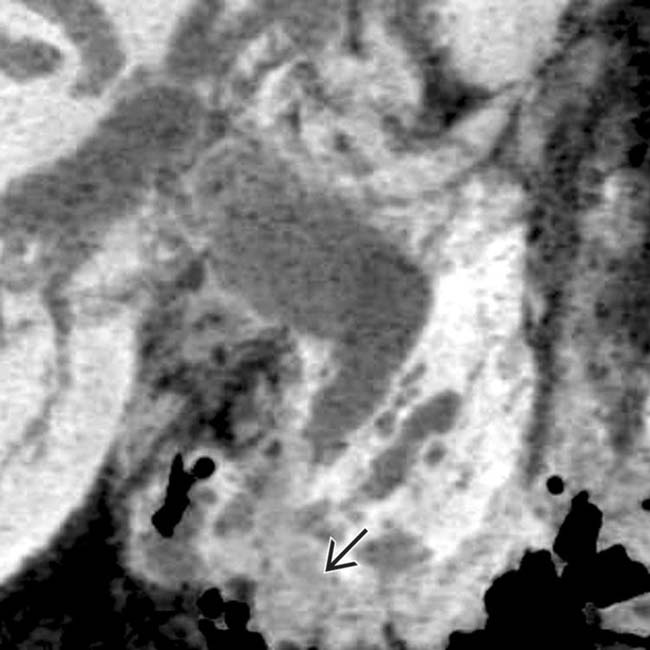
 .
.
 .
.
 obstructing the pancreatic and common bile ducts.
obstructing the pancreatic and common bile ducts.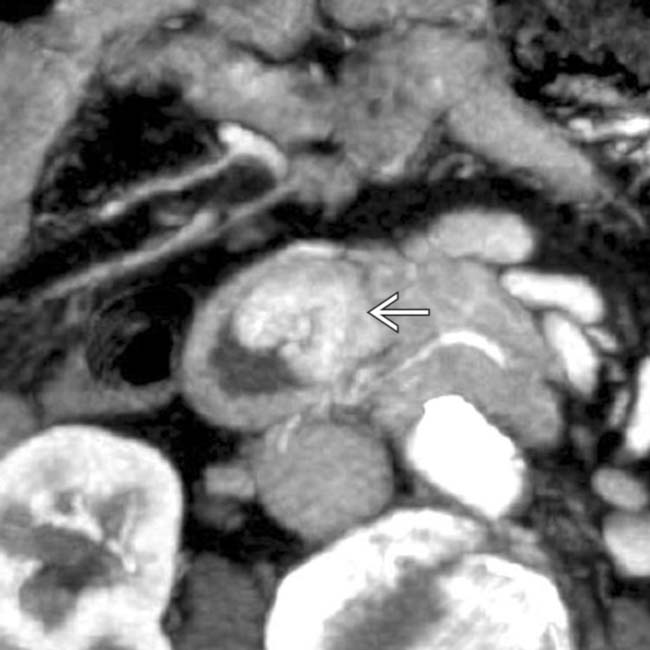
 with an unusual degree of increased vascularity.
with an unusual degree of increased vascularity.































































 .
.
 centered in the duodenum at the level of the ampulla, found to represent an ampullary carcinoma at histology. Note the presence of a biliary stent
centered in the duodenum at the level of the ampulla, found to represent an ampullary carcinoma at histology. Note the presence of a biliary stent  due to biliary obstruction by the mass.
due to biliary obstruction by the mass.