Chapter 67 Amebiasis and other parasitic infections
Amebic Liver Abscess
History
The earliest report of amebiasis is probably the Sanskrit document Brigu-samhita, written about 3000 bce, referring to bloody mucoid diarrhea (Vaidya & Ray, 1982). Assyrian and Babylonian texts refer to blood in the feces, suggesting the presence of amebiasis in the Tigris-Euphrates basin before the sixth century bce (Bray, 1996), and it is possible that hepatic and perianal abscesses described in both Epidemics and Aphorisms in the Corpus Hippocratorum refer to amebiasis (Jones et al, 1948; Jones & Whithington, 1953). After the death of Hippocrates in 356 bce, Alexander the Great became king of Macedonia. In Alexander’s eastern campaign, he reached an area where amebiasis was endemic, and he died on the return trip at the age of 33 years, probably of an amebic liver abscess (Saville, 1964).
In 1818, Ballingall described a surgical technique to drain liver abscesses. When Ballingall practiced in Madras, India, Napoleon Bonaparte was exiled on the tropical island of St. Helena by the British after his defeat at Waterloo, where bloody diarrhea—possibly amebiasis—was common. Napoleon died in 1821 after an attack suggestive of amebic liver abscess, with symptoms of fever, diarrhea, and painful, tender swelling in the epigastrium. At the postmortem, an ulcer was found at the small curve of the stomach, communicating with the liver, suspected to be due to rupture of an amebic liver abscess of the left lobe into the stomach (Chaplin, 1913). In 1828, James Annesley gave detailed descriptions of “hepatic dysentery” (Kapoor, 1979). The connection between amebic dysentery and liver abscesses was described by the English physician William Budd (1857), but Charles Morehead, professor of Medicine and first principal of Grant Medical College, Bombay, India, was the first to report a case of hepatic abscess in 1848 (Martinez Baez, 1986).
Entamoeba histolytica was discovered by Friedrich Lösch in 1873 in Russia (Lösch, 1975). Lösch recognized amebae in the colon and terminal ileum accompanying acute dysentery (Martinez Baez, 1986). He gave descriptions of amebae, including structure, size, motility, intracytoplasmic elements, and drawings. Lösch named the amebae after his patient; Amoeba coli was proved later on sequencing of the genome (Tovar et al, 1999), and a calreticulin-like protein and Golgi apparatus were detected in the amebae (Gonzalez et al, 2002).
Stephanos Kartulis, a Greek physician, found amebae in intestinal ulcers in patients from Egypt in 1885 and noted that he never found amebae from nondysenteric cases (Kartulis, 1886). In 1890, Osler reported a young physician who died of amebic liver abscess (ALA) after an attack of dysentery. The report by William Thomas Councilman and Henri Lafleur, working at Johns Hopkins in 1891, represents a definitive statement about the pathology of amebiasis, amebic dysentery, and ALA at the end of the nineteenth century (Faust, 1931). Schaudinn did differentiate between harmless Entamoeba coli and pathogenic E. histolytica (Martinez Baez, 1986). In 1901, Harris produced ALAs by intrarectal infection of puppies with E. histolytica. Musgrave and Clegg (1904) cultivated E. histolytica in vitro, introducing the term amebiasis. In 1952, Hoare reported that E. histolytica had three phases. Species of amebae found in human host; E. gingivalis, Entamoeba coli, Iodamoeba bütschlii, and E. hartmanni were discovered between 1849 and 1919 (Martinez Baez, 1986).
A plant alkaloid, concessine, was found to kill E. histolytica (Vaidya & Ray, 1982). The first effective treatment came from Brazil in the form of ipecac; emetine was isolated from ipecac in the nineteenth century. Leonard Rogers (1912), professor of pathology at Medical College Hospital in Calcutta, India, reported successful treatment of both intestinal and hepatic amebiasis by injectable salts of emetine. The 1930s witnessed the introduction of two important hydroxyquinolines introduced by Anderson and Koch in 1931 and by a number of others. Although largely replaced by imidazoles in the 1980s, hydroxyquinolines remain useful today. In 1966, Powell and his colleagues demonstrated the effectiveness of metronidazole as an amebicidal agent in both intestinal and extraintestinal amebiasis.
Epidemiology
Approximately one tenth of the world population is believed to be infected with E. histolytica, with 100,000 deaths worldwide each year due to invasive amebiasis (Haque et al, 2003; Petri et al, 2000; Walsh, 1986; World Health Organization [WHO] et al, 1997b). Amebiasis is the third most common parasitic cause of death worldwide (Li & Stanley, 1996). The prevalence of E. histolytica could be an overestimate because it dates before the separation of E. histolytica from the morphologically identical, nonpathogenic E. dispar (Diamond & Clark, 1993) and E. moshkovskii (Beck et al, 2008; Fotedar et al, 2008). However, recent prevalence and morbidity data obtained through molecular techniques allow construction of more reliable map of endemic regions of amebiasis around the world, such as on the Asian subcontinent (India, Bangladesh), Africa, Asian Pacific (Thailand, Japan), and South and Central America (Mexico, Colombia) (Ximenez et al, 2009).
Disease expression varies geographically; invasive disease seen in Egypt, predominantly amebic colitis, may vary in schistosomiasis-endemic versus nonendemic areas (Mansour et al, 1997), whereas in South Africa an excessive rate of amebic liver abscess is noted. Although amebic liver abscess has been encountered in epidemic form in temperate climates, as it was in the water-borne epidemic in Chicago in 1933 (Munoz, 1986), it is a problem chiefly of the tropics and developing countries (Sepulveda, 1982). In developed countries, it continues to be encountered sporadically in immigrants or travelers from endemic zones, low socioeconomic groups, residents of institutions, and male homosexuals (Ravdin & Stauffer, 2005).
In the United States and Europe, homosexual males are principally colonized with E. dispar, and patients infected with the human immunodeficiency virus (HIV) had no increased risk of either intestinal or extraintestinal amebiasis (Petri & Singh, 2006). Invasive extraintestinal amebiasis (hepatic abscesses) are more frequent in HIV-infected patients in these countries (Hung et al, 2005), whereas the same is not true elsewhere (Moran et al, 2005). E. histolytica remains a significant cause of morbidity and mortality in developing countries, where infection is common through flies and food handlers. The annual incidence of amebic dysentery in children is 2.2% in Bangladesh (Haque et al, 2003), and annual incidence of amebic liver abscess averaged 21 cases per 100,000 inhabitants in Hue City, Vietnam (Blessmann et al, 2002). Ingestion of E. histolytica occurs where poverty facilitates deficiencies in sanitation and sewage treatment. In addition, a recent report suggests that parasite genotype plays a role in determining outcome of infection by E. histolytica (Ali et al, 2007).
Organism
E. histolytica is a protozoan with two forms: trophozoite and cyst. Cysts constitute the infective form through fecal-oral transmission via food, water, or direct person-to-person contact. Cysts survive the acid of the stomach and travel through the small intestine, and within the terminal ileum or colon, trophozoites emerge to complete the life cycle (Guerrant, 1986). Cysts can survive for 45 minutes in feces lodged under fingernails and for 1 month in soil at 10° C. They remain infective in fresh water, seawater, and sewage but are destroyed by drying, iodine, and heat. They are not killed by chlorination used to purify drinking water (Munoz, 1986). The genus Entamoeba includes the pathogenic species E. histolytica and nonpathogenic E. hartmanni, E. coli, E. polecki (in swine), and E. moshkovskii (from sewage) (Guerrant, 1986). E. histolytica is morphologically similar to E. dispar; therefore the study of zymodemes, patterns of the electrophoretic mobility of isoenzymes; genetic differences using RNA and DNA probes; and the use of polymerase chain reaction (PCR) amplification became more reliable in their identification (Tannich et al, 1989). Encoding genes for transcription factors have been cloned for E. histolytica (Castanon-Sanchez et al, 2009).
Community Screening Procedures
An effective community screening protocol includes 1) microscopic examination of unfixed fecal samples, 2) Ritchie’s fecal concentration, 3) staining of alcohol-fixed stools, 4) Robinson’s in vitro culture, 5) screening stool antigens, 6) serology (indirect hemoagglutination test [IHAT], enzyme-linked immunosorbent assay [EIA], and immunofluorescence test [IFT]), and 7) isoenzyme electrophoresis of stool for zymodeme identification (Thomas & Garg, 2007).
Direct microscopic examination is found to be more sensitive than serology; however, the sensitivity of both is lower than Robinson’s culture and zymodeme identification, the gold standards (Gatti et al, 2002). DNA-based PCR is rapid and sensitive to detect cysts in the stool (<5) and in fluids aspirated from amebic liver abscess (Rivera et al, 1998). A PCR test for E. moshkovskii was developed with a high sensitivity and specificity using DNA from stool samples (Fotedar et al, 2007). In 2007, Helmy used nested PCR and restriction enzyme digestion (RED) to distinguish E. histolytica from E. dispar.
Host Factors
The major reservoir for Entamoeba spp. is the human being. Male homosexuals transmit the infection but usually harbor nonpathogenic E. dispar, which is more common in females (Gatharim & Jackson, 1987). E. histolytica can be transmitted by heterosexual activity as well as male and female homosexual activity (Salit et al, 2009), although menstruating women are protected against invasive infection. Breastfed neonates have a low incidence as a result of the presence of immunoglobin A (IgA) and low iron content in breast milk (Thomas & Ravindra, 2000). A diet rich in iron and carbohydrates predisposes to invasive amebiasis (Gatharim & Jackson, 1987), and HLA-DR3 gene expression is an independent risk factor for amebic liver abscess (Arellano et al, 1996).
Although immunosuppression is considered an important risk factor for invasive amebiasis in Asian Pacific countries (Hsu et al, 2008; Hung et al, 2008), others maintain there is no particular susceptibility for developing invasive forms of amebiasis in immunosuppressed individuals. Nevertheless, some HIV infections have been detected during admission of amebic liver abscess patients. On the other hand, the natural history of the disease seems to be the same as in nonimmunosuppressed patients (Kershenobish & Corona, 2008).
Pathogenesis
The disease course is determined by three virulence factors: lectin (a surface protein), amebapores (small peptides), and cysteine proteases. Trophozoite adhesion to the colonic wall is mediated by lectin, which results in persistent infection, and caspase 3 activation, which is a crucial step in cell necrosis and abscess formation (Huston et al, 2003). Amebapores are inserted by the trophozoite into the host cell, where they puncture the lipid bilayer and form a portal of entry into the host. Amebapores result in colloid osmotic lysis of the cell (Leippe et al, 1991). Cysteine proteases contribute to degradation of the extracellular matrix proteins and disruption of cell monolayers (Que & Reed, 1997).
It is anticipated that antiamebic antibodies protective against invasive infection would block lectin binding and neutralize amebapore and cysteine proteases. It has been suggested that the proteophosphoglycans (PPGs) in the amebic glycocalyx may participate in E. histolytica pathogenicity because the closely related, nonpathogenic E. dispar lacks a significant glycocalyx surface layer (Bhattacharya et al, 2000). Furthermore, antibodies that bind to PPGs neutralize liver abscess formation (Marinets, 1997). PPGs are anchored into the parasite cell membrane by a glycosylphosphatidylinositol (GPI) moiety. Synthesis of the GPI anchor requires a cascade of enzymes, including mannosyl transferase 1 (PIG-M1), whose blockage reduces GPI synthesis and PPGs in trophozoites (Weber, 2008). In addition, experimental evidence suggests that liver cell necrosis is increased when neutrophils are present along with E. histolytica (Aikat et al, 1979).
Normal blood flow in the portal vein is about 1.4 L/min, blood pressure is 12 to 15 mm Hg, and erythrocyte velocity is 8 to 18 cm/s. In comparison, sinusoidal blood flow is extremely low (3.4 to 0.16 mL/min), as is red blood cell velocity (0.1 mm/s; Puhl et al, 2003). Forces exerted on a parasite that adheres to the endothelium are thus much lower in the sinusoids and may partly explain why the parasite crosses the endothelium within these structures. Lack of tight junctions in liver sinusoidal endothelial cells (LSECs) can facilitate crossing by the parasite, creating a larger breach when reaching the hepatic parenchyma (Blazquez et al, 2007).
Publication of the E. histolytica genome (Loftus et al, 2005) facilitated transcriptome studies of the parasite. Microarrays have been used to compare virulent and avirulent trophozoites (those unable to form liver abscesses) from the same strain (Santi-Rocca et al, 2008). Overexpression of peroxiredoxin and rubrerythrin genes in virulent parasites is of interest for ALA development because inflammatory cell recruitment and subsequent inflammation are features of liver infection by E. histolytica (Choi et al, 2005a).
Molecular Genetics
The Institutes for Genomic Research have recognized that E. histolytica has a small, highly repetitive genome rich in adenosine-thymidine and densely packed sequences, but it lacks introns (Mann, 2002). Although some regions of the genome encode highly conserved proteins, other areas exhibit high degrees of polymorphism (Haghighi et al, 2002). Sequencing of the E. histolytica genome has revealed at least 44 genes (Clark et al, 2007). The purification of trophozoites from different organs of the same patients revealed that their tropism was linked to different genotypes (Ali et al, 2007).
Host Defense and Potential for Vaccine Development
It has not been definitively established which mechanism is responsible for invasion or recurrence (Ravdin & Guerrant, 1982). The first line of defense is the innate immunity that recognizes pathogen-associated molecular patterns (PAMPs) that trigger an inflammatory response. Natural killer T cells activated by E. histolytica are important in the control of amebic liver abscess (Lotter et al, 2009). Interferon (IFN)-γ initiates inflammation through macrophage production of tumor necrosis factor (TNF) and nitrous oxide (NO) synthesis by polymorphonuclear cells and macrophages. In vitro, the effects of IFN-γ can be bypassed by the recognition of PPGs of E. histolytica by Toll-like receptors (TLR) 2 and 4, which results in direct production of TNF and interleukins (ILs)-12 and -8 (Maldonado-Bernal, 2005). This shows the importance of early recognition of PPGs and inflammatory cell recruitment during ALA onset (Ivory & Chadee, 2007).
Complement cannot prevent invasion because it is absent from gut mucosal secretions (Braga et al, 1992), and amebic cysteine proteases degrade C3a and C5a (Reed et al, 1995). Neutrophils fail in initial host defense (Salata et al, 1989), and trophozoites can infect the human liver for months before abscesses are diagnosed. The second line of adaptive immune response constituted by activated lymphocytes and macrophages is the important effector mechanism against E. histolytica (Vohra et al, 2003). E. histolytica infection elicits mucosal IgA and serum IgG response to lectin protein shown to be protective against infection (Abd-Alla et al, 2004; Haque et al, 2002) in addition to epitope-specific antibodies that inhibit adherence to target cells (Pillai et al, 1999). The galactose/GalNAc lectin isolates from three distinct areas of the world—Bangladesh, Republic of Georgia, and Mexico—retain remarkable sequence conservation (Beck et al, 2002), and is recognized as a potential vaccine target (Thomas & Garg, 2007). Serum immunoglobulin (Ig) G response to the lectin protein does not provide protection against infection (Haque et al, 2002). Oral vaccines that use amebic antigens have been developed and tested in animals (Lotter et al, 2003; Mann et al, 1997; Snow & Stanley, 2006). A codon-optimized DNA vaccine has been tested in a murine model and was found to be useful in stimulating type 1 cellular immune response and serum antibodies (Gaucher & Chadee, 2002). Cell-mediated immunity (CMI) may be sufficient for vaccine protection from intestinal amebiasis with significant IFN-γ, interleukin (IL)-2, IL-12, IL-10, and IL-17 production with recombinant vaccines (Guo et al, 2009). Gram-negative bacteria expressing E. histolytica antigens may constitute a suitable oral vaccine carrier against invasive ameobiasis (Lotter et al, 2008).
Pathology
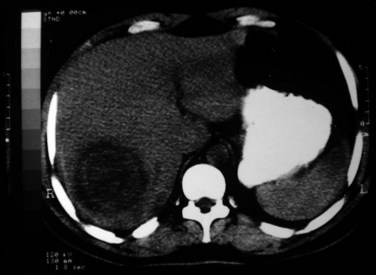
The development of ALAs is a fatal feature of infection by E. histolytica and is the most common extraintestinal form of invasive amoebiasis. Indeed, an estimated 100,000 people succumb to ALA each year (WHO, 1997a). Trophozoites that successfully penetrate the colonic mucosal barrier cause invasive disease, enter the portal system, and travel to the liver. Amebic colitis and amebic liver abscess rarely occur simultaneously, and the colonic lesions are usually silent; direct extension to the liver and lymphatic spread do not occur. The cecum is the most common site of amebic colitis, and the right lobe of the liver is more commonly affected because of drainage of the right portal branch from the right side of the colon. The condition usually starts as diffuse amebic hepatitis; liver cells undergo liquefactive necrosis, starting in the center and spreading peripherally to produce a cavity full of blood (Fig. 67.1) and liquefied liver tissue resembling anchovy sauce; it has no odor and is sterile. The fluid itself is free from any amebae, which may be found at the expanding edge of the abscess cavity with little inflammation. Amebae are known to lyse neutrophils, and the release of neutrophilic mediators may promote hepatocyte death and extension of the abscess. Secondary bacterial infection may occur spontaneously, altering the color, odor, and consistency of the pus. Lack of fibrotic response by the surrounding tissue with centrifugal extension results in extention of the abscess to the Glisson capsule, which is resistant to the amebae. Typically, amebic liver abscesses are solitary, large, and located in the right liver. Left lobe abscesses are less common, but because of the smaller volume of the left liver, abscesses in this location are more prone to rupture the capsule (Thomas & Ravindra, 2000). Vascular and biliary structures may traverse the abscess cavity; because of the intrahepatic covering of the Glisson capsule, such structures are resistant to the process of liquefactive necrosis. However, these structures can be mistaken for septa within the abscess cavity, and fracturing of these strands can lead to hemorrhage or biliary leak, or it can create a communication between the vascular and biliary channels and result in hemobilia and jaundice (Singh et al, 2008). The abscess wall is typically ill defined with a minimal host response of fibrous tissue, but longstanding abscesses may develop a fibrous wall and may even calcify (Rogers et al, 1980). In treated cases, complete resolution is the rule, but it may take 6 months to 2 years or longer (Thomas & Ravindra, 2000) than the usual time for pyogenic abscesses to resolve (Sudhamshu & Sharma, 2009).
Clinical Presentation
The peak incidence of amebic liver abscess is between 20 and 60 years of age; thus it is predominantly a disease of young men. Amebic liver abscess is 10 times more common in adults than in children and is three to 10 times more common in males (Sepulveda & Manzo, 1986; Wells & Arguedas, 2004). Population groups that are not commonly affected—such as children (especially neonates), pregnant women, and women in the postpartum period—have an increased risk of severe disease and death. Treatment with steroids, malignancy, men having sex with men, advanced age, and malnutrition could be considered risk factors for severe disease (Guerrant, 1986; Li & Stanley, 1996).
Most E. histolytica infections are asymptomatic or present as mild, “noninvasive” disease. Asymptomatic carriers, or cyst passers, may excrete cysts for a short period, but the majority of these patients clear the infection within 12 months. Patients with confirmed E. histolytica infection, even if they are asymptomatic, should be treated to eliminate the organism and prevent further transmission (Stanley, 2003). The time between penetration of colonic mucosa and damage to hepatic parenchyma is unknown. Active diarrhea usually occurs in less than 30% of patients at any time before presentation despite intestinal infection by E. histolytica. In most cases, standard stool microscopy results are negative, but in research studies, cultures of stool were positive for E. histolytica in more than 75% of patients with amebic liver abscess (Irusen et al, 1992).
Concomitant hepatic abscess is found in only one third of patients with amebic colitis. The duration of symptoms is usually 10 days. In nonendemic regions, such as Western Europe and the United States, patients usually report travel to an endemic area in the previous 2 to 5 months (median, 3 months), although a prolonged latency may occur (Johnston et al, 2009; Wells & Arguedas, 2004). Patients with amebic liver abscess start reporting nonspecific symptoms such as anorexia, nausea, vomiting, and acute colitic illness. Abdominal pain and fever are the cardinal symptoms of the disease, seen in 90% of patients or more. Fever typically is between 38° C and 40° C and is seen in 87% to 100% of patients; rigors occur in 36% to 69%. Other signs and symptoms vary according to the site and the size of the abscess. The chief sympton is typically the abrupt onset of right hypochondrial pain radiating to the right shoulder and scapular area. The pain increases with coughing, deep breathing, and walking. If the abscess is in the left hepatic lobe, the pain may be epigastric, precordial, or retrosternal and may radiate to the left shoulder. Abscesses located on the inferior aspect of the liver may present in a manner similar to peritonitis resulting from any upper abdominal cause. On occasion, the presentation is insidious, lasting 2 or more weeks; in such patients, significant weight loss may occur (Thomas & Ravindra, 2000). One report has suggested that amebic liver abscesses can be silent and asymptomatic (Blessmann et al, 2003).
Abdominal examination usually reveals a tender, soft hepatomegaly accompanied by overlying muscle guarding and intercostal tenderness. On occasion, increased warmth and cutaneous edema may occur. Jaundice is often seen, although it was previously reported to be a prominent feature in only 5% to 8% of patients (Pitt, 1990); biliary communication of amebic liver abscess has been reported in up to 27% (Agarwal et al, 1995; Bayraktar et al, 1996). Because the right posterior-superior surface is the most common site of amebic liver abscess, it is always accompanied by right basal lung signs as a result of either pleural effusion, empyema, or lung abscess (Loulergue & Mir, 2009). On the other hand, left lobe abscess may be complicated by pericardial friction, and abscesses in this location can extend into the pericardium, a sign associated with a very high mortality rate (Wiwanitkit, 2008). Hepatic failure, ascites, and splenomegaly may occur in 15% of patients who have multiple abscesses. They present with fever, toxemia, deep jaundice, and encephalopathy; toxemia is suggestive of an added bacterial infection, leading to more severe disease. Esherichia coli and Klebseilla pneumoniae are the most commonly cultured organisms, and patients are seen with a clinical picture indistinguishable from hepatic encephalopathy as a result of acute hepatocellular failure. Hepatic encephalopathy in patients with amebic liver abscess may result from a combination of right hepatic vein occlusion, pylephlebitis, and occlusion of several portal vein radicles (Kapoor & Joshi, 1992). Clinically, the usual differential diagnosis includes acute cholecystitis, hepatitis resulting from viral or other causes, and pyogenic liver abscess. With atypical presentation, hepatocellular carcinoma, a hepatic hydatid cyst, or a simple cyst may be considered (Thomas & Ravindra, 2000). Approximately three quarters of patients with an amebic liver abscess have leukocytosis. Leukocytosis most likely appears if symptoms are acute or complications have developed. Eosinophilia is rare, but mild anemia may occur in half of patients and is multifactorial. Hyperbilirubinemia is present in only a small proportion of cases.
In acute liver abscess, aspartate aminotransferase (AST) levels are high. In chronic liver abscess, the alkaline phosphatase level tends to be elevated, and the AST level tends to be within normal limits. Overall, the alkaline phosphatase level is elevated in about 70% of patients with amebic liver abscess. Similar complete blood count (CBC) and liver test abnormalities are found in patients with pyogenic liver abscesses and are not specific (Ravdin & Stauffer, 2005). Prolonged prothrombin time may occur. Chest radiography typically shows elevation of the right dome of the diaphragm with an anterior bulge on the lateral view (Debakey & Ochsner, 1951), atelectasis of the right lung, and pleural effusion. The liver shadow on plain abdominal radiographs is diffusely enlarged, and gas in the biliary tree or liver parenchyma is unusual unless there is communication of the hepatic abscess cavity with the lung or hollow viscus (Thomas & Garg, 2007).
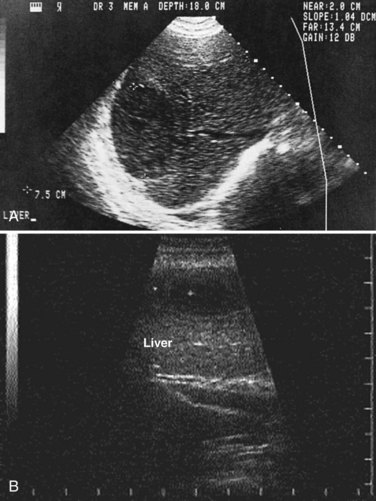
Ultrasonography
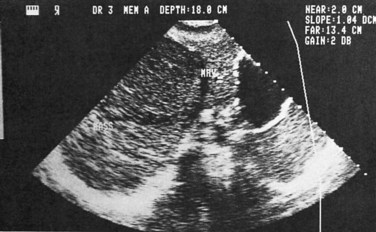
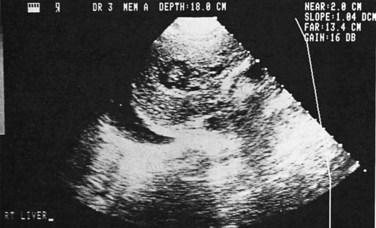
Ultrasonography (US) is the preferable diagnostic test, with an accuracy of 90% (see Chapter 13; Sepulveda & Manzo, 1986). Abscesses are usually located in contact with the liver capsule (see Fig. 67.1) and are 2 to 21 cm in size, round or oval, with well-defined margins; they are hypoechoic and clearly defined from normal liver parenchyma with distal enhancement (Figs. 67.2 to 67.4; Ralls et al, 1982; Sukougj et al, 1989). In 80% of patients, the abscess is single in the right lobe; 10% in the left lobe and 6% in the caudate lobe are single, and the remaining are multiple abscesses (Ralls et al, 1979).
In early stages, amebic abscess appears as a subtle area of decreased echogenicity, and US diagnosis is not pathognomonic; complicated cysts, hematoma, metastases, and amebic abscess may mimic each other (Elzi et al, 2004; Kapoor, 1989). Only 40% of patients have typical US features of amebic liver abscess. Serial scanning tends to show no change despite adequate treatment with amebicidal drugs, complete aspiration of the abscess, or both (Ralls et al, 1979; Sukov et al, 1980). The mean resolution time is 7 months, and complete resolution may take up to 2 years. On occasion, percutaneous diagnostic aspiration may be needed to differentiate amebic from pyogenic liver abscess (Kurland & Brann, 2004). Although pyogenic liver abscesses tend to resolve earlier (within 2 to 4 months), amebic liver abscesses acquire a more echogenic and fibrous wall in 8 to 16 weeks and begin to resemble, but must be differentiated from, an encapsulated tumor. With time, resolution may be complete, or the result may be a residual cystic cavity that resembles a simple cyst of the liver (Ralls et al, 1983; Sheen et al, 1989).
Other Imaging Modalities
Computed tomography (CT) does not add to the diagnostic accuracy of US in acute stages, except in evaluation of imminent rupture of an abscess or for detection of small lesions (see Chapter 16; Kimura et al, 1997). Amebic liver abscesses usually appear on CT with contrast as rounded, well-defined lesions with complex fluid (Radin et al, 1988). The most characteristic finding is an enhancing wall with a peripheral zone of edema around the abscess (Fig. 67.5). The abscess cavity may show multiple septa (more with pyogenic abscesses), fluid and debris levels, air bubbles, or hemorrhage. CT may detect extension of amebic liver abscesses to other organs (Radin et al, 1988).
Magnetic resonance imaging (MRI; see Chapter 17) is not superior to CT in the diagnosis of amebic liver abscess, but it may be useful in differentiating it from a hepatic neoplasm. Amebic liver abscesses appear as heterogeneous cavities that are hypointense on T1-weighted images and hyperintense on T2-weighted MR images. The abscess margin may show incomplete hyperintense rings with perilesional edema on T2-weighted images. Following treatment, the abscess cavity becomes homogeneous, and complete concentric rings appear as a result of periabscess fibrosis and hemosiderin deposits (Mortelé & Ros, 2001).
Angiography is rarely required. When performed, it reveals a hypovascular or avascular mass displacing the hepatic artery and portal vein branches but may show portal vein thrombosis (Viana, 1975). Although its role is currently limited with gallium scanning, amebic liver abscesses appear as cold spots, whereas pyogenic lesions are seen as warm spots (Kapoor, 1989).
Amebic Serology
Amebic serology is highly sensitive and specific in the differentiation between pyogenic and amebic hepatic abscess. EIA is simple, rapid, inexpensive, and more sensitive, and it has now largely replaced IHAT and counterimmunoelectrophoresis (Restrepo et al, 1996). EIA detects antibodies specific for E. histolytica in approximately 95% of patients with extraintestinal amebiasis, 70% with active intestinal infection, and 10% who are asymptomatic cyst passers. Antibody response is related to the duration of illness and may be detectable 7 to 10 days after the onset of symptoms. Titers peak by the second and third months, decreasing to lower levels by 9 months; they revert to negative by 12 months (Munoz, 1986). Monoclonal antibody-based tests allow differentiation between invasive and noninvasive parasites (Kimura et al, 1997). Enzyme-linked immunosorbent assay (ELISA) and IHAT cannot differentiate acute from remote infection in endemic areas (Thomas & Garg, 2007).
Currently, ELISA for detection of the galactose-inhibitable adherence protein in serum and feces and IHAT appear to be the most reliable tests, with sensitivity and specificity greater than 95% (Hira et al, 2001), with a reversal rate of 82% after 1 week of treatment with metronidazole (Tanyuksel & Petri, 2003). Efforts are ongoing to identify antigens specific for acute infection (Ravdin, 1995). Rapid antigen and antibody tests are being evaluated and seem very promising (Leo et al, 2006).
Role of Aspiration
US-guided aspiration (Fig. 67.6) is often justified on the basis that the diagnosis would be “more certain,” or that the abscess can be “aspirated to dryness” at the time of therapeutic aspiration. PCR may detect E. histolytica DNA in amebic liver abscess pus as well as in the saliva of patients (Khairnar & Parija, 2008; Khan et al, 2006). No randomized controlled trial has ever shown that aspiration is beneficial in survival, length of hospitalization, or time to become afebrile compared with treatment with antiamebic drugs alone (Chavez-Tapia et al, 2009; Sharma et al, 1989; Stanley, 2003; Van Allan et al, 1992), and aspiration may only confuse the diagnosis by revealing atypical pus or blood. The belief that aspiration hastens clinical recovery and may not involve significant procedure-related morbidity is widespread in clinical practice, however. This approach is supported by a small prospective study (Tandon et al, 1997) and continues to be advocated in reviews (Haque et al, 2002). Clinical improvement invariably occurs with antiamebic therapy alone in uncomplicated cases. When the differential diagnosis in a given case includes operable neoplasm or hydatid disease, aspiration is risky and may be contraindicated (Thomas & Garg, 2007).
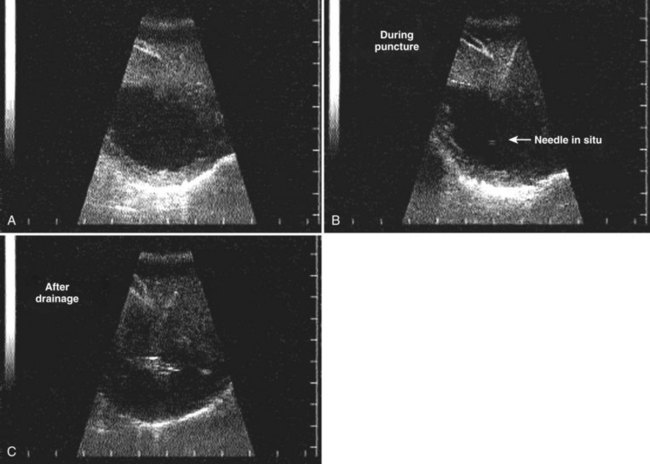
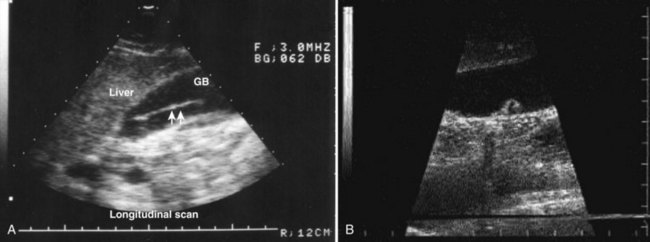
FIGURE 67.6 Ultrasound showing a typical liver abscess. A, Before drainage. B, During drainage. C, After drainage.
(Courtesy Professor A.K. El Dory, Ain Shams University, Cairo.)
Therapeutic aspiration should be reserved for the following situations (Ralls et al, 1982):
1 Serology is inconclusive and differential diagnosis is pyogenic liver abscess.
2 A therapeutic trial with antiamebic drugs is deemed inappropriate, as in pregnancy.
3 The liver abscess is secondarily infected, estimated to be true in 15% of cases (McDermott, 1995).
4 Fever and pain persist for more than 5 to 7 days after starting appropriate therapy.
5 Rupture is imminent in an extremely large abscess (>10 cm), especially if pericardial rupture from a left lobe abscess appears likely.
The following factors are predictive of the need for aspiration: 1) age 55 years or older, 2) an abscess 5 cm or more in diameter, and 3) failure of medical therapy after 7 days (Khan et al, 2008). In endemic areas, because of late presentation and existence of multiple abscesses, up to 50% of patients may require aspiration (Khanna et al, 2005). A single aspiration may be sufficient for diagnostic purposes, but it is inaccurate to recommend it because the characteristic “anchovy paste” characteristic of amebic abscess may not be found; when performed as part of therapy, it will likely be inadequate. Percutaneous catheter drainage (PCD) is better than percutaneous needle aspiration (PNA) for management of large liver abscesses (>10 cm) in terms of duration to attain clinical relief and duration for which parenteral antibiotics are needed (Singh et al, 2009).
Complications
Peritoneal and Visceral Involvement
Peritonitis with amebiasis is due to rupture of an amebic liver abscess in 78% of patients and perforated or necrotizing amebic colitis in the other 22% (Ken et al, 1989; Monga et al, 1976). Spontaneous rupture of amebic liver abscess may occur in 2.7% to 17% of cases (Eggleston et al, 1982; Monga et al, 1976; Sarda et al, 1989). Adherence of the liver abscess to the diaphragm, abdominal wall, omentum, or bowel tends to confine contamination and lead to rupture into hollow viscera, such as the stomach or colon (Angel et al, 2000).
A hepatogastric, hepatoduodenal, or hepatocolonic fistula and acute hepatic failure may occur. Free rupture into the peritoneal cavity is uncommon and usually only occurs in a nutritionally depleted patient (Rao et al, 2009). Patients present with abdominal pain, a palpable mass, or generalized distension. Bloody diarrhea may occur in colonic rupture, and hematemesis may occur in cases of hepatogastric fistula. Signs of peritonitis with tender hepatomegaly, intercostal tenderness, right basal lung signs, and clinical jaundice may lead to suspicion of the diagnosis, which can be confirmed on US. The diagnosis may be made only at laparotomy, with risk of bleeding from decreased prothrombin levels (Thomas & Garg, 2007). US and CT often show a perihepatic fluid collection in cases of amebic liver abscess that are reactive or with actual leaks from the abscess cavity (Radin et al, 1988). Management of abscesses that extended into the peritoneal cavity with aggressive surgical approaches were associated with increased mortality rates (Balasegaram, 1981; Eggleston et al, 1982) and have now been replaced by increasingly successful attempts at percutaneous drainage of the liver abscess and the extravasated pus (Ken et al, 1989; Sarda et al, 1989).
Absolute indications for laparotomy include a doubtful diagnosis; concomitant hollow viscus perforation with fistulization, resulting in life-threatening hemorrhage or sepsis; and failure of conservative management. At laparotomy, the liver abscess, which usually appears as a tan-colored bulge on the surface, must be handled gently. Septa running across the cavity are usually blood vessels and bile ducts traversing the abscess cavity. Hemorrhage can be difficult to control, and postoperative bile leaks may result. Endoscopic stenting or nasobiliary drainage may be required in cases of biliary communication (Sandeep et al, 2006). Irrigation of the abscess cavity with saline is usually sufficient and may be followed by the installation for 3 to 5 minutes of a solution of 65 mg of emetine hydrochloride in 100 mL of normal saline. Tube drains are inserted and retained as necessary. Hollow viscus perforations must be dealt with on an individual basis, with exteriorization, proximal diversion, or serosal patch closure (Thomas & Garg, 2007). Postoperative intravenous metronidazole is combined with broad-spectrum antibiotics, and dehydroemetine is added if no cardiac contraindication exists. The mortality rate of viscus perforation ranges from 12% to 50% (Sarda et al, 1989).
Thoracic and Pleuropulmonary Involvement
Pulmonary complications occur in 7% to 20% of patients with amebic liver abscess (Stanley, 2003). A sympathetic right-sided effusion is the most common pulmonary complication and usually does not require treatment itself. Other thoracic complications include rupture of the abscess into the pleural cavity or into the bronchial tree. This condition manifests as dyspnea and dry cough with right basal crepitations and collapse of the right lung in addition to the abdominal signs. A pleural rub may also be found. Sudden onset of coughing with expectoration of copious quantities of chocolate-colored sputum occurs if the abscess ruptures into bronchi (Guarner, 1986). There are several routes of pulmonary infection, including direct hematogenous and inhalational amebiasis (Shamsuzzaman & Hashiguchi, 2002). Thoracocentesis is the main line of treatment. In case of aspiration and intercostal tube drainage, care is taken to go high on the right lateral side of the chest near the axilla. Ineffective early drainage of the amebic empyema is usually complicated by secondary infection that requires aggressive surgical procedures, such as pulmonary decortication, because the fibrous tissue can be dense enough to complicate thoracoscopic surgical techniques (Thomas & Garg, 2007).
Lung abscess rarely occurs, and it is walled off from the pleural and peritoneal cavities; surgical intervention is not required because postural drainage, bronchodilators, and antiamebic drugs may suffice. Metronidazole is effective, but emetine produces a rapid response and may be required in cases of metronidazole resistance (Jain et al, 1990).
Vascular and Pericardial Involvement
Some cases of vascular complications have been reported, such as Budd-Chiari syndrome (Mechai et al, 2009) and right atrial and inferior vena cava thrombosis, complicating multiple large amebic liver abscesses (Khan & Ameen, 2009; Sodhi et al, 2009). Surgical removal of the thrombus may be required.
Abscess rupture into pericardium is rare but serious, occurring in 1.3% to 2% of patients, with mortality rates from 30% to 60% (Shamsuzzaman & Hashiguchi, 2002). Abscesses of the left lobe of the liver or those more centrally located are more prone to pericardial complications that range from asymptomatic pericardial effusion to cardiac tamponade. Although left lobe abscesses resolve equally well with antiamebic drugs, as do right-sided abscesses (Thompson et al, 1985), the detection of pericardial thickening or pericardial effusion may constitute an indication for aspiration of a left-sided amebic liver abscess (Radin et al, 1988). In cardiac tamponade, pericardiocentesis must be performed, along with drainage of the liver abscess followed by antiamebic drugs, namely metronidazole. Dehydroemetine is used with caution because of its cardiotoxicity (Thomas & Garg, 2007).
Chemotherapy
Metronidazole
Metronidazole is the drug of choice for amebic liver abscess. The oral dose is 500 to 750 mg three times daily for 7 to 10 days, the same dose and duration used for intestinal amebiasis in adults, and 35 to 50 mg/kg in three divided doses for 10 days in children, with a cure rate of more than 90% (Li & Stanley, 1996). Hepatopulmonary amebiasis was found to respond equally to doses of 400 and 800 mg three times daily given over 5 days (Jain et al, 1990). The intravenous route is also highly effective at a recommended dose of 500 mg every 6 hours. Metronidazole reaches high concentrations in the liver and intestine, and it crosses the placenta and the blood-brain barrier. Its use is contraindicated in the first trimester of pregnancy and must be used cautiously in the second and third trimesters; breastfeeding should be discontinued during its use. The response of patients with amebic liver abscess to metronidazole is profound, with improvement in symptoms within 72 to 96 hours. However, a luminal agent such as paromomycin (30 mg/kg three times a day for 5 to 7 days), iodoquinol (650 mg orally three times a day for 20 days), or diloxanide furoate (500 mg orally three times a day for 10 days) should also be used to eradicate intestinal colonization (Stanley, 2003).
Medical therapy has a disulfiram-like action. The most common side effects are nausea, a metallic taste in the mouth, vomiting, and dark brown discoloration of the urine. Over 5 days, an 85% cure rate is achieved, which may increase to 95% after 10 days (Guarner, 1986). From 5% to 15% of patients with amebic liver abscess may be resistant to metronidazole (Thompson et al, 1985), but this may not be a major clinical problem (Li & Stanley, 1996); most reports of “drug resistance” reflect delayed resolution of either clinical symptoms or US findings and not a true resistance documented by drug failure. Experimental resistance may be related to inducing superoxide dismutase in vitro (Wells & Arguedas, 2004). Alternate medical therapy, percutaneous aspiration, or surgical intervention usually must be considered in patients who do not respond or show only modest improvement in 72 hours (Thompson et al, 1985). In countries where they are available, tinidazole, ornidazole, and nitazoxanide are alternative agents for the treatment of amebic liver abscess; these drugs are administered for only a few days (Lasserre et al, 1983; Quaderi et al, 1978; Vanijanonta et al, 1985). One study of a single 2-g dose of either tinidazole or ornidazole gave a success rate of 94% in both treatment arms (Lasserre et al, 1983). Other studies have shown a success rate of almost 100% for patients treated with tinidazole for 2 to 3 days (Khokhani et al, 1978; Quaderi et al, 1978; Vanijanonta et al, 1985). Tinidazole has been approved by the Food and Drug Administration (FDA) for the treatment of amebiasis, including amebic liver abscess. Secnidazole has a longer half-life, and a single daily dose of 2 g for 5 days is effective (Salles et al, 2003). Satranidazole showed lower incidence of side effects and better tolerance than metronidazole in a randomized, single-blind trial of 49 patients with amebic liver abscess (Muzaffar et al, 2006).
Other Medications
Emetine hydrochloride is effective against trophozoites and reaches amebicidal concentrations in tissues rather than intestine. Emetine is administered by intramuscular or deep subcutaneous injection in a dose of 1 mg/kg/day (maximum 60 mg/day) for 10 days. The patient must be placed on complete bed rest. Tissue levels persist for 40 to 60 days, and readministration should be avoided for 6 weeks. Side effects include myositis at the injection site, hypotension, tachycardia, chest pain, dyspnea, and abnormalities on electrocardiogram, including T-wave inversion and prolonged Q-T interval. The drug is contraindicated in renal, cardiac, and muscular disease and is used cautiously in children and elderly patients (Thomas & Garg, 2007). Emetine or dehydroemetine is valuable in treatment of hepatopulmonary amebiasis (Guarner, 1986; Jain et al, 1990; Thompson et al, 1985).
Chloroquine phosphate is antimalarial and is effective in patients with resistance to emetine and pulmonary amebiasis (Guarner, 1986) but has no luminal amebicidal activity. Chloroquine is administered orally in a dose of 1 g (600 mg base) per day for 2 days followed by 500 mg (300 mg base) per day for 2 to 3 weeks. It is contraindicated in patients with retinopathy, but it has been used in pregnant patients (Guarner, 1986). Diloxanide is ineffective in invasive amebiasis and is used for treatment of asymptomatic carriers. The recommended dose is 500 mg three times a day for 10 days. No serious side effects are reported.
Nitazoxanide was effective in treating invasive amebiasis and eliminating colonization in intestine. Further studies are warranted in hepatic amebiasis (Rossignol et al, 2007). Trifluoromethionine is a lead compound, but a single subcutaneous or oral dose prevented the formation of amebic liver abscess in a rodent model (Sato et al, 2010).
Therapeutic Strategy
Oral metronidazole is administered as a single drug, with concomitant correction of hypoprothrombinemia, hypoproteinemia, and anemia. If dramatic improvement occurs in 48 to 72 hours, no therapy other than the complete course of metronidazole is required. A luminal agent, such as diloxanide furoate (500 mg three times a day for 10 days) or paromomycin (30 mg/kg/day in three doses for 10 days), must be administered after metronidazole therapy for eradication of intestinal infection as part of a complete treatment regimen (Irusen et al, 1992).
In patients who do not respond satisfactorily, emetine or dehydroemetine is added. Evidence of pulmonary, peritoneal, or pericardial extension is an indication for aspiration of the liver abscess with an intercostal tube or catheter drainage. Percutaneous catheter drainage is usually recommended for abscesses larger than 10 cm in diameter and for left lobe liver abscess, failure of medical treatment for 7 days, and failure to differentiate the abscess from a pyogenic abscess (Singh et al, 2009). The following are predictive of the need for aspiration: 1) age older than 55 years, 2) abscess greater than 10 cm in diameter, and 3) failure of medical therapy after 7 days (Khan et al, 2008). Late presentation with the existence of multiple abscesses may require aspiration (Khanna et al, 2005), but prompt medical care decreases the need. Laparotomy is usually reserved for patients with suspected peritonitis, fistulization, or secondary infection with sepsis after failure of above measures.
A meta-analysis of patients with amebic liver abscess showed that 4% died (Pitt, 1990). In patients treated with amebicidal drugs alone, the mortality rate was 2%. Independent risk factors for death include serum bilirubin greater than 3.5 mg/dL; encephalopathy; hypoalbuminemia, defined as less than 2 mg/dL; and multiple abscess cavities (Sharma et al, 1996) or total abscess volume greater than 500 mL (Wells & Arguedas, 2004). Rupture into the peritoneal cavity and the pericardium is responsible for most deaths. Patients treated with early and aggressive surgery as advocated by some authors (Balasegaram, 1981; Eggleston et al, 1982) did not show a remarkable improvement in mortality rate, although in Balasegaram’s series, the hospital stay was probably reduced. Ruptured amebic liver abscess occurs in 2% to 17% of patients, with reported mortality rate of 6% to 50%. These patients usually constitute a major risk for surgery and anesthesia.
Prevention
In addition to regular sanitary measures that include proper washing of vegetables and fruits as well as drinking only bottled water in endemic areas, modern biologic techniques have helped characterize amebic antigens that show great promise in the development of a vaccine. No prophylactic vaccine is currently available for amebiasis, but efforts to better define antigenic candidates and wider use of animal models are encouraging. For example, a serine-rich E. histolytica protein (SREHP) has been expressed in avirulent vaccine strains of Salmonella (Stanley, 2006). E. histolytica galactose/N-acetyl-D-galactosamine (Gal/GalNAc) (Houpt et al, 2004) and a synthetic, enhanced, intranasal lectin-based amebiasis subunit have been extensively studied as attractive candidates for vaccine development (Abd Alla et al, 2007). In addition, Gal-inhibitable lectin shows promise in animal studies (Ivory & Chadee, 2007).
Liver Fluke Disease
Fasciola hepatica and Clonorchis sinensis are the primary liver flukes (see Chapter 45). They are unsegmented leaf-shaped trematodes. Although both have common features with respect to structure and life cycle, with accidental infection of humans, there are some epidemiologic differences. C. sinensis is endemic in East Asia, regions bordered by the South China Sea, and along the great rivers and streams of China, North Vietnam, and Korea. Disease results from eating contaminated raw freshwater fish. These areas also are endemic for viral hepatitis, and epidemiologic surveys of the population yield patients with common symptoms in whom the differential diagnosis must be made (Wang et al, 2004). Opisthorchis viverrini and Opisthorchis felineus are other liver flukes transmitted by the consumption of raw freshwater fish, but most of these cases are asymptomatic. The Western Hemisphere is free of the disease, but clinical manifestations may occur in natives of these areas several years after emigration to other countries. The first report of C. sinensis infection was described in a Chinese carpenter residing in Calcutta, India (McConnell, 1875).
Fascioliasis
Zoonotic disease is caused by F. hepatica (temperate) and F. gigantica (tropical), the causative agents of fascioliasis in cattle and sheep (see Chapter 45). Human infections with F. hepatica are found in areas where sheep and cattle are raised and where humans consume raw watercress, including Europe, the Middle East, and Asia (Thomas & Garg, 2007). Infections with F. gigantica have been reported in Asia, Africa, and Hawaii. Prevalence is high in Bolivia (65% to 92%), Ecuador (24% to 53%), Egypt (2% to 17%), and Peru (10%). In hyperendemic areas of Bolivia, 68% of children are infected; 11% of Ethiopians who emigrated to Israel are also infected (Thomas & Garg, 2007). An estimated 2.4 million people are infected with liver flukes worldwide (Mas-Coma et al, 1999). The incidence in humans is estimated to be only 1%, and most patients are encountered as sporadic cases or in small community outbreaks (Ashton et al, 1970).
Morphology and Life Cycle
F. hepatica is a flat, leaflike, hermaphrodite trematode 15 to 30 mm long, 10 mm thick, and 3 mm wide that inhabits the bile ducts and gallbladder of the host. When the eggs in mammalian stool are deposited in tepid water (22° C to 26° C), miracidia appear, develop, and hatch in 9 to 14 days. These miracidia then invade many species of freshwater snails, in which they multiply as sporozoites and redia for 4 to 7 weeks. They leave as free-swimming cercaria that subsequently attach to watercress, water lettuce, mint, parsley, or khat. Free-swimming cercaria may remain suspended in the water and encyst over a few hours. When humans consume contaminated plants or water, larvae excyst in the duodenum, migrate through the bowel wall and peritoneal cavity, and penetrate the Glisson capsule, actions that initiate the acute larval, hepatic, and invasive stages of human infection, in which the parasites eat their way down to the bile ducts. Larvae sometimes also travel to ectopic body sites. This stage may last 3 to 4 months, during which the larvae mature and migrate through liver into large hepatic and common bile ducts. Mature flukes consume hepatocytes and duct epithelium and reside for years in the hepatic and common bile ducts and occasionally in the gallbladder; this is the chronic adult biliary stage of infection. Adult fluke worms produce eggs about 4 months (range, 3 to 18 months) after infection; these eggs traverse the sphincter of Oddi and intestine and then continue the cycle of infection. Acute and chronic stages can overlap, particularly in a high-level infection. The life cycle takes about 5 months, 3 months of which are required for the journey through the human host. The adult worms that develop in the bile ducts at the end of this period have a life span of 3 to 4 years. The parasite may invade other organs during migration, such as the urinary tract, and cause hematuria. Ectopic fascioliasis may affect the peritoneum, muscles, brain, and subcutaneous tissues (Jones et al, 1977).
Pathology
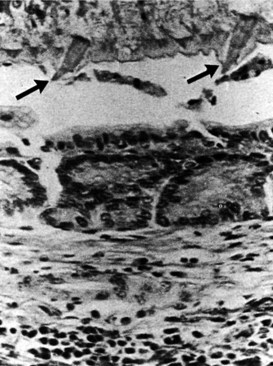
Early during infection, hepatic lesions may be white or gray nodules on the liver capsule, subcapsular tracts, and, rarely, a localized hepatic mass. Later, the worm enters the biliary system. F. hepatica is a relatively large parasite. In the intrahepatic ducts, the worms move to and fro, and the spinous nature of the integument causes extensive destruction of the biliary epithelium; in this way, the presence of the parasite stimulates fibrosis in the bile duct wall (Fig. 67.7). Serious infection may be associated with hepatic and biliary fibrosis, hepatic necrosis, abscess formation, cholangiohepatitis, impaired nutrition, and death. A liver biopsy may reveal a nonspecific round cell infiltration of portal tracts, and cholangiocarcinoma may occur in association with fascioliasis (Thomas & Garg, 2007).
Clinical Features
Eating watercress and drinking contaminated water in an endemic area are the likely routes of infection. Clinical symptoms and signs, together with radiologic findings, are the mainstay of diagnosis. The acute phase usually lasts 4 months and is caused by the migration of the immature fluke through the hepatic parenchyma, leading to hemobilia. Patients usually develop right upper quadrant pain, hepatomegaly, fever, vomiting, diarrhea, anemia, hypergammaglobulinemia (especially IgG and IgE), and allergic reactions (Aksoy et al, 2006).
On US examination of the intrahepatic and extrahepatic bile ducts, no motile echogenic images are seen (Saba et al, 2004). The findings in the liver parenchyma are nonspecific, although suspected nodules or cavitary lesions might warrant further imaging. CT is the most useful method for diagnosis; it can show peripherally located hypodense lesions, usually on the right lobe, with peripheral enhancement on contrast scans, arranged in a track like fashion with no coalescence into a larger abscess cavity, such as is seen in pyogenic abscesses. MRI usually adds nothing and cannot show the tracklike appearance of the lesions (Oto et al, 1999). After infection of the biliary tract or gallbladder, an asymptomatic clinical phase starts during which the flukes mature into adult form. This is followed by the chronic phase, caused by the adult fluke within the biliary system, during which the symptoms persist more than 4 months, are more discrete, and reflect intermittent biliary obstruction and inflammation. The main symptoms include recurrent episodes of biliary colic, cholangitis, and cholecystitis accompanied by constitutional upset, jaundice, anemia, hypoproteinemia, and edema. The liver may be tender and enlarged with splenomegaly. Motile echogenic images with biliary mud can be seen in the gallbladder and bile ducts on US during this phase (Fig. 67.8). Real-time sonography may show the worm moving inside the gallbladder. Biliary duct dilation is common, and MRI cholangiography may show signal loss in the large bile ducts consistent with flukes or edema (LaPook et al, 2000). Diagnosis of fascioliasis by endoscopic ultrasound (EUS) (Fig. 67.9) has also been reported (Sotoudehmanesh & Yoonessi, 2003). Liver biopsy is not indicated for diagnosis and usually reveals necrotic debris, tracklike destruction of parenchyma, polymorphonuclear infiltration with abundant eosinophils, Charcot-Leyden crystals, granulomas with or without eggs, fibrosis, and bile duct proliferation (Acosta-Ferreira et al, 1979). Eosinophilia may be 50% or more of the total white blood cell count and is seen regardless of the clinical stage of infection; it may also be seen in asymptomatic contacts of the patient, who have latent infection (Saba et al, 2004; El-Shabrawi et al, 1997). It has been reported that eosinophilia could be absent in chronic cases in endemic areas (Marcos et al, 2008), and anemia is usually present (Bassiouny et al, 1991). Liver function tests show features of cholestasis. Hemoglobinuria is often present because the flukes can penetrate and lodge in the kidneys during migration through the peritoneal cavity. Adult flukes and eggs are absent from the feces and bile during the acute phase, and even in the chronic phase, so stool examination may not be diagnostic. Repeated stool analysis is required because of intermittent secretion of ova. Serologic modalities include complement fixation test (CFT), indirect fluorescent antibody test (IFAT), IHAT, and counterimmunoelectrophoresis (CIE) may be used with neither low specificity nor sensitivity (Marcos et al, 2008).
A specific serologic test to detect IgG antibodies to a crude Fasciola worm antigen preparation has been found to be positive in 97% to 100% of patients (Maher et al, 1999). Fas2-ELISA is more specific than Western blot and Arc II (Espinoza et al, 2005). The largest human diagnostic study has been carried out in Peru, where the Fas2-ELISA (cathepsin L1-based antibody) was validated, including 634 infected children from endemic areas. The sensitivity was 92.4% and specificity was 83.6%, with a negative predictive value of 97.2% (Espinoza et al, 2007).
The findings on imaging have already been described, and a differential diagnosis from necrotic hepatic neoplasms and liver abscesses must be made (see Chapter 66; LaPook et al, 2000). At laparoscopy or laparotomy, the characteristic features of hard, grayish white nodules on the liver surface with subcapsular channels may be seen in 55% to 75% of patients. Portal lymph nodes are often enlarged, and the common bile duct may be dilated. The diagnosis of fascioliasis should be considered in patients with a history of ingestion of raw watercress or other aquatic plants or those who have recently traveled to an endemic area and develop prostration, cholangitis, hepatosplenomegaly, anemia, and eosinophilia. The acute parenchymal phase is usually difficult to diagnose except by serologic tests for Fasciola. Pseudofascioliasis refers to the presence of eggs in the stool, which results not from an actual infection but from recent ingestion of infected liver containing eggs. This can be avoided by eating a liver-free diet several days before stool examination (Thomas & Garg, 2007).
Consequences of Chronicity of Infection in Fascioliasis
Recent studies have shown a strong association between fascioliasis and liver fibrosis (see Chapter 6). It seems that hepatic fibrosis may evolve in some susceptible hosts depending on the time and burden of infection. Liver cirrhosis has been reported in infected children (Marcos et al, 2005) and adults (Sanchez-Sosa et al, 2000), especially those with high-density infections (see Chapter 70A, Chapter 70B ). New data on the pathogenesis of hepatic involvement associated with F. hepatica infection have implicated cathepsin L1 and its collagenolytic function, associated with tissue invasion (Stack et al, 2007) with proteolytic activity, leading to collagen type I expression and ultimately hepatic fibrosis (Marcos, unpublished observation). On the contrary, chronic infection may also cause a persistent immunosuppressed status, suggesting that the infected host may be susceptible during chronic infection to other Th2-associated pathogens (Gironès et al, 2007).
Treatment
Emetine and chloroquine be may be successful in the short term, but recurrence is the rule because these drugs have no effect on the parasite. Fasciolae are also usually refractory to praziquantel. The drug of choice for both phases of Fasciola infection is triclabendazole, 10 mg/kg orally, repeated after 12 hours (El-Tantawy et al, 2007; Marcos et al, 2008), and it can be used even in the presence of biliary obstruction. Cure rates of 90% have been reported with no significant drug-related side effects. The most frequent adverse event is biliary colic caused by the passage of dead or dying parasites passing through the bile ducts. Bithionol is an alternative, available as 200-mg tablets at a daily dose of 30 mg/kg/day, to a total therapeutic dose of 150 mg/kg. The drug is administered on alternate days in three divided doses after meals. Nausea, abdominal pain, diarrhea, and photosensitivity are the most common side effects. Clinical improvement is gradual and occurs over 2 to 3 weeks (Thomas & Garg, 2007).
Surgical treatment may be indicated for diagnosis or for cholangitis (see Chapter 43). On occasion, a live worm may be found in the gallbladder or the bile duct, or the worm may find its way out through the T-tube placed at surgery (Nicholas, 1970). Endoscopic retrograde cholangiopancreatography (ERCP) may be used to relieve obstruction with removal of the worm. Rarely, percutaneous transhepatic cholangiography (PTC) may be an alternative with failed ERCP (Fig 67.10; see Chapter 18).
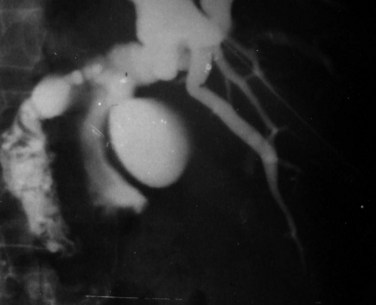
FIGURE 67.10 Percutaneous transhepatic cholangiography of Fasciola hepatica inside the common bile duct.
(Courtesy Professor A.K. El Dory, Ain Shams University, Cairo.)
Proteomics and genomics have led to identification of molecules, including cathepsin L, glutathione S-transferase, leucine aminopeptidase, and fatty acid binding proteins (Dalton et al, 2003). The feasibility of inducing protective responses has been proven only in laboratory and large animals; human studies are lacking. Development of a vaccine for fasciolaisis still requires analysis of the protective effector mechanism provided by different vaccine candidates, vaccine formulations, doses, and route of administration.
Clonorchiasis
Epidemiology
C. sinensisis is endemic in northeast China, southern Korea, Japan, Taiwan, northern Vietnam, and the far eastern part of Russia (Lun et al, 2005; Rim, 2005). In general, the infection is acquired by eating raw or uncooked cyprinoid fish products in rural areas.
Morphology and Life Cycle
C. sinensis is 10 to 25 mm long, 3 to 5 mm wide, pink, and transparent when alive but hard, friable, and black when dead (Hou, 1956). Adult worms inhabit the intrahepatic ducts and may migrate down the common bile duct but tend to die in the gallbladder. In heavy infection, they may be found in the pancreatic duct. Cysts from infected fish are digested by gastric juice; larvae then migrate from the duodenum into the common bile duct upward until they lodge in a duct that is too small for further passage. Eggs laid in bile ducts reach the intestine to be excreted in feces, and the life cycle continues in fresh water in the snail, grass, or black carp, the intermediate hosts. The cercarial forms that emerge from the snail in fresh water attach to and penetrate the fish, with encysted metacercariae developing in the muscles; humans acquire the infection by ingesting the fish (Thomas & Garg, 2007).
Pathology
Irritation by the worm causes the biliary epithelium to undergo hyperplasia, which may progress to metaplasia and adenomatous hyperplasia. In the initial stages, periductal inflammation is mild, and dilation of the small- and medium-sized ducts in the periphery of the liver may occur (Choi et al, 2004; Hou, 1956). Metaplastic changes occur early in biliary epithelium, transforming normal cells into mucin-producing cells, which results in highly mucinous bile. This results in stagnation of bile with repeated cholangiohepatitis that eventually kills the worms. This may lead to ductal fibrosis and stricturing with formation of calcium bilirubinate calculi (Choi et al, 2004) and sepsis resulting from infection by Escherichia coli and anaerobic streptococci (Fung, 1961; Tandon, 1988). Progression to biliary cirrhosis is unusual, but there is an increased incidence of cholangiocarcinoma in patients infected with C. sinensis (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ; Choi et al, 2004; Flavell, 1981; Hou, 1956). The etiology of cholangiocarcinoma is multifactorial. Carcinogens in infected individuals may be prone to malignant changes when hyperplasia of bile duct cells occurs (Flavell, 1981).
Relationship to Recurrent Pyogenic Cholangitis (See Chapter 44)
Of patients with Oriental cholangiohepatitis, 50% are infected with C. sinensis (Augustine et al, 1988; Tandon, 1988; Teoh, 1963). It is prudent to investigate patients with cholangitis or cholangiocarcinoma who reside in endemic areas for evidence of Clonorchis infection because screening for latent infection may be appropriate in relatives and close contacts of patients who test positive for Clonorchis (Thomas & Garg, 2007).
Clinical Features
The clinical features are independent of the severity of infestation. In an endemic area, 86% of 1091 patients screened by stool examination were found to have clonorchiasis. Only one patient had intrahepatic stones, and the 8% incidence of gallbladder stones was related more to ethnicity than to infection with C. sinensis (Hou et al, 1989). The classic signs associated with clonorchiasis are those of recurrent pyogenic cholangitis (Park & Son, 2008; Stunell et al, 2006). When clonorchiasis is acalculous, it is usually asymptomatic. Early symptoms include general malaise, abdominal discomfort, and diarrhea. Screening investigations may show eosinophilia with fluctuating jaundice or elevated liver enzymes (Choi et al, 2004). Clonorchiasis occurs equally in males and females, with peak incidence in the second and third decades of life (Fung, 1961). Another study showed that men are more commonly affected (Choi et al, 2004). Anemia and hypoproteinemia are common. Jaundice varies in intensity, and progression to cirrhosis results in splenomegaly. Serum bilirubin and alkaline phosphatase levels are high, and transaminase levels are slightly elevated. Evidence of pancreatitis may also be found. Plain radiographs of the abdomen may reveal gas in the biliary tree, but stones are rarely visualized. US and CT detect ductal dilation and calculi; however, contrast imaging of the biliary tract is essential to delineate the distribution of stones and strictures and to plan operative therapy. The common bile duct and hepatic ducts are usually dilated (Fung, 1961), but definite stenosis is seen in only 5%. Cholangiocarcinoma may be intrahepatic, hilar, or extrahepatic (Choi et al, 2006). The diagnosis of clonorchiasis with cholangitis depends on high index of suspicion. Elevated eosinophil counts warrant a search for the parasite, and fecal examination for eggs has been used for diagnosis of clonorchiasis (Choi et al, 2005b). Detection of specific antibodies (Kim, 1998; Lin et al, 1995) and circulating antigen by Dot-ELISA has been described (Wang et al, 2004). Other methods for diagnosis include intradermal test with diluted antigens of C. sinensis (Shin & Choi, 2000), radiologic studies of the liver (Choi et al, 2004), and bile examination for eggs, metacercariae, and cercariae. Of these, the intradermal test is the easiest to perform, but it has low specificity (Shin & Choi, 2000). Real-time PCR appears to be a powerful tool for both the detection and quantification of C. sinensis infections (Kim et al, 2009). Paramyosin (CsPmy) is a myofibrillar parasitic protein whose gene has been identified. Induction of host IgG suggests that CsPmy may be a diagnostic antigen or a vaccine for clonorchiasis (Park et al, 2009).
Treatment
Praziquantel (Biltricide) is the drug of choice. The dosage schedule is usually 25 mg/kg/day three times at 5-hour intervals in 1 day (total dose, 75 mg/kg) for 1 or 2 days, with a cure rate of 83% to 85% (Kaewpitoon et al, 2008). Antibiotics may be needed for associated pyogenic cholangitis, and interventional procedures are required in cases complicated by calculus formation and depend on the location of ductal stones and strictures. Endoscopic and minimally invasive techniques have the advantage of reduced morbidity over open surgery (Zhi et al, 2004). These procedures are usually adequate only in simple strictures, and patients referred to surgery are complicated cases in whom multiple endoscopic (ERCP) or percutaneous (PTC) procedures have failed.
Biliary Ascariasis
Epidemiology
Ascaris lumbricoides is a roundworm that commonly infects more than a quarter of the population in Asia, Africa, and Central America as well as those who have emigrated to the United States from these areas (Misra & Dwivedi, 2000). Clinical symptoms occur in individuals who have a heavy worm burden (Khuroo, 2001). When confined to the intestine, Ascaris infestation is usually asymptomatic, although ascariasis is an important cause of biliary disease in areas of the world where the rate of Ascaris infection is high. Tropical and subtropical regions with poor socioeconomic conditions and fecal contamination of the soil around dwellings and farms are main sources of infection (Khuroo et al, 1989a; Louw, 1966). Infection becomes symptomatic when the worms enter the biliary tract (Khuroo et al, 1989a, 1989b). Kamiya and colleagues (1993) reported that more than 11% of patients with gallbladder or biliary tract complications had Ascaris worms in their biliary tract. The incidence of gallstones in developing and developed countries is similar (Hou et al, 1989; Khuroo et al, 1989a), and it is unnecessary to consider ascariasis as an etiologic factor in all patients diagnosed with gallstone disease who hail from an endemic area.
The adult worm in humans inhabits the small intestine, mainly the jejunum, where the fertilized female lays eggs. These ova are excreted in host feces and can survive environmental conditions. In warm moist soil, the ova undergo maturation and are able to infect humans if the egg is swallowed. Mature ova hatch in the duodenum, releasing larvae that penetrate the mucosa of the proximal intestine to enter the portal venous blood, in which they are carried through the liver and via the right side of the heart to the pulmonary capillary bed. Here, larvae develop further, penetrating alveoli to travel up the trachea, enter the esophagus, and return to the intestine. In the jejunum, they mature to adults, reach 20 to 30 cm in length, and have a life span of 1 to 2 years (Thomas & Garg, 2007).
Pathology
The adult Ascaris reaches the duodenum either because of increased worm burden in the jejunum or as a consequence of increased intestinal motility (Khuroo, 1996). The ability of A. lumbricoides to enter the common bile duct, especially with heavy duodenal infection, is well known. Usually only one or two worms enter the biliary system, but massive infection may occur. The worm head moves first through the ampulla of Vater to the common bile duct, cystic duct, and intrahepatic ducts; because the length of the extrahepatic tree is only 4 to 10 cm, part of the worm may remain in the duodenum (Davies & Rode, 1982). In 2002, Kamiya and colleagues reported that 95% of the worms found in the biliary tract were located in the hepatocholedochus, whereas only 3% were found in the gallbladder and even more rarely in the intrahepatic ducts. Migration into the biliary tract has been reported to be frequent after cholecystectomy, sphincterotomy, sphincteroplasty, choledochostomy, or biliary enteric anastomosis, presumably from increased space in the bile duct after gallbladder removal and increased release of cholecystokinin and secretin, which relaxes the sphincter of Oddi (Wani et al, 2000). Biliary migration is commonly seen in women, especially pregnant women; this is due to high levels of progesterone, which also relaxes the sphincter of Oddi (Shah et al, 2005). Children are at lower risk for biliary migration because of the small diameter of their ductal systems (Zargar & Khuroo, 1990).
The worm tends to move out of the biliary tract within 24 to 36 hours of inducing biliary or pancreatic symptoms, and the detection of live ascarids in the duodenal lumen is considered strong evidence of biliary ascariasis. The impacted worm causes spasm of the sphincter of Oddi, resulting in partial biliary obstruction and colicky pain, and cholangitis can occur after mechanical obstruction (see Chapter 43). Suppurative cholangitis may extend to intrahepatic ducts and cause multiple cholangiolitic liver abscesses. Acalculous cholecystitis, empyema of the gallbladder and, less commonly, necrosis and perforation of the bile duct may occur (Chang & Han, 1966). Dead worms cannot leave the bile duct; rather they disintegrate, providing a nidus for stones and provoking a chronic inflammatory response that leads to ductal strictures. Calculi associated with ascariasis are of the calcium bilirubinate type (Khuroo et al, 1989b; Maki, 1966), with the exception that ascarid ova or debris usually can be identified in the calculus. Escherichia coli, the pathogen usually identified with calcium bilirubinate stones, is frequently encountered in suppurative cholangitis (Maki, 1966). Another factor that may facilitate stone formation is the albuminoid membrane that covers most of the eggs and is not found in any other parasite. Worms reaching the intrahepatic ducts may become impacted or invade the liver parenchyma to form nests of worms (Lloyd, 1982). Unable to extricate themselves, the worms die. The resultant cholangiolitic abscess may enlarge and present on the surface or rupture into the peritoneal cavity. Extension of the abscess through the diaphragm can cause bronchopleural fistula and lung abscess, a well-known and potentially fatal complication (Chang & Han, 1966; Lloyd, 1981). The presence of dead worms in the bile duct also induces an intense eosinophilic inflammation that precipitates a fibromatous reaction. Eventually, the dead worms are surrounded by calcification, and the ongoing inflammation results in biliary stricture formation (Shah et al, 2006). Based on the aforementioned pathologic features, biliary ascariasis has been classified as uncomplicated and complicated (Thomas & Garg, 2007).
Clinical Features
Biliary ascariasis in the pediatric age group is most commonly seen in children aged 2 to 8 years. In endemic areas, adults are frequently affected, and mean age at presentation is 35 years. For unknown reasons, women are three times more likely to be affected than men; in children, a similar sex difference has not been noted. The mean duration of symptoms varies from 4 to 6 years, and 40% of patients may give a previous history of biliary surgery. In one large series, 80% of patients had undergone either cholecystectomy or endoscopic sphincterotomy (Sandouk et al, 1997a). Microscopic examination of stool and bile samples in these patients gives a positivity rate for A. lumbricoides of nearly 100% (Khuroo et al, 1990). Vomiting of worms is common, as well as a history of passing worms in the stool (Lloyd, 1981; Louw, 1966).
The most common presentation is usually with sudden severe upper abdominal colic with tenderness and guarding localized to the right upper quadrant or epigastrium. If present, fever is usually low grade, and jaundice, marked toxemia, and hepatomegaly are usually absent. Worms are rarely seen in the gallbladder, and the US finding of distension, wall edema, and intraluminal sludge completely resolves, usually within 2 weeks. Endoscopic examination is difficult to perform and unnecessary in the acute phase, but if done it may show duodenal or ampullary worms. ERCP has been commonly used for both diagnosis and removal of worms from the biliary tract (Fig. 67.11) and is particularly valuable when used with real-time US, with a sensitivity of 100% (Sanai & Al-Karawi, 2007).
Acute Suppurative Cholangitis
Worms in the bile duct may block the cystic duct, leading to acute acalculous cholecystitis or empyema of the gallbladder. Biliary stasis as a result of spasm of the sphincter of Oddi coupled with intestinal contents carried by the worm into biliary tract lead to pyogenic cholangitis (Shah et al, 2006). This appears with right hypochondrial pain, fever, jaundice, tender hepatomegaly, and leukocytosis. Apathy, hypotension, metabolic acidosis, and electrolyte disturbances signify a grave prognosis. US may show worms in the biliary tree, and endoscopy may visualize pus, a tubular filling defect not suggestive of a tumor or stone on cholangiography, or a worm emerging from the ampulla (Fig. 67.12).
Hepatic Ascariasis
No clinical features distinguish hepatic ascariasis from suppurative cholangitis. The diagnosis is usually made at US, and aspirated pus might yield the ova of A. lumbricoides. The right lobe is more commonly affected, and rupture into the peritoneal cavity is a recognized complication (Shah et al, 2006).
Acute Pancreatitis
The character of the pain with acute pancreatitis varies, but an increase in serum amylase confirms diagnosis. Although encountered half as often as the purely biliary presentation (Louw, 1966), the condition was seen in only 31 of 500 patients in the series by Khuroo and colleagues (1990). Severe pancreatitis with progression to hemorrhagic pancreatitis or pancreatic fluid collections occurred in only one tenth of these cases. Sandouk and colleagues (1997a) reviewed 300 patients with pancreatic ascariasis in Syria and showed that US, along with clinical findings, was the mainstay of diagnosis, similar to another review of 14 patients (Misra & Dwivedi, 2000).
Diagnosis
Laboratory Studies
Stool analysis is usually the investigation of choice in the search for ascarid ova or remnants of dead worms, and results may approach 100% (Khuroo et al, 1990). Leukocytosis greater than 12,000/mm3 usually denotes suppurative biliary and hepatic complications. The eosinophil count is seldom elevated more than 5%. Hyperbilirubinemia occurs in hepatopancreatobiliary ascariasis (Lloyd, 1982). Elevation of alkaline phosphatase and transaminases may occur when cholangitis supervenes, and pancreatitis is suggested if serum amylase is greatly elevated. Normal liver enzymes and amylase levels have been reported in a case of recurrent ascaris related pancreatitis as a result of worm migration (Lee et al, 2009). Specific serologic tests are available that can be used to monitor successful treatment (Santra et al, 2001) but are not commonly used in clinical practice.
Diagnostic Imaging
Abdominal radiographs may confirm the presence of intestinal worms in 90% of children. On occasion, air is seen in the biliary tree or in hepatic or subphrenic abscesses (Lloyd, 1982). US is the imaging modality of choice because it shows dilated bile ducts containing linear or round areas of increased echogenicity representing worms (Desai & Tobin, 1995; Akhter et al, 2006’). Other findings were gallbladder distension with sludge inside, an edematous wall, and a mildly dilated biliary tree (Akhter et al, 2006). Real-time scanning may reveal the diagnostic movement of active worms within the biliary system. Multiple bile duct worms have a spaghetti-like appearance, with alternating echogenic and echolucent strips; if densely packed in the bile duct, they may appear as a hyperechoic pseudotumor (Shah et al, 2006). On transverse scanning, the biliary worm produces a bulls-eye appearance (Cremin, 1982). Other characteristic appearances include single or multiple long, linear echogenic strips within the bile duct without acoustic shadowing (Khuroo et al, 1987). US is useful for identifying intrahepatic worms and abscesses and for monitoring the response of lesions to therapy. Ascarid liver abscesses characteristically contain echogenic worm debris; CT does not offer significant advantage over real-time US, and it is less sensitive (Sandouk et al, 1997a). A thin line of dye is occasionally seen within the corpus of worm that has swallowed dye, and the worm is easily visualized on an unenhanced scan within the biliary tree (Sherman & Weber, 2005).
Endoscopy may reveal a worm in the duodenum, and occasionally a worm may be seen impacted in or protruding from the ampulla of Vater. ERCP has been done safely, even in the acute setting, and it shows worms in the extrahepatic and intrahepatic biliary tree, including liver abscesses (Lloyd, 1981) and cases of recurrent pancreatitis (Lee et al, 2009). Biliary strictures can be studied, and endoscopic extraction of worms and biliary decompression may succeed in aborting an attack of severe cholangitis (Khuroo et al, 1990). Magnetic resonance cholangiopancreatography (MRCP) is a useful modality in pancreaticobiliary ascariasis. The worms are described as linear, hyperintense tubular structures with a central hypointense area (Arya et al, 2005). EUS has been shown to document biliary ascariasis (Sriram et al, 2006). Percutaneous transhepatic cholangiography has been used in cases of failed ERCP to visualize and relieve obstruction in the abnormal biliary tree containing worms (Lloyd, 1982). Adult worms or debris may be identified in the aspirated bile.
Management
The management strategy for ascariasis includes conservative measures, endoscopic extraction, or surgical intervention. Without appropriate nonoperative management, worms spontaneously return to the duodenum in 98% of children (Chang & Han, 1966; Louw, 1966) and 94% of adults (Khuroo et al, 1990). Parenteral antispasmodic drugs and analgesics to relax the sphincter of Oddi and relieve biliary colic, nasogastric decompression, and intravenous fluids are recommended in the acute stage. Successful elimination of an adult Ascaris worm from the gallbladder 7 days after killing it with albendazole has been documented (Cha et al, 2002); however, oral antihelmintic drugs may not be helpful in the treatment of biliary ascariasis because of poor enterohepatic circulation (Khuroo, 1996). Administration of piperazine citrate through the nasobiliary catheter has been shown to be effective (Kamath et al, 1986). However, some investigators believe that this approach should not be used because it has been reported to cause paralysis or death of the worm in the bile duct, and this dead worm may act as a nidus for future stone formation (Shah et al, 2006). Albendazole (400 mg/day for 1 day) or mebendazole (100 mg twice daily for 3 days) and pyrantel pamoate (single dose of 11 mg/kg to a maximum of 1 g) are the preferred agents, and cure can be achieved in nearly 100% of cases. The safety of these drugs in pregnant women and children younger than 2 years is not established; in this situation, piperazine is safe in a single dose of 75 mg/kg (maximum 3.5 g) on 2 consecutive days. Stool examination is required to confirm eradication of the infection, and follow-up US is needed to look for intraductal worms. ERCP is recommended if symptoms or US abnormalities persist beyond 2 weeks with suspected pancreatobiliary ascariasis. To prevent reinfection in endemic areas, antihelmintic therapy must be repeated every 2 months in patients who are cured (Khuroo et al, 1990).
The problem of reinfection of the bile ducts after operative or endoscopic removal of worms has led to important modifications in the interventional approach previously advocated (Chang & Han, 1966; Louw, 1966). Preoperative eradication of intestinal worms ideally should be done before endoscopic or surgical manipulation of the common bile duct. Severe persistent pain unrespondive to antihelmintics is an indication for ERCP and endoscopic removal of intrabiliary worms. In an endemic area, multiple reinvasions of the bile ducts by worms is seen in patients who have undergone endoscopic sphincterotomy (Khuroo et al, 1990).
It would seem to be of paramount importance to preserve the sphincter of Oddi (Shah et al, 2006; Khuroo & Zargar, 1985; Khuroo et al, 1990). Failure of response to conservative management with increasing jaundice may indicate coexisting stones in the common bile duct (Khuroo & Zargar, 1985; Khuroo et al, 1990). When skilled endoscopic intervention has failed or is unavailable, PTC or surgery is recommended, a situation more likely to occur in children than in adults (Hangloo et al, 1989). Laparoscopic common bile duct exploration, with removal of the worm and a cholecystectomy, was reported by Astudillo and colleagues (2008).
Worm extraction with biliary drainage may be lifesaving in patients with severe cholangitis, and it is successful in all patients from the papillary opening and in more than 90% of patients from the bile ducts (Shah et al, 2006). Biliary cannulation in these patients is easier to achieve because the passage of worms across the sphincter makes it patulous (Khuroo et al, 1990). Worms can be extracted across the papillary opening by using a grasping forceps or a dormia basket; polypectomy snares should be avoided because the snare may cut the worm, leaving a remnant that may lead to stone formation. The worms in the bile duct may protrude out of the papilla after injection of the contrast and thus may be extracted. If the worms are unable to come out or are dead, they can be pulled out using a dormia basket or a biliary occlusion balloon (Reddy et al, 2003). Total clearance of bile ducts may require multiple endoscopic sessions. Endoscopic papillary balloon dilation has been described in nonendemic areas to remove the worm and associated calculi (Misra & Dwivedi, 1998).When the worm cannot be reached by conventional ERCP, Sandouk and colleagues (1997b) have suggested using the whirlpool jet technique. Early ERCP should be performed when pancreatobiliary ascariasis is suspected in cases of repeated pancreatitis following cholecystectomy and sphincterotomy, even with normal liver enzymes, because of worm migration (Lee et al, 2009).
Surgical intervention is required in patients after an unsuccessful endoscopic attempt; if the Ascaris organisms enter the intrahepatic ducts, leading to the formation of stones, strictures and abscesses; and when gallbladder ascariasis is present (see Chapters 39 and 44; Shah et al, 2006). Laparoscopic extraction of live worms from the bile duct has also been reported in the literature (Yoshihara et al, 2000). Overall, 20% of patients with biliary ascariasis need surgery (Wani & Chrungoo, 1992). An initial operative cholangiogram helps define the extent of the infection. Worms are removed through a longitudinal choledochotomy because compression of the liver often propels intrahepatic worms toward the common bile duct. Choledochoscopy is also valuable, especially if worms, debris, or stones are impacted in the intrahepatic ducts.
Postoperatively, a broad-spectrum antibiotic is prescribed. Worms identified in the bile duct at this point should be managed conservatively because the worm may spontaneously return to the intestine. Irrigation of the T-tube with saline may help, but antihelmintics should not be administered through the T-tube; this would kill the worm in the common bile duct and prevent spontaneous evacuation. Endoscopic extraction may be successful if the worm is impacted around the T-tube or can be removed by applying suction to a T-tube and gently withdrawing it with the worm. If these measures fail, reoperation is necessary with preoperative oral antihelmintics (Thomas & Garg, 2007).
Full recovery is the rule, with a mortality rate of 1% or less. Worm reinvasion after successful initial management is always symptomatic and may occur in 30% of those treated (Khuroo et al, 1989b, 1990). A careful search for biliary lithiasis in the intrahepatic and extrahepatic bile ducts and management of sepsis and biliary strictures should result in a good prognosis. MRCP and ERCP should always be considered in cases of repeated pancreatitis. Recurrent pyogenic cholangitis, liver abscesses, and granuloma necessitate surgery.
Biliary Opisthorchiasis (See Chapter 45)
Opisthorchiasis is caused by Opisthorchis viverrini and O. felineus, the latter of which is endemic in the Soviet Union, Kazakhstan, and Ukraine. The infection is acquired by eating raw or uncooked cyprinoid fish products in rural areas or dishes as koi-pla (Sayasone et al, 2007).
These small flukes migrate to the liver through the duodenum via the ampulla of Vater into the bile ducts, where they mature into adult worms in less than a month. The adult flukes reside in medium-sized and small intrahepatic bile ducts and occasionally in extrahepatic bile ducts, the gallbladder, and pancreatic duct, where they can live for many years; in the liver, they can live for decades (Marcos et al, 2008).
Clinical Features
Opisthorchis Viverrini
In acute infection, only 5% to 10% of heavily infected patients have symptoms, such as right upper quadrant pain, flatulence, fatigue, and a hot sensation over the abdomen. In the chronic phase, mild hepatomegaly occurs. Jaundice and splenomegaly are rare, but intrahepatic duct stones and recurrent cholangitis are common. Whenever jaundice and ascending cholangitis are detected, fluke-related cholangiocarcinoma should be suspected (see Chapter 50B).
Opisthorchis Felineus
In acute infection, symptoms occur 2 to 4 weeks after eating raw fish and include fever, nausea, vomiting, abdominal pain, malaise, arthralgia, lymphadenopathy, and skin rash (Tselepatiotis et al, 2003). Eosinophilia is common during the initial 2 to 6 weeks of infection, with raised liver enzyme levels. In chronic infection, eosinophilia is milder. Patients may come to medical attention with suppurative cholangitis and liver abscess because of biliary obstruction.
Consequences of Chronicity of Infection in Opisthorchiasis
The higher the intensity and the higher the anti–O. viverrini antibody titres, the higher the risk for cholangiocarcinoma (Honjo et al, 2005). The International Agency for Research on Cancer recognizes this parasite as a category I carcinogen. The lesions that predispose to malignant changes in O. viverrini are dilation of subcapsular medium and large bile ducts with prominent fibrotic wall, periductal inflammatory cell infiltration, goblet cell metaplasia, epithelial and adenomatous hyperplasia, and periductal fibrosis. The pathogenesis of O. viverrini–mediated hepatobiliary changes may be due to mechanical irritation or its metabolic products (Sriamporn, et al, 2004). Several N-nitrosol compounds and their precursors occur at low levels in fermented food, such as preserved mud fish paste (pla ra), a condiment that is a ubiquitous component of the cuisine of northeastern Thailand and Laos (Sripa et al, 2007).
The study of O. viverrini genes should expedite molecular studies of cholangiocarcinogenesis and accelerate research focused on developing new interventions, drugs, and vaccines that might help in controlling O. viverrini and related flukes (Laha et al, 2007).
Diagnosis of Opisthorchiasis
Opisthorchiasis is diagnosed by detection of eggs in feces. In light infections, those with fewer than 10 adult worms in the biliary tract, a PCR detecting the DNA of the adult parasite in stools may be helpful (Duenngai et al, 2008). Ruangsittichai and colleagues (2006) reported high sensitivity and specificity using a recombinant eggshell protein with potential for the serodiagnosis of human opisthorchiasis.
Blood Flukes
Schistosomiasis
Schistosomiasis is sometimes referred to as bilharziasis after Theodor Bilharz, who first identified the parasite in 1852. Schistosomiasis is caused by infection with blood flukes known as schistosomes. It can be associated with serious morbidity and mortality and may cause anemia, chronic pain, diarrhea, exercise intolerance, malnutrition, bladder cancer, portal hypertension, and central nervous system complications (King et al, 2005). Chronic complications usually occur in individuals who live in endemic areas and have recurrent exposure. However, schistosomiasis can also cause complications in people with even brief exposures, such as travelers (Blanchard, 2004). Three major species and two less common species produce infection in humans. Of the major species, Schistosoma mansoni is common in tropical and subtropical areas of sub-Saharan Africa, the Middle East, South America, and the Caribbean. S. japonicum, prevalent in Asia, can provoke intestinal and hepatic complications. S. haematobium is common in North Africa, sub-Saharan Africa, the Middle East, Turkey, and India and predominantly leads to renal and bladder sequelae, although on occasion it results in liver disease. The minor species include S. mekongi and S. intercalatum, both of which can also induce intestinal and liver disease. More than 200 million people worldwide are estimated to have schistosomiasis, a disease responsible for more than 200,000 deaths annually (Chistulo et al, 2004).
Life Cycle
The life cycle of schistosomiasis is complex and requires both intermediate and definitive hosts. The adult worms are approximately 1 to 2 cm in length with a cylindrical body, two terminal suckers, a blind digestive tract, and reproductive organs (Gryseels et al, 2006). The male schistosome forms a groove, where the female resides. Females produce hundreds to thousands of eggs per day. After contact with water, the ovum releases miracidium, which seeks an intermediate host in the snail. After 4 to 6 weeks, the miracida multiply asexually into sporocysts and later into cercariae. The cercariae leave the snail and seek a definitive host, where they develop into adult worms. As the definitive host, humans acquire schistosomiasis via contact with fresh water. Cercariae penetrate the intact skin of humans and become schistosomulae, which migrate from the skin into blood and lymph vessels and are carried to the heart and lungs. They then migrate through pulmonary capillaries into the left side of the heart and then into the arterial circulation, where they are carried to the mesenteric arteries, splanchnic arteries, and portal veins; they subsequently reach the liver, where they mature into adults over a period of 1 to 4 weeks.
Different species have a propensity to affect different organs. The adult worms migrate against portal blood flow to varying destinations: mesenteric venules of the small intestine (S. japonicum and S. mekongi), mesenteric venules of the colon (S. mansoni and S. intercalatum), and the vesical venous plexus (S. haematobium). Adults remain in these blood vessels for life, residing in permanent copulation and adhering to wall of blood vessels. Worms survive for 5 to 7 years but can persist up to 30 years (Arnon, 1990). Female worm begins to produce eggs, which travel hematogenously to other sites or traverse from vascular space through host tissues to the lumina of intestine or urinary bladder. The eggs are then variably excreted in the feces (S. mansoni, S. japonicum, S. haematobium, S. intercalatum, and S. mekongi) or the urine (S. haematobium).
Pathogenesis
Unless adult worms migrate to an unusual location, such as the spinal cord or brain, little damage occurs to the host, whereas cellular infiltrate is consistently found around the eggs (Keating et al, 2006). Eggs released into the bloodstream can invade local tissues, release toxins and enzymes, and provoke a Th-2–mediated immune response. Inflammation and granuloma formation occur around deposited eggs and lead to fibrosis of affected tissues (Cheever et al, 2000). S. mansoni, S. japonicum, S, mekongi, and S. intercalatum eggs tend to either penetrate the bowel adjacent to the mesenteric vessels or travel via the portal venous system to the liver. In the bowel, granulomatous inflammation around the invading eggs can result in intestinal schistosomiasis characterized by ulceration and scarring (Strickland, 1994).
The interaction between the parasite and host immune response is complex. Immunity to infection with any schistosome species is both innate and acquired. It is the host’s immune response to invading eggs that is the major cause of clinical disease. A host-mediated type 2 Th2 fibrogranulomatous inflammatory response occurs, which may result in activation of hepatic stellate cells, the mediators of fibrosis (Bartley et al, 2006).
Clinical Picture
Most patients are asymptomatic, and symptoms depend on the stage of disease. The different species of Schistosoma cause varying clinical complications. Acute symptoms may present as swimmer’s itch or Katayama fever. Symptoms are also more likely to occur in travelers and other nonimmune hosts. Lymphadenopathy, eosinophilia, and hepatosplenomegaly may be prominent findings. The diagnosis of Katayama fever relies upon appropriate epidemiology and consistent clinical findings (Gryseels et al, 2006).
Symptoms of chronic infection often begin insidiously and are progressive without treatment. Intestinal schistosomiasis may cause chronic or intermittent abdominal pain and diarrhea with iron-deficiency anemia secondary to ulceration and polyps. Acute appendicitis has also been described in one case report (Gabbi et al, 2006). Hepatic schistosomiasis can lead to hepatomegaly and severe splenomegaly in children and adolescents. Chronic hepatic schistosomiasis develops years later in young and middle-aged adults, with a long duration of intense infection, splenomegaly, and portal hypertension. However, hepatocellular function remains normal. Leading causes of morbidity and mortality include the formation of ascites and esophageal bleeding from varices. Urinary schistosomiasis may be asymptomatic or may cause hematuria, and symptoms related to anemia may be present. Squamous cell carcinoma of the bladder and nephritic syndrome may occur (Ross et al, 2002).
Schistosomiasis can sometimes be associated with serious neurologic complications. Pulmonary manifestations can also be seen in people with hepatosplenic disease as a result of heavy infections with S. mansoni, S. japonicum, or S. haematobium infection. Presinusoidal portal hypertension fosters the development of portosystemic collateral vessels that allow Schistosoma eggs to embolize into the pulmonary circulation, leading to granulomatous pulmonary endarteritis. Pulmonary hypertension and cor pulmonale gradually ensue with dyspnea as the principal symptom. As disease evolves, the heart enlarges and pulmonary arteries dilate to aneurysmal proportions, representing end-stage, irreversible alterations (Sarwat et al, 1986). Recurrent urinary tract infections or bacteremia as a result of Salmonella infection are classic complications of schistosomiasis (Elliott, 1996). It has been postulated that infection with S. haematobium may increase the risk of HIV infection (De Silva et al, 2006). Persons with hepatitis B or C viruses have more severe disease.
Treatment
Praziquantel is the drug of choice for treatment of schistosomiasis. S. haematobium, S. mansoni, and S. intercalatum can be treated with 40 mg/kg in one or two doses, whereas the dosage for S. japonicum and S. mekongi is 60 mg/kg in two or three doses at least 3 hours apart. No serious side effects are reported, and treatment is efficacious in about 85% to 90% (Ross et al, 2002). In addition to antihelmintic therapy, patients with severe portal hypertension and esophageal varices may also benefit from treatment with propranolol and/or sclerotherapy band ligation or shunt procedures (see Chapter 70A, Chapter 70B ; Elliott, 1996).
Despite its demonstrated efficacy in decreasing recurrent variceal hemorrhage, enthusiasm for surgery has declined over the last 2 decades, due in part to the more easily administered techniques of endoscopic therapy or intervention. Orthotopic liver transplant is the only treatment that corrects the portal hypertension and also corrects the liver failure when it occurs. Endoscopic therapy or intervention and distal splenorenal shunting do not hinder transplantation but lengthen the time taken for surgery (Bismuth et al, 1995).
Acknowledgment
The authors acknowledge the contributions of P.G. Thomas and N. Garg to this chapter.
Abd Alla MD, et al. Identification of the Entamoeba histolytica galactose-inhibitable lectin epitopes recognized by human immunoglobulin A antibodies following cure of amebic liver abscess. Infect Immun. 2004;72:3974-3980.
Abd Alla MD, et al. Adherence-inhibitory intestinal immunoglobulin a antibody response in baboons elicited by use of a synthetic intranasal lectin-based amebiasis subunit vaccine. Infect Immun. 2007;75:3812-3822.
Acosta-Ferreira W, et al. Fasciola hepatica human infection: histopathological study of sixteen cases. Virchows Arch A Pathol Anat Histol. 1979;383:319-327.
Agarwal DK, et al. Percutaneous catheter drainage of amebic liver abscesses, with and without intrahepatic biliary communication: a comparative study. Eur J Radiol. 1995;20:61-64.
Aikat BK, et al. The pathology and pathogenesis of fatal hepatic amoebiasis: a study based on 79 autopsy cases. Trans R Soc Trop Med Hyg. 1979;73:188-192.
Akhter N, et al. Prevalence of biliary ascariasis and its relation to biliary lithiasis. J Med Ultrasound. 2006;33(1):55-56.
Aksoy DY, et al. Fasciola hepatica infection: clinical and computerized tomographic findings of ten patients. Turk J Gastroenterol. 2006;17:40-45.
Ali IKM, et al. Evidence for a link between parasite genotype and outcome of infection with Entamoeba histolytica. J Clin Microbiol. 2007;45:285-289.
Anderson JH, Koch DA. lodochloroxyqiunoline (Vioform N.N.R.) as an amebacide in macaques. Proc Soc Exper Biol Med. 1931;28:828-844.
Angel C, et al. Gastric wall erosion by an amebic liver abscess in a 3-year-old girl. Pediatr Surg Int. 2000;16:429-430.
Arellano J, et al. Increased frequency of HLA-DR3 and complotype SCO1 in Mexican mestizo children with amebic abscess of the liver. Parasite Immun. 1996;18:491-498.
Arnon R. Life span of parasite in schistosomiasis patients. Isr J Med Sci. 1990;26:404.
Arya PK, et al. Magnetic resonance appearance of gall bladder ascariasis. Indian J Med Sci. 2005;59:208-210.
Ashton WL, et al. Human fascioliasis in Shropshire. Br Med J. 1970;3:500-502.
Astudillo JA, et al. Ascariasis in the hepatobiliary system: laparoscopic management. J Am Coll Surg. 2008;207:527-532.
Augustine P, et al. Recurrent pyogenic cholangitis (Oriental cholangiopathy) in Kerla. J Gastoenterol Hepatol. 1988;3:515.
Balasegaram M. Management of hepatic abscess. Curr Probl Surg. 1981;18:282-340.
Ballingall G. Practical observations on fever, dysentery and liver complaints as they occur amongst the European troops in India. Edinburgh, UK: Balfour and Clark; 1818.
Bartley PB, et al. A contributory role for activated hepatic stellate cells in the dynamics of Schistosomia japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993-1001.
Bassiouny HK, et al. Human fascioliasis in Egypt: effect of infection and efficacy of bithionol treatment. J Trop Med Hyg. 1991;94:333-337.
Bayraktar Y, et al. Percutaneous drainage of hepatic abscess: therapy does not differ for those with identifiable biliary fistula. Hepatogastroenterology. 1996;43:620-626.
Beck DL, et al. Entamoeba histolytica: sequence conservation of the gal/galnac lectin from clinical isolates. Exp Parasitol. 2002;101:157-163.
Beck DL, et al. High prevalence of Entamoeba moshkovskii in a Tanzanian HIV population. Acta Trop. 2008;107:48-49.
Bhattacharya A, et al. Absence of lipophosphoglycan-like glycoconjugates in Entamoeba dispar. Parasitology. 2000;120:31-35.
Bismuth H, et al. Liver transplantation in the treatment strategy of portal hypertension. Chirurg. 1995;66:574-581.
Blanchard TJ. Schistosomiasis. Travel Med Infect Dis. 2004;2:5-11.
Blazquez S, et al. Initiation of inflammation and cell death during liver abscess formation by Entamoeba histolytica depends on activity of the galactose/N-acetyl-D-galactosamine lectin. Int J Parasitol. 2007;37:425-433.
Blessmann J, et al. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am J Trop Med Hyg. 2002;66:578-583.
Blessmann J, et al. Hepatic ultrasound in a population with high incidence of invasive amoebiasis: evidence for subclinical, self-limited amoebic liver abscess. Trop Med Int Health. 2003;8:231-233.
Braga LL, et al. Inhibition of the complement membrane attack complex by the galactose-specific adhesion of Entamoeba histolytica. Arch Med Res. 1992;23:133.
Bray RS. Amoebiasis. In: Cox, FEG, editor. The Wellcome Trust Illustrated History of Tropical Diseases. London: The Wellcome Trust; 1996:170-177.
Budd W. On Diseases of the Liver. London: Churchill; 1857.
Castanon-Sanchez C, et al. Entamoeba histolytica: a unicellular organism containing two active genes encoding for members of the TBP family. Protein Expr Purif. 2009;70:48-59.
Cha DY, et al. Successful elimination of Ascaris lumbricoides from the gallbladder by conservative medical therapy. J Gastroenterol. 2002;37:758-760.
Chang CC, Han CT. Biliary ascariasis in childhood. Chin Med J. 1966;85:167-171.
Chaplin A. The Illness and Death of Napoleon Bonaparte. London: Hirschfeld Brothers; 1913.
Chavez-Tapia NC, et al. Image-guided percutaneous procedure plus metronidazole versus metronidazole alone for uncomplicated amebic liver abscess. Cochrane Database Syst Rev. 1, 2009. CD004886
Cheever AW, et al. Immunopathology of Schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465.
Chistulo L, et al. Disease watch: schistosomiasis. TDR Nat Rev Microbiology. 2004;2:12.
Choi BI, et al. Clonorchiasis and cholangiocarcinoma: etiological relationship and imaging diagnosis. Clin Microbiol Rev. 2004;17:540-552.
Choi MH, et al. An unusual surface peroxiredoxin protects invasive E. histolytica from oxidant attack. Mol Biochem Parasitol. 2005;143:80-89.
Choi MH, et al. Correlation of egg counts of Clonorchis sinensis by three methods of fecal examination. Korean J Parasitol. 2005;43:115-117.
Choi D, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006;44:1066-1073.
Clark CG, et al. Structure and content of the Entamoeba histolytica genome. Adv Parasitol. 2007;65:51-190.
Cremin BJ. Real-time ultrasound in paediatric biliary ascariasis. S Afr Med J. 1982;61:914-916.
Dalton JP, et al. Fasciola hepatica cathepsin L–like proteases: biology, function, and potential in the development of first-generation liver fluke vaccines. Int J Parasitol. 2003;33:1173-1181.
Davies MR, Rode H. Biliary ascariasis in children. Prog Pediatr Surg. 1982;15:55-74.
Debakey ME, Ochsner A. Hepatic amebiasis: a 20-year experience and analysis of 263 cases. Surg Gynecol Obstet. 1951;92:209-231.
Desai S, Tobin K. Biliary ascariasis: sonographic findings. AJR Am J Roentgenol. 1995;164:767-768.
De Silva S, Walsh J, Brown M. Symptomatic Schistosoma mansoni infection as an immune restoration phenomenon in a patient receiving antiretroviral therapy. Clin Infect Dis. 2006;42:303-304.
Diamond LS, Clark CG. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol. 1993;40:340-344.
Duenngai K, et al. Improvement of PCR for detection of Opisthorchis viverrini DNA in human stool samples. J Clin Microbiol. 2008;46:366-368.
Eggleston FC, et al. Amoebic peritonitis secondary to amoebic liver abscess. Surgery. 1982;91:46-48.
Elliott DE. Schistosomiasis: pathophysiology, diagnosis, and treatment. Gastroenterol Clin North Am. 1996;25:599-625.
El-Shabrawi M, et al. Human fascioliasis: clinical features and diagnostic difficulties in Egyptian children. J Trop Pediatr. 1997;43:162-166.
El-Tantawy WH, et al. Effect of fascioliasis on the pharmacokinetic parameters of triclabendazole in human subjects. Pharm World Sci. 2007;29:190.
Elzi L, et al. Low sensitivity of ultrasonography for the early diagnosis of amebic liver abscess. Am J Med. 2004;117:519-522.
Espinoza JR, et al. Fas2-ELISA in the detection of human infection by Fasciola hepatica. J Helminthol. 2005;79:235-240.
Espinoza JR, et al. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg. 2007;76:977-982.
Faust EC. The Incidence and significance of infestation with Entamoeba histolytica in New Orleans and the American tropics. Am J Trop Med Hyg. 1931;s1-s11:231-237.
Flavell DJ. Liver-fluke infection as an aetiological factor in bile-duct carcinoma of man. Trans R Soc Trop Med Hyg. 1981;75:814-824.
Fotedar R, et al. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511-532.
Fotedar R, et al. Entamoeba moshkovskii infections in Sydney, Australia. Eur J Clin Microbiol Infect Dis. 2008;27:133-137.
Fung J. Liver fluke infestation and cholangio-hepatitis. Br J Surg. 1961;48:404-415.
Gabbi C, et al. Acute abdomen associated with schistosomiasis of the appendix. Dig Dis Sci. 2006;51:215-217.
Gatharim V, Jackson TF. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S Afr Med J. 1987;72:669-672.
Gatti S, et al. Amebic infections due to the Entamoeba histolytica–Entamoeba dispar complex: a study of the incidence in a remote rural area of Ecuador. Am J Trop Med Hyg. 2002;67:123-127.
Gaucher D, Chadee K. Construction and immunogenicity of a codon-optimized Entamoeba histolytica gal-lectin–based DNA vaccine. Vaccine. 2002;20:3244-3253.
Gironès N, et al. Immune suppression in advanced chronic fascioliasis: an experimental study in a rat model. J Infect Dis. 2007;195:1504-1512.
Gonzalez EG, et al. Calreticulin-like molecule in trophozoites of Entamoeba histolytica HM1:IMSS (Swissprot: accession P83003). Am J Trop Med Hyg. 2002;67:636-639.
Gryseels B, et al. Human schistosomiasis. Lancet. 2006;23(368):1106-1118.
Guarner V. Treatment of amebiasis. In: Martinez-Palomo, A, editor. Amebiasis: Human Parasitic Diseases, No. 2. Amsterdam: Elsevier; 1986:189-211.
Guerrant RL. Amebiasis: introduction, current status, and research questions. Rev Infect Dis. 1986;8:218-227.
Guo X, et al. Protection against intestinal amebiasis by a recombinant vaccine is transferable by T cells and mediated by gamma interferon. Infect Immun. 2009;77:3909-3918.
Haghighi A, et al. Remarkable genetic polymorphism among Entamoeba histolytica isolates from a limited geographic area. J Clin Microbiol. 2002;40:4081-4090.
Hangloo VK, et al. Biliary ascariasis. Indian J Gastroenterol. 1989;8:265-266.
Haque R, et al. Innate and acquired resistance to amebiasis in Bangladeshi children. J Infect Dis. 2002;186:547-552.
Haque R, et al. Amebiasis. N Engl J Med. 2003;348:1565-1573.
Helmy M. Detection and differentiation of Entamoeba histolytica and Entamoeba dispar isolates in clinical samples by PCR. J Egypt Soc Parasitol. 2007;37:257-274.
Hira PR, et al. Invasive amebiasis: challenges in diagnosis in a non-endemic country (Kuwait). Am J Trop Med Hyg. 2001;65(4):341-345.
Hoare A, et al. The feed habits of Entamoeba histolytica in its commensal phase. Parasitology. 1952;42(1-2):43-47.
Honjo S, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, Northeast Thailand. Int J Cancer. 2005;117:854-860.
Hou MF, et al. The ultrasound survey of gallstone diseases of patients infected with Clonorchis sinensis in Southern Taiwan. J Trop Med Hyg. 1989;92:108-111.
Hou PC. The pathology of Clonorchis sinesis infestation of the liver. J Pathol Bacteriol. 1956;70:53-60.
Houpt E, et al. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine. 2004;22:611-617.
Hsu MS, et al. Association between amebic liver abscess and human immunodeficiency virus infection in Taiwanese subjects. BMC Infect Dis. 2008;8:48.
Hung CC, et al. Invasive amebiasis as an emerging parasitic disease in patients with human immunodeficiency virus type 1 infection in Taiwan. Arch Intern Med. 2005;28(165):409-415.
Hung CC, et al. Increased risk for Entamoeba histolytica infection and invasive amebiasis in HIV seropositive men who have sex with men in. Taiwan. PLoS Negl Trop Dis. 2008;2:e175.
Huston CD, et al. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun. 2003;71:964-972.
Irusen EM, et al. Asymptomatic intestinal colonization by pathogenic Entamoeba histolytica in amebic liver abscess: prevalence, response to therapy, and pathogenic potential. Clin Infect Dis. 1992;14:889-893.
Ivory CP, Chadee K. Activation of dendritic cells by the Gal-lectin of Entamoeba histolytica drives Th1 responses in vitro and in vivo. Eur J Immunol. 2007;37:385-394.
Jain NK, et al. Hepatopulmonary amoebiasis: efficacy of various treatment regimens containing dehydroemetine and/or metronidazole. J Assoc Physicians India. 1990;38:269-271.
Johnston V, et al. Fever in returned travelers presenting in the United Kingdom: recommendations for investigation and initial management. J Infect. 2009;59:1-18.
Jones EA, et al. Massive infection with Fasciola hepatica in man. Am J Med. 1977;63:836-842.
Jones WH, Whithington ET. Works of Hippocrates. Loeb Classical Library. London: Heinemann; 1948.
Kaewpitoon N, et al. Opisthorchiasis in Thailand: Review and current status. World J Gastroenterol. 2008;14:2297-2302.
Kamath PS, et al. Biliary ascariasis: ultrasonography, endoscopic retrograde cholangiopancreatography and biliary drainage. Gastroenterology. 1986;91:230-232.
Kamiya T, et al. Duodenoscopic management in biliary ascariasis. Dig Endosc. 1993;5:179-182.
Kamiya T, et al. Biliopancreatic ascariasis: endoscopic approach. J Gastroenterol. 2002;37(Suppl XIII):97-99.
Kapoor OP. Amoebic Liver Abscess. Bombay: SS Publishers; 1979.
Kapoor OP. Imaging of an amoebic liver abscess. Bombay Hosp J. 1989;31:123-125.
Kapoor OP, Joshi VR. Multiple amoebic liver abscess: a study of 56 cases. J Trop Med Hyg. 1992;75:4-6.
Kartulis S. Zur Aetiologie der Dysenterie in Aegyptien. Arch Pathol Anat. 1886;105:521-531.
Keating JH, et al. No overt cellular inflammation around intravascular schistosomes in vivo. J Parasitol. 2006;92:1365-1369.
Ken JG, et al. Perforated amebic liver abscesses: successful percutaneous treatment. Radiology. 1989;170:195-197.
Kershenobish D, Corona DL. Amibiasis hepatica. In: Sociedad Mexicana de Parasitologia, Amibiasis en el Siglo XXI. Mexico City. IDISA Press; 2008:44-56.
Khairnar K, Parija SC. Detection of Entamoeba histolytica DNA in the saliva of amoebic liver abscess patients who received prior treatment with metronidazole. J Health Popul Nutr. 2008;26:418-425.
Khan R, et al. Predictive factors for early aspiration in liver abscess. World J Gastroenterol. 2008;14:2089-2093.
Khan S, Ameen R. Amoebic liver abscess complicated by inferior vena cava and right atrium thrombus. Trop Doct. 2009;39:177-180.
Khan U, et al. Detection of Entamoeba histolytica using polymerase chain reaction in pus samples from amebic liver abscess. Indian J Gastroenterol. 2006;25:55-57.
Khanna S, et al. Experience with aspiration in cases of amebic liver abscess in an endemic area. Eur J Clin Microbiol Infect Dis. 2005;24:428-430.
Khokhani RC, et al. Treatment of amoebic liver abscess with tinidazole and metronidazole. Drugs. 1978;15(Suppl 1):23-25.
Khuroo MS. Ascariasis. Gastroenterol Clin N Am. 1996;25:553-577.
Khuroo MS. Hepatobiliary and pancreatic ascariasis. Indian J Gastroenterol. 2001;20:C28-C32.
Khuroo MS, Zargar SA. Biliary ascariasis: a common cause of biliary and pancreatic disease in an endemic area. Gastroenterology. 1985;88:418-423.
Khuroo MS, et al. Sonographic appearances in biliary ascariasis. Gastroenterology. 1987;93:267-272.
Khuroo MS, et al. Prevalence of biliary tract disease in India: a sonographic study in adult population in Kashmir. Gut. 1989;30:201-205.
Khuroo MS, et al. Biliary and pancreatic ascariasis: a long-term follow up. Natl Med J India. 1989;2:4-7.
Khuroo MS, et al. Hepatobiliary and pancreatic ascariasis in India. Lancet. 1990;335:1503-1506.
Kim EM, et al. Detection of Clonorchis sinensis in stool samples using real-time PCR. Ann Trop Med Parasitol. 2009;103:513-518.
Kim SI. A Clonorchis sinensis–specific antigen that detects active human clonorchiasis. Korean J Parasitol. 1998;36:37-45.
Kimura K, et al. Amebiasis: modern diagnostic imaging with pathological and clinical correlation. Semin Roentgenol. 1997;32:250-275.
King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561-1569.
Kurland JE, Brann OS. Pyogenic and amebic liver abscesses. Curr Gastroenterol Rep. 2004;6(4):273-279.
Laha H, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189.
LaPook JD, et al. Sheep, watercress, and the internet. Lancet. 2000;356:218-220.
Lasserre R, et al. Single-day drug treatment of amebic liver abscess. Am J Trop Med Hyg. 1983;32:723-726.
Lee KH, et al. Recurrent pancreatitis secondary to pancreatic ascariasis. Singapore Med J. 2009;50:e218-e219.
Leippe M, et al. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991;88:7659-7663.
Leo M, et al. Evaluation of Entamoeba histolytica antigen and antibody point-of-care tests for the rapid diagnosis of amebiasis. J Clin Microbiol. 2006;44:4569-4571.
Li E, Stanley SLJr. Protozoa: amebiasis. Gastroenterol Clin North Am. 1996;25:471-492.
Lin YL, et al. Antibodies in serum of patients with clonorchiasis before and after treatment. Southeast Asian J Trop Med Public Health. 1995;26:114-119.
Lloyd DA. Massive hepato-biliary ascariasis in childhood. Br J Surg. 1981;68:468-476.
Lloyd DA. Hepato-biliary ascariasis in children. Surg Ann. 1982;14:277-297.
Loftus B, et al. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865-868.
Lösch FA. Massive development of amebas in the large intestine: translation from the original in Russian, 1875. Am J Trop Med Hyg. 1975;24:383-392.
Lotter H, et al. Oral vaccination with recombinant Yersinia expressing hybrid type III proteins protects gerbils from amebic liver abscess. Infect Immune. 2004:7318-7321.
Lotter H, et al. Attenuated recombinant Yersinia as live oral vaccine carrier to protect against amebiasis. Int J Med Microbiol. 2008;298:79-86.
Lotter H, et al. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog. 2009;5:e1000434.
Loulergue P, Mir O. Pleural empyema secondary to amebic liver abscess. Int J Infect Dis. 2009;13:e135-e136.
Louw JH. Abdominal complications of Ascaris lumbricoides infestation in children. Br J Surg. 1966;53:510-521.
Lun ZR, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31-41.
Maher K, et al. Parasite-specific antibody profile in human fascioliasis: application for immunodiagnosis of infection. Am J Trop Med Hyg. 1999;61:738-742.
Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966;164:90-100.
Maldonado-Bernal C. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by Toll-like receptors 2 and 4. Parasite Immunol. 2005;27:127-137.
Mann BJ. Entamoeba histolytica genome project: an update. Trends Parasitol. 2002;18:147-148.
Mann BJ, et al. Protection in a gerbil model of amebiasis by oral immunization with Salmonella expressing the galactose/N-acetyl D-galactosamine inhibitable lectin of Entamoeba histolytica. Vaccine. 1997;15:659-663.
Mansour N, et al. Amebiasis in schistosomiasis endemic and non-endemic areas in Egypt. J Egypt Soc Parasitol. 1997;27:617-628.
Marcos LA, et al. Report of cases of fascioliasis in the Specialized Children Health Institute in Peru (1988-2003). Rev Gastroenterol Peru. 2005;25:198-205.
Marcos LA, et al. Update on hepatobiliary flukes: fascioliasis, opisthorciasis and chlonorchiasis. Curr Opin Infect Dis. 2008;21:523-530.
Marinets A. Protection against invasive amebiasis by a single monoclonal antibody directed against a lipophosphoglycan antigen localized on the surface of Entamoeba histolytica. J Exp Med. 1997;186:1557-1565.
Martinez Baez M. Historical introduction. In: Martinez-Palomo, A, editor. Amebiasis: Human Parasitic Diseases, No. 2. Amsterdam: Elsevier; 1986:1-9.
Mas-Coma MS, et al. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ. 1999;77:340-346.
McConnell JFP. Remarks on the anatomical and pathological revelation of a new species of liver fluke. Lancet. 1875;2:271-273.
McDermott VG. What is the role of percutaneous drainage for treatment of amebic abscesses of the liver? AJR Am J Roentgenol. 1995;165:1005-1006.
Mechai F, et al. Budd Chiari syndrome as a vascular complication of amebic liver abscess. Am J Trop Med Hyg. 2009;81:768-769.
Misra SP, Dwivedi M. Removal of Ascaris lumbricoides from the bile duct using balloon sphincteroplasty. Endoscopy. 1998;30:S6-S7.
Misra SP, Dwivedi M. Clinical features and management of biliary ascariasis in a non-endemic area. Postgrad Med J. 2000;76:29-32.
Monga NK, et al. Amebic peritonitis. Am J Gastroenterol. 1976;66:366-373.
Moran P, et al. Infection by human immunodeficiency virus-1 is not a risk factor for amebiasis. Am J Trop Med Hyg. 2005;73:296-300.
Mortelé K, Ros P. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895-910.
Musgrave WE, Clegg MT. Amebas: Their Cultivation and Etiological Significance. Manila: Bur. Govt Laboratories; 1904.
Munoz O. Epidemiology of amebiasis. In: Martinez-Palomo, A, editor. Amebiasis: Human Parasitic Diseases, No. 2. Amsterdam: Elsevier; 1986:213-239.
Muzaffar J, et al. Randomized, single-blind, placebo-controlled multicenter trial to compare the efficacy and safety of metronidazole and satranidazole in patients with amebic liver abscess. Dig Dis Sci. 2006;51(12):2270-2273.
Nicholas JL. Obstruction of the common bile duct by Fasciola hepatica: occurrence in a boy of 12 years. Br J Surg. 1970;57:544-546.
Oto A, et al. Focal inflammatory diseases of the liver. Eur J Radiol. 1999;32:61-75.
Park DH, Son HY. Clonorchis sinensis, images in clinical medicine. N Engl J Med. 2008;358:16.
Park TJ, et al. Molecular cloning and characterization of a paramyosin from Clonorchis sinensis. Korean J Parasitol. 2009;47:359-367.
Petri WA, et al. Estimating the impact of amebiasis on health. Parasitol Today. 2000;16:320-321.
Petri WA, Singh U. Enteric amebiasis. In: Guerrant, R, Walker, DH, Weller, PF. Tropical Infectious Diseases: Principles, Pathogens, and Practice. 2nd ed. Philadelphia: Elsevier; 2006:967.
Pillai DR, et al. The cysteine-rich region of the Entamoeba histolytica adherence lectin (170-kilodalton subunit) is sufficient for high-affinity Gal/GalNAc-specific binding in vitro. Infect Immun. 1999;67:3836-3841.
Pitt HA. Surgical management of hepatic abscesses. World J Surg. 1990;14:498-504.
Powell SJ, et al. Metronidazole in amoebic dysentery and amoebic liver abscess. Lancet. 1966;2:1329-1331.
Puhl G, et al. Noninvasive in vivo analysis of the human hepatic microcirculation using orthogonal polarization spectral imaging. Transplantation. 2003;75:756-761.
Quaderi MA, et al. Amoebic liver abscess and clinical experiences with tinidazole in Bangladesh. J Trop Med Hyg. 1978;81:16-19.
Que X, Reed SL. The role of extracellular cysteine proteinases in pathogenesis of Entamoeba histolytica invasion. Parasitol Today. 1997;13:190-194.
Radin DR, et al. CT of amebic liver abscess. AJR Am J Roentgenol. 1988;150:1297-1301.
Ralls PW, et al. Gray-scale ultrasonography of hepatic amoebic abscesses. Radiology. 1979;132:125-129.
Ralls PW, et al. Sonographic findings in hepatic amebic abscess. Radiology. 1982;145:123-126.
Ralls PW, et al. Patterns of resolution in successfully treated hepatic amebic abscess: sonographic evaluation. Radiology. 1983;149:541-543.
Rao S, et al. Hepatic amebiais: a reminder of the complications. Curr Opin Pediatr. 2009;21:145-149.
Ravdin JI. Amebiasis. Clin Infect Dis. 1995;20(6):1453-1464.
Ravdin JI, Guerrant RL. A review of the parasite cellular mechanisms involved in the pathogenesis of amebiasis. Rev Infect Dis. 1982;4:1185-1207.
Ravdin JI, Stauffer W. Entamoeba histolytica (amebiasis, 6th ed. Gl Mandell, Bennett J, Dolin R, editors. Principles and Practice of Infectious Diseases, Vol 2. Philadelphia: Elsevier. 2005:3097-3111. part III H
Reddy DN, et al. Endoscopic diagnosis and management of tropical parasitic infestations. Gastrointest Endosc Clin N Am. 2003;13:765-773.
Reed SL, et al. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins c3a and c5a. J Immunol. 1995;155:266-274.
Restrepo MI, et al. Diagnostic tests for amoebic liver abscess: comparison of enzyme-linked immunosorbent assay (ELISA) and counterimmunoelectrophoresis (CIE). Rev Soc Bras Med Trop. 1996;29:27-32.
Rim HJ. Clonorchiasis: an update. J Helminthol. 2005;79:269-281.
Rivera WL, et al. Application of the polymerase chain reaction (PCR) in the epidemiology of Entamoeba histolytica and Entamoeba dispar infections. Tokai J Exp Clin Med. 1998;23:413-415.
Rogers L. The rapid cure of amoebic dysentery and hepatitis by hypodermic injections of soluble salts of emetine. Br Med J. 1912;1:14-24.
Rogers WF, et al. Amebiasis: unusual radiographic manifestations. Am J Roentgenol. 1980;135:1253-1257.
Ross AG, et al. Schistosomiasis. N Engl J Med. 2002;346:1212-1220.
Rossignol J, et al. Nitazoxanide in the treatment of amoebiasis. Trans R Soc Trop Med Hyg. 2007;101:1025-1031.
Ruangsittichai J, et al. Opisthorchis viverrini: identification of a glycine-tyrosine rich eggshell protein and its potential as a diagnostic tool for human opisthorchiasis. Int J Parasitol. 2006;36:1329-1339.
Saba R, et al. Human fascioliasis. Clin Microbiol Infect. 2004;10:385-387.
Salata RA, et al. Chemoattractant activity of Entamoeba histolytica for human polymorphonuclear neutrophils. J Parasitol. 1989;75:644-646.
Salit IE, et al. A possible cluster of sexually transmitted Entamoeba histolytica: genetic analysis of a highly virulent strain. Clin Infect Dis. 2009;49:346-353.
Salles JM, et al. Hepatic amebiasis. Braz J Infect Dis. 2003;7:96-110.
Sanai FM, Al-Karawi MA. Biliary ascariasis: report of a complicated case and literature review. Saudi J Gastroenterol. 2007;13:25-32.
Sanchez-Sosa S, et al. Massive hepatobiliary fascioliasis. Rev Gastroenterol Mex. 2000;65:179-183.
Sandeep SM, et al. Endoscopic biliary drainage with amebic liver abscess and biliary communication. Indian J Gastroenterol. 2006;25:125-127.
Sandouk F, et al. Pancreatic-biliary ascariasis: experience of 300 cases. Am J Gastroenterol. 1997;92:2264-2267.
Sandouk F, et al. The whirlpool jet technique for removal of pancreatic duct ascaris. Gastrointest Endosc. 1997;46:180-182.
Santi-Rocca JC, et al. The lysine- and glutamic acid–rich protein KERP1 plays a role in Entamoeba histolytica liver abscess pathogenesis. Cell Microbiol. 2008;10:202-217.
Santra A, et al. Serodiagnosis of ascariasis with specific IgG4 antibody and its use in an epidemiological study. Trans R Soc Trop Med Hyg. 2001;95:289-292.
Sarda AK, et al. Intraperitoneal rupture of amoebic liver abscess. Br J Surg. 1989;76:202-203.
Sarwat AK, et al. Schistosomiasis of the lung. J Egypt Soc Parasitol. 1986;16:359.
Sato D, et al. Cytotoxic effect of amide derivatives of trifluormethionine against the enteric protozoan parasite Entamoeba histolytica. Int J Antimicrob Agents. 2010;35:56-61.
Saville TD. Saville’s System of Clinical Medicine, 14th ed. London: Edward Arnold Publishers; 1964.
Sayasone S, et al. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101:40-47.
Sepulveda B. Amebiasis: host–pathogen biology. Rev Infect Dis. 1982;4:1247-1253.
Sepulveda B, Manzo NTG. Clinical manifestations and diagnosis of amebiasis. In: Martinez-Palomo, A, editor. Amebiasis: Human Parasitic Diseases, No. 2. Amsterdam: Elsevier; 1986:169-187.
Shah OJ, et al. Management of biliary ascariasis in pregnancy. World J Surg. 2005;29:1294-1298.
Shah OJ, et al. Biliary ascariasis: a review. World J Surg. 2006;30:1500-1506.
Shamsuzzaman SM, Hashiguchi Y. Thoracic amebiasis. Clin Chest Med. 2002;23:479-492.
Sharma MP, et al. Needle aspiration of amoebic liver abscess. Br Med J. 1989;299:1308-1309.
Sharma MP, et al. Prognostic markers in amebic liver abscess: a prospective study. Am J Gastroenterol. 1996;91:2584-2588.
Sheen IS, et al. Resolution of liver abscesses: comparison of pyogenic and amebic liver abscesses. Am J Trop Med Hyg. 1989;40:384-389.
Sherman SC, Weber JM. The CT diagnosis of ascariasis. J Emerg Med. 2005;28:471-472.
Shin BM, Choi KI. Diagnostic significance of intradermal test compared with radiologic findings for clonorchiasis. Korean. J Clin Pathol. 2000;20:81-86.
Singh O, et al. Comparative study of catheter drainage and needle aspiration in management of large liver abscesses. Indian J Gastroenterol. 2009;28:88-92.
Singh V, et al. Pathophysiology of jaundice in amoebic liver abscess. Am J Trop Med Hyg. 2008;78:556-559.
Snow MJ, Stanley SL. Recent progress in vaccines for amebiasis. Arch Med Res. 2006;37:280-287.
Sodhi KS, et al. Right atrial and inferior vena caval thrombosis in a case of amebic liver abscess. J Emerg Med. 2009;41:397-399.
Sotoudehmanesh R, Yoonessi A. Diagnosis of Fasciola hepatica by endoscopic ultrasound. Endoscopy. 2003;35:1038.
Sriamporn S, et al. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588-594.
Sripa B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:201.
Sriram PVJ, et al. Endoscopic ultrasound features of biliary ascariasis. Indian J Gastroenterol. 2006;25:50.
Stack CM, et al. The major secreted cathepsin L1 protease of the liver fluke, Fasciola hepatica: a leu-12 to pro-12 replacement in the nonconserved C-terminal region of the prosegment prevents complete enzyme autoactivation and allows definition of the molecular events in prosegment removal. J Biol Chem. 2007;282:16532-16543.
Stanley SLJr. Amebiasis. Lancet. 2003;361:1025-1034.
Stanley SLJr. Vaccines for amoebiasis: barriers and opportunities. Parasitology. 2006;133(Suppl):S81-S86.
Strickland G. Gastrointestinal manifestations of schistosomiasis. Gut. 1994;35:1334-1337.
Stunell H, et al. Recurrent pyogenic cholangitis due to chronic infestation with Clonorchis sinensis. Eur Radiol. 2006;16:2612-2614.
Sukougj CJ, et al. Sonography of hepatic amoebiasis. AJR Am J Roentgenol. 1989;134:911-915.
Sudhamshu KC. Long-term follow-up of pyogenic liver abscess by ultrasound. Eur J Radiol. 2009;74(1):195-198.
Sukov RJ, Cohen CJ, Sample WF. Sonography of hepatic amebic abscesses. AJR Am J Roentgenol. 1980;134:911-915.
Tandon A, et al. Needle aspiration in large amoebic liver abscess. Trop Gastroenterol. 1997;18:19-21.
Tandon R. Pigment gallstones. Indian J Gastroenterol. 1988;7:5-6.
Tannich E, et al. Genomic DNA differences between pathogenic and nonpathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1989;86:5118-5122.
Tanyuksel M, Petri WA. Laboratory diagnosis of amebiasis. Clin Microbiol Rev. 2003;16:713-729.
Teoh TB. A study of gall-stones and included worms in recurrent pyogenic cholangitis. J Pathol Bacteriol. 1963;86:123-129.
Thomas P, Garg N. Amebiasis and other parasitic infections. Blumgart, LH, et al. Surgery of the Liver, Biliary Tract, and Pancreas, 4th ed, Philadelphia: Saunders, 2007.
Thomas PG, Ravindra KV. Amebiasis and biliary infection. In: Blumgart, W, et al. Surgery of the Liver, Biliary Tract, and Pancreas. 4th ed. Philadelphia: Saunders; 2000:1147-1166.
Thompson JEJr, et al. Amebic liver abscess: a therapeutic approach. Rev Infect Dis. 1985;7:171-179.
Tovar J, et al. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol Microbiol. 1999;32:1013-1021.
Tselepatiotis E, et al. A case of Opisthorchis felineus infestation in a pilot from Greece. Infection. 2003;6:430-432.
Vaidya AB, Ray DK. Amoebiasis: the tropical scourge. Sci Today. 1982;16:21-26.
Van Allan RJ, et al. Uncomplicated amebic liver abscess: prospective evaluation of percutaneous therapeutic aspiration. Radiology. 1992;183:827-830.
Vanijanonta S, et al. Low dose tinidazole in the treatment of amoebic liver abscess. Southeast Asian J Trop Med Public Health. 1985;16:253-256.
Viana RL. Selective arteriography in the diagnosis and evaluation of amebic abscess of the liver. Am J Dig Dis. 20, 1975. 632–628
Vohra H, et al. Effective human defense against E. histolytica: high amoebicidal activity of lymphocytes and monocytes in amoebic liver abscess patients until 3 months follow-up. Parasitol Int. 2003;52:193-202.
Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228-238.
Wang KX, et al. Clinical and epidemiological data of patients with clonorchiasis. World J Gastroenterol. 2004;10:446-448.
Wani NA, Chrungoo RK. Biliary ascariasis: surgical aspects. World J Surg. 1992;16:976-979.
Wani NA, et al. Postoperative biliary ascariasis: presentation and management experience. World J Surg. 2000;24:1143-1145.
Weber CS. Bioinformatics and functional analysis of an Entamoeba histolytica mannosyltransferase necessary for parasite complement resistance and hepatic infection. PLoS Negl Trop Dis. 2008;2:e165.
Wells CD, Arguedas M. Amebic liver abscess. South Med J. 2004;97:673-682.
1997 WHO/PAHO/UNESCO report: a consultation with experts on amoebiasis. Epidemiol Bull PAHO. 1997;18:13-14.
Wiwanitkit V. Amebic pericarditis: a summary of Thai cases. Anadolu Kardiyol Deg. 2008;8:305.
World Health Organization. WHO/PAHO/UNESCO report of a consultation of experts on amoebiasis. Wkly Epidemiol Rec. 1997;72:97-100.
Ximenez C, et al. Reassessment of the epidemiology of amebiasis: state of the art. Infect Genet E. 2009;9:1023-1032.
Yoshihara S, et al. Laparoscopic treatment for biliary ascariasis. Surg Laparosc Endosc Percut Tech. 2000;10:103-105.
Zargar SA, Khuroo MS. Management of biliary ascariasis in children. Indian J Gastroenterol. 1990;9:321.
Zhi FC, et al. Treatment of severe Clonorchis sinensis by endoscopic nasobiliary drainage and oral praziquantel. World J Gastroenterol. 2004;10:2150-2152.