Chapter 69 Ambulatory Electrocardiography
Long-Term Monitors and Event Recorders
Types of Ambulatory Electrocardiography Monitors
Continuous Short-Term Monitors
The Holter monitor, invented by Neil Holter in 1941, is the prototype for continuous ambulatory ECG monitors. Modern continuous monitors provide three to 12 high-quality surface ECGs with full disclosure for a period of 24 to 48 hours. These are battery-operated, self-contained devices that comprise electrodes and leads connected to a compact, lightweight box that can be worn on the patient’s belt or placed in a pouch. These devices are capable of storing high-quality ECG data on a cassette tape, which can then be turned in and analyzed at a workstation. More recently, continuous monitors store high-fidelity digital ECGs on a flash card or a PC card. Time stamps correlating patient symptoms to the ECG, extended battery and memory capacity (up to 96 hours), ability to transmit digital recordings over the Internet, and software packages facilitating “advanced” digital ECG analyses are also available. This type of monitor is ideal for patients who have symptoms that occur frequently (at least once per day) but has limited application in situations where patients experience rare and sporadic symptoms. An example of a Holter monitor is shown in Figure 69-1.
Event Monitors
Event monitors are ambulatory ECG monitors that are designed to be worn or carried for up to 30 days. Two types of event monitors are available: (1) Loop recorders (before symptom onset) constantly store and dump ECG data for 30 seconds to 4.5 minutes (depending on the manufacturer); when activated by the patient, ECG data from this buffer are stored for analysis, along with an interval of ECG that is recorded after activation. This type of monitor requires that the patient wear electrodes and leads constantly. (2) Nonlooping event recorders (after symptom onset) do not require that the patient wear electrodes continuously. These are small recording devices that are kept by the patient nearby (pocket or purse), and when the patient has symptoms, the electrodes on the device are placed on the chest, and the ECG is then recorded. These devices typically have 5 to 10 minutes of memory, but more advanced devices may have up to 30 minutes of memory capacity. Event recorders are ideal monitors for identifying a symptom/rhythm correlation for intermittent symptoms that are long enough to allow time for recording. The limitations of event recorders are that patient-triggered recorders cannot detect asymptomatic arrhythmias. Looping event recorders with automatic triggers may allow the detection of very slow or very rapid arrhythmias but may “miss” some arrhythmias such as rate-controlled atrial fibrillation (AF) or supraventricular tachycardia (SVT) that are slower than the detection rate of the device. The primary limitations of looping event recorders are patient discomfort, poor patient compliance, and premature termination of monitoring (since patients often complain about having to wear electrodes and leads for extended periods). The limitations of nonlooping event recorders include their inability to detect short-lived arrhythmias or event initiation and occasional poor ECG quality. Examples of a looping event recorder and a nonlooping event recorder are shown in Figures 69-2 and 69-3, respectively.
Implantable Loop Recorders
Implantable loop recorders (ILRs) are small, lightweight devices that are placed in the subcutaneous space in a minor procedure and provide high-quality ECGs. The earlier-generation ILRs were equipped with patient-activated storage only (Medtronic Reveal; Medtronic, Inc., Minneapolis, MN). Subsequent models have implemented programmable auto-triggering based on high and low heart rate thresholds (Reveal Plus [Medtronic]; Confirm [St Jude Medical, St. Paul, MN]; Sleuth [Transoma Medical, Arden Hills, MN]). The most-recent-generation ILRs are equipped with AF detection algorithms (Reveal XT, AF [Medtronic]). These devices provide 1 to 3 years of monitoring with 48 to 630 minutes of storage and can be patient activated or automatically activated by programming a low rate and a high rate for detection. In the case of patient-activated recordings, these devices store 60 to 240 seconds of ECGs before (usually 60 seconds of ECGs) and after the patient has triggered the device. The ECGs corresponding to these events can be reviewed noninvasively. Some devices are equipped with remote recording capabilities, by which ECGs from the recorder can be sent transtelephonically to a receiving station (Confirm) or can be manually or automatically transmitted from a home-based station to a secure Web site (Reveal Plus and Carelink [Medtronic]) or to a monitoring station manned by trained ECG technicians (Sleuth). These devices are ideal for rare, but significant, events that are worrisome in the case of a cardiac arrhythmia such as syncope. The limitations of these monitors include failure of the patient to activate the device during symptoms, availability of only one lead for analysis, discomfort associated with the implantation and removal of the device, a small risk of infection, and minor cosmetic alteration of the chest wall. An example of an ILR is shown in Figure 69-4.
Implantable Pacemakers and Implantable Defibrillators
Implantable defibrillators and pacemakers are placed for therapeutic, rather than diagnostic, reasons, but they do have sophisticated diagnostic capabilities that allow for storage of intracardiac ECGs during tachyarrhythmias. Diagnostic information available from implantable devices includes rate histograms, atrial high-rate episodes, ventricular high-rate episodes, and stored intracardiac ECGs (depending on the model and vendor of the device). The accuracy of the information available in the device depends on appropriate sensing parameters and programmed detection parameters. Diagnostic information about arrhythmic events that are available in cardiac pacemakers and implantable defibrillators is discussed in Chapters 22 and 92 and therefore will not be covered in this chapter.
Diagnostic Efficacy of Ambulatory Electrocardiography Monitors
Palpitations
Palpitations, defined here as an awareness of one’s heart beat, are a symptom commonly encountered in clinical practice. Although possibly a manifestation of arrhythmias, palpitations are often caused by noncardiac factors. When arrhythmias are the cause of palpitations, the most common rhythms recorded during symptoms are premature atrial contractions, premature ventricular contractions, SVTs, and AF. In a prospective cohort study, 190 patients who presented at an academic medical center with palpitations were evaluated and followed up for 1 year. A diagnosis of the etiology of their palpitations was established in 84%. Among these patients, 40% experienced palpitations because of cardiac arrhythmias and 33% because of anxiety and panic disorder, and the remaining patients had nonarrhythmic causes.1 Patients with palpitations associated with structural heart disease (including complex congenital heart disease), presyncope, or syncope are at higher risk of having a cardiac arrhythmia, which may even be life threatening.
A limitation of some studies evaluating the diagnostic efficacy of continuous 24- to 48-hour monitors for the workup of palpitations is that some of these studies report the number of times these monitors provide an arrhythmia diagnosis but not the number of times the palpitations are associated with sinus rhythm. This underestimates the diagnostic yield of the 24-hour Holter monitor because multiple recordings of sinus rhythm associated with palpitations may help rule out cardiac arrhythmia as the cause of the palpitations (Tables 69-1, 69-2, and 69-3).
Diagnostic Yield of 24-Hour Continuous Electrocardiography Monitors
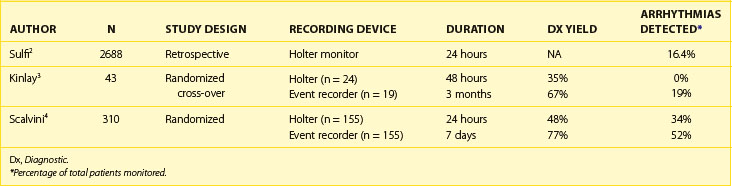
In general, the likelihood of a 24-hour Holter monitor documenting arrhythmias in patients with intermittent palpitations is low. In a large retrospective study of consecutive patients who received Holter monitors for symptoms of palpitations, the 24-hour Holter monitor demonstrated sinus rhythm in 2247 of 2688 Holter recordings (83.6%). Two hundred seventy-six patients had a second Holter monitoring performed, and 210 of 276 were negative (76.1%). Among patients with arrhythmias corresponding to palpitations detected on 24-hour Holter monitoring, 4.4% had frequent ectopic beats (>15 per 10,000 beats), 6.6% had AF, 2.8% had narrow-complex tachycardia, and 2.6% had ventricular tachycardia (VT). The frequency of cardiac arrhythmias detected by 24-hour Holter monitoring is especially low among patients younger than 50 years, in whom 93.1% of Holter monitors demonstrated no arrhythmias. Cardiac arrhythmias were observed in 29% of patients older than 70 years, with 15% of recordings demonstrating AF.2 This study provided information about the type of arrhythmias recorded during continuous 24-hour monitoring among patients with palpitations. The study did not provide insights into the diagnostic yield of the Holter monitor (the ability to provide a symptom/rhythm correlation).
Diagnostic Yield of Continuous Monitors and Event Recorders
In a randomized cross-over study of 43 patients with intermittent palpitations, patients were randomized to a 48-hour Holter monitor versus a 3-month (nonlooping) event recorder and then crossed over to the other monitor. The 48-hour Holter monitor recorded ECGs during symptoms in 15 (35%) of 43, and none of these recordings demonstrated an arrhythmia. The event recorder was able to record ECGs during symptoms in 29 (67%) of 43 and diagnosed an arrhythmia in 8 (28%) of 29. Among the arrhythmias recorded on the event recorder, SVT, premature ventricular beats, and AF or atrial flutter accounted for 18%, 12%, and 6% of arrhythmias, respectively.3 Scalvini et al randomized 310 patients with palpitations to initial monitoring with a 24-hour Holter monitor (n = 155) or a looping event recorder for 7 days (n = 155). The diagnostic yield of the Holter monitor was 48% (34% of patients had arrhythmias detected) and 77% for the event recorder (52% of patients had arrhythmias detected, P < .01).4 In a review of studies evaluating the diagnostic yield of ambulatory ECG monitors for palpitations, Zimetbaum et al reported a diagnostic yield (providing a symptom/rhythm correlation) of 66% to 83% for event recorders and 33% to 35% for a Holter monitor.5
In noncomparison studies, nonlooping event recorders have demonstrated a diagnostic yield for palpitations of up to 66% with arrhythmias detected in 33% to 54% of patients.6–8 Studies of looping event recorders have demonstrated a diagnostic yield of 66% to 83% with arrhythmias detected in 28% to 42% of patients.9,10
Olsen et al evaluated the role of mobile cardiac outpatient telemetry (MCOT, Cardionet, Philadelphia, PA) in the evaluation of palpitations, syncope, and presyncope. In 18 patients in this study, MCOT was the first monitoring system applied for the evaluation of palpitations. The diagnostic yield was 73% (14 of 18) with all 14 patients recording arrhythmias. In another 58 patients, a previous rhythm diagnosis associated with palpitations was already established with another recording system prior to MCOT monitoring. During monitoring, 34 (59%) of 58 of patients had a symptom/rhythm correlation, with 27 (79%) of 34 demonstrating an arrhythmia associated with palpitations.11 These data demonstrate the limitations in evaluating patients for palpitations. Only 59% of patients with a previously established “diagnosis” of an arrhythmia had recurrent palpitations during a long-term monitoring period, and an arrhythmia different from the original “diagnosis” was responsible for palpitations in many patients. These observations should be considered when making clinical decisions about arrhythmias detected by any ambulatory monitor and in the interpretation of studies evaluating the diagnostic yield of these monitors for palpitations.
Optimal Duration of Monitoring for Palpitations
Data on the optimal duration of monitoring in providing a symptom/rhythm correlation demonstrate that 2 weeks of monitoring yields the majority of diagnoses. In a study by Reiffel et al, the weekly yield of an event recorder was evaluated retrospectively in 5052 patients. During the first 2 weeks of monitoring, 87% of patients had transmitted an ECG recording during symptoms. The additional diagnostic yield during the following 2 weeks (weeks 2 to 4) was 9%.12 Zimetbaum et al demonstrated that 83% of patients had a diagnostic transmission from their event recorder within 2 weeks of monitoring and that the diagnostic yield was low beyond this monitoring period.13 On the basis of this evidence, a monitoring period of at least 2 weeks and up to 4 weeks is considered to provide a symptom/rhythm correlation in the majority of cases of diagnosis likely to be achieved by an event recorder.
To summarize this section, studies of the etiology of palpitations demonstrate that nonarrhythmic causes of palpitations are common. ECG recordings during palpitations may demonstrate different arrhythmias during different episodes of palpitations within the same patient. With these data in mind, event recorders with patient-triggered events provide better diagnostic yield than does a 24- to 48-hour Holter monitor and will provide a diagnosis in up to 87% of patients within the first 2 weeks of monitoring (Figure 69-5). The continuous 24-hour monitor is associated with a relatively low diagnostic yield (approximately 35%) among patients with intermittent palpitations, and the second 24-hour recording is of especially low yield. ILRs and mobile cardiac telemetry are not indicated in most cases of intermittent palpitations, as they are not likely to provide additional diagnostic yield to external event recorders.
Syncope
Syncope is a common and complex clinical situation with multiple etiologies. Syncope is responsible for 3% of emergency room visits and for 1% of all hospital admissions.14 Common causes of syncope include reflex-mediated syncope (vasovagal syncope, cough syncope, and micturition syncope), carotid sinus hypersensitivity, orthostatic hypotension, transient ischemic attacks, seizures, aortic stenosis, pulmonary embolism, and cardiac arrhythmias. When syncope is associated with structural heart disease or an ion channelopathy, a higher risk of sudden cardiac death from an arrhythmia is present.15 Some specific clinical situations that are associated with a high risk of sudden death when accompanied by a history of syncope include long QT syndrome (LQTS), Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy, familial cardiomyopathy, hypertrophic cardiomyopathy, repaired tetralogy of Fallot, family history of sudden death, and severe left ventricular dysfunction. Among patients with structurally normal hearts, however, in most cases, syncope (excluding patients with ion channelopathies) is caused by nonarrhythmic factors, and the majority of these are reflex-mediated (vasovagal) syncope.
Establishing the etiology for syncope can be challenging. A detailed history, physical examination, orthostatic vital signs, and a 12-lead ECG are important for patients with syncope. If all of these are performed thoughtfully, this should lead to a diagnosis in 50% of patients presenting for the evaluation of syncope.16 In an unselected population of patients with syncope, many remain undiagnosed despite thorough evaluation. Patients with cardiac syncope are a high-risk group, with a 5-year mortality rate of up to 51%.15 Studies evaluating the diagnostic yield of ambulatory ECG monitors vary widely, largely because of differences in the patient population, such as number of syncopal episodes, age of the patient population studied, presence of structural heart disease, and diagnostic evaluation performed before placement of the monitor.
In a study evaluating the incidence and prognosis of syncope in the Framingham study population, 822 of 7814 patients reported an episode of syncope and had an average of 17 years of follow-up. The etiology of syncope was vasovagal in 29.9%, cardiac in 9.5%, orthostatic in 9.4%, neurologic (stroke, transient ischemic attack, or seizure disorder) in 9%, medication related in 6.8%, and unknown in 36.6%. During follow-up, 78.4% had only incidental episodes of syncope, 7.6% had 1 recurrence, 3.3% had 2 recurrences and 0.9% had 3 or more recurrences. Patients with syncope from a cardiac cause had a higher mortality rate compared with patients without a history of syncope (hazard ratio, 2.01; confidence interval, 1.48 to 2.73). In contrast, patients with vasovagal syncope had a good prognosis.17
Because of the unpredictable nature and the infrequency of syncope recurrence, the ability of the ambulatory ECG recording to provide a symptom/rhythm correlation is proportional to the duration of the monitoring period and the pretest suspicion for cardiac arrhythmias (structural heart disease, ion channelopathy, congenital heart disease, etc.). In the study by Linzer et al, 78.4% of patients (unselected population) had only one episode of syncope.16 In general, the most useful monitoring strategy in patients with infrequent episodes of syncope is an ILR and, to a significantly lesser degree, a looping event recorder. The 24-hour Holter monitor and the nonlooping event recorder are unlikely to provide a diagnosis in this setting.
Continuous Electrocardiography Monitors for Syncope
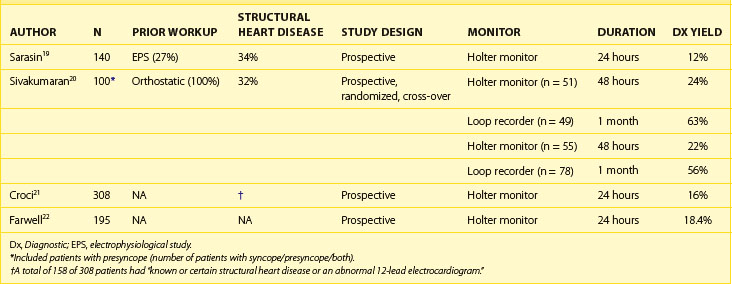
Studies of the efficacy of the 24-hour Holter monitor for the evaluation of syncope have demonstrated that the likelihood of establishing a symptom/rhythm correlation and demonstrating an arrhythmic cause of syncope with this monitoring strategy is low. In a review by DiMarco et al, a symptom/rhythm correlation among patients with syncope could only be established in 22% of patients on a Holter Monitor.18 Sarasin et al evaluated the diagnostic yield of Holter monitors among 140 patients with a high likelihood of arrhythmias. Overall, the diagnostic yield for the 24-hour Holter monitor was 11.4% (16 of 140 patients had syncope [n = 7] or presyncope [n = 15] during the 24-hour monitoring period). Nine of the 16 patients who had symptoms during the monitoring period had a serious arrhythmia recorded.19 Sivakumaran et al evaluated 100 patients with syncope (n = 21), presyncope (n = 29), or both (n = 50) by first randomizing them to either a 24-hour Holter monitor or to a looping event recorder. The diagnostic yield for patients randomized to a loop recorder first was 63%, with 8% of these patients demonstrating an arrhythmia as a cause of their symptoms. For patients randomized to a 24-hour Holter monitor, the diagnostic yield was 24% with no abnormal arrhythmias recorded as a cause of their syncope. When the initial monitoring strategy did not yield a diagnosis, cross-over to the other monitor was performed in some. Four patients were crossed over to the 24-hour Holter monitor after nondiagnostic loop recording, and 29 patients were crossed over to a loop recorder after nondiagnostic Holter monitoring. The overall diagnostic yield for the Holter monitor (n = 56) and the loop recorder (n = 78) was 22% and 56%, respectively.20 Taken together with the review by Dimarco et al, these studies and two other studies established the low diagnostic yield (15% to 24%) of Holter monitors in providing a diagnosis among patients with unexplained syncope (Table 69-4).17,18,21,22 The primary reason for the poor performance of the Holter monitors is that, in general, it is unlikely that a syncopal episode will recur during the relatively short monitoring period. For this reason, a long-term monitoring strategy is more likely to provide a diagnosis among patients who have a high clinical probability of cardiac arrhythmias.
Event Recorders
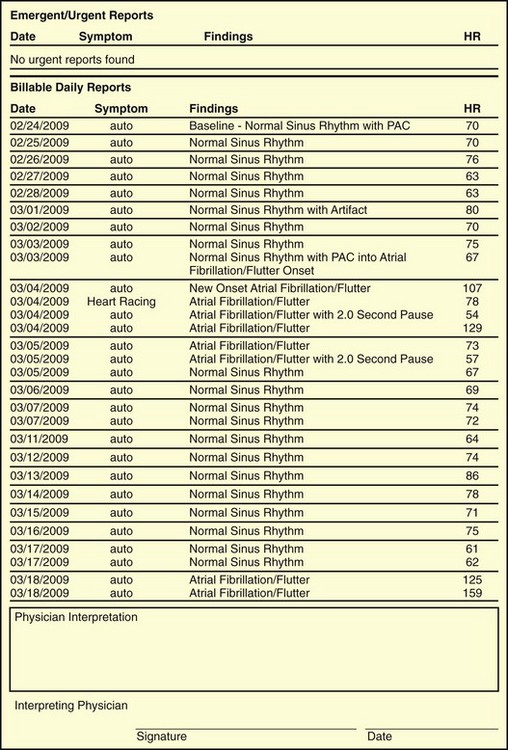
Nonlooping event recorders are unlikely to be useful in the evaluation of syncope because the patient would not be conscious to self-record the event. However, a looping event recorder allows patients to activate the monitor on regaining consciousness. Furthermore, looping event recorders with auto-triggering would automatically store bradyarrythmias or tachyarrhythmias in the event that the patient is not able to trigger the monitor himself or herself (Figure 69-6). Although looping event recorders can provide much longer monitoring periods than a 24-hour Holter monitor can, studies of loop recorders have been disappointing. Inherent limitations diminish the diagnostic yield of this monitoring strategy. In many patients, a 30-day monitoring period may not be long enough to capture a recurrent event. Another significant limitation is poor patient tolerance and compliance (failure to activate the loop recorder or failure to wear the device consistently).
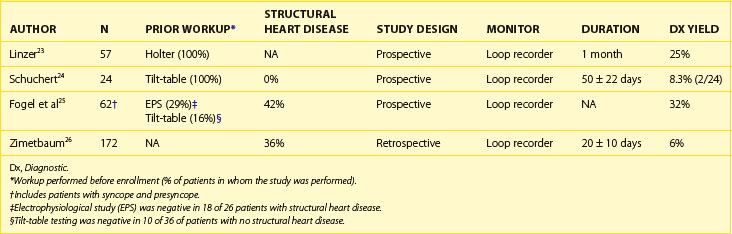
Linzer et al evaluated 57 patients with syncope who had a negative Holter monitor recording and compared them with the recording from a looping event recorder.23 After a monitoring period of 1 month, 14 (25%) of 57 patients had a definitive diagnosis for the cause of syncope. Four (28%) of 14 patients had a primary cardiac arrhythmia diagnosed as the cause of their syncope, including VT (1 patient), high-grade atrioventricular (AV) block (2 patients), and SVT (1 patient). Neurally mediated syncope was observed in 3 patients, and normal cardiac rhythm was recorded in 7 patients. Schuchert et al evaluated 24 patients (50 ± 14 years) with a history of 3 ± 4 recurrent syncopal events over the last 6 months, no structural heart disease, and a negative tilt-table test with a looping event recorder. The average monitoring period was 50 ± 22 days. During the monitoring period, 26 device activations occurred in 14 patients, but only 1 of these was caused by syncope (and this was associated with sinus tachycardia recorded on the monitor). Overall, 8 patients had 90 episodes (1 patient had 80 episodes) of recurrent syncope with 2 episodes prior to monitoring, 2 during monitoring, and 4 after monitoring (15 ± 10 months after termination of monitoring). Of the 2 patients who had syncope during monitoring, 1 patient inadvertently erased the stored ECGs before transmitting. Overall, the diagnostic yield of the event recorder was only 1 (4%) of 24 in this highly selected population of patients with a structurally normal heart and a history of multiple episodes of syncope within the previous 6 months.24
In general, studies evaluating the diagnostic efficacy of looping event recorders reported that these devices provided a symptom/rhythm correlation in 4% to 24% of patients presenting with syncope of unknown cause (Table 69-5).23–26 A major limitation of the looping event recorder is related to patient compliance. Up to 25% of patients may fail to activate the loop recorder while experiencing symptoms during the monitoring period, and early termination of monitoring may occur because of patient intolerance or adverse skin reactions to the electrodes.20,23,24
Implantable Loop Recorders
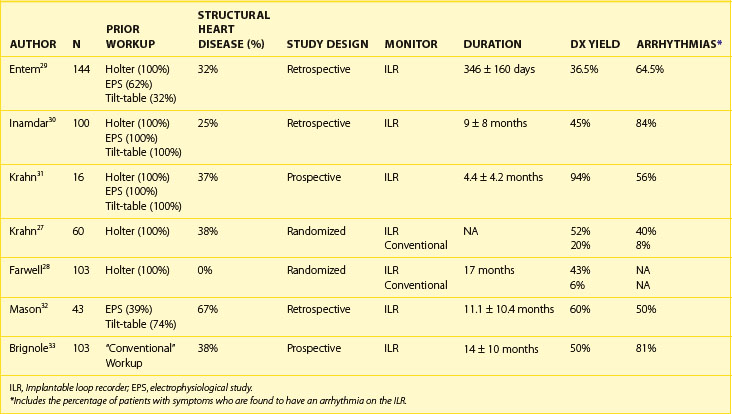
Because of its long-term monitoring capabilities and its improved tolerability (no external electrodes, wires, and boxes), the ILR has emerged as an important tool for establishing or excluding an arrhythmic cause for syncope. Table 69-6 provides data on some recent studies evaluating the efficacy of ILRs in diagnosing or rejecting arrhythmias as a cause of syncope.27–33 In the Randomized Assessment of Syncope Trial (RAST), Krahn et al randomized patients referred to their arrhythmia center to “conventional” testing versus an ILR with 1-year follow-up. Patients with a left ventricular ejection fraction (LVEF) of less than 35%, those with a history consistent with vasovagal syncope, and those unlikely to survive 1 year were excluded. The etiology of syncope was diagnosed in 52% of patients randomized to an ILR versus 20% in the “conventional” testing arm (P < .012).27 In an observational study of “high-risk” patients (high risk defined in this study as syncopal episodes being recurrent, unpredictable, or frequent, or occurring during high-risk activities), ILRs were implanted in 103 patients, who were followed up for 14 ± 10 months. Overall, a symptom/rhythm correlation was demonstrated in 52 patients (50%). Four additional patients had recurrent syncope during follow-up, but they were not able to activate the recorder. Among patients who were older than 65 years (n = 78), 56% had a symptom/rhythm correlation provided by the ILR, and 42 of 44 of these episodes were caused by an arrhythmia. Among patients younger than 65 years (n = 25), 32% had a symptom/rhythm correlation provided by the ILR, and 20% of the patients were found to have an arrhythmia during their syncopal event. Arrhythmias recorded were AV block in 23%, sinus node dysfunction in 14%, atrial arrhythmias in 4%, and ventricular tachycardia or ventricular fibrillation (VT/VF) in 3% of patients older than 65 years. Among patients younger than 65 years, recorded arrhythmias were AV block in 12% and sinus node dysfunction in 8%. No atrial or ventricular tachyarrhythmias were recorded among patients less than 65 years. During follow-up, 4 patients (all >65 years) died during monitoring; in 1 patient, recording was unavailable because of the occurrence of sudden death, and the others had noncardiac causes. Five syncope-related traumatic episodes occurred in 3 patients during follow-up.28
Outpatient Telemetry
Outpatient telemetry is a new monitoring technology that provides continuous, full-disclosure ECGs with patient and auto-triggering algorithms, which can be worn for up to 21 to 31 days. This technology also provides continuous surveillance monitoring (by trained ECG technicians, 24 hours per day) of ECGs that are transmitted daily and instantly by a hand-held cellular device when events are triggered. The unique features of this device, as compared with current looping event recorders, are as follows: (1) Outpatient telemetry provides full disclosure of ECGs during the monitoring period, (2) it can provide information about the AF burden, and (3) ECGs can be evaluated in real time and physicians notified about significant arrhythmias. Retrospective studies evaluating the ability of continuous outpatient telemetry to provide a symptom/rhythm correlation among patients with a variety of indications have established it as a useful tool.29 However, published studies on the ability of outpatient telemetry to significantly improve the diagnostic yield, as compared with auto-triggered looping event recorders, are lacking. In a retrospective study, Olson et al evaluated the diagnostic efficacy of MCOT in 122 consecutive patients in a variety of clinical settings (palpitations, presyncope, syncope, drug efficacy monitoring).10 Ten (59%) of the 17 patients who were evaluated for presyncope or syncope in this study had a diagnosis made with MCOT. In a randomized, multi-center study, 305 patients were randomized to a looping event monitor versus MCOT (Cardionet, Philadelphia, PA). Patients with syncope, presyncope, or severe palpitations occurring less frequently than 1 per 24 hours and a nondiagnostic Holter monitor recording were included in this study. Patients with severe heart failure symptoms, myocardial infarction within 3 months, unstable angina, history of sustained VT or VF, and an ejection fraction of less than 35% were excluded. The primary endpoint of the study was confirmation or exclusion of an arrhythmic cause for the symptoms. Of the 305 patients randomized, results from 266 patients were analyzed (MCOT [n = 134], loop recorder [n = 132]). The overall diagnostic yield reported in the study was 88% for patients monitored by MCOT and 75% for patients monitored by a loop recorder (P = .008). In the subset of patients with syncope (n = 43) and presyncope (n = 91), the diagnostic yield for MCOT was 89% versus 69% for the loop recorder (P = .008).34 The diagnostic yield was significantly better than that of a loop recorder despite the fact that the reported diagnostic yield of the loop recorder was higher in this study than reported by others. A potential explanation for this is that patients who could not tolerate the MCOT monitor or the loop recorder were not included in the analysis. Among the 39 patients who did not complete the protocol, 20 patients (MCOT [n = 13], loop recorder [n = 7]) were noncompliant and did not wear the device for the entire duration of the study. These patients were not accounted for in the reported diagnostic yield of this study. An accurate assessment of a monitoring system should take into account all the factors impacting the diagnostic yield of a monitor. Another important shortcoming of this study is that the majority of loop recorders did not have the auto-triggering feature. Previous studies had demonstrated that auto-triggered loop recorders are superior to loop recorders without this feature in diagnosing the underlying symptom provoking arrhythmia and in the detection of asymptomatic episodes.35 Although MCOT has some theoretical advantages (continuous monitoring) over the loop recorder, currently evidence that MCOT provides significant improvements over the diagnostic yield of looping event recorders with auto-triggering for the evaluation of syncope is insufficient.
To summarize the role of ambulatory ECG monitors for the evaluation of syncope, clinical evidence consistently demonstrates that the 24-hour Holter monitor is associated with a low diagnostic yield for patients with unexplained syncope. Looping event recorders may have a limited role in select patients who can reliably activate the monitor and who have very frequent episodes of presyncope or syncope. Studies evaluating the role of outpatient telemetry (MCOT, ECAT) in syncope are promising, but it has not been established whether outpatient telemetry provides significant improvements in diagnostic yield over currently available auto-triggered loop recorders. In most cases of unexplained syncope, the literature supports the use of an ILR as the monitoring strategy of choice after conventional testing fails to provide a diagnosis. A selective list of studies evaluating the diagnostic capabilities of various ambulatory ECG monitoring strategies in syncope is provided in Tables 69-4, 69-5, and 69-6.
Monitoring in Atrial Fibrillation
AF is the most common cardiac rhythm disturbance encountered in clinical practice. Ambulatory ECG monitors have numerous potential roles in the diagnosis and management of patients with AF. Patients may have symptoms of palpitations, fatigue, lightheadedness, and shortness of breath, but because of its paroxysmal nature, AF may escape diagnosis. Asymptomatic AF is common even among patients with known symptomatic bouts of AF; in one study, AF was found to be 12 times more common than symptomatic recurrences.36–38 When continuous monitoring is possible with an implantable pacemaker, the incidence of asymptomatic episodes among patients with a history of AF may be as high as 50%. Israel et al evaluated asymptomatic episodes of AF in 110 patients with implanted atrial defibrillators for the treatment of AF. These devices have sophisticated algorithms with excellent sensitivity and specificity for detecting AF. In this study, 38% of patients had asymptomatic bouts of AF during continuous monitoring. Furthermore, 1 in 6 patients had asymptomatic AF that persisted for 48 hours or longer.39 These data confirm that symptoms are a poor indication of the onset, frequency, and duration of episodes among patients with AF. The best monitoring strategy for the detection of asymptomatic AF in a variety of clinical situations has not yet been established (see Figure 69-6).
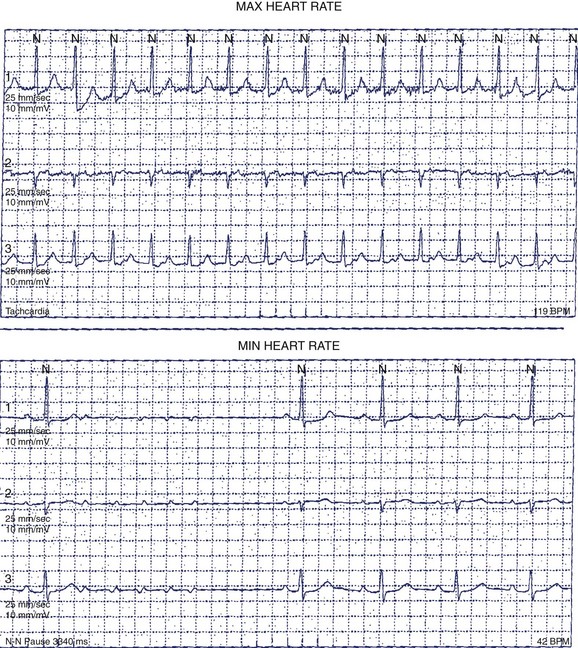
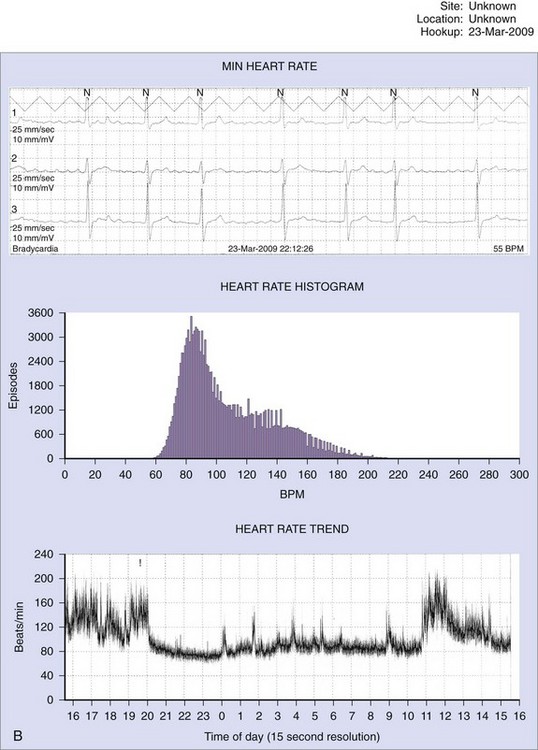
Patients with AF may suffer symptoms of exercise intolerance, dyspnea on exertion, and fatigue caused by inadequate rate control. Achieving rate control in patients with AF is important because inadequate rate control may contribute to, or be the sole cause of, depressed left ventricular function from a tachycardia-mediated cardiomyopathy. Grogan et al reported significant improvement in left ventricular (LV) function in 10 patients who presented with severe LV dysfunction and AF with poor rate control. After treatment with rate control, the mean LVEF improved from 25% (range, 12% to 30%) to 52% (range, 40% to 64%).40 In the assessment of rate control among patients with AF, a resting heart rate alone is inadequate. The goal of rate control is to improve symptoms, optimize hemodynamics through a range of activities, and prevent tachycardia-mediated cardiomyopathy. The target heart rate defining adequate rate control at rest and with exercise has not yet been well established. In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, patients randomized to the rate control arm of the study were defined as adequately rate controlled when their resting heart rate was less than 80 beats/min and less than 110 beats/min during a 6-minute walk test.41 Optimal rate control targets are controversial. In general, goals are to alleviate symptoms and prevent tachycardia-mediated cardiomyopathy. Among asymptomatic patients with normal LV systolic function, a more lenient strategy (resting heart rate <110 beats/min) may be reasonable.42 A 24-hour Holter monitor may be a useful tool in assessing rate control in patients with persistent or chronic AF throughout a range of patient activities. The 24-hour Holter reports minimum, maximum, and average heart rates and can provide useful clinical information to guide treatment. Among patients with LV dysfunction or symptoms, a reasonable goal is to achieve a 24-hour average heart rate of less than 90 beats/min and a maximum heart rate of less than 120 beats/min (Figure 69-7). Elderly patients with normal LV function and minimal symptoms may benefit from a more lenient rate control target (<110 beats/min at rest). The usefulness of a 24-hour Holter monitor for the assessment of rate control is limited in patients with paroxysmal AF, especially among patients who experience infrequent episodes.
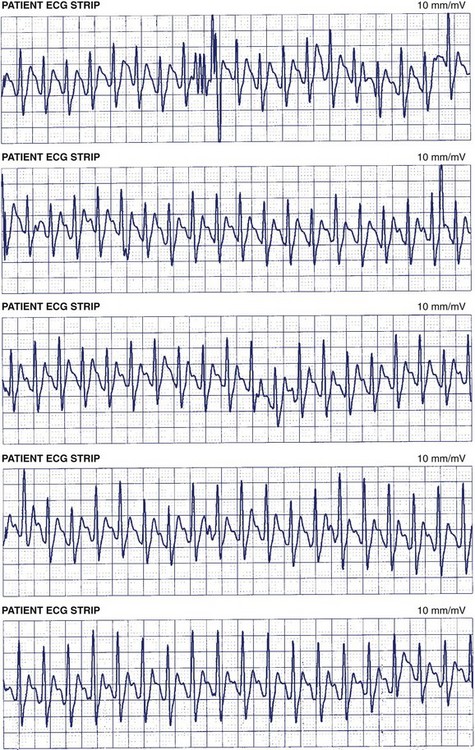
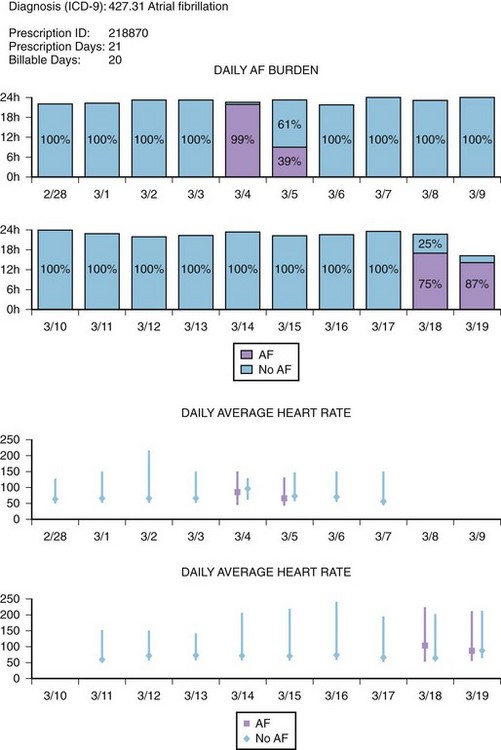
Recent studies have demonstrated the important role of ambulatory ECG monitoring in evaluating the efficacy of rhythm control strategies. Although the major goal in any rhythm control strategy is the alleviation of symptoms, for both therapy and ablation, it is clinically useful to establish whether, and to what degree, both symptomatic AF and asymptomatic AF are recurrent. In a study by Senatore et al, 72 patients were followed up for 30 to 120 days after an AF ablation procedure, with scheduled daily event recordings (30 seconds) along with recordings during any symptoms. All these patients were also evaluated with a 24-hour Holter monitor on days 14, 30, and 120 following the procedure. A detailed history was obtained from the patients, and 12-lead ECG was administered during each follow-up visit. A total of 5585 thirty-second transmissions (mean, 77.5 per patient) were obtained. When patients were evaluated with a Holter monitor and 12-lead ECGs, AF recurrence was detected in 10 patients (13.9%). However, when additional diagnostic information from the event recorder was included, 20 patients were found to have AF recurrences (27.8%).43 In another study of 114 patients undergoing AF ablation, a continuous 7-day Holter recording was obtained before ablation, immediately following ablation, and then at 3, 6, and 12 months. On the 7-day Holter monitor before the ablation, episodes of AF were recorded in 92 (81%) of 114 patients. Episodes were entirely symptomatic in 35 (38%) of 114, symptomatic and asymptomatic episodes in 52 (57%) of 114, and entirely asymptomatic in 5 patients (4%). After the ablation, the percentage of patients with only asymptomatic episodes of AF increased from 4% to 37%.44 These studies demonstrate that a symptom-based follow-up strategy for AF recurrence is inadequate and highlight the importance of long-term ambulatory monitoring in detecting asymptomatic AF following radiofrequency (RF) ablation of AF. Some current ambulatory monitoring systems (MCOT, ECAT, Medtronic Reveal AF, etc.) have sophisticated AF algorithms that may not only detect episodes of AF but also provide the percentage of time a patient is in AF (AF burden) (Figure 69-8). These monitoring systems have not been established as the standard of care for AF surveillance; however, in the future, specific roles for such monitors may be established in AF management, such as guiding antiarrhythmic drug therapy and determining continuation of anticoagulation in patients with paroxysmal AF.
Key References
Farwell DJ, Sulke AN. Does the use of a syncope diagnostic protocol improve the investigation and management of syncope? Heart. 2004;90:52-58.
Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation. Circulation. 2005;112:307-313.
Israel CW, Gronefeld G, Ehrlich JR, et al. Long term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: Implications for optimal patient care. J Am Coll Cardiol. 2004;43:47-52.
Kapoor WN, Karpf M, Weant S, et al. A prospective evaluation and follow-up of patients with syncope. N Engl J Med. 1983;309:197-203.
Kinlay H, Leitch JW, Neil A, et al. Cardiac event recorders yield more diagnoses and are more cost-effective than 48-hour Holter monitoring in patients with palpitations: A controlled clinical trial. Ann Int Med. 1996;124:16-20.
Krahn AD, Klein GJ, Norris C. The etiology of syncope in patients with negative tilt-table and negative electrophysiological testing. Circulation. 1995;92:1819-1824.
Krahn AD, Klein GJ, Yee R, et al. Randomized Assessment of Syncope Trial: Conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46-51.
Linzer M, Yang EH, Estes NA, et al. Diagnosing syncope. Part 1: Value of history, physical examination and electrocardiography. Clinical efficacy assessment project of the American College of Physicians. Ann Intern Med. 1997;126:989-996.
Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18:241-247.
Senatore G, Stabile G, Bertaglia E. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2005;45:873-876.
Sivakumaran S, Krahn AD, Klein GJ, et al. A prospective randomized comparison of loop recorders versus Holter monitors in patients with syncope or presyncope. Am J Med. 2003;115:1-5.
Zimetbaum P, Josephson ME. Evaluation of patients with palpitations. N Engl J Med. 1998;338:1369-1373.
Zimetbaum P, Josephson M. The evolving role of ambulatory arrhythmia monitoring in clinical practice. Ann Intern Med. 1999;130:848-856.
Zimetbaum P, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: A cost-effectiveness analysis. Ann Int Med. 1998;128:890-895.
1 Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
2 Sulfi S, Balami D, Kapur A, et al. Limited clinical utility of Holter recordings in patients with palpitations and altered consciousness: An analysis of 8973 recordings in 7394 patients. Ann Noninvasive Electrocardiol. 2008;13:39-43.
3 Kinlay H, Leitch JW, Neil A, et al. Cardiac event recorders yield more diagnoses and are more cost-effective than 48-hour Holter monitoring in patients with palpitations: A controlled clinical trial. Ann Int Med. 1996;124:16-20.
4 Scalvini S, Zanelli E, Martinelli G, et al. Cardiac event recording yields more diagnoses than 24 hour Holter monitoring in patients with palpitations. J Telemed and Telecare. 2005;11:14-16. S1
5 Zimetbaum P, Josephson M. The evolving role of ambulatory arrhythmia monitoring in clinical practice. Ann Intern Med. 1999;130:848-856.
6 Ringqvist I, Jonason T, Nilsson G, et al. Diagnostic value of long term ambulatory ECG in patients with syncope, dizziness, or palpitations. Clin Physiol. 1989;9:47-55.
7 Wu CC, Hsieh MH, Tai CT, et al. Utility of patient-activated event recorders in the detection of cardiac arrhythmias. J Int Cardiol Electrophysiol. 2003;8:117-120.
8 Barrionuevo J, Baron-Esquivias G, Rodriguez AN, et al. Utility of cardiac event recorders in diagnosing arrhythmic etiology of palpitations in patients without structural heart disease. Rev Exp Cardiol. 2002;55:107-112.
9 Zimetbaum P, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: A cost-effectiveness analysis. Ann Int Med. 1998;128:890-895.
10 Fogel RI, Evans JJ, Prystowsky EN, et al. Utility and cost of event recorders in the diagnosis of palpitations, presyncope, and syncope. Am J Cardiol. 1997;79:207-208.
11 Olson JA, Fouts AM, Padanilam BJ, et al. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol. 2007;18:473-477.
12 Reiffel JA, Schulhof E, Joseph B, et al. Optimal duration of transtelephonic ECG monitoring when used for transient symptomatic event detection. J Electrocardiol. 1991;24:165-168.
13 Zimetbaum P, Josephson ME. Evaluation of patients with palpitations. N Engl J Med. 1998;338:1369-1373.
14 Day SC, Cook EF, Funkenstein H, et al. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am J Med. 1982;73:15-23.
15 Kapoor WN, Karpf M, Weant S, et al. A prospective evaluation and follow-up of patients with syncope. N Engl J Med. 1983;309:197-203.
16 Linzer M, Yang EH, Estes NA, et al. Diagnosing syncope. Part 1: Value of history, physical examination and electrocardiography. Clinical efficacy assessment project of the American College of Physicians. Ann Intern Med. 1997;126:989-996.
17 Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878-885.
18 DiMarco JP, Philbrick JT. Use of ambulatory electrocardiographic (Holter) monitoring. Ann Intern Med. 1990;113:53-68.
19 Sarasin FP, Carballo D, Slama S, et al. Usefulness of 24H Holter monitoring in patients with unexplained syncope and a high likelihood of arrhythmias. Int J Cardiol. 2004;101:203-207.
20 Sivakumaran S, Krahn AD, Klein GJ, et al. A prospective randomized comparison of loop recorders versus Holter monitors in patients with syncope or presyncope. Am J Med. 2003;115:1-5.
21 Croci F, Brignole M, Alboni P, et al. The application of a standardized strategy of evaluation in patients with syncope referred to three syncope units. Europace. 2002;4:351-355.
22 Farwell DJ, Sulke AN. Does the use of a syncope diagnostic protocol improve the investigation and management of syncope? Heart. 2004;90:52-58.
23 Linzer M, Pritchett EL, Pontinen M, et al. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol. 1990;662:214-219.
24 Schuchert A, Maas R, Krezschmar C, et al. Diagnostic yield of external electrocardiographic loop recorders in patients with recurrent syncope and negative tilt table test. PACE. 2003;26:1837-1840.
25 Fogel RI, Evans JJ, Prystowsky EN. Utility and cost of event recorders in the diagnosis of palpitations, presyncope, and syncope. Am J Cardiol. 1997;79:207-208.
26 Zimetbaum P, Kim KY, Ho KK, et al. Utility of patient-activated cardiac event recorders in general clinical practice. Am J Cardiol. 1997;79:207-208.
27 Krahn AD, Klein GJ, Yee R, et al. Randomized Assessment of Syncope Trial: Conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46-51.
28 Farwell DJ, Freemantle N, Sulke N. The clinical impact of implantable loop recorders in patients with syncope. Eur Heart J. 2006;27:351-356.
29 Entem FR, Enriquez SG, Cobo M, et al. Utility of implantable loop recorders for diagnosing unexplained syncope in clinical practice. Clin Cardiol. 2009;32:28-31.
30 Inamdar V, Mehta S, Juang G, et al. The utility of implantable loop recorders for diagnosing unexplained syncope in 100 consecutive patients: Five-year, single-center experience. J Invasive Cardiol. 2006;18:313-315.
31 Krahn AD, Klein GJ, Norris C. The etiology of syncope in patients with negative tilt-table and negative electrophysiological testing. Circulation. 1995;92:1819-1824.
32 Mason P, Wood MA, Reese DB. Usefulness of implantable loop recorders in office-based practice for evaluation of syncope in patients with and without structural heart disease. Am J Cardiol. 2003;92:1127-1129.
33 Brignole M, Menozzi C, Maggi R, et al. The usage and diagnostic yield of the implantable loop-recorder in detection of the mechanism of syncope and in guiding effective antiarrhythmic therapy in older people. Europace. 2005;7:273-279.
34 Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18:241-247.
35 Reiffel JA, Schwarzberg R, Murry M. Comparison of autotriggered memory loop recorders versus standard loop recorders versus 24-hour Holter monitors for arrhythmia detection. Am J Cardiol. 2005;95:1055-1059.
36 Rho RW, Page RL. Asymptomatic atrial fibrillation. Prog Cardiovasc Dis. 2005;48:79-87.
37 Page RL, Tilsh TW, Connolly SJ, et al. Asymptomatic or “silent” atrial fibrillation: Frequency in untreated patients and patients receiving azimilide. Circulation. 2003;107:1141-1145.
38 Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994:224-227.
39 Israel CW, Gronefeld G, Ehrlich JR, et al. Long term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: Implications for optimal patient care. J Am Coll Cardiol. 2004;43:47-52.
40 Grogan M, Smith HC, Gersh BJ, et al. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1570-1573.
41 The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42 Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43 Senatore G, Stabile G, Bertaglia E. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2005;45:873-876.
44 Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation. Circulation. 2005;112:307-313.