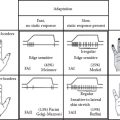
FIGURE 6.1 Overview of neurogenic inflammation.
CGRP predominantly induces arteriole vasodilation and stimulates the proliferation of keratinocytes and vascular endothelial cells via interaction with a CGRP receptor. It is one of the most potent vasodilators and generally mediates anti-inflammatory and neurotrophic action. However, under certain circumstances, CGRP increases the adhesion of neutrophils and monocytes, enhances neutrophil accumulation and edema formation, and promotes the release of TNF-a from mast cells, indicating its proinflammatory role. It inhibits the degradation of SP by neutral endopeptidase (NEP) and triggers SP release (8,10,16).
Moreover, there are about 30 other neurotransmitters identified in the skin, for example, NK A, neurotensin, vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, neuropeptide Y, somatostatin, gastrin-releasing hormone, β-endorphin, enkephalin, and galanin. There are excellent review papers on other neuropeptides in the skin elsewhere (8,10,30–34). Collectively, neural mediators from nociceptors promote (a) chemotaxis and activation of neutrophils, macrophages, and lymphocytes and degranulation of mast cells; (b) increased blood flow, vascular leakage, and edema; and (c) priming of dendritic cells for T cell differentiation (11).
Moreover, many cells with neuropeptide receptors also produce neuropeptide-degrading enzymes such as NEP or angiotensin-converting enzyme to limit the extent or duration of neurogenic inflammation (8,10).
The coordinated interaction between the peripheral nervous system and the immune system is mediated by different types of peripheral nerves and target cells in the skin such as keratinocytes, mast cells, endothelial cells, fibroblasts, and other immune cells (10,14). These interactions are implicated in cutaneous disease states such as urticaria, psoriasis, atopic dermatitis, hypersensitivity reactions, rosacea, UV-induced inflammation, and sensitive skin as well as normal homeostasis (8,10,16,19,29,33,35–41).
Taken together, neurogenic inflammation is a multidirectional and interactive cross talk between the peripheral nervous system, the immune system, and skin cells such as keratinocytes. Targeting neurogenic inflammation is a promising target of novel therapeutic approaches for sensitive skin.
The Role of Vanilloid Receptors in Sensitive Skin
Many subjects with sensitive skin report untoward sensory feelings such as pruritus, burning, and pain to changes in temperature (1). This phenomenon led to the notion that receptors and pathways that mediate both temperature sensation and sensory symptoms are implicated in the pathogenesis of sensitive skin. Moreover, the stinging test using capsaicin or lactic acid has been employed as a robust method to diagnose sensitive skin objectively (2,6,42).
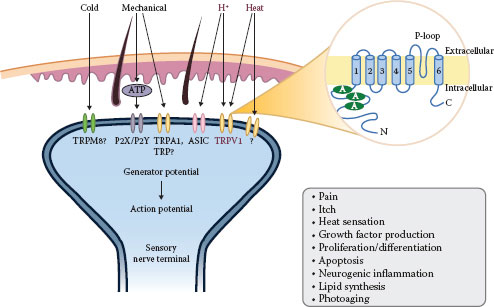
Capsaicin, the main pungent ingredient of hot chili pepper, is a natural agonist of TRPV1. Since the successful cloning of TRPV1 in 1997 (43), significant progress has been made in the field of TRPV1 biology (8,22,44–46). Six related subfamilies (TRPV [vanilloid], TRP canonical, TRP melastatin, TRP polycystin, TRP mucolipin, and the TRP ankyrin groups) based on the amino acid sequence homology comprise mammalian TRP channels. TRP channels are composed of six putative transmembrane domains, a pore-forming loop between the fifth and sixth domains, and intracellular NH2 and COOH termini that assemble as homo- or heterotetramers to form cation-permeable channels (22,47). TRPV1 forms cation channels with varying selectivity to diverse cations. For example, capsaicin-activated TRPV1 channels have roughly a 10:1 selectivity (permeability ratio) of Ca2+ over Na+, whereas heat-activated TRPV1 channels have a 4:1 selectivity (22,48). In addition to Ca2+ influx, Ca2+ release from internal stores, such as the Golgi apparatus, the endoplasmic reticulum, or the sarcoplasmic reticulum in muscle cells, contributes to changes in intracellular Ca2+ (22).
TRPV1 is a key molecular sensor and signaling integrator of thermal, chemical, and other sensory stimuli (49). TRPV1 is expressed on keratinocytes, fibroblasts, mast cells, endothelial cells, and sensory C and A-δ fibers (50–53). TRPV1 is a crucial contributor to pain, itch, and neurogenic inflammation (54–56). TRPV1 can be activated by low pH (<5.9), noxious heat (>43°C), and various chemicals such as adenosine triphosphate (ATP), anandamide, leukotriene B4, and resiniferatoxin (22) (Table 6.1; Figure 6.2). Long-term application of capsaicin causes TRPV1-mediated depletion of neuropeptides, leading to the desensitization of nerves (long-lasting refractory state unresponsive to further seemingly innocuous stimuli), and the amelioration of inflammatory responses (8,44).
The channel activity of TRPV1 can be markedly enhanced by low pH or inflammatory mediators via the activation of protein kinase A (PKA), PKC, PLC, and Ca2+/CaMKII pathways, leading to receptor sensitization, which lowers sensory perception thresholds (22,57–59). Pain sensation is augmented by acidic milieu during ischemia or inflammation. A-δ and C fiber neurons transduce extracellular protons via at least two different classes of cation-selective channels, TRPV1 and acid-sensing ion channels (ASICs) (60). On the contrary, TRPV1 can be inhibited by phosphorylation by cyclic adenosine monophosphate-dependent protein kinase (61).
The transmembrane influx of cations into the cytoplasm depolarizes the cells and elicits neuronal action potential propagation and muscle contraction. In nonexcitable cells such as keratinocytes and fibroblasts, membrane depolarization by TRPV1 leads to the stimulation of voltage-dependent channels and is associated with various physiological and pathological functions such as proliferation, differentiation, apoptosis, and inflammation (22,44,47,48). Previously, our group has identified the role of TRPV1 in intrinsic and extrinsic skin aging (induced by UV irradiation and heat) as well as UV-induced inflammation (62–66).
TABLE 6.1
Properties of TRPV1 Proteins
|
Expression in the skin |
Keratinocytes, fibroblasts, mast cells, dermal blood vessels, hair follicles, sebocytes, sweat glands, smooth muscle, skeletal muscle, Langerhans cells, C, A-δ fibers |
|
Direct activation |
Noxious heat (>43°C) |
|
Vanilloid compounds |
|
|
Capsaicin, resiniferatoxin, olvanil |
|
|
Endocannabinoid lipids |
|
|
Anandamide, arachidonylethanolamide, 2-arachidonoyl glycerol |
|
|
Eicosanoids |
|
|
5-(S)-HETE, 12-(S)-HETE, 5-(S)-HPETE, 2-(S)-HPETE |
|
|
Leukotriene B4 |
|
|
Extracellular proton (pH <5.9) |
|
|
Allicin |
|
|
ATP (via protein kinase C [PKC]) |
|
|
Bradykinin (via PKC) |
|
|
Camphor |
|
|
Eugenol |
|
|
NGF (via PKC) |
|
|
Oleoylethanolamide |
|
|
PGE2/PGI2 (via PKA) |
|
|
Piperine (black pepper) |
|
|
Serotonin (via PKC) |
|
|
Zingerone (ginger) |
|
|
Sensitizing pathways |
PKA |
|
PKC |
|
|
Phospholipase C (PLC) |
|
|
Ca2+/calmodulin-dependent kinase II (CaMKII) |
|
|
Inhibitors |
Capsazepine, ruthenium red, iodoresiniferatoxin, (N-(4-tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropryazine-1(2H)-carbox-amide), phosphatidylinositol-4,5-bisphosphate |
|
Functions |
Pain, noxious temperature sensation, bladder distension sensing, neurogenic inflammation |
Source: Nilius, B., Owsianik, G., Voets, T., and Peters, J. A., Physiol Rev, 87, 165–217, 2007; Ramsey, I. S., Delling, M., and Clapham, D. E., Annu Rev Physiol, 68, 619–647, 2006; Veldhuis, N. A., Poole, D. P., Grace, M., McIntyre, P., and Bunnett, N. W., Pharmacol Rev, 67, 36–73, 2015.
Note: HETE: hydroxyeicosatetraenoic acid; HPETE: hydroperoxyeicosatetraenoic acid.
FIGURE 6.2 Overview of TRPV1.
Kueper et al. (2) showed that selective TRPV1 antagonist trans-4-tert-butylcyclohexanol could inhibit capsaicin-induced human TRPV1 (hTRPV1) activation in vitro in hTRPV1-overexpressing HEK293 cells and oocytes. Moreover, in a clinical study involving 30 women, the compound was effective in reducing capsaicin-induced burning in vivo (2). These findings strongly suggest that TRPV1 activation is important in the pathogenesis of sensitive skin and that TRPV1 antagonists can be used for the treatment of sensitive skin.
Novel Pathomechanism of Sensitive Skin
As previously summarized, sensitive skin is related to altered somatosensory systems, especially lowered pain threshold and enhanced pain induction elicited by neurogenic inflammation and TRPV1 activation. In addition, sensitive skin is associated with impaired skin barrier function and altered immune responsiveness (3,67). More recently, we employed an unbiased microarray analysis of skin samples obtained from subjects with sensitive or nonsensitive skin and identified the unexpected gene signature in sensitive skin, which is closely associated with the dysfunction of muscle contraction, metabolic homeostasis, and ion balance. These alterations may result in decreased synthesis of ATP and enhanced proton, leading to skin sensitivity (29,68).
Sensitive Skin Showed Decreased Expression of Muscle Contraction-Related Genes
Healthy volunteers with sensitive or nonsensitive skin were classified based on self-assessment questionnaires and a 10% lactic acid stinging test. Those with underlying skin diseases such as rosacea were excluded. Microarray analyses using the skin from volunteers revealed an unexpected and distinct gene expression signature that while 17 upregulated genes in sensitive skin are associated with the inflammatory and immune responses, 29 downregulated genes in sensitive skin represent muscle composition/contraction, carbohydrate/lipid metabolism, and ion transport/ionic balance (Tables 6.2 and 6.3).
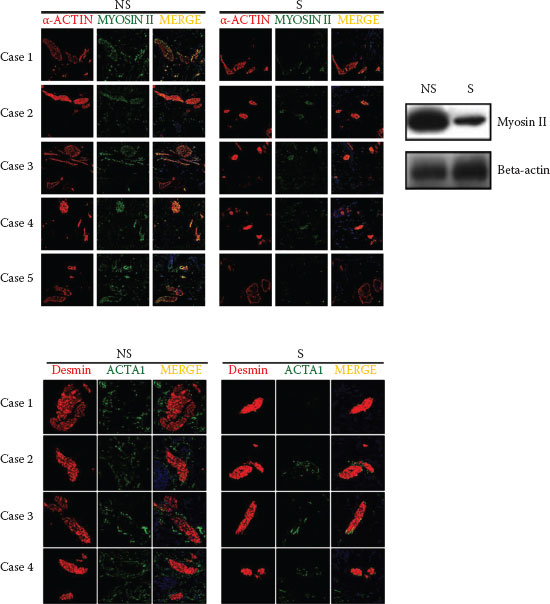
Many downregulated genes are associated with muscle contraction and relaxation process as well as muscle structure. In human facial skin, striated muscle fibers are found in the reticular dermis and subcutis (69), along with smooth muscles accompanying the hair follicles (arrector pili). The sarcomere, a functional unit of muscle, is composed mainly of thick filaments (myosin, slow-type myosin-binding protein C), thin filaments (actin, troponin, alpha tropomyosin 1, nebulin), and elastic components (titin). In the presence of Ca2+ from sarcoplasmic reticulum and ATP, the myosin head binds to the actin that enables the thin filament to slide along the thick filament, allowing for the shortening of the sarcomere (cross-bridge cycling) (70). Actin-bound myosin cross bridges in sensitive skin had more compacted shape than those in nonsensitive skin, indicating more contracted cross-bridge state in sensitive skin tissues (Figure 6.3). Further supporting experiments demonstrated that the decreased expressions of muscle-related genes in sensitive skin were not due to either a sampling bias or differences in anatomical sites. Our results suggest that sensitive skin may be associated with abnormal muscle contraction/relaxation process (29).
Sensitive Skin Showed Dysfunction of Metabolic Homeostasis, Impaired Aerobic ATP Synthesis, and Abnormal Muscle Contraction/Relaxation
Muscle contraction and relaxation require ATP regeneration through metabolic pathways such as phosphocreatine, anaerobic glycolysis, or oxidative metabolism. Carbohydrate and fat are the principal substrates for oxidative metabolism (71). The genes related to carbohydrate (genes for enolase 3; glycogenin 2; phosphorylase, glycogen, muscle; phosphoenolpyruvate carboxykinase 1) and fat metabolism (genes for lipase E, hormone-sensitive type, perilipin 1, glycerol-3-phosphate acyltransferase, mitochondrial, fatty acid-binding protein 4, lipoprotein lipase) were downregulated in sensitive skin. Myoglobin and carbonic anhydrase III (muscle-specific), which are involved in aerobic ATP synthesis, were also downregulated in sensitive skin. Consequently, sensitive skin stored significantly less ATP than nonsensitive skin. Lack of ATP in muscles leads to abnormal muscle contraction/relaxation, fatigue, and pain (72). Thus, the lower expression of genes involved in metabolic pathways could result in a lower level of ATP, which may result in abnormal muscle contraction and pain in sensitive skin.
TABLE 6.2
Downregulated Genes in Human Sensitive Skin
|
Gene Title |
Gene Symbol |
Entrez Gene |
S/NS(−) |
S/NS(+) |
|
Structural Constituent of Muscle and Muscle Contraction |
||||
|
Titin |
TTN |
7273 |
0.08 |
0.08 |
|
Actin, alpha 1, skeletal muscle |
ACTA1 |
58 |
0.18 |
0.09 |
|
Myosin binding protein C, slow type |
MYBPC1 |
4604 |
0.22 |
0.10 |
|
Myozenin 1 |
MYOZ1 |
58529 |
0.26 |
0.14 |
|
Tropomyosin 1 (alpha) |
TPM1 |
7168 |
0.28 |
0.16 |
|
Nebulin |
NEB |
4703 |
0.33 |
0.29 |
|
Kelch like family member 41 |
KLHL41 |
10324 |
0.10 |
0.08 |
|
Carbohydrate Metabolism |
||||
|
Enolase 3 |
ENO3 |
2027 |
0.29 |
0.25 |
|
Glycogenin 2 |
GYG2 |
8908 |
0.31 |
0.27 |
|
Protein phosphatase 1, regulatory inhibitor subunit 1A |
PPP1R1A |
5502 |
0.42 |
0.28 |
|
Glycogen phosphorylase, muscle associated |
PYGM |
5837 |
0.24 |
0.28 |
|
Phosphoenolpyruvate carboxykinase 1 |
PCK1 |
5105 |
0.58 |
0.35 |
|
Lipid Metabolism |
||||
|
Lipase E, hormone sensitive type |
LIPE |
3991 |
0.41 |
0.31 |
|
Perilipin 1 |
PLIN1 |
5346 |
0.34 |
0.32 |
|
Glycerol-3-phosphate acyltransferase, mitochondrial |
GPAM |
57678 |
0.52 |
0.34 |
|
Fatty acid binding protein 4 |
FABP4 |
2167 |
0.43 |
0.34 |
|
Lipoprotein lipase |
LPL |
4023 |
0.43 |
0.35 |
|
Ion Transport and Ionic Balance |
||||
|
Calsequestrin 1 |
CASQ1 |
844 |
0.45 |
0.23 |
|
Myoglobin |
MB |
4151 |
0.16 |
0.14 |
|
Carbonic anhydrase III, muscle specific |
CA3 |
761 |
0.42 |
0.18 |
|
ATPase H+ transporting V1 subunit B1 |
ATP6V1B1 |
525 |
0.58 |
0.51 |
|
Signaling Pathway |
||||
|
Adiponectin, C1Q and collagen domain containing |
ADIPOQ |
9370 |
0.42 |
0.39 |
|
Phosphodiesterase 3B |
PDE3B |
5140 |
0.48 |
0.40 |
|
Activin A receptor, type IC |
ACVR1C |
130399 |
0.43 |
0.42 |
|
Others |
||||
|
G0/G1 switch 2 |
G0S2 |
50486 |
0.36 |
0.28 |
|
Cell death inducing DFFA like effector c |
CIDEC |
63924 |
0.45 |
0.35 |
|
Tenomodulin |
TNMD |
64102 |
0.59 |
0.46 |
|
Retinol binding protein 4 |
RBP4 |
5950 |
0.35 |
0.26 |
|
Cysteine rich secretory protein 3 |
CRISP3 |
10321 |
0.33 |
0.58 |
Source: J Dermatol Sci, 76, Kim, E. J., Lee, D. H., Kim, Y. K., Kim, M. K., Kim, J. Y., Lee, M. J. et al., Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin, 214–221, Copyright (2014), with permission from Elsevier.
Note: S: sensitive skin; NS: nonsensitive skin; (−): − lactic acid; (+): + lactic acid.
TABLE 6.3
Upregulated Genes in Human Sensitive Skin
|
Gene Title |
Gene Symbol |
Entrez Gene |
S/NS(−) |
S/NS(+) |
|
Immune Response |
||||
|
Immunoglobulin light chain variable region complementarity determining region (CDR3) mRNA |
– |
– |
2.11 |
6.60 |
|
Major histocompatibility complex, class I, C |
HLA-C |
3107 |
1.65 |
3.86 |
|
Immunoglobulin heavy constant alpha 1 /// immunoglobulin heavy constant alpha 2 |
IGHA1 /// IGHA2 |
3493 /// 3494 |
1.89 |
2.79 |
|
Inflammation Response |
||||
|
S100 calcium binding protein A8 |
S100A8 |
6279 |
1.49 |
2.63 |
|
Others |
||||
|
Transferrin receptor |
TFRC |
7037 |
1.32 |
2.65 |
|
Cadherin 1 |
CDH1 |
999 |
1.90 |
2.23 |
|
Serpin family B member 13 |
SERPINB13 |
5275 |
1.28 |
2.38 |
|
Actin related protein 2 homolog |
ACTR2 |
10097 |
1.55 |
2.19 |
|
TNF receptor superfamily member 19 |
TNFRSF19 |
55504 |
1.40 |
2.07 |
|
GM2 ganglioside activator |
GM2A |
2760 |
1.68 |
2.06 |
|
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta |
YWHAZ |
7534 |
1.31 |
2.01 |
|
FK506 binding protein 5 |
FKBP5 |
2289 |
1.37 |
2.01 |
|
Peptidase inhibitor 3 |
P13 |
5266 |
2.11 |
1.97 |
|
Phosphoserine phosphatase |
PSPH |
5723 |
1.56 |
1.96 |
|
HORMA domain containing 1 |
HORMAD1 |
84072 |
2.25 |
1.88 |
|
ETS homologous factor |
EHF |
26298 |
1.21 |
2.37 |
|
Transcription elongation factor A (SII), 1 |
TCEA1 |
6917 |
1.88 |
2.84 |
|
Tumor protein p63 |
TP63 |
8626 |
1.63 |
2.29 |
|
Small proline-rich protein 2G |
SPRR2G |
6706 |
1.83 |
1.76 |
Source: J Dermatol Sci, 76, Kim, E. J., Lee, D. H., Kim, Y. K., Kim, M. K., Kim, J. Y., Lee, M. J. et al., Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin, 214–221, Copyright (2014), with permission from Elsevier.
Note: S: sensitive skin; NS: nonsensitive skin; (−): − lactic acid; (+): + lactic acid.
Enhanced Acidity May Cause Skin Sensitivity and Abnormal Muscle Contraction in Human Sensitive Skin
Muscle exposed to anaerobic state triggers the overproduction of carbon dioxide and H+, leading to enhanced acidity (73), which is known to elicit pain via TRPV1 and ASIC3 (43,74,75). Subjects with sensitive skin showed impaired pH homeostasis after lactic acid stimulation. The expressions of TRPV1, ASIC3, and CGRP were significantly induced in human sensitive skin. Moreover, rhabdomyosarcoma (RD) (skeletal muscle) cells treated with low pH showed significantly increased expressions of TRPV1, ASIC3, and CGRP. Finally, by using a well-established muscle contraction model in vitro, we confirmed that low pH could induce a state of abnormal muscle contraction, which was similar to that of sensitive skin. Collectively, our results suggest that sensitive skin may be associated with pain provocation through TRPV1, ASIC3, and CGRP due to impaired acidic homeostasis.
FIGURE 6.3 Actin-bound myosin cross bridges in sensitive skin. (Reprinted from J Dermatol Sci, 76, Kim, E. J., Lee, D. H., Kim, Y. K., Kim, M. K., Kim, J. Y., Lee, M. J. et al., Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin, 214–221, Copyright (2014), with permission from Elsevier.)
ADIPOQ Mediates Sensitivity in Human Skin
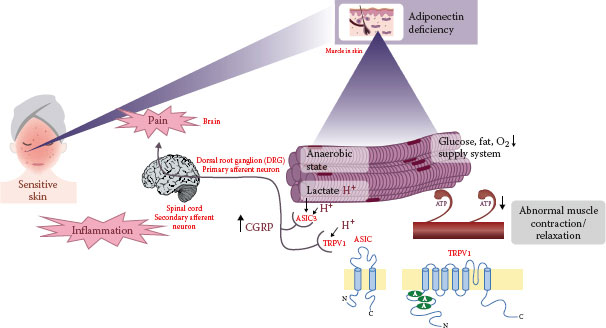
Adiponectin, C1Q and collagen domain containing (ADIPOQ) is an adipocyte-derived adipokine with multiple salutary effects, such as antiapoptotic and anti-inflammatory activities (76). The expression of ADIPOQ and adiponectin receptor was markedly downregulated in sensitive skin. Adenosine monophosphate-activated protein kinase, a downstream regulator of glucose and lipid metabolism, was also downregulated in sensitive skin (77). Intriguingly, the transient knockdown of ADIPOQ in vitro recapitulated the distinct gene expression signature in human sensitive skin in vivo (29) and showed abnormal muscle contraction, and lower ATP concentration, lower pH, but greater expression of pain-related transcripts such as TRPV1, ASIC3, and CGRP than control small interfering ribonucleic acid-transfected cells. Conversely, the treatment of RD cells with ADIPOQ induced a substantial reduction in the expressions of pain-related transcripts, suggesting a potential therapeutic role of ADIPOQ supplementation in sensitive skin.
FIGURE 6.4 A schematic model of putative pathway from reduced adiponectin to sensitive skin.
The disruption of metabolic homeostasis can cause variable diseases such as obesity, diabetes, and metabolic syndrome, which are closely associated with reduced ADIPOQ production (78). Little is known about the relationship between sensitive skin and metabolic disorders. Sensitive skin is also linked to ADIPOQ deficiency (Figure 6.4), and reduced ADIPOQ in sensitive skin may influence metabolic disorders or vice versa. Further preclinical and clinical studies are required to confirm its role in the treatment of sensitive skin in vivo.
REFERENCES
1. Farage MA, Maibach HI. Sensitive skin: Closing in on a physiological cause. Contact Dermatitis. 2010;62:137–149.
2. Kueper T, Krohn M, Haustedt LO, Hatt H, Schmaus G, Vielhaber G. Inhibition of TRPV1 for the treatment of sensitive skin. Exp Dermatol. 2010;19:980–986.
3. Stander S, Schneider SW, Weishaupt C, Luger TA, Misery L. Putative neuronal mechanisms of sensitive skin. Exp Dermatol. 2009;18:417–423.
4. Kim SJ, Lim SU, Won YH, An SS, Lee EY, Moon SJ et al. The perception threshold measurement can be a useful tool for evaluation of sensitive skin. Int J Cosmet Sci. 2008;30:333–337.
5. Querleux B, Dauchot K, Jourdain R, Bastien P, Bittoun J, Anton JL et al. Neural basis of sensitive skin: An fMRI study. Skin Res Technol. 2008;14:454–461.
6. Primavera G, Berardesca E. Sensitive skin: Mechanisms and diagnosis. Int J Cosmetic Sci. 2005;27:1–10.
7. Boulais N, Misery L. The epidermis: A sensory tissue. Eur J Dermatol. 2008;18:119–127.
8. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379.
9. McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev. 2010;34:148–159.
10. Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488.
11. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067.
12. Hendrix S, Peters EM. Neuronal plasticity and neuroregeneration in the skin—The role of inflammation. J Neuroimmunol. 2007;184:113–126.
13. Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901;26:173–209.
14. Gouin O, Lebonvallet N, L’Herondelle K, Le Gall-Ianotto C, Buhe V, Plee-Gautier E et al. Selfmaintenance of neurogenic inflammation contributes to a vicious cycle in skin. Exp Dermatol. 2015.
15. Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53.
16. Zegarska B, Lelinska A, Tyrakowski T. Clinical and experimental aspects of cutaneous neurogenic inflammation. Pharmacol Rep. 2006;58:13–21.
17. Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845.
18. Harvima IT, Nilsson G, Naukkarinen A. Role of mast cells and sensory nerves in skin inflammation. G Ital Dermatol Venereol. 2010;145:195–204.
19. Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol. 2008;121:955–961.
20. Quatresooz P, Pierard-Franchimont C, Pierard GE. Vulnerability of reactive skin to electric current perception—A pilot study implicating mast cells and the lymphatic microvasculature. J Cosmet Dermatol. 2009;8:186–189.
21. Reilly DM, Parslew R, Sharpe GR, Powell S, Green MR. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm Venereol. 2000;80(3):171–174.
22. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217.
23. Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266.
24. Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758.
25. Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768.
26. Brain SD, Williams TJ. Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin. Br J Pharmacol. 1989;97:77–82.
27. Brain SD, Tippins JR, Morris HR, MacIntyre I, Williams TJ. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Invest Dermatol. 1986;87:533–536.
28. Saria A. Substance P in sensory nerve fibres contributes to the development of oedema in the rat hind paw after thermal injury. Br J Pharmacol. 1984;82:217–222.
29. Kim EJ, Lee DH, Kim YK, Kim MK, Kim JY, Lee MJ et al. Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin. J Dermatol Sci. 2014;76:214–221.
30. Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC et al. Neuropeptide control mechanisms in cutaneous biology: Physiological and clinical significance. J Invest Dermatol. 2006;126:1937–1947.
31. Madva EN, Granstein RD. Nerve-derived transmitters including peptides influence cutaneous immunology. Brain Behav Immun. 2013;34:1–10.
32. Mikami N, Fukada S, Yamamoto H, Tsujikawa K. Neuronal derivative mediators that regulate cutaneous inflammations. Crit Rev Immunol. 2012;32:307–320.
33. Cevikbas F, Steinhoff A, Homey B, Steinhoff M. Neuroimmune interactions in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2007;7:365–373.
34. Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63.
35. Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011;15:33–39.
36. Gutwald J, Goebeler M, Sorg C. Neuropeptides enhance irritant and allergic contact dermatitis. J Invest Dermatol. 1991;96:695–698.
37. Misery L. Atopic dermatitis and the nervous system. Clin Rev Allergy Immunol. 2011;41:259–266.
38. Bak H, Lee WJ, Lee YW, Chang SE, Choi JH, Kim MN et al. Expression of neuropeptides and their degrading enzymes in ACD. Clin Exp Dermatol. 2010;35:318–323.
39. Yin S, Luo J, Qian A, Du J, Yang Q, Zhou S et al. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest. 2013;123:3941–3951.
40. Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161.
41. Scholzen TE, Brzoska T, Kalden DH, O’Reilly F, Armstrong CA, Luger TA et al. Effect of ultraviolet light on the release of neuropeptides and neuroendocrine hormones in the skin: Mediators of photodermatitis and cutaneous inflammation. J Investig Dermatol Symp Proc. 1999;4:55–60.
42. Farage MA, Katsarou A, Maibach HI. Sensory, clinical and physiological factors in sensitive skin: A review. Contact Dermatitis. 2006;55:1–14.
43. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824.
44. Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372.
45. Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: Targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68.
46. Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein-coupled receptor-transient receptor potential channel axis: Molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev. 2015;67:36–73.
47. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006; 68:619–647.
48. Song MY, Yuan JX. Introduction to TRP channels: Structure, function, and regulation. Adv Exp Med Biol. 2010;661:99–108.
49. Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524.
50. Kim SJ, Lee SA, Yun SJ, Kim JK, Park JS, Jeong HS et al. Expression of vanilloid receptor 1 in cultured fibroblast. Exp Dermatol. 2006;15:362–367.
51. Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139.
52. Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002;291:124–129.
53. Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285:1250–1252.
54. Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005;451:151–159.
55. Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313.
56. Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187.
57. Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288.
58. Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990.
59. Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962.
60. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177.
61. Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731.
62. Lee YM, Kang SM, Chung JH. The role of TRPV1 channel in aged human skin. J Dermatol Sci. 2012;65:81–85.
63. Lee YM, Kang SM, Lee SR, Kong KH, Lee JY, Kim EJ et al. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Arch Dermatol Res. 2011;303:727–736.
64. Lee YM, Kim YK, Kim KH, Park SJ, Kim SJ, Chung JH. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J Cell Physiol. 2009;219:766–775.
65. Lee YM, Kim YK, Chung JH. Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp Dermatol. 2009;18:431–436.
66. Li WH, Lee YM, Kim JY, Kang S, Kim S, Kim KH et al. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J Invest Dermatol. 2007;127:2328–2335.
67. Pinto P, Rosado C, Parreirao C, Rodrigues LM. Is there any barrier impairment in sensitive skin?: A quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Res Technol. 2011;17:181–185.
68. Kim EJ, Lee DH, Kim YK, Eun HC, Chung JH. Adiponectin deficiency contributes to sensitivity in human skin. J Invest Dermatol. 2015;135:2331–2334.
69. Yus ES, Simon P. Striated muscle: A normal component of the dermis and subcutis in many areas of the face. Am J Dermatopathol. 2000;22:503–509.
70. Kho AL, Perera S, Alexandrovich A, Gautel M. The sarcomeric cytoskeleton as a target for pharmacological intervention. Curr Opin Pharmacol. 2012;12:347–354.
71. van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536(Pt 1):295–304.
72. MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue—Regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125(Pt 9):2105–2114.
73. Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: A mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194.
74. Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35.
75. Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009;194:283–332.
76. Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568.
77. Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—Development of the energy sensor concept. J Physiol. 2006;574(Pt 1):7–15.
78. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792.
* Dong Hun Lee and Jin Ho Chung share senior authorship.