Algorithmic Approach to Diagnosis of Liver Disorders
Neil D. Theise
Romil Saxena
Introduction
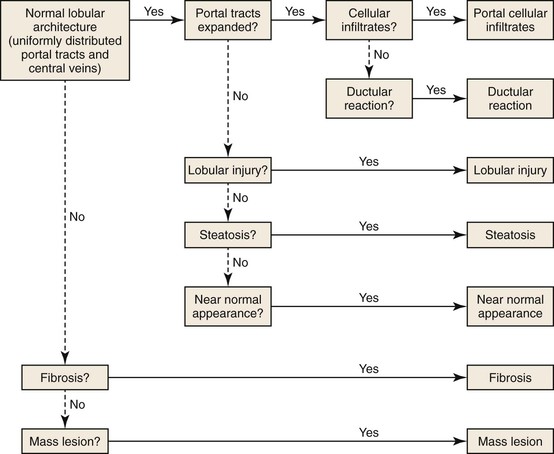
Assessment of liver architecture is the first step in assessment of a liver biopsy specimen. Unlike other organs that have well-defined anatomic compartments (e.g., glomeruli and tubules in the kidney), the liver consists of sheets of hepatocytes interrupted, at discrete intervals, by portal tracts and central veins of various sizes. Fibrous tissue is especially scarce in normal liver. It is limited to the portal tracts and central veins, and the amount varies according to the size of these structures. Therefore, delineation of normal architecture is challenging.
Preservation of normal architecture is indicated by the presence of portal tracts and central veins occurring at regular intervals. Alteration of architecture is suspected when portal tracts and central veins either are not present in the biopsy specimen or are distorted by fibrous tissue. After assessment of architecture, examination of portal tracts and parenchyma helps identify a dominant pattern of injury. The major patterns of liver injury include predominantly portal inflammation, predominantly lobular injury, ductular reactions, steatosis, and fibrosis. In some instances, histologic changes are minimal or absent. An algorithm to facilitate interpretation of liver biopsies is shown in Figure 42.1.
Helpful Diagnostic Tips
There is considerable overlap in morphologic patterns of injury among the various types of liver diseases. Therefore, clinical correlation is essential to establishing a correct diagnosis. For example, viral, autoimmune, and drug-induced hepatitis all show portal inflammation, and distinction among these disorders often requires clinical and serologic data (see Evaluation of Clinical and Serologic Information).
More than one pattern of injury may be present in a single liver biopsy specimen. For instance, a prominent ductular reaction can indicate biliary disease, but a mild ductular reaction can also indicate chronic viral hepatitis. Similarly, significant portal inflammation may be present in patients with lobular hepatitis.
Liver injury caused by drugs or toxins can mimic any type of liver disease. Drugs and toxins are the most frequent cause of a mixed, or “atypical,” pattern of liver injury. Establishing a diagnosis of drug- or toxin-induced liver injury relies heavily on determination of a temporal relationship between liver injury and drug use (including disappearance of symptoms on withdrawal of the drug and reappearance on rechallenge), as well as exclusion of competing causes of liver injury.
Pathognomonic features of certain diseases may be focal and therefore may not be present in a liver biopsy specimen. Examples include granulomatous duct destruction characteristic of primary biliary cirrhosis (PBC) and occluded central veins in patients with sinusoidal obstruction syndrome (formerly termed “veno-occlusive disease”). In such cases, other “soft” findings may be helpful in establishing the diagnosis in the right clinical context.
Some diseases, such as Wilson disease and α1-antitrypsin deficiency exhibit several different patterns of liver injury that can mimic other diseases. For example, Wilson disease may manifest as chronic hepatitis or steatohepatitis.
In some disorders, such as focal nodular hyperplasia, the features observed in a liver biopsy specimen depend on the portion of the lesion that was sampled.
Identification of Major Pattern of Injury
Predominantly Portal Inflammation
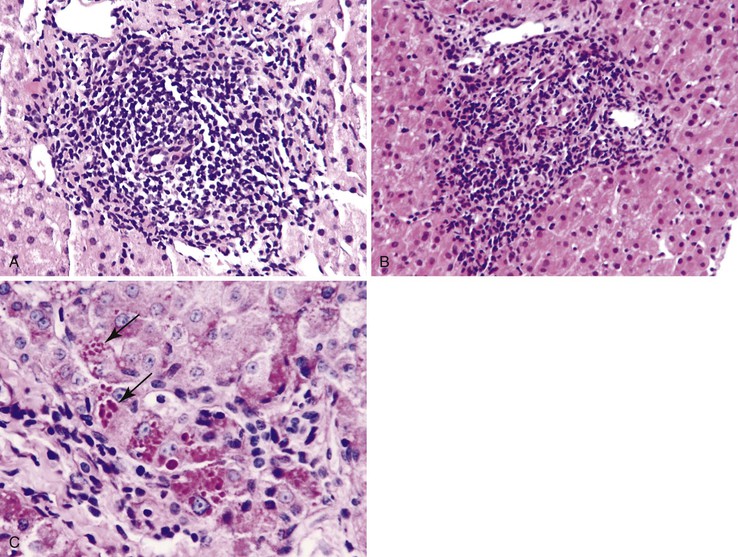
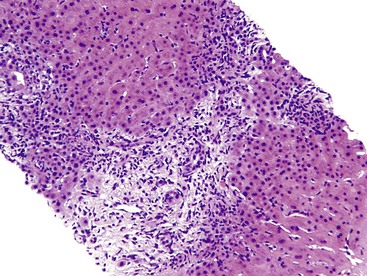
Inflammation located predominantly in the portal tracts (Fig. 42.2) occurs in a wide variety of liver diseases, such as chronic viral hepatitis, autoimmune hepatitis, drug- or toxin-induced hepatitis, chronic biliary diseases (PBC, primary sclerosing cholangitis [PSC], chronic biliary strictures), metabolic diseases (Wilson disease, α1-antitrypsin deficiency), and neoplastic conditions (lymphomas, leukemia).
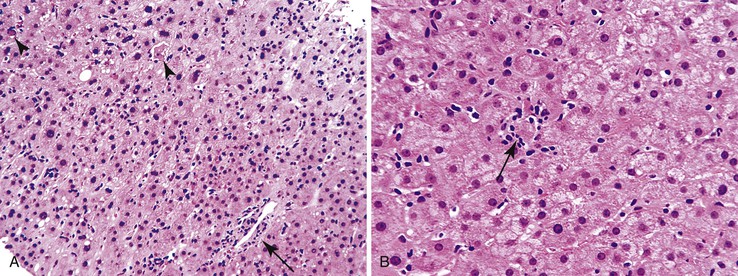
The infiltrate may be limited to the portal tracts, or it may extend into the periportal parenchyma (“interface hepatitis”).1 Even in this pattern of injury, there may be some degree of lobular inflammation with hepatocyte injury (Fig. 42.3). The presence of interface hepatitis indicates a chronic disorder that, ultimately, may lead to fibrosis. However, the rate of progression of fibrosis varies among the various disorders, and even among individuals with the same disease.2,3
The type of infiltrate, the pattern of involvement, and the presence or absence of associated findings (e.g., ductular reaction in chronic biliary diseases, intracytoplasmic globules in α1-antitrypsin deficiency) provide clues to the correct diagnosis.
Predominantly Lymphocytic Inflammation in Portal Tracts
Chronic Hepatitis C
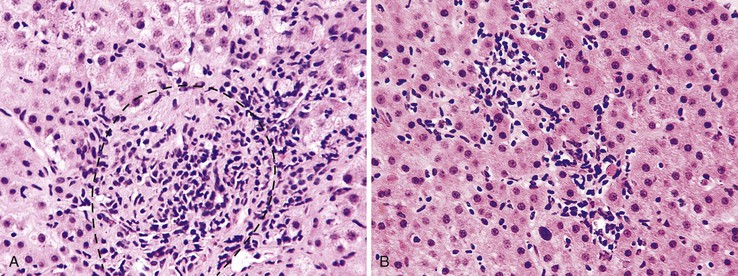
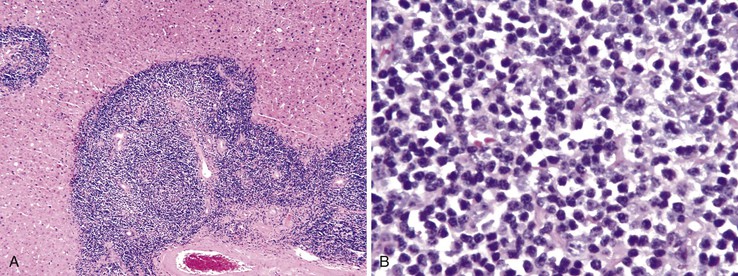
Lymphoid follicles (lymphoid aggregates with germinal centers) are a characteristic feature of hepatitis C.4 Although bile ducts may be associated within lymphoid follicles, they are not the primary target of injury. Nevertheless, bile ducts may show evidence of mild epithelial damage, such as loss, overlapping, and irregularity of nuclei. This may be caused by direct infection of cholangiocytes. Although lymphoid aggregates and lymphoid follicles are most often associated with hepatitis C virus (HCV) infection, they are not specific for this disorder.4 They also may occur in chronic hepatitis B virus (HBV) infection and in autoimmune hepatitis (Fig. 42.4).
Chronic hepatitis C is also characterized by the presence of macrovesicular steatosis, lipogranulomas, or both.4,5 Lipid droplets are essential for viral assembly, and the hepatitis C core protein has been shown to be localized to lipid droplets.6 Also, HCV is able to induce transcription of genes involved in lipid metabolism, most notably sterol regulatory element binding protein 1 (SREBP1) and peroxisome proliferator–activated receptor-γ (PPAR-γ). Chronic HCV infection also induces insulin resistance, which correlates with viral load. HCV infection causes insulin resistance by interfering with the function of the insulin receptor and insulin signaling pathways.7 Both steatosis and insulin resistance are associated with treatment resistance and progression of fibrosis.
Chronic Hepatitis B
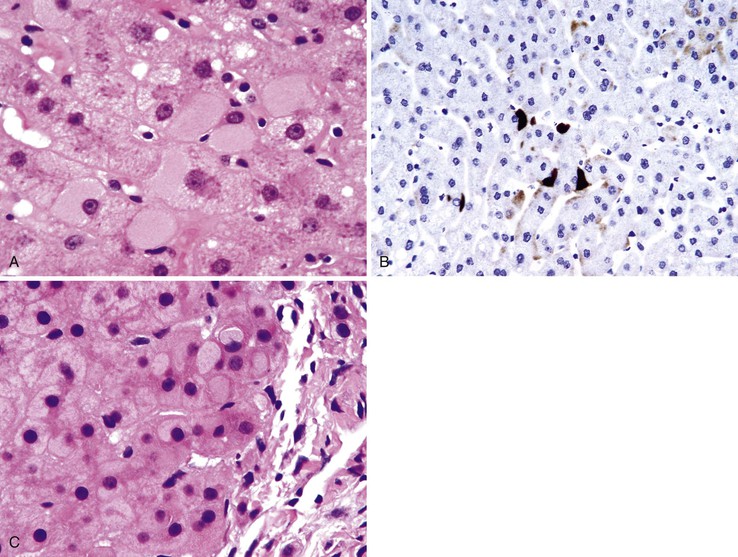
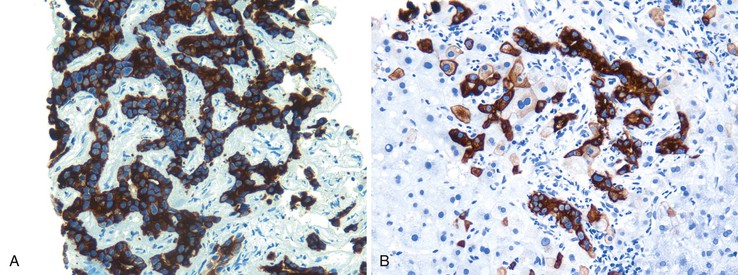
Chronic HBV infection shows predominantly portal mononuclear inflammation. Lymphoid aggregates are not as frequently present as with HCV infection. The presence of ground-glass hepatocytes is suggestive of chronic hepatitis B. However, ground-glass cells have been described in a wide variety of other conditions, such as use of certain drugs, and in patients who have received total parenteral nutrition.8 For this reason, immunohistochemical staining for the hepatitis B surface antigen (HBsAg) is important to confirm HBV infection (Fig. 42.5).
Autoimmune Hepatitis
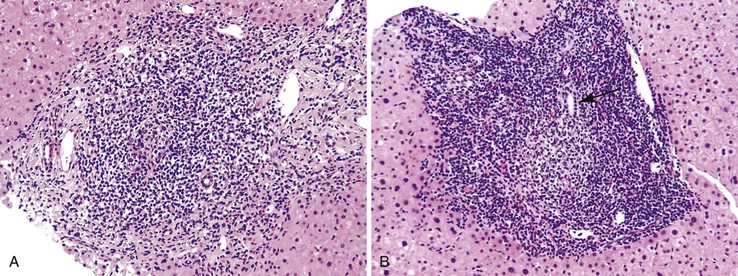
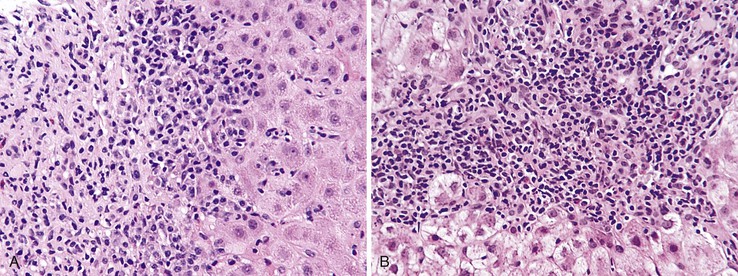
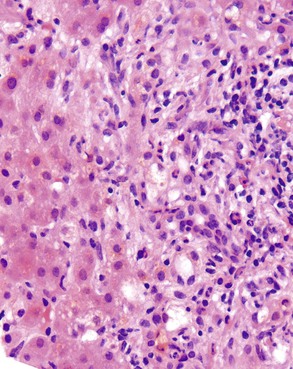
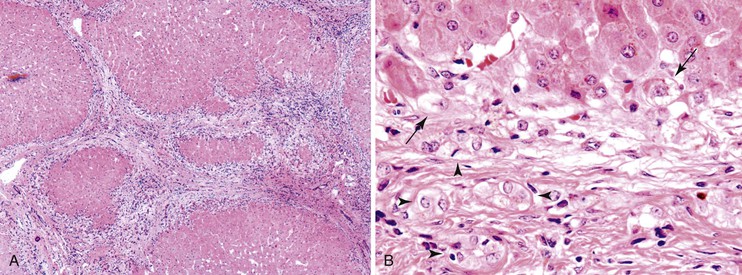
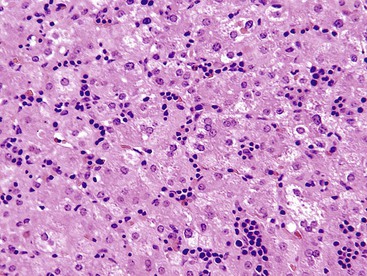
Autoimmune hepatitis is characterized by a mixture of portal and periportal inflammation and by marked lobular activity. Although plasma cells are also characteristic, they are not always prominent (Fig. 42.6). Some cases show predominant lymphocytic inflammation without prominent plasma cells.9 Eosinophils are usually present, albeit in very small numbers. Lymphoid aggregates and lymphoid follicles may be present as well. If follicles or aggregates surround bile ducts that show evidence of epithelial damage, then the possibility of an overlap syndrome with PBC should be considered.10
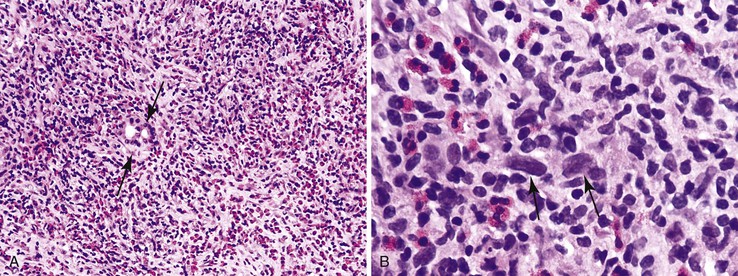
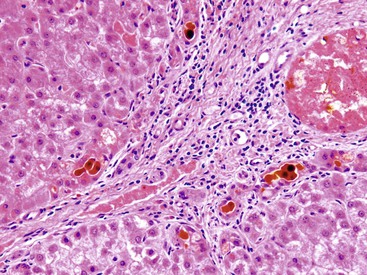
Most patients with autoimmune hepatitis, before treatment, show severe inflammatory activity. This is characterized by the presence of marked interface and lobular activity with hepatocyte necrosis and hepatocyte loss and, in particular, by confluent necrosis (Fig. 42.7). The latter is often localized around the central veins, or may bridge central–portal or portal–portal areas (“bridging necrosis”) or involve entire lobules (“parenchymal collapse”).11 Regions of confluent necrosis show loss of hepatocytes, cellular debris, macrophages, and extravasated red blood cells. When the areas of confluent necrosis extend to portal tracts, a ductular reaction often becomes prominent. Because bridging necrosis may mimic fibrous septa on hematoxylin and eosin (H&E) staining, a trichrome stain should be used to distinguish between bridging necrosis and bridging fibrous septa.
Acute Hepatitis
Prominent lobular inflammation and hepatocyte necrosis are characteristic features of acute hepatitis. However, this type of infiltrate is usually also accompanied by a variable amount of portal inflammation, which in some cases is quite prominent. The most common etiologic agents of acute hepatitis include hepatotropic viruses (hepatitis A virus [HAV], hepatitis B virus [HBV] with or without hepatitis D virus [HDV], HCV, and hepatitis E virus [HEV]),12 nonhepatotropic viruses, and drugs or toxins. Although most nonhepatotropic viruses (e.g., cytomegalovirus [CMV], herpes simplex virus [HSV], and adenovirus) can cause acute hepatitis, these organisms are not generally associated with prominent portal inflammation. However, Epstein-Barr virus (EBV) infection is associated with portal mononuclear infiltration to such as degree that it may mimic chronic hepatitis C or B.
Chronic Biliary Diseases
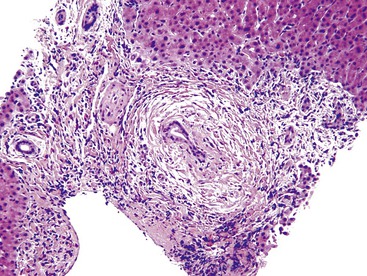
Chronic biliary diseases also demonstrate prominent portal lymphocytic inflammation. For instance, PBC, in its earliest stage (stage I), shows portal lymphocytic inflammation, lymphoid aggregates, and follicles. The latter are often associated with damaged bile ducts.13 The pathognomonic feature of PBC is the florid duct lesion (non-necrotizing granuloma surrounding a damaged bile duct), but florid duct lesions tend to be focal and therefore may not always be present in biopsy specimens (Fig. 42.8).13 Similarly, concentric periductal inflammation, or fibrosis, and bile duct scars are diagnostic of PSC (Fig. 42.9) but may not be present in a biopsy specimen. Nonspecific findings such as ductular reaction or copper accumulation in periportal hepatocytes may be observed in chronic biliary diseases, but mild ductular proliferation is also present in late stages of viral hepatitis (e.g., chronic hepatitis C), particularly as fibrous septa become more frequent and cirrhosis begins to develop.
Miscellaneous Conditions
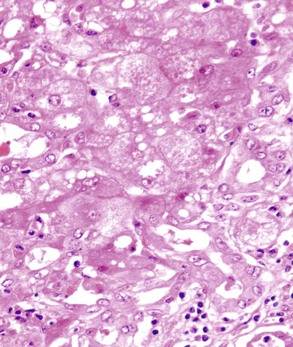
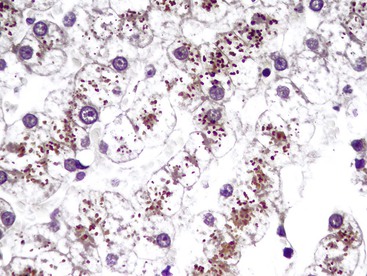
A prominent portal inflammatory infiltrate may be present in patients with α1-antitrypsin deficiency or Wilson disease. In the former, eosinophilic globules within periportal hepatocytes provide a clue to the diagnosis (see Fig. 42.2, B and C). However, globules may also represent an acute phase reaction during concomitant chronic viral hepatitis.14 Wilson disease may show prominent portal lymphocytes. Accompanying findings are relatively nonspecific and include mild steatosis, binucleated hepatocytes, and clear (glycogenated) nuclei.15
Predominantly Plasma Cell Inflammation in Portal Tracts
The main differential diagnoses in patients with abundant plasma cells in portal tracts are autoimmune hepatitis and PBC.9 Autoimmune hepatitis is more likely to show extensive interface hepatitis and/or confluent necrosis, features that are not common in PBC (see Fig. 42.7). In contrast, bile duct centric granulomas and predominant mononuclear infiltration are not features of autoimmune hepatitis. If hepatitic features predominate, autoimmune hepatitis is more likely; if biliary features (e.g., ductular reaction, chronic cholestasis) predominate, PBC is more likely, even in the absence of a duct-destructive lesion. If both sets of features are present and the clinical features of the patient reflect aspects of both diseases, then an autoimmune hepatitis–PBC overlap may be present.
Immunoglobulin G4 (IgG4) disease–associated sclerosing cholangitis may also show a predominance of plasma cells.16 In liver allografts, a plasma cell–predominant infiltrate is often indicative of recurrent autoimmune hepatitis, recurrent PBC, or late cellular rejection.17
Predominantly Mixed Portal Inflammation in Portal Tracts
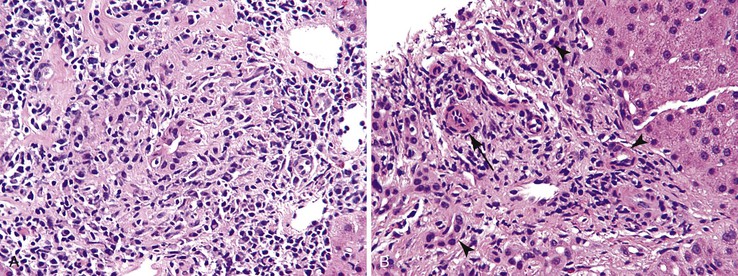
Acute cellular rejection is the prototype for a mixed portal inflammatory infiltrate that includes lymphocytes neutrophils, macrophages, and eosinophils (Fig. 42.10). Caution should be exercised in patients who have undergone liver transplantation for hepatitis C, because the portal mononuclear infiltrate characteristic of that disease, when recurrent, may be difficult to appreciate in the presence of rejection, particularly when the recurrent hepatitis C is mild.
Other diseases that may reveal a mixed infiltrate include Hodgkin disease. The presence of Reed-Sternberg cells, combined with immunohistochemical typing of the inflammatory infiltrate, helps reveal the malignant nature of the lesion. Extramedullary hemopoiesis may also have a mixed pattern if it occurs within portal tracts.
Predominantly Eosinophilic Inflammation in Portal Tracts
A pure eosinophilic infiltrate does not represent a common pattern of liver injury, but when it occurs, parasitic infection should be suspected. Of these, schistosomiasis targets the portal venous system. Larva migrans can cause eosinophilic abscesses.18 If the biopsy is obtained from a mass lesion, the presence of numerous eosinophils raises the possibility of an inflammatory myofibroblastic tumor, inflammatory pseudotumor, Langerhans cell histiocytosis, or Hodgkin disease (Fig. 42.11).
Eosinophils are also plentiful, although they are not the predominant cell type, in autoimmune diseases such as autoimmune hepatitis, PBC, and PSC and in drug-induced liver injury (see Figs. 42.4, B and 42.9).
Predominantly Granulomatous Inflammation in Portal Tracts
Granulomas may be divided into four basic diagnostic categories, characterized as “see the cause,” “know the cause,” “suspect the cause,” and “don’t know the cause.”19
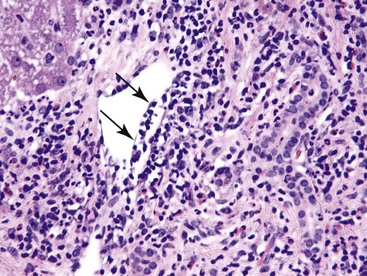
• Know the cause: The patient has a known clinical history of infectious (mycobacterial or fungal) granulomatous disease, sarcoidosis (Fig. 42.12), or other granuloma-associated disease, such as PBC (see Fig. 42.8).
Atypical Lymphoid Infiltrate in Portal Tracts
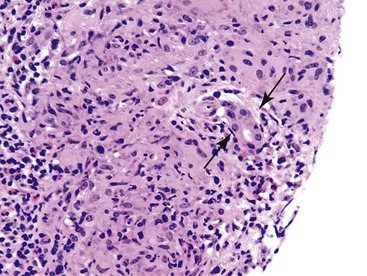
The differential diagnosis includes hemopoietic malignancies such as leukemia, lymphoma, and posttransplantation lymphoproliferative disorder (Fig. 42.13).20 The infiltrate in EBV hepatitis tends to appear atypical.
Evaluation of Clinical and Serologic Information
After a specific pattern of inflammation has been identified in portal tracts, the next step is to obtain the necessary clinical and serologic data. Most patients with this predominantly portal inflammation have chronic hepatitis B or C or autoimmune hepatitis. If these are excluded clinically, then drug- or toxin-induced hepatitis and Wilson disease are important entities to consider.
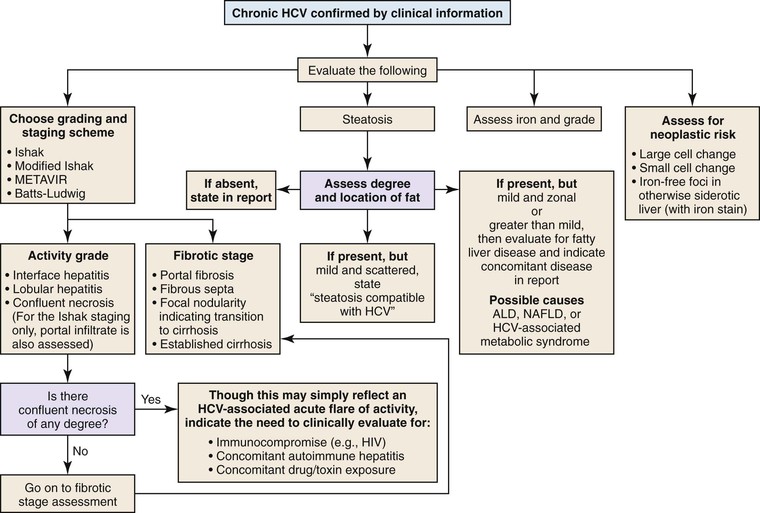
If the clinical history indicates either chronic viral hepatitis or autoimmune hepatitis, several points are worth emphasizing: There are several grading and staging schemes available, and all have advantages and disadvantages.21 When a particular grading or staging system is used, the name of the system (e.g., METAVIR, Ishak, Batts-Ludwig, modified Ishak) should be stated in the diagnosis or in a comment.11,21
The presence of confluent necrosis (perivenular necrosis, bridging necrosis, or parenchymal collapse; see Fig. 42.7) is typical of autoimmune hepatitis, particularly early in the course of disease. However, in viral hepatitis, it should trigger an evaluation for other, potentially important concomitant diseases, such as super infection with another type of hepatotropic virus or the presence of an immunodeficiency state (Table 42.1).
Table 42.1
Possible Implications of Confluent Necrosis in Biopsy Specimens from Patients with Viral Hepatitis
| Hepatitis B | Hepatitis C |
| Acute flare from HBeAg to HBeAb seroconversion | Acute flare |
| Super-infection with HDV | |
| Immunocompromise (HIV-associated or other) | Immunocompromise (HIV-associated or other) |
| Concomitant autoimmune hepatitis | Concomitant autoimmune hepatitis |
| Concomitant drug/toxin-induced liver injury | Concomitant drug/toxin-induced liver injury |
HBeAb, Antibody to the hepatitis B e antigen; HBeAg, hepatitis B e antigen; HDV, hepatitis D virus; HIV, human immunodeficiency virus.
Chronic Hepatitis C
If the clinical and serologic data confirm chronic hepatitis C, then the biopsy should be graded and staged with the most useful system at each clinician’s institute.
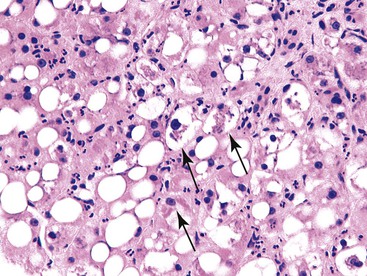
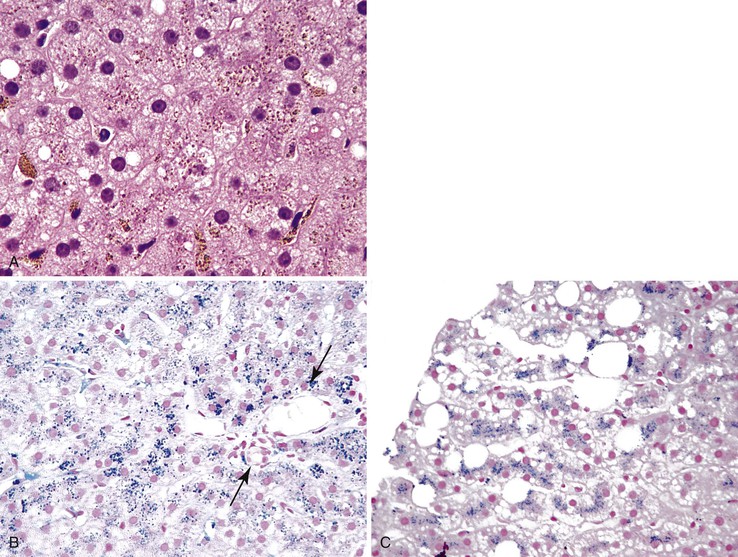
Steatosis and iron accumulation may be present in biopsy specimens from patients with chronic hepatitis C.11 Steatosis associated with chronic hepatitis C should be distinguished from primary fatty liver disease (alcoholic or nonalcoholic).11,22 Although mild hemosiderin may be associated with chronic hepatitis C, marked hemosiderin may indicate concomitant hereditary hemochromatosis.11,23 In all cases of chronic hepatitis, the biopsy should also be evaluated for the presence or absence of large cell change, small cell change, and iron-free foci (in an otherwise siderotic biopsy specimen) (Fig. 42.14 and Table 42.2).24
Table 42.2
Beyond Grading and Staging: Other Features to Evaluate in Biopsy Specimens from Patients with Viral Hepatitis
| Feature | Implication |
| Steatosis | HCV-associated steatosis vs. concomitant alcoholic or nonalcoholic fatty liver disease |
| Iron | HCV-associated vs. treatment-associated vs. hereditary hemochromatosis |
| Confluent necrosis | Severe activity of viral hepatitis vs. concomitant autoimmune hepatitis vs. concomitant drug/toxin-induced liver injury (see Table 42.1) |
| Large cell change | Malignancy-associated lesion indicating increased risk for development of HCC |
| Small cell change | Premalignant lesion indicating increased risk for development of HCC |
| Iron-free foci in siderotic liver | Malignancy-associated lesion indicating increased risk for development of HCC |
HCC, Hepatocellular carcinoma; HCV, hepatitis C virus.
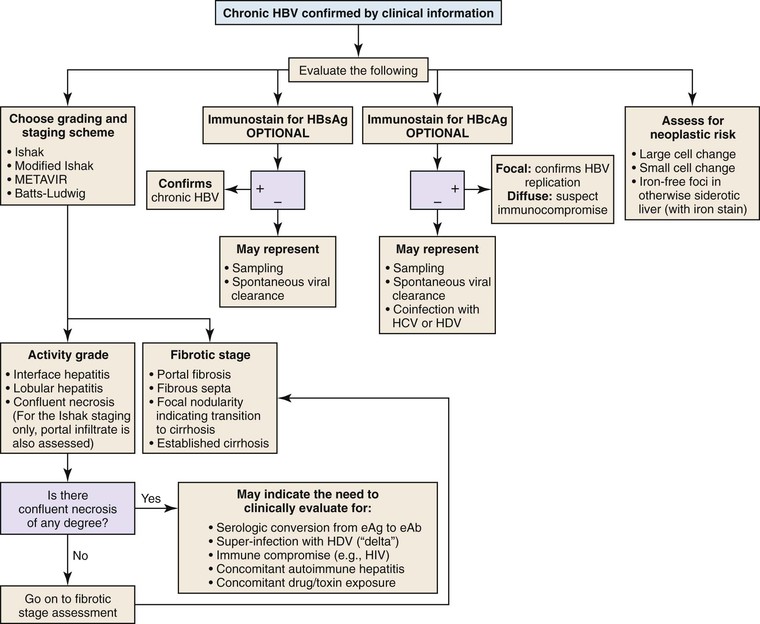
Chronic Hepatitis B
The presence of HBV infection can be confirmed by evaluation of a liver biopsy specimen; this is in contrast to the situation in HCV infection, in which the virus cannot be visualized in a histology specimen. In chronic hepatitis B, ground-glass hepatocytes, which indicate accumulation of HBsAg within endoplasmic reticulum of cells that have viral DNA integrated into the host genome, helps confirm the diagnosis. However, ground-glass changes are not always easy to identify, and their appearance is highly variable and dependent on the quality and type of fixation and staining. They are sometimes highlighted by a trichrome stain, but an immunostain for HBsAg is almost always positive, even in cases without overt ground-glass inclusions.25
An immunohistochemical stain for nuclear HBV core antigen (HBcAg) provides valuable clinical information.25 A positive stain confirms the presence of active viral replication and correlates with the presence of HBV DNA in the patient’s serum. A negative stain may be a sampling artifact, but it may also indicate spontaneous clearance of the viral infection (in which case, HBV DNA is absent) or coinfection with HCV or HDV (“delta hepatitis”). Therefore, this finding should be reported, particularly in geographic regions in which HDV infection is endemic. In cases with diffuse expression of HBVcAg, the patient may be immunocompromised, and coinfection with human immunodeficiency virus (HIV) should be considered. Steatosis and iron accumulation are not usually associated with chronic hepatitis B. Therefore, fatty liver disease should always be considered in chronic hepatitis B patients with steatosis. Similarly, concomitant hereditary hemochromatosis should be considered if significant hemosiderin deposition is present, particularly in patients of European descent (Fig. 42.15; see Table 42.2).
Autoimmune Hepatitis
In most patients with hepatitis B or C, the disease is confirmed by clinical and serologic correlation; biopsies are used to assess the grade and stage of infection and to rule out associated conditions. In contrast, in autoimmune hepatitis, the goal of the liver biopsy is confirmation of the disease in a patient with elevated serum transaminases and positive autoantibodies that are characteristic of autoimmune hepatitis, such as antinuclear antibodies (ANA), anti–smooth muscle antibody (ASMA), and anti–liver-kidney microsomal antibody type I (anti-LKM1).9 However, biopsy specimens may reveal features of other diseases, such as fatty liver disease, in which case the autoantibody levels may represent a reflection of some other disorder, such as rheumatologic conditions in other organs. Therefore, establishing a definite diagnosis of autoimmune hepatitis can be challenging. The following points may be helpful.
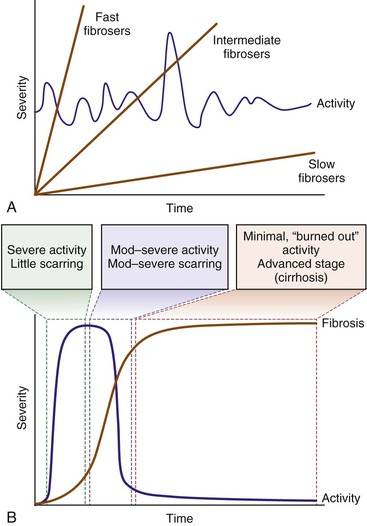
In chronic viral hepatitis, necroinflammatory activity is typically present at low levels throughout the natural history of the disease. It may diminish in late (“burned out”) stages, and there may occasionally be acute flares (particularly in hepatitis C) (Fig. 42.16, A). Fibrosis develops regardless of inflammation. In contrast, patients with autoimmune hepatitis have bouts of severe activity followed by long periods of inactivity (see Fig. 42.16, B). Fibrosis is a direct result of necrosis, so progression to cirrhosis is swift compared with chronic viral hepatitis. As a result, cases of autoimmune hepatitis may demonstrate any of the following features9,11: (1) cirrhosis with little or no necroinflammatory activity; (2) intermediate-stage disease with moderate to severe activity, combined with scarring and nodularity; or (3) early-stage disease with marked activity (at least focal confluent necrosis), combined with parenchymal collapse. However, patients with autoimmune hepatitis are unlikely to show periods of mild activity combined with mild or absent fibrosis. Therefore, biopsies that reveal both mild activity and mild fibrosis are unlikely to represent autoimmune hepatitis. In such cases, viral hepatitis and drug- or toxin-induced hepatitis should be excluded clinically.
As mentioned earlier, plasma cells are often absent in patients with autoimmune hepatitis.9,26 Their absence should not negate the possibility of autoimmune hepatitis if all other histologic and clinical features are compatible with that disorder. Chronic hepatitis caused by drugs or toxins may reveal prominent plasma cells. Therefore, in patients with no positive autoantibodies but with abundant plasma cells in portal tracts, a drug- or toxin-induced injury is more likely than autoimmune hepatitis.
Autoimmune hepatitis may overlap with PBC, which may also show abundant plasma cells.10 If serologic features typical of PBC and/or markedly elevated serum alkaline phosphatase are also present, the clinician may suspect the presence of both diseases. However, symptoms of both are just as likely to be seen in a biopsy specimen that shows very prominent activity, particularly with confluent necrosis. It is often not possible to be certain unless all clinical and pathologic features indicate the presence of both diseases. If the pathologist recognizes only scattered foci of interface and lobular hepatitis (akin to what one might see in hepatitis C), then it is likely not autoimmune hepatitis. Plasma cells are also not diagnostic of autoimmune hepatitis because they are almost always present in PBC as well. However, confluent necrosis or widespread interface and lobular hepatitis (that is easily recognized at low power), strongly suggest an overlap syndrome.
Ductular Reaction
A ductular reaction is defined by proliferated bile ductules present at the interface of the portal tracts and parenchyma. The term ductular reaction is preferred to “ductular proliferation,” because this phenomenon is associated with stromal, vascular, and inflammatory changes as well as ductular proliferation (Fig. 42.17).27 Ductular reactions develop as a result of bile duct or hepatocyte injury and are derived from stem cells located in the canals of Hering. A ductular reaction may also develop directly from intralobular bile ducts or even from extrahepatic bone marrow–derived precursor cells. A ductular reaction should be distinguished from biliary metaplasia of hepatocytes. The latter may occur at the portal-parenchymal interface as well. Biliary metaplasia represents a degenerative rather than a regenerative process. Ductular reactions are separated into acute and chronic forms and also by the primary target of injury (whether the injury occurs primarily to hepatocytes or bile ducts).
Acute Bile Duct Obstruction
Except in patients who have undergone liver transplantation, the finding of features of acute bile duct obstruction in a liver biopsy specimen is rare. In most instances, bile duct obstruction is diagnosed clinically, by radiologic tests. Ductular reactions associated with acute bile duct obstruction are typically diffuse, occurring in many or all portal tracts (Fig. 42.18). The ductular reaction may link portal tracts together. The reaction consists of anastomosing ductules associated with edematous stroma and neutrophils. Therefore, the presence of neutrophils is not necessarily indicative of acute (bacterial) ascending cholangitis. Ductular reactions are not normally associated with cholestasis or bile stasis within the lumina of the bile ductules. Their presence, termed cholangitis lenta, often indicates sepsis in the patient (Fig. 42.19).28 Acute bile duct obstruction is also often associated with bile infarcts, which are caused by the degenerating and detergent action of bile on hepatocytes.
Ascending Cholangitis
Patients with extrahepatic bile duct obstruction who have a secondary bacterial infection can develop ascending cholangitis. Ascending cholangitis is characterized by neutrophilic bile duct injury (acute cholangitis) combined with a ductular reaction located at the portal-parenchymal interface. In these cases, bile may be present within cholangiocytes and within the lumen of the bile ducts. Bile infarcts occur more commonly in patients with acute cholangitis. In patients with acute cholangitis and sepsis, cholangitis lenta may be present as well.
Cholangitis Lenta (Ductular Cholestasis)
Cholangitis lenta is characterized by the presence of dilated bile ductlike structures containing inspissated biliary concretions (see Fig. 42.19).28 These are located at the portal-parenchymal interface, and the condition is also commonly referred to as “ductular cholestasis.” This reaction is most often associated with sepsis, cardiac decompensation with poor hepatic perfusion, or severe dehydration, particularly in postsurgical patients. It is believed that ductular cholestasis related to sepsis is caused by circulating endotoxins. The finding of cholangitis lenta in biopsy specimens from patients with hepatitis should prompt the pathologist to notify the clinician of a potentially serious medical disorder that can result in sepsis. In some cases, cholangitis lenta may occur before clinical sepsis, the latter of which may not be evident clinically at the time of the liver biopsy.
Acute Hepatitis with Submassive or Massive Hepatic Necrosis
Ductular reactions may represent a prominent histologic feature in patients with severe acute hepatitis that has resulted in massive or submassive hepatic necrosis. In these instances, the ductular reaction develops from activation of stem or progenitor cells, as a result of injury to hepatocytes.29 Ductular reactions associated with acute hepatitis appear different from those that develop in patients with acute bile duct obstruction (Fig. 42.20). The former is associated with a mild mononuclear infiltrate, rather than neutrophils, and shows less of an edematous stroma. Proliferating bile ductules appear morphologically similar to hepatocytes and less similar to bile duct epithelium. In patients with submassive or massive hepatic necrosis, diffuse areas of bridging necrosis may surround regenerating liver nodules, mimicking an appearance of established cirrhosis. In this circumstance, a trichrome stain will help identify the presence of collagen in patients with cirrhosis and distinguish it from the lack of collagen in patients with acute hepatitis and submassive necrosis. The latter is characterized by necrosis with cellular debris, abundant pigment-laden macrophages, and inflammatory cells. A trichrome stain may also illustrate collapsed reticulin fibers, and this feature can be highlighted with a reticulin stain. In these cases, if a ductular reaction is present, it is typically located within necrotic septa, rather than at the border between the parenchyma and necrotic tissue.
Chronic Bile Duct Obstruction
Patients with chronic bile duct obstruction may develop a ductular reaction as well. Ductular reactions associated with chronic bile duct obstruction show scattered mononuclear cells and dense fibrosis instead of edema and neutrophils. The ductular reaction may link portal tracts in patients with cirrhosis due to chronic obstruction. The fibrous septa may show a halo effect, consisting of a band of edematous stromal tissue located directly beneath the ductular reaction. that may be associated with cholate stasis, which is defined by the presence of swollen hepatocytes sometimes associated with Mallory-Denk bodies (Fig. 42.21). The presence of a halo effect may be observed in many forms of chronic biliary tract injury, including PBC and PSC.
Primary Biliary Cirrhosis
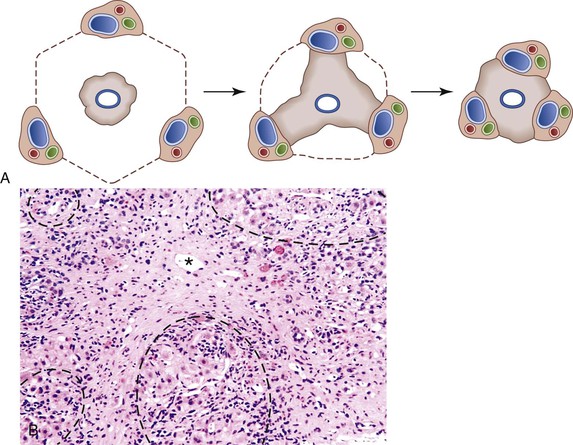
PBC is associated with destruction of small to medium-sized bile ducts, often with granulomatous inflammation. PBC is often associated with a bile ductular reaction that appears to represent a compensatory mechanism to allow maintenance of bile flow through the hepatic biliary system.30 Ductular reactions associated with PBC are usually accompanied by fibrosis and a mild plasma cell–rich mononuclear infiltrate. In this condition, it is believed that the ductular reaction leads to scar formation via release of cytokines and chemokines. The histologic changes of PBC are often patchy and segmental. Diagnostic bile duct destructive lesions may not be present in biopsy specimens from patients with PBC; specimens from deeper levels may be needed to demonstrate them. When typical lesions are not present, the ductular reaction and other changes may be described as being “compatible with PBC.” Direct consultation with the clinician is often necessary.
Thus, the pathologic stage of PBC may not accurately reflect overall disease progression, because the disease is patchy in nature.31 It reflects the biologic process that results in end-stage liver disease: Duct destruction (stage 1) leads to stem or progenitor cell activation, which leads to a ductular reaction (stage 2); the ductular reaction evokes scar formation (stage 3) through release of cytokines and chemokines, and scarring eventually leads to cirrhosis (stage 4).31
Primary Sclerosing Cholangitis
PSC is characterized by destruction of small, medium, and large-sized bile ducts, both within and outside the liver parenchyma.32 Extrahepatic bile duct injury related to PSC may lead to the development of acute and chronic bile duct obstruction within the liver parenchyma. In patients with PSC who have small bile duct obstruction only, chronic bile duct–type ductular reactions similar to PBC may develop. Most patients with PSC show a combination of small and large bile duct lesions. Therefore, the finding of a ductular reaction suggestive of both acute small and large bile duct obstruction should raise the possibility of PSC. This can be confirmed by imaging of the biliary tree by endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP). Most patients with PSC do not need a biopsy, because the diagnosis is established clinically and radiologically. However, patients with the small duct variant of PSC may show normal imaging results, and therefore are more likely to have a liver biopsy for diagnostic purposes. The characteristic findings of PSC, such as “onion skinning” (characterized by the presence of degenerative bile ducts surrounded by concentric bands of fibrosis) or “tombstone scars” (in which bile ducts are replaced by fibrous scars), are, in fact, quite rare. Often the presence of a ductular reaction is the only histologic manifestation of the disorder in liver biopsies.
Mass Lesions
Ductular reactions may be associated with primary hepatocellular tumors, such as (HCC), focal nodular hyperplasia (FNH), or hepatocellular adenoma (HCA). If a patient has a mass lesion in a noncirrhotic liver and the lesion is composed of hepatocytes, then the differential diagnosis includes these three entities.
In patients with suspected HCC, a ductular reaction should be distinguished from malignant bile ductules associated with combined hepatocellular cholangiocarcinoma. HCC is not associated with ductular reaction because this tumor does not contain a biliary component. In combined hepatocellular cholangiocarcinoma, the cholangiocarcinoma component is not difficult to distinguish from a ductular reaction.
In cases in which the needle biopsy missed the suspected lesion and samples were obtained from the adjacent parenchyma, the latter may show a ductular reaction either with or without sinusoidal dilatation.
Focal Nodular Hyperplasia
FNH is characterized by the presence of broad fibrous septa, often associated with a central stellate scar containing abnormal arteries, veins, and arteriovenous malformations.33 A ductular reaction is commonly located within the fibrous septa associated with this condition. Ductular reactions associated with FNH resemble chronic bile duct obstruction. The ductular reaction is associated with scant mononuclear cells, little or no edema, and, occasionally, cholestasis of adjacent hepatocytes. The ductular reaction may be highlighted with a keratin 7 stain. The presence of a maplike pattern with glutamine synthetase helps confirm a diagnosis of FNH in diagnostically difficult cases.34,35
Inflammatory Hepatocellular Adenoma
Although most HCAs do not contain a prominent biliary component, the inflammatory subtype of HCA may be associated with a ductular reaction.36 Inflammatory HCAs often demonstrate telangiectatic blood vessels and are termed telangiectatic adenomas. Ductular reactions associated with HCAs may also be highlighted with a keratin 7 stain, but the diagnosis is established by use of C-reactive protein or serum amyloid A immunostains, both of which are upregulated in almost all tumor cells. These proteins are usually elevated in the serum as well.
Space-Occupying Lesion
Liver parenchyma adjacent to space-occupying lesions may show features of bile duct obstruction combined with features of hepatic venous outflow obstruction. The liver parenchyma may show centrilobular sinusoidal dilatation, hepatocyte atrophy and congestion, and a periportal ductular reaction. The typical setting of this constellation of findings is a specimen from a patient with a mass in a noncirrhotic liver in which the biopsy needle missed the lesion. In this circumstance, a combination of centrilobular sinusoidal dilatation, hepatocyte atrophy and congestion (venous outflow obstruction), and periportal ductular reaction (biliary obstruction) helps confirm the presence of a nearby lesion even if not sampled in the biopsy specimen.
Cholangiocarcinoma
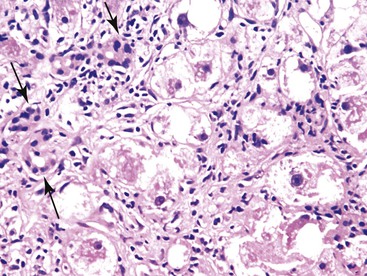
Proliferating bile ductules associated with benign ductular reactions can occasionally be difficult to distinguish from well-differentiated cholangiocarcinoma, particularly in frozen section specimens. Benign ductular reactions are typically located at the interface of the portal tracts and hepatic parenchyma, whereas malignant bile ductules have a more random, nongeographic distribution. Cholangiocarcinomas show cells with an increased nucleus-to-cytoplasm ratio (N : C ratio) and pleomorphism. Atypical mitoses may be present. The nuclei in benign ductular reactions are more uniform in appearance and lack atypical mitoses (Fig. 42.22).
Other Chronic Liver Diseases
A benign ductular reaction may develop in patients with any type of chronic liver disease that results in fibrosis and cirrhosis, such as nonalcoholic or alcoholic fatty liver disease, α1-antitrypsin deficiency, hereditary hemochromatosis, and chronic viral infections. At present, it is unclear whether fibrous tissue results in the development of a ductular reaction or whether the latter is important in the actual formation of fibrous tissue.
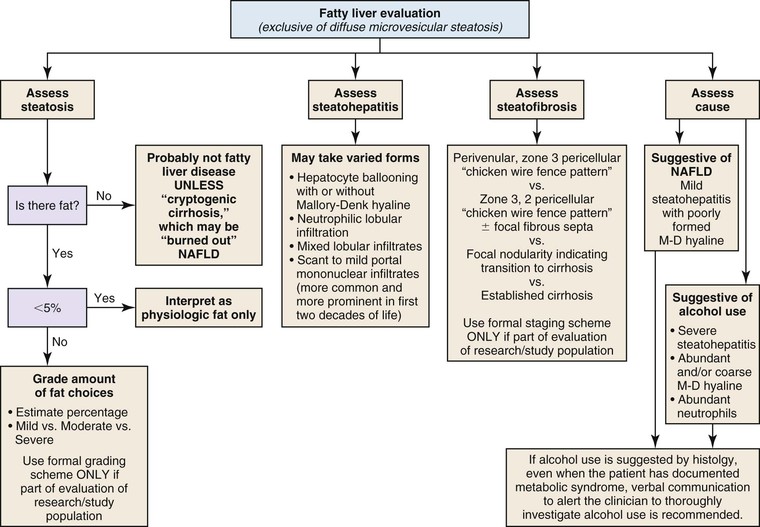
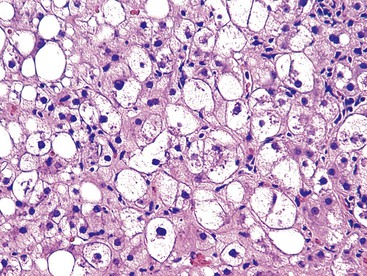
Steatosis
Steatosis in the liver is categorized into two types: large droplet and small droplet.37 The large droplet type is characterized by the presence of intracytoplasmic lipid vacuoles that enlarge the size of hepatocytes and result in compression of the nucleus against the cell membrane (Fig. 42.23). Small droplet steatosis refers to the presence of multiple small fat droplets within the cell cytoplasm that does not result in displacement of the nucleus to the edge of the cell membrane. The type and pattern of fat accumulation, with the accompanying histologic findings, help provide clues to the specific type of disorder.38 Steatosis is most commonly caused by nonalcoholic fatty liver disease (NAFLD), alcohol-induced liver disease (ALD), Wilson disease, drugs (e.g., amiodarone, methotrexate, anti-HIV medications), and viral infections (e.g., HCV, HIV). Metabolic diseases that cause fat accumulation because of defective fat utilization, or protein/calorie deprivation because of malabsorption, are termed NAFLD. Metabolic diseases most often associated with steatosis are cystic fibrosis, galactosemia, hereditary fructose intolerance, glycogen storage disease I and III, urea cycle defects, fatty oxidation defects, and a variety of mitochondrial disorders of the liver. Accumulation of fat in children is common in cases of calorie deprivation.
Three classes of histologic changes may be seen in fatty liver disease of any kind: steatosis, steatohepatitis, and steatofibrosis (Fig. 42.24). The most common causes of steatotic liver disease are ALD and NAFLD. There are no pathognomonic features that distinguish ALD from NAFLD reliably and consistently, but there are minor features that favor one diagnosis instead of the other. For instance, the severity of changes correlates more consistently with alcohol use. The specific changes associated with ALD and NAFLD are covered in detail later in this chapter.39
A comment about alcohol use in a biopsy report has medicolegal and ethical implications. If the clinician provides a written statement documenting the cause of the fatty liver disease (e.g., long term alcohol use, morbid obesity, uncontrolled diabetes mellitus, dyslipidemia), then the diagnosis may indicate “compatible with alcoholic liver disease” or “compatible with nonalcoholic fatty liver disease.” However, in the absence of such information, it is best to indicate that the liver disease is “compatible with alcoholic or nonalcoholic fatty liver disease.” There are no pathognomonic findings that can reliably distinguish ALD from NAFLD. However, the severity of changes may suggest alcohol use.40
Steatosis with Ballooned Hepatocytes or Mallory-Denk Hyalin
Nonalcoholic Fatty Liver Disease
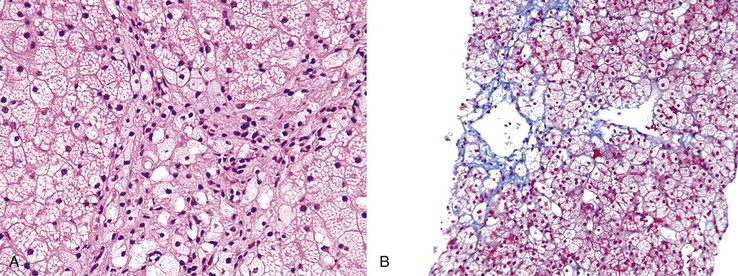
NAFLD is the most common cause of steatosis in the general population.37,38 This is related to the increasing incidence of obesity and metabolic syndrome in Western populations. NAFLD typically consists of a mixture of small, medium, and large droplet fat. NAFLD is categorized as either simple steatosis or steatosis associated with hepatocyte damage (steatohepatitis) (Fig. 42.25). Steatosis is believed to represent a nonprogressive type of disorder, whereas steatohepatitis may progress to fibrosis and cirrhosis. A diagnosis of NAFLD is based on the finding of ballooned hepatocytes (with or without Mallory-Denk hyalin) or parenchymal neutrophilic infiltrates associated with hepatocyte necrosis. Both steatosis and steatohepatitis may be associated with a mild portal inflammatory infiltrate composed almost exclusively of lymphocytes, as well as scattered foci of lymphocytes in the liver parenchyma. Apoptotic bodies and giant mitochondria may be present in NAFLD. In most patients, these features are present most notably in the centrilobular region surrounding central veins (zone 3) (Fig. 42.26, A). NAFLD-associated fibrosis begins in the centrilobular (perivenular) location and progresses through the sinusoids to create a sinusoidal pattern of fibrosis (see Fig. 42.26, B). Risk factors associated with progression to bridging fibrosis and cirrhosis are variable and largely unknown. In fact, NAFLD-associated cirrhosis may be devoid of fat, and this disease may account for a certain proportion of patients with apparent cryptogenic cirrhosis.
NAFLD in children may reveal a different morphologic pattern of injury than in adults and is sometimes referred to as NASH2.38 In children, fat accumulation occurs preferentially in periportal hepatocytes (Fig. 42.27), and fibrosis may preferentially involve the periportal parenchyma as well. Hepatocyte ballooning and Mallory-Denk hyalin is less common in children than in adults, and the degree of portal lymphocytic inflammation may be higher. The presence of ballooned hepatocytes and Mallory-Denk hyalin in periportal hepatocytes should raise suspicion for drug-induced NAFLD (e.g., amiodarone; Fig. 42.28) or Wilson disease. The metabolic syndrome (diabetes, hypertension, hyperlipidemia, insulin resistance, and hyperinsulinemia) usually underlies the development of NAFLD.
Alcohol-Induced Liver Disease
The histologic features of liver injury in ALD are similar to those of NAFLD.39,40 They include steatosis, ballooned hepatocytes (with or without Mallory-Denk hyalin), giant mitochondria, and sinusoidal fibrosis. These features occur preferentially in the centrilobular region, as in NAFLD. Although the features of these two disorders overlap considerably, the presence of well-formed or numerous Mallory-Denk hyalin bodies, prominent neutrophilic infiltration of the parenchyma, and prominent mitochondria are more suggestive of ALD than NAFLD (Fig. 42.29). ALD-associated Mallory-Denk hyalin is usually located within ballooned hepatocytes and is often surrounded by neutrophils. Perivenular fibrosis is more consistently present, and more prominent, in ALD compared with NAFLD.
Chronic Hepatitis C
As mentioned earlier, chronic hepatitis C is often associated with a mild degree of steatosis in the liver.11 Chronic hepatitis C–associated steatosis is usually macrovesicular (large droplet) and nonzonal in distribution. Mallory-Denk hyalin, ballooned hepatocytes, and giant mitochondria are not features of chronic hepatitis C–related steatosis. If these features are present in a patient with established hepatitis C, concomitant NAFLD should be suspected. There is a high prevalence rate of concurrent NAFLD (or ALD) and chronic hepatitis C in the general population. The relationship between chronic hepatitis C and NAFLD is not fully understood. There is some evidence that chronic hepatitis C promotes induction of the metabolic syndrome and, consequently, NAFLD, but this is controversial.
Wilson Disease
Patients with Wilson disease may show a variety of morphologic patterns of liver injury, including fatty liver disease, chronic hepatitis, and even fulminant liver failure.41 Steatosis associated with Wilson disease is often mild and of the large droplet type; it may be associated with Mallory-Denk hyalin. Other features of Wilson disease include binucleated hepatocytes, prominent glycogenetic nuclei, and mild portal and lobular inflammation.
Steatosis without Mallory-Denk Hyalin or Ballooned Hepatocytes
Steatosis without prominent Mallory-Denk hyalin or ballooned hepatocytes can be caused by a variety of different disorders, including several inherited metabolic syndromes (e.g., galactosemia, fructosemia). Liver biopsy specimens from patients with cystic fibrosis or α1-antitrypsin deficiency often show a variable amount of steatosis.42,43 In cystic fibrosis, steatosis may be related to malabsorption. Dilated ductules containing pink, eosinophilic material are observed. In α1-antitrypsin deficiency, eosinophilic intracytoplasmic globules are present in periportal hepatocytes; they are periodic acid–Schiff (PAS) positive and resist digestion with diastase. The diagnosis can be confirmed by immunohistochemistry.
Diffuse Microvesicular (Small Droplet) Steatosis
The presence of diffuse small droplet fat is termed diffuse microvesicular steatosis.44–46 Metabolic diseases associated with this relatively uncommon pattern of steatosis include fatty acid oxidation defects and mitochondrial disorders of the liver. Acute fatty liver of pregnancy is one example of a mitochondrial disorder that is triggered during pregnancy.45 It occurs when a woman has a heterozygous mutation for long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) and is carrying a fetus homozygous for this mutation. Fatty acids that cannot be metabolized by the fetus reach the maternal circulation and overwhelm the metabolizing pathway that is already deficient because of the heterozygous mutation.
Reyes syndrome, although rare, can lead to diffuse microvesicular steatosis in the liver.44 Other rare causes are alcoholic foamy degeneration and the effects of drugs such as valproate and tetracycline. Consumption of unripened ackee fruit, which contains the hepatotoxin hypoglycin A, may result in diffuse microvesicular steatosis, known as “vomiting sickness of Jamaica.”
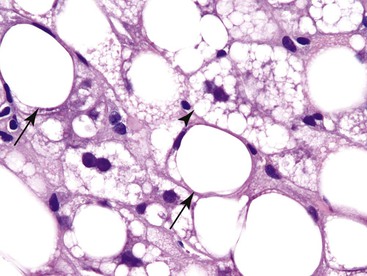
Patients with Niemann-Pick disease may develop finely vesiculated hepatocytes that resemble microvesicular steatosis (Fig. 42.30). This is actually caused by the accumulation of myelin figures within the cytoplasm of cells.46
Steatosis Associated with Tumors
Some types of primary liver tumors, such as FNH, HCA, and even HCC, can produce steatosis.47 HCAs with abundant steatosis are often associated with biallelic inactivation of the HNF1A gene and loss of liver-type fatty acid binding protein 1 (FABP1). This can be demonstrated by immunohistochemistry. In these cases, the surrounding nontumor liver tissue shows normal expression of FABP1. These adenomas have few or no cytologic abnormalities, and there is no risk of malignant progression.
Prominent Lobular Inflammation
Nonsinusoidal Pattern of Lymphocytic Infiltration
A nonsinusoidal pattern of lymphocytic infiltration is characterized by the presence of prominent inflammation in the hepatic parenchyma that is disproportionately high compared with the degree of inflammation in the portal tracts (Fig. 42.31, A). This pattern of liver injury may be associated with a wide variety of disorders, including hepatotropic and nonhepatotropic viral infections, lymphoproliferative disorders, and drug toxicity (hypervitaminosis A).
Acute Hepatitis
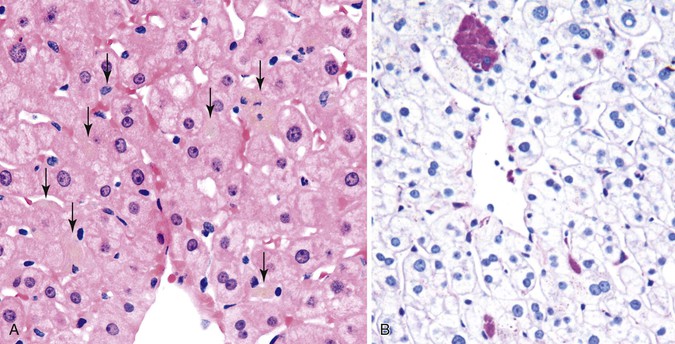
Many causes of acute hepatitis, including HAV and HBV infection and many drugs and toxins, are associated with prominent lobular inflammation that is disproportionately high in comparison with the portal tracts.21 When mild and focal, this is sometimes called “spotty necrosis” (see Fig. 42.31, B), which refers to the presence of isolated acidophilic bodies associated with mononuclear cells. In some circumstances, acidophilic bodies may be “naked,” implying an absence of mononuclear inflammation.4 This is characteristic of damage from certain drugs and toxins as well as HCV infection. In areas of spotty necrosis, a PAS stain with diastase digestion (d-PAS) may be used to highlight the presence of macrophages that have digested cellular debris. In many of these instances, the degree of portal inflammation is minimal or mild. Cases that show prominent lobular inflammation associated with equally severe or more prominent portal inflammation are more often associated with chronic viral hepatitis or autoimmune hepatitis. Of course, many types of drugs and toxins produce a mixed pattern of inflammation.
Features of Recent Acute or Subacute Resolving Hepatitis
Patients who have mildly elevated serum liver transaminase levels and no known liver disease may undergo a liver biopsy for diagnosis.21 In this situation, one may see clusters of pigment-laden macrophages that are silvery-gray on H&E stain but bright magenta with the d-PAS stain (Fig. 42.32). These may be centrilobular, midzonal or periportal, or in the portal tracts. The macrophages represent tissue repair from prior injury, such as acute viral hepatitis, environmental toxin, medications, or dietary supplements. Because macrophages exit via the lymphatics of the portal tract, their distribution provides information on disease repair. There may also be regenerative hepatocellular changes (increased binucleation, slightly thickened plates) providing more evidence of resolving liver injury. These patients do not typically progress to chronic liver disease or suffer recurrence. One appropriate diagnostic statement for such cases is “features of largely resolved acute or subacute hepatitis, cause unknown.”
Nonhepatotropic Virus Hepatitis
Cytomegalovirus.
CMV is a rare cause of acute hepatitis. It occurs almost exclusively in immunocompromised hosts. CMV hepatitis was more common in the late 1980s and early 1990s, when it was associated with transplantation, and in patients with untreated HIV infection. Characteristic features include the presence of cytomegalic cells combined with microabscesses.48,49 CMV may infect hepatocytes, cholangiocytes, or endothelial cells. Microabscesses are defined as small foci of neutrophils within the liver parenchyma. Microabscesses may be associated with cytomegalic cells. Cytomegalic cells and microabscesses occur randomly throughout the lobule, without a preferential zone of involvement. Immunostains for CMV help confirm the diagnosis.
Herpes Simplex.
HSV infection of the liver is rare.49 It results in randomly distributed, sharply delineated (“punched-out”) foci of necrosis. Necrotic areas may be filled with cellular debris, including macrophages, and may be lined by viable hepatocytes containing herpes-type nuclear inclusions.
Adenovirus.
Most common in children, adenoviral hepatitis may appear similar to infection by HSV.50 Well-delineated punched-out areas of hepatocyte loss develop, but in adenovirus-related cases, areas of parenchymal loss are often larger and more confluent than in HSV infection. Virus-related inclusions are large and basophilic and impart a smudgy appearance to the cell nuclei.
Sinusoidal Pattern of Lymphocytic Infiltration
Several diseases may be associated with a prominent sinusoidal pattern of lymphocytic infiltration in the liver, including celiac disease, EBV infection, and some types of lymphoproliferative disorders. Stellate cell lipidosis associated with hypervitaminosis A may also simulate this pattern of liver injury.
Celiac Disease
Patients with celiac disease may have mild, nonspecific changes in the liver characterized by an increase in the number of mononuclear cells within the sinusoids and rare scattered acidophilic bodies.51 They may also have a mild degree of portal mononuclear inflammation. The cause of this pattern of liver injury is unknown. Of course, there is a well-known association between celiac disease and other autoimmune disorders, such as autoimmune hepatitis and PBC.
Epstein-Barr Virus
EBV-related hepatitis occurs predominantly in immunocompromised patients.52 However, in immunocompetent patients, infected livers may show increased lymphocytic infiltration in the liver sinusoids, with or without associated acidophilic bodies. Sinusoidal lymphocytes may appear larger and slightly more atypical than mature lymphocytes. The diagnosis can be confirmed with in situ hybridization for Epstein-Barr encoded RNA (EBER).
Lymphoma and Leukemia
Lymphoproliferative disorders such as lymphoma and leukemia may involve the liver with a variety of patterns. Although some cases show predominantly portal infiltration, others show prominent lobular involvement, particularly within the sinusoids. All types of leukemia and both B- and T-cell lymphomas may involve the liver in this fashion. The degree of sinusoidal infiltration is typically much more pronounced than in cases of nonhepatotropic virus hepatitis.
Extramedullary Hematopoiesis
Extramedullary hematopoiesis may also involve the liver in a sinusoidal pattern (Fig. 42.33). It may occur in isolation or in association with a lymphoproliferative disorder. In some hematopoietic malignancies in the liver, it is easy to recognize complete hematopoiesis (megakaryocytes, myelocyte, and erythroid lineages). In such situations, the hematopoietic reaction may reflect a direct effect of the malignancy in the liver through “reawakening” extramedullary hematopoiesis that the liver produces in utero and in early postnatal life. It may also reflect marrow replacement, suppression by toxins, or a malignancy elsewhere in the body. Finally, some patients with congestive heart failure develop extramedullary hematopoiesis. Therefore, the finding of extramedullary hematopoiesis in liver, in the absence of other findings, should also trigger a workup for cardiac disease and hematologic disorders.
Stellate Cell Lipidosis Associated with Hypervitaminosis A
Finally, although it is not a primary lymphocytic disorder, patients with hypervitaminosis A may develop abundant stellate-shaped cells within the sinusoids that can mimic other sinusoidal infiltrative disorders.53 When marked, this may produce a “starry sky” pattern of injury. In patients with high-dose vitamin A consumption over a long period, stellate cells may get activated and produce collagen, which results in the development of cirrhosis. Elimination of vitamin A from the diet can result in regression of lipidosis and reversal of fibrosis.
Patients who have stellate cell lipidosis detected on staging biopsies for other types of liver diseases, particularly ALD or chronic viral hepatitis, may develop synergy between scarring of the two disease processes.
Of note, not all patients with hypervitaminosis A have used vitamin A; this finding also may result from use of other retinoid compounds, either orally or in skin creams or ointments. Patients should diminish or cease their use of these products.
Pigments in the Liver
Lipofuscin
Lipofuscin normally accumulates in the liver with advancing age.54 It is most prominent in the fifth or sixth decades of life. Lipofuscin is a golden, granular pigment that is often most prominent in centrilobular hepatocytes. Its primary distribution is in the midzonal portions of the liver (zone 2), and it does not stain with Prussian blue or Perls iron stain. Any disorder that results in chronic injury to the liver may be associated with increased lipofuscin deposition.
Iron
In contrast to lipofuscin, iron is a refractile pigment that stains positive with Prussian blue or Perls stain (Fig. 42.34). Iron deposition may be primary (hereditary hemochromatosis) or, more often, secondary.11,23 The most common secondary causes are NAFLD and chronic hepatitis C (see Fig. 42.34).
Copper
Increased copper deposition within the liver is difficult to identify on routinely stained tissue sections.41 It is usually present within periportal hepatocytes and can be highlighted with a rhodanine (Fig. 42.35), Victoria blue, or Orcein stain. These stains highlight copper-associated proteins. Copper accumulation in the liver may occur in a variety of chronic cholestatic disorders or in Wilson disease. In chronic cholestatic disorders, interference with copper secretion into bile leads to accumulation of the pigment within the liver. Wilson disease is protean in its manifestations. Rarely, this disorder may show completely normal liver or mild copper deposition without any other associated findings. However, some patients with Wilson disease develop an acute or chronic form of hepatitis, steatosis, steatohepatitis, and steatofibrosis. Other features include glycogenated hepatocyte nuclei and ballooned hepatocytes with Mallory-Denk bodies. Wilson disease–associated cirrhosis often shows a prominent ductular reaction as well.
Fibrosis (and Cirrhosis)
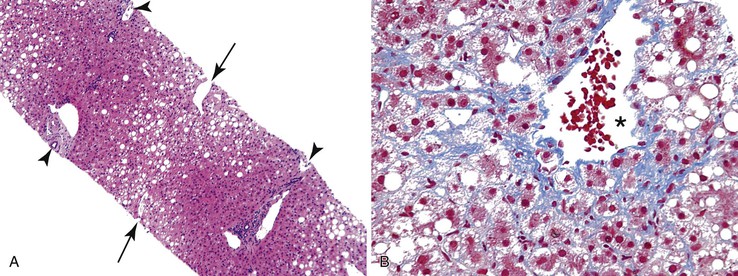
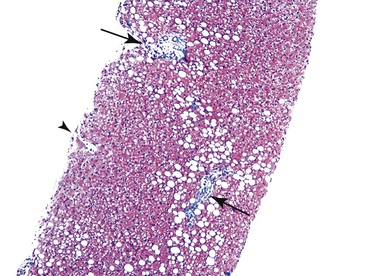
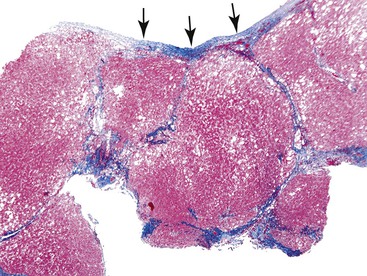
Fibrosis, the end result of most forms of chronic liver disease, is not considered to be a primary pattern of injury of the liver. However, the pattern of fibrosis and associated histologic findings may provide clues to the patient’s diagnosis. When evaluating liver specimens for fibrosis, pathologists should be aware of several pitfalls. For example, the subcapsular region of the liver normally contains fibrous tissue, and this should not be overinterpreted as pathologic fibrosis. Also, portal tracts and central veins may show more prominent fibrous tissue within 5 mm of the liver capsule. In this area, one may see bridges of fibrous tissue between portal tracts or between portal tracts and central veins (Fig. 42.36). The amount of fibrous tissue in normal portal tracts depends on the size of the tracts: Large portal tracts contain a more significant amount of fibrous tissue.
Fragmented Biopsy Specimen
Liver tissue that contains bridging fibrosis and regenerating nodules may result in liver biopsy specimens that are fragmented.54 Therefore, biopsies that are fragmented should always be considered suspicious for cirrhosis. However, fragmentation may also occur in the absence of cirrhosis. Distinction between fragmentation associated with cirrhosis and normal fragmentation caused by the technical aspects of obtaining a liver biopsy specimen may be difficult. Evaluation of the borders of the fragments of tissue and the architecture of the hepatic plates (by reticulin stain) can help differentiate these two conditions. For instance, the finding of multiple round fragments of liver tissue is more commonly observed in cirrhosis than in normal liver. In addition, collapse of the normal connective tissue framework of the liver in patients with bridging necrosis and multiacinar loss of hepatic parenchyma may be mistaken for true fibrosis. In these instances, a trichrome stain can be helpful. Finally, demonstration of wide bands of fibrosis, either with or without nodules, may be associated with FNH.
Overall, fibrosis should be evaluated with regard to its primary location. Predominantly portal-based fibrosis is more common in chronic biliary diseases, chronic viral hepatitis, and autoimmune hepatitis, In contrast, prominent perivenular and sinusoidal fibrosis is associated with ALD and NAFLD and with a wide variety of other drugs and toxins.37–40 Outflow tract obstruction is also associated with perivenular and perisinusoidal fibrosis (Fig. 42.37).
Portal-Based Fibrosis
As mentioned previously, predominantly portal and periportal fibrosis occurs in many types of chronic liver diseases, such as chronic viral hepatitis, autoimmune hepatitis, and chronic biliary diseases (PSC and PBC).21,31,32 All of these disorders are characterized by pathology that is located in the portal tracts and portal and periportal fibrosis in the early stage of disease, before the development of cirrhosis. An initial expansion of the portal tracts by fibrous tissue is followed by extension into the surrounding parenchyma. The inflammatory, and subsequently fibrotic, process involves the limiting plate of hepatocytes and creates the “leading edge” for progression of fibrosis. Eventually, portal-to-portal bridging fibrosis results in the development of cirrhosis. This pattern of fibrosis may be modified by disease flares, which cause bridging necrosis to progress to the development of central-portal fibrous septa.
Chronic biliary disorders may be associated with strands of fibrous tissue that parallel bile ductules.13,31,32 These diseases are associated with cholestasis, marked by an accumulation of intracellular bile and copper in periportal hepatocytes, and associated with hepatocyte necrosis. As mentioned earlier, cirrhosis associated with chronic biliary diseases may show a halo surrounding cirrhotic nodules. This represents a zone of edematous, lighter-appearing tissue containing proliferating bile ductules and ballooned periportal hepatocytes.
In general, hepatofibrosis and Caroli disease cause expansion of the portal tracts due to deposition of fibrous tissue, which may result in the development of fibrous septa between adjacent portal tracts.55 Fibrous tissue may contain ductal structures, with open lumina, containing inspissated bile. These are often arranged in a configuration reminiscent of fetal ductal plates. Caroli disease is also associated with dilatation of the native intrahepatic bile ducts.
Perivenular and Perisinusoidal Fibrosis
The presence of prominent fibrous tissue in the perivenular region of the liver parenchyma and within sinusoids is characteristic of steatohepatitis.37–40 In ALD and NAFLD, fibrosis begins in the perivenular region of the liver and extends into other areas of the parenchyma as the disease progresses. Sinusoidal fibrosis is often associated with pericellular fibrosis, in which fibrous tissue surrounds individual hepatocytes. Perivenular fibrosis tends to be particularly prominent in patients with ALD, in which case it may result in occlusion of the central veins (Fig. 42.38). Diseases that may be associated with sinusoidal and pericellular fibrosis include copper deposition diseases, tyrosinemia, galactosemia, citrin deficiency, hereditary fructose intolerance, Niemann-Pick disease, and Gaucher disease. Infections such as congenital syphilis and visceral leishmaniasis also cause sinusoidal fibrosis. Finally, disorders that cause chronic venous outflow tract obstruction, such as right-sided heart decompensation, veno-occlusive disease/sinusoidal obstruction syndrome, and Budd-Chiari syndrome, characteristically produce perivenular and perisinusoidal fibrosis as well.
Diagnosis of Solid and Cystic Masses
Tumors of the liver may be cystic or solid, primary or metastatic.56 Furthermore, any solid tumor can develop necrosis and thus appear cystic on imaging. These tumors usually retain their characteristic features in noncystic areas.
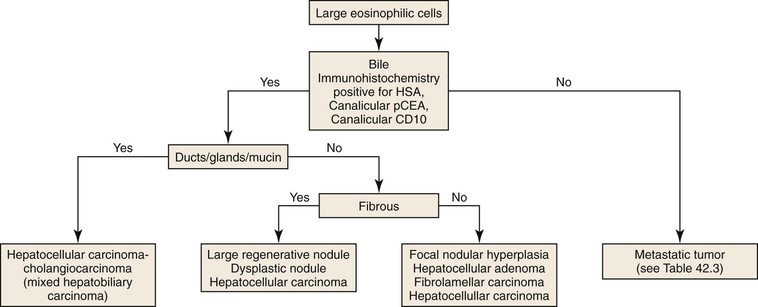
Most solid tumors demonstrate one of four general histologic patterns in liver biopsies: (1) tumors consisting of large eosinophilic cells, which may or may not represent hepatocytes; (2) tumors consisting of ductal/glandular structures, either with or without mucin; (3) tumors consisting of both hepatocytes and glandular/ductal structures; and (4) tumors consisting of small, immature-appearing cells.56
Tumor with Eosinophilic Cells
Tumors consisting of large eosinophilic cells with abundant cytoplasm and centrally located nuclei may represent a hepatocellular neoplasm or a hepatoid metastatic tumor (Fig. 42.39). The most frequent “hepatoid” neoplasms are adrenocortical carcinoma, renal cell carcinoma, neuroendocrine carcinoma, angiomyolipoma, and melanoma. However, any type of adenocarcinoma can demonstrate hepatoid features.
The presence of bile provides definitive evidence of hepatocellular differentiation and essentially rules out a metastatic tumor. Hepatocellular neoplasms demonstrate a trabecular pattern of growth. However, this feature is shared with a wide variety of nonhepatocellular tumors. Neoplastic hepatocytes may, on occasion, be arranged in a glandular (pseudogland) configuration. Pseudoglands do not contain mucin. The lumen represents malformed bile canaliculi and therefore may contain bile. The tumor cells may also contain one of a variety of inclusions, such as Mallory-Denk hyalin, eosinophilic inclusions, pale bodies, and fat droplets. Immunohistochemically, hepatocellular neoplasms are positive for hepatocyte-specific antigen (HSA) in a punctate cytoplasmic pattern. They are also positive for arginase-1 in the cytoplasm, either with or without nuclear staining, and positive for polyclonal anti–carcinoembryonic antigen (anti-CEA) and CD10 in a canalicular pattern. The latter pattern may coexist with a membranous pattern of staining. The diagnosis of HCC cannot be established in the absence of a canalicular pattern of staining. Therefore, to establish an accurate diagnosis in nonhepatocellular tumors composed of large eosinophilic cells, it is necessary to use appropriate immunohistochemical markers (Table 42.3).
Table 42.3
Immunohistochemical Markers for Diagnosis of Tumors with Large Eosinophilic Cells
| Diagnosis | Helpful Markers |
| Hepatocellular carcinoma | Hepatocyte-specific antigen (HepPar1), arginase 1, canalicular pattern with polyclonal anti-CEA or CD10 |
| Adrenocortical carcinoma | Inhibin, melan A, calretinin, vimentin, synaptophysin |
| Renal cell carcinoma | PAX8, carbonic anhydrase IX, glutamine S-transferase-α, CD10 (membranous pattern), vimentin, keratin 8/18 |
| Neuroendocrine tumor | Synaptophysin, chromogranin |
| Angiomyolipoma | HMB-45, melan A, smooth muscle actin |
| Melanoma | Melan A, HMB-45, S100 |
CEA, Carcinoembryonic antigen.
Because cirrhosis is the most important risk factor for the development of HCC, a tumor consisting of large eosinophilic cells arising in a liver with severe fibrosis or cirrhosis is most likely a hepatocellular neoplasm, regardless of the age of the patient. A dominant nodule found in a patient with cirrhosis (or advanced fibrosis) that demonstrates well-differentiated hepatocytes may represent a large regenerative nodule, a dysplastic nodule, or HCC. Overlapping features make distinctions among these lesions difficult.56–59 Helpful distinguishing features are shown in Table 42.4 and in Chapter 55.
Table 42.4
Features of Hepatocellular Neoplasms in Cirrhotic or Markedly Fibrotic Livers*
| Histologic Features | Diagnosis |
| Cell plates more than three cells thick, moderate nuclear contour irregularities, stromal or vascular invasion, widespread loss of reticulin framework; immunohistochemical positivity for two of three markers (i.e., HSP70, glypican 3, glutamine synthetase58) | HC carcinoma |
| Unpaired arteries, “clonal” appearance because of prominent steatosis, resistance to iron accumulation or prominent hepatocellular siderosis | At least dysplastic nodule |
| Dense cellularity, pseudogland formation, nuclear hyperchromasia, expansile nodules (nodule-in-nodule appearance), mild nuclear irregularities, cytoplasmic basophilia suggest HGD | HGD |
| Absence of the above | Large regenerative nodule |
* Portal tracts may be found in all these lesions but are uncommon in HCC.
HCC, Hepatocellular carcinoma; HGD, high-grade dysplasia; HSP70, heat shock protein 70.
In the absence of significant fibrosis or cirrhosis, a tumor consisting of large eosinophilic cells may represent a primary hepatocellular neoplasm or a metastatic lesion. Hepatocellular neoplasms that may arise within a nonfibrotic liver include FNH, HCA, HCC, fibrolamellar carcinoma, and hepatoblastoma.57 A significant proportion of HCCs occur in nonfibrotic livers, especially in geographic areas that are endemic for hepatitis B, where the infection is often transmitted vertically. HCC also occurs in patients with hemochromatosis, and in these patients, it may not be associated with cirrhosis.60,61
FNH is a well-circumscribed, unencapsulated nodule. It contains a central (or eccentric) scar with fibrous septa that radiate toward the periphery of the lesion. The scar tissue contains abnormal blood vessels that show eccentric thickening. The presence of these types of blood vessels in a liver biopsy specimen facilitates a diagnosis of FNH. A ductular reaction is often found at the edges of fibrous septa as well, and this can be highlighted by an immunohistochemical stain for keratin 19 or keratin 7. Characteristically, an immunohistochemical stain for glutamine synthetase highlights large, irregularly shaped (“maplike”) areas of positivity.35,36,62
Fibrolamellar carcinoma may also demonstrate a central scar. Sheets of large, deeply eosinophilic cells (oncocytes packed with mitochondria) are separated by thick bands of lamellar collagen. The cells contain numerous eosinophilic inclusions and pale bodies, which stain for α-fetoprotein and fibrinogen, respectively. In contrast to conventional HCC, keratin 7 is diffusely positive in fibrolamellar carcinoma.63
The distinction between adenoma and well-differentiated carcinoma can be difficult.36 The distinction relies heavily on the thickness of the hepatic plates and the loss of reticulin framework, among other features. Hepatic plates in adenomas are uniformly less than three cells thick and are supported by an abundant reticulin framework. Hepatic plates in carcinoma are typically more than three cells thick and display focal or diffuse reticulin loss. Isolated arteries can be found in both adenomas and carcinomas. A markedly steatotic, well-differentiated hepatocellular lesion arising in a nonsteatotic, nonfibrotic liver is likely to be an adenoma. This subtype of adenoma results from inactivation of the HNF1A gene. The diagnosis can be confirmed by showing loss of LABP1 within the nodule, with retention of this marker in the surrounding liver. The finding of significant sinusoidal dilatation and telangiectasia, often accompanied by an inflammatory infiltrate and ductular reaction, suggests the inflammatory subtype of HCA. These lesions are positive for C-reactive protein and serum amyloid A. The presence of pseudoglands in a well-differentiated hepatocellular lesion is more common in carcinoma, and the β-catenin–inactivated subtype of HCA. This subtype of adenoma is more likely to undergo malignant transformation. Immunohistochemically, there is overexpression of glutamine synthetase and aberrant nuclear localization of β-catenin.36,62
HCCs may show a mixture of glandular and ductal elements. These tumors represent combined hepatocellular-cholangiocarcinoma. Hepatocellular differentiation is confirmed by the presence of bile or immunohistochemical positivity for HSA, arginase 1, or canalicular polyclonal anti-CEA and CD10. Cholangiocytic differentiation is confirmed by the presence of true glands or ducts with mucin or diffuse and strong positivity for keratin 7.
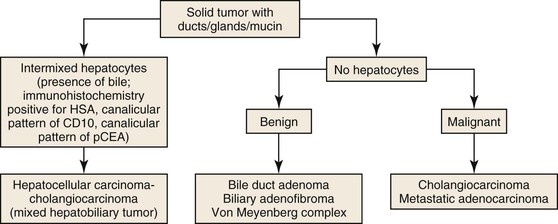
Solid liver tumors that consist entirely of ductal elements (Fig. 42.40) may represent a benign lesion, such as a bile duct adenoma, biliary adenofibroma, or biliary hamartoma (von Meyenburg complex). These lesions consist of benign ductal elements. Some show nuclear pleomorphism and contain abundant fibrous stroma. Because these lesions may appear as small, firm nodules, they are often biopsied during surgery to rule out a metastasis. Malignant tumors consisting entirely of ductal and glandular elements include intrahepatic cholangiocarcinoma and metastatic adenocarcinoma. Identification of the primary site of origin of a metastasis often requires the use of immunohistochemical markers in conjunction with clinical information. Differentiation of intrahepatic cholangiocarcinoma from metastatic pancreaticobiliary carcinoma can be extremely difficult. Keratin 19 staining is useful in this regard. A negative stain suggests that a pancreaticobiliary tumor is unlikely. Therefore, evaluation of adenocarcinomas in the liver should include immunostaining with keratin 19, in addition to keratin 7 and keratin 20.
A solid tumor of the liver that consists of small undifferentiated cells may represent a hepatoblastoma, a rhabdomyosarcoma, or a metastasis from a “small blue cell” tumor such as lymphoma or neuroblastoma. Analysis of appropriate immunohistochemical markers, in conjunction with clinical information, provides clues to the correct diagnosis. Hepatoblastoma is positive for HSA and α-fetoprotein. Hepatoblastoma occurs mainly in childhood and shows a broad range of morphologic patterns. At the well-differentiated end is the fetal subtype. This type may require distinction from well-differentiated HCC. Hepatoblastoma occurs in children younger than 5 years, whereas HCC is very rare at that age. Serum α-fetoprotein is elevated in both of these tumors, but the levels are much higher in patients with hepatoblastoma. Pure fetal hepatoblastoma consists entirely of fetal hepatoblasts arranged in “light” and “dark” areas. The former consists of hepatoblasts that contain glycogen, which imparts a clearness to the cytoplasm, whereas hepatoblasts in the “dark” areas do not share this characteristic. Extramedullary hemopoiesis is typically present in fetal hepatoblastomas.
The embryonal subtype of hepatoblastoma consists of cells with a high N : C ratio, abundant mitotic figures, and tubular structures. This variant may mimic metastatic neuroblastoma. Clinical information and positivity for neural rather than hepatocellular markers helps in this differential diagnosis. Light and dark areas typical of fetal hepatoblastoma are often present. The small cell or undifferentiated variant of hepatoblastoma may mimic other types of small blue cell tumors. However, the small cell variant of hepatoblastoma rarely occurs in a pure form. and areas with typical morphology are usually present. The presence of an undifferentiated small cell component, even focally, is a bad prognostic feature in hepatoblastoma.
Cystic Tumors
True cystic neoplasms of the liver (i.e., fluid-containing cysts lined by neoplastic epithelium) constitute a rather limited proportion of liver neoplasms. Because of the clinical and histologic similarity of these lesions to cystic tumors of the pancreas, the World Health Organization has revised the terminology of liver lesions to align it with that of pancreatic cystic tumors.56 In contrast to cystic tumors of the pancreas, cystic tumors of the liver are uncommon. Most cysts in the liver are either developmental or inflammatory/infectious in nature. The latter include hydatid cysts, amoebic abscesses, pyogenic abscesses, and necrotizing eosinophilic granulomas.
A liver cyst may or may not show an epithelial lining. The epithelium may be cuboidal/low columnar, ciliated, or tall columnar either with or without papillae; tall columnar epithelium may be biliary, mucinous, or oncocytic (Fig. 42.41). Solitary cysts lined by cuboidal or low columnar epithelium are relatively common. They arise from biliary epithelium and may represent a localized cystic dilatation of the biliary tree caused by obstruction or a biliary cyst caused by localized developmental arrest of the ductal plate. Numerous developmental cysts are seen in fibropolycystic diseases and may or may not involve other organs such as kidney and pancreas. The presence of ciliated respiratory epithelium suggests a ciliated hepatic foregut cyst, which is thought to arise from sequestration of respiratory epithelium during embryologic development of the liver.
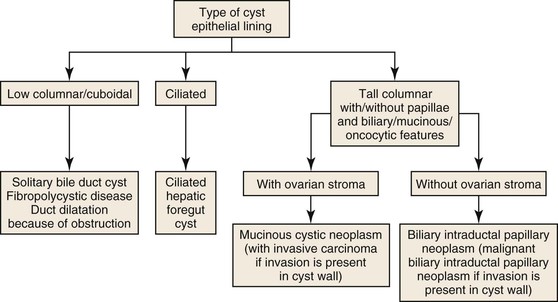
Cysts lined by tall columnar epithelium may show biliary, mucinous, or oncocytic differentiation. These cysts may or may not contain ovarian-like stroma. When present, the ovarian-like stroma is characteristic of mucinous cystic neoplasm of the liver.64 Similar to its counterpart in the pancreas, this type of cystic tumor occurs in young to middle-aged women. The stroma is positive for estrogen receptor, progesterone receptor, and inhibin. Most of these lesions are benign. Malignant transformation is characterized by invasion into the cyst wall. This lesion is best referred to as “mucinous cystic neoplasm with invasive carcinoma,” and not by the previous term, “biliary cystadenocarcinoma.”64,65
Cysts lined by tall columnar epithelium with biliary, mucinous, or oncocytic differentiation and an absence of ovarian stroma represent intraductal papillary biliary neoplasms (IPBNs). These tumors arise within the ductal system, and most form papillae. Because these lesions represent the hepatic counterpart of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas, the term “IPBN” is preferred to “biliary papilloma/papillomatosis.” In contrast to pancreatic lesions, which are most often lined by mucinous epithelium, most cysts in the liver are lined by biliary epithelium. Pure oncocytic variants are rare in both liver and pancreas, although foci of oncocytic epithelium are not infrequently present in otherwise biliary or mucinous epithelium. As in the pancreas, the cysts are connected to the main ductal system but may lose this connection when the cysts enlarge. Malignant lesions are characterized by invasion of the cyst wall and are referred to as “malignant IPBN.”64,66–68
Mesenchymal tumors of the liver are similar to those that occur elsewhere in the body except for mesenchymal hamartoma, which is specific to the liver. Mesenchymal hamartomas are found in early childhood, many cases manifesting at birth and most before 3 years of age, and are often cystic due to degeneration. Cavernous hemangioma is the most common mesenchymal, sometimes cystic lesion of the liver. Other, more uncommon mesenchymal tumors that occur preferentially in the liver include infantile hemangioma, undifferentiated embryonal sarcoma, inflammatory pseudotumor, angiomyolipoma, epithelioid hemangioendothelioma, and angiosarcoma (in adults). Cavernous hemangioma is the most common type, most of which are asymptomatic.