Airway
Pathophysiology
Failure to Maintain or Protect the Airway
A patent airway is essential for adequate ventilation and oxygenation. If the patient is unable to maintain an open airway, patency should be established by artificial means, such as repositioning, chin lift, jaw thrust, or insertion of an oral or nasal airway. Likewise, the patient must be able to protect against aspiration of gastric contents, which carries significant morbidity and mortality. Historically, the presence or absence of a gag reflex has been advocated as a reliable indicator of the patient’s ability to protect the airway, but the gag reflex is absent in 12 to 25% of normal adults, and there is no evidence that its presence or absence corresponds to airway protective reflexes or the need for intubation.1 The patient’s ability to swallow or handle secretions is a more reliable indicator of airway protection. The recommended approach is to evaluate the patient’s level of consciousness, the ability to phonate in response to voice command or query (which provides information about both the integrity of the upper airway and the level of consciousness), and the ability to manage his or her own secretions (e.g., pooling of secretions in the oropharynx, absence of swallowing spontaneously or on command.) In general, a patient who requires a maneuver to establish a patent airway or who easily tolerates an oral airway probably requires intubation for protection of that airway, unless a temporary or readily reversible condition, such as opioid overdose, is present.
Failure of Ventilation or Oxygenation
Regardless of the underlying cause, the need for mechanical ventilation generally mandates intubation. External mask devices increasingly have been used to provide assisted mechanical ventilation without intubation (see Chapter 2), but despite these advances, most patients who need assisted ventilation or positive pressure to improve oxygenation require intubation.2
Anticipated Clinical Course
Certain conditions indicate the need for intubation even in the absence of frank airway, ventilatory, or oxygenation failure. These conditions are characterized by a moderate to high likelihood of inevitable intubation to facilitate the patient’s workup and treatment or predictable airway deterioration that would require intervention. Intubation may be indicated relatively early in the course of certain overdoses. Although the patient initially may be protecting the airway and exchanging gas adequately, intubation often is advisable to guard against the strong likelihood of clinical deterioration, which can occur after the initial phase of care when the patient no longer is closely observed. Significant multiple trauma, with or without head injury, may be an indication for intubation even if the patient is ventilating normally through a patent airway and has adequate oxygen levels.3,4 Multiple trauma with hypotension, an open femur fracture, and diffuse abdominal tenderness warrant early intubation even if the patient is initially awake and alert without airway injury or hypoxemia. Aggressive resuscitation, pain control, the need for invasive procedures and imaging outside of the emergency department (ED), and inevitable operative management argues strongly for early airway control. In addition, a patient with penetrating neck trauma may have a patent airway and adequate gas exchange. Nevertheless, early intubation is advisable with any evidence of vascular or direct airway injury because these patients tend to deteriorate and increasing hemorrhage or swelling in the neck tend to both compromise the airway and confound later attempts at intubation.5,6
Clinical Features
Identification of the Difficult Airway
In most patients, intubation is technically easy and straightforward. In large ED studies, overall intubation failure rates resulting in rescue cricothyrotomy are 0.9% for all intubations and 1.7% for trauma patients.7–9 Intubation failure occurs in approximately 1 in 200 to 1 in 2000 elective general anesthesia cases.10 Bag-mask ventilation (BMV) is difficult in approximately 1 in 50 general anesthesia patients and impossible in approximately 1 in 600.11,12 BMV is difficult, however, in up to one third of patients in whom intubation failure occurs, and difficult BMV makes the likelihood of difficult intubation four times higher and the likelihood of impossible intubation 12 times higher.11,12 The combination of failure of intubation and failure of BMV in elective anesthesia practice is estimated to be exceedingly rare: 1 in 5000 to 1 in 20,000 elective anesthesia patients.12,13 These numbers cannot be extrapolated to populations of ED patients who are acutely ill or injured and for whom intubation is both urgent and unavoidable. Although patient selection cannot occur (as with a preanesthetic visit), a preintubation analysis of factors predicting difficult intubation can provide the operator with the information necessary to formulate a safe and effective plan for intubation.
Preintubation assessment should evaluate the patient for difficult intubation, difficult BMV, difficult ventilation with an extraglottic device (EGD; see later discussion), and difficult cricothyrotomy. Knowledge of all four domains is crucial to successful planning.10
Difficult Direct Laryngoscopy: LEMON
Most of the difficult airway markers discussed in the anesthesia and emergency medicine literature have not been scientifically validated.14 Nevertheless, a methodical approach can be used to evaluate the patient, based on the accepted hallmarks of difficult intubation by direct laryngoscopy. Videolaryngoscopy, on the other hand, so rarely fails to provide excellent laryngeal visualization that characterization of difficult videolaryngoscopy predictors may not be possible. When direct laryngoscopy is planned, a standard screening process for difficulty should be undertaken with every patient. It is probably useful to perform this screen when videolaryngoscopy is planned, as well, because it may help to identify challenges, such as a very large tongue or poor mouth access. One such approach uses the mnemonic LEMON (Box 1-1).10,15
L—Look externally. The patient first should be examined for external markers of difficult intubation, which are determined based simply on the intubator’s clinical impression. For example, the severely bruised and bloodied face of a combative trauma patient, immobilized in a cervical collar on a spine board, should (correctly) invoke an immediate appreciation of anticipated difficult intubation. Subjective clinical judgment can be highly specific (90%) but insensitive and so should be augmented by other evaluations.12
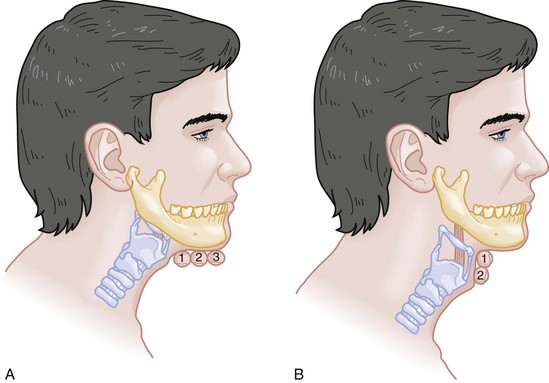
E—Evaluate 3-3-2. The second step in the evaluation of the difficult airway is to assess the patient’s airway geometry to determine his or her suitability for direct laryngoscopy. Glottic visualization with a direct laryngoscope requires that the mouth open adequately, that the submandibular space be adequate to accommodate the tongue, and that the larynx be positioned low enough in the neck to be accessible. These relationships have been explored in various studies by external measurement of mouth opening, oropharyngeal size, neck movement, and thyromental distance.16 The “3-3-2 rule” is an effective summary of these assessments.10,15 The 3-3-2 rule requires that the patient be able to place 3 of his or her own fingers between the open incisors, 3 of his or her own fingers along the floor of the mandible beginning at the mentum, and 2 fingers from the laryngeal prominence to the floor of the mandible (Fig. 1-1). A patient with a receding mandible and high-riding larynx can be impossible to intubate using direct laryngoscopy because the operator cannot adequately displace the tongue and the angle that must be overcome for a direct view of the glottic aperture is too acute. In practice, the operator compares the size of his or her fingers with the size of the patient’s fingers and then performs the three tests.
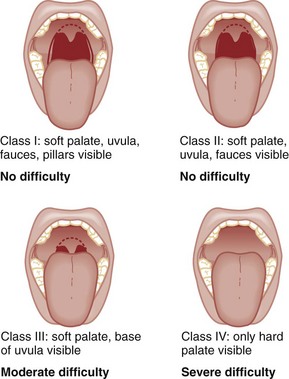
M—Mallampati scale. Oral access is assessed with the Mallampati scale (Fig. 1-2). Visibility of the oral pharynx ranges from complete visualization, including the tonsillar pillars (class I), to no visualization at all, with the tongue pressed against the hard palate (class IV). Class I and class II predict adequate oral access, class III predicts moderate difficulty, and class IV predicts a high degree of difficulty.16,17 A meta-analysis confirmed that the four-class Mallampati score performs well as a predictor of difficult laryngoscopy (and, less so, of difficult intubation) but that the Mallampati score alone is not a sufficient assessment tool.18 A Mallampati score may have to be improvised with the direct laryngoscope blade as a tongue depressor in obtunded or uncooperative patients.19
O—Obstruction or obesity. Upper airway (supraglottic) obstruction may make visualization of the glottis, or intubation itself, mechanically impossible. Conditions such as epiglottitis, head and neck cancer, Ludwig’s angina, neck hematoma, or glottic polyps can compromise laryngoscopy, passage of the endotracheal tube (ETT), BMV, or all three. Examine the patient for airway obstruction and assess the patient’s voice to satisfy this evaluation step. There is conflicting evidence regarding whether obesity is itself an independent marker of difficult intubation or whether patients with obesity simply are more likely to have other markers of difficult intubation.20,21 Regardless, obese patients generally are more difficult to intubate than their nonobese counterparts, and preparations should account both for this and for the more rapid oxyhemoglobin desaturation and increased difficulty with ventilation using bag and mask or an EGD (see later) that will occur.
N—Neck mobility. Neck mobility is desirable for any intubation technique and is essential for positioning the patient for optimal direct laryngoscopy. Neck mobility is assessed with the patient’s flexion and extension of the head and neck through a full range of motion. Neck extension is the most important motion, and simple extension may be as effective as the “sniffing” position in achieving an optimal laryngeal view.22 One study found that the “extension-extension” position, in which the neck is extended on the body (opposite of the flexion used for the sniffing position) with the head extended on the neck, provides superior laryngeal views to the sniffing position.23 Modest limitations of motion do not seriously impair direct laryngoscopy, but severe loss of motion, as can occur in ankylosing spondylitis or rheumatoid arthritis, for example, may render direct laryngoscopy impossible. Cervical spine immobilization in trauma artificially reduces cervical spine mobility, but direct laryngoscopy is still highly successful in this group of patients.7
Identification of a difficult intubation does not preclude use of an RSI technique. The crucial determination is whether the clinician judges that the patient has a reasonable likelihood of intubation success, despite the difficulties identified, and that ventilation with a bag and mask or an EGD will be successful in the event that intubation fails (hence the value of the BMV and EGD assessments; see Boxes 1-2 and 1-3).
Difficult Bag-mask Ventilation: MOANS
Attributes of difficult BMV have largely been validated and can be summarized with the mnemonic MOANS (see Box 1-2).10–12
Mask seal compromise or difficulty; Obstruction (particularly supraglottic obstruction, but it can be present anywhere in the airway) or Obesity (because of redundant upper airway tissues, chest wall weight, and resistance of abdominal mass); advanced Age (best judged by the physiologic appearance of the patient, but age older than 55 years increases risk); edentulousness (“No teeth”), which independently interferes with mask seal; and “Stiffness” or resistance to ventilation (e.g., asthma, chronic obstructive pulmonary disease, pulmonary edema, restrictive lung disease, term pregnancy) may each contribute to increased difficulty with BMV. The difficulty with BMV of the edentulous patient is the basis of the advice “Teeth out to intubate, teeth in to ventilate.” Multiple studies have validated this advice.24 A new approach involves placing the mask inside the patient’s lower lip. This may limit air leak in patients without teeth and eliminates the risk of aspiration associated with dental prosthetics or rolled gauze (Fig. 1-3).25 Difficult BMV is not uncommon, but with proper technique it usually is successful. A recent review of more than 50,000 patients undergoing elective anesthesia found that impossible BMV was exceptionally rare (0.2%) and was associated with neck changes secondary to radiation therapy, presence of a beard, male sex, history of sleep apnea, and a Mallampati class III or IV airway. Impossible BMV was five times more likely if one of these attributes was present and 25 times more likely with four or more.26
Difficult Extraglottic Device Placement: RODS
Placement of an EGD, such as a laryngeal mask airway (LMA), a Combitube, or a similar upper airway device, often can convert a “can’t intubate, can’t oxygenate” situation to a “can’t intubate, can oxygenate” situation, which allows time for rescue of a failed airway (see following section.) Difficulty achieving placement or ventilation with an EGD is predicted by the mnemonic RODS. Fortunately, if the clinician has already performed the LEMON and MOANS assessments, only the D for distorted anatomy remains to be evaluated (see Box 1-3). EGDs are placed blindly and have either a mask or a balloon structure that, when inflated, obstructs the oropharynx proximally and the esophageal inlet distally, permitting indirect ventilation. Distorted upper airway anatomy can result in a poor seal and ineffective ventilation.
Difficult Cricothyrotomy: SMART
Difficult cricothyrotomy can be anticipated whenever there is disturbance of the ability to locate and access the landmarks of the anterior airway via the neck and is remembered by the SMART mnemonic (Box 1-4). Prior surgery; hematoma, tumor, or abscess; scarring (as from radiation therapy or prior injury); local trauma; obesity; edema; or subcutaneous air each has the potential to make cricothyrotomy more difficult. Perform an examination for the landmarks needed to perform cricothyrotomy as part of the preintubation difficult airway assessment of the patient.
Measurement and Incidence of Intubation Difficulty
The actual degree to which an intubation is “difficult” is highly subjective, and quantification is challenging. The most widely used system for grading laryngoscopic view of the glottis is that of Cormack and Lehane (CL),26a which grades laryngoscopy according to the extent to which laryngeal and glottic structures can be seen. In grade 1 laryngoscopy, all or nearly all of the glottic aperture is seen. Grade 2 laryngoscopy visualizes only a portion of the glottis (arytenoid cartilages alone or arytenoid cartilages plus part of the vocal cords). Grade 3 laryngoscopy visualizes only the epiglottis. In grade 4 laryngoscopy, not even the epiglottis is visible.
Research conducted on elective anesthesia patients undergoing direct laryngoscopy suggests that true grade 4 laryngoscopy, which is associated with impossible intubation, occurs in less than 1% of patients. Grade 3 laryngoscopy, which represents extreme intubation difficulty, is found in less than 5% of patients. Grade 2 laryngoscopy, which occurs in 10 to 30% of patients, can be subdivided further into grade 2a, in which arytenoids and a portion of the vocal cords are seen, and grade 2b, in which only the arytenoids are seen. Intubation failure occurs in 67% of grade 2b cases but in only 4% of grade 2a cases.27 Outside of the operating room, the rate of difficulty may be higher. In a recent review of emergency adult inpatient intubations, as many as 10% were considered difficult (either a grade 3 or 4 CL direct view or more than three attempts required).28 The incidence of difficult ED intubations is unknown but is likely much higher, with some estimates between 4 and 26%.9,29 Approximately 80% of all grade 2 laryngoscopies are grade 2a; the rest are grade 2b. A grade 1 view is associated with virtually 100% intubation success. An alternative system, POGO (percentage of glottic opening), also has been proposed and validated but is not widely used or studied.30
Confirmation of Endotracheal Tube Placement
The most serious complication of endotracheal intubation is unrecognized esophageal intubation with resultant hypoxic brain injury. Although direct visualization of the ETT passing through the vocal cords generally is a reliable indicator of tracheal intubation, such clinical anatomic observations are fallible, and additional means are required to ensure correct placement of the tube within the trachea. Traditional methods, such as chest auscultation, gastric auscultation, bag resistance, exhaled volume, visualization of condensation within the ETT, and chest radiography, all are prone to failure as means of confirming tracheal intubation.31 Other clinical techniques are readily available for detecting tracheal or esophageal intubation.
Immediately after intubation, the intubator should apply an end-tidal carbon dioxide (ETCO2) detection device to the ETT and assess it through six manual ventilations. Disposable, colorimetric ETCO2 detectors are highly reliable, convenient, and easy to interpret, indicating adequate CO2 detection by color change (Figs. 1-4 and 1-5). ETCO2 detection is highly reliable in determining tracheal and esophageal intubation in patients with spontaneous circulation.32 These devices indicate the carbon dioxide (CO2) content in exhaled air either qualitatively or quantitatively. The persistence of detected CO2 after six manual breaths indicates that the tube is within the airway, although not necessarily within the trachea. CO2 is detected with the tube in the mainstem bronchus, the trachea, or the supraglottic space. Correlation of ETCO2 detection with the depth markings on the ETT (particularly important in pediatric patients) confirms tracheal placement. Rarely, BMV before intubation or ingestion of carbonated beverages may lead to release of CO2 from the stomach after esophageal intubation, causing a transient false indication of tracheal intubation. Washout of this phenomenon occurs within six breaths, however, so persistence of CO2 detection after six breaths indicates tracheal intubation.
Although colorimetric ETCO2 measurement is highly sensitive and specific for detecting esophageal intubation, caution is required for patients with cardiopulmonary arrest. Insufficient gas exchange may hamper CO2 detection in the exhaled air, even when the tube is correctly placed within the trachea.32 In patients with cardiopulmonary arrest, a CO2 level greater than 2%, which is the threshold for color change on colorimetric capnometers, should be considered definitive evidence of correct ETT placement, but the absence of such CO2 cannot be used reliably as an indicator of esophageal intubation. This circumstance arises in approximately 25 to 40% of intubated cardiac arrest patients.32,33 In all other patients, absence of CO2 detection indicates failure to intubate the trachea, and rapid reintubation is indicated. When possible, continuous quantitative capnography is more accurate and yields more information than capnometry (including colorimetric devices).
When ETCO2 detection is not possible, tracheal tube position can be confirmed with other techniques. One novel approach involves bedside ultrasound. In both live patient and cadaver studies, ultrasonography performed over the cricothyroid membrane or upper trachea accurately confirmed ETT position in the trachea, especially during the act of intubation.34,35
Bulb or syringe aspiration devices may be used in patients with cardiac arrest who have no detectable CO2, but although such devices are highly reliable at detecting esophageal intubation (sensitivity > 95%), false positives, in which a correctly placed tracheal tube is incorrectly identified as esophageal, can occur in up to 25% of cardiac arrest patients.32 Aspiration devices may be useful in the out-of-hospital setting when poor lighting hampers colorimetric ETCO2 determination. They also are good backup devices when cardiac arrest confounds attempts to assess placement with ETCO2. Detection of expired CO2 is more reliable and should be considered the standard for confirmation of tracheal placement of an ETT and for early detection of accidental esophageal intubation. Aspiration devices have a valuable but secondary role.
Accordingly, ETCO2 detection, with either aspiration or ultrasound techniques as backup, should be considered the primary means of ETT placement confirmation. Secondary means include physical examination findings, oximetry, and radiography. The examiner should auscultate both lung fields and the epigastric area. Pulse oximetry is indicated as a monitoring technique in all critically ill patients, not just those who require intubation. Oximetry is useful in detecting esophageal intubation but may not show a decreasing oxygen saturation for several minutes after a failed intubation because of the oxygen reservoir (preoxygenation) created in the patient before intubation.36 Although chest radiography is universally recommended after ETT placement, its primary purpose is to ensure that the tube is well positioned below the cords and above the carina. A single anteroposterior chest radiograph is not sufficient to detect esophageal intubation, although esophageal intubation may be detected if the ETT is clearly outside the air shadow of the trachea. In cases in which doubt persists, a fiberoptic scope can be passed through the ETT to identify tracheal rings, another “gold standard” for confirmation of tracheal placement.
Management
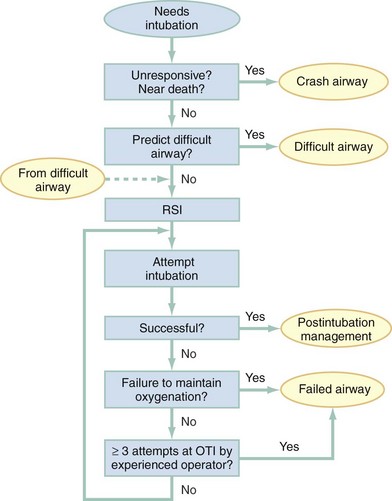
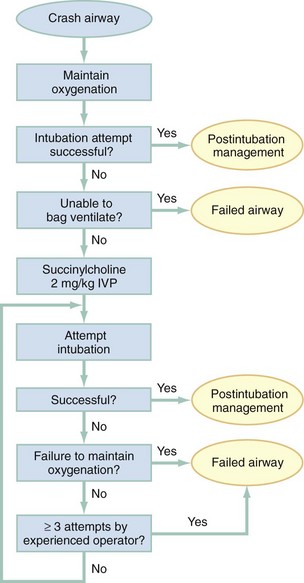
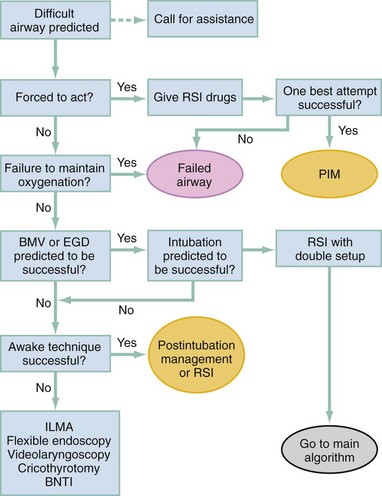
Algorithms for emergency airway management have been developed and provide a useful guide, both for planning intubation and for rescue in the event of intubation failure. The algorithm assumes that a decision to intubate has been made and outlines such an approach. The approach is predicated on two key determinations that must be made before active airway management is begun (Fig. 1-6). The first determination is whether the patient is in cardiopulmonary arrest or a state of near arrest and is predicted to be unresponsive to direct laryngoscopy. Such a patient (agonal, near death, in circulatory collapse) is called a “crash airway” patient for the purposes of emergency airway management and is treated with the crash airway algorithm by an immediate intubation attempt without use of drugs, supplemented by a single, large dose of succinylcholine if the attempt to intubate fails and the patient is thought not to be sufficiently relaxed (Fig. 1-7). Next, it must be determined whether the patient represents a difficult intubation as determined by the LEMON, MOANS, RODS, and SMART evaluations, and if so, the difficult airway algorithm is used (Fig. 1-8).
For patients who require emergency intubation but who have neither a crash airway nor a difficult airway, we recommend RSI. RSI provides the safest and quickest method of achieving intubation in such patients.7,14,37,38 After administration of the RSI drugs, intubation attempts are repeated until the patient is intubated or a failed intubation is identified. If more than one intubation attempt is required, oxygen saturation is monitored continuously, and if saturation falls to 90% or less, BMV is performed until saturation is recovered for another attempt. If the clinician cannot maintain oxygen saturation despite optimal use of BMV or an EGD, a failed airway exists. This is referred to as a “can’t intubate, can’t oxygenate” situation. In addition, if three attempts at laryngoscopy have been unsuccessful, a failed airway exists because subsequent attempts at laryngoscopy by the same clinician are unlikely to succeed. The three failed laryngoscopy attempts are defined as attempts by an experienced clinician using the best possible patient positioning and technique. If available, videolaryngoscopes should be used for one or all of these attempts. Also, if the clinician ascertains after even a single attempt that intubation will be impossible (e.g., grade 4 laryngoscopic view with a direct laryngoscope despite optimal patient positioning and use of external laryngeal manipulation), and no alternative laryngoscope (e.g., videolaryngoscope) is available, a failed airway is present. The failed airway is managed according to the failed airway algorithm (Fig. 1-9).
Difficult Airway
The perception of a difficult airway is relative, and many emergency intubations could be considered “difficult.” The judgment regarding whether to treat the airway as a typical emergency airway or whether to use the difficult airway algorithm is based on the degree of perceived difficulty, operator experience, the airway management devices available, and the individual circumstances of the case.39 The LEMON, MOANS, RODS, and SMART assessments provide a systematic framework to assist in identifying the potentially difficult airway.
When preintubation evaluation identifies a potentially difficult airway (see Fig. 1-8), the approach is based on the premise that NMBAs generally should not be used unless the clinician believes that (1) intubation is likely to be successful and (2) oxygenation via BMV or EGD is likely to be successful if a first intubation attempt does not succeed and oxygenation is required.10 The one exception to this recommendation occurs in the “forced to act” scenario.
Therefore, in the difficult airway algorithm, the first determination is whether the operator is “forced to act.” If so, RSI drugs are given, a best attempt at laryngoscopy is undertaken, and, if intubation is not successful, the airway is considered failed and the operator moves immediately to the failed airway algorithm. In the vast majority of difficult airway situations, however, the operator is not forced to act, and the first step is to ensure that oxygenation is sufficient to permit a planned, orderly approach (see Fig. 1-8). If oxygenation is inadequate and cannot be made adequate by supplementation with bag and mask, the airway should be considered a failed airway. Whereas inadequate oxygenation should be defined on a case-by-case basis, oxygenation saturation at or below 90% is the accepted threshold because this represents the point at which hemoglobin undergoes a conformational change, more readily releasing oxygen and increasing the pace of desaturation. Oxyhemoglobin saturations in the 80s, if holding steady, might be considered adequate in some circumstances, particularly if the patient is chronically hypoxemic. When oxygenation is inadequate, the failed airway algorithm should be used because the predicted high degree of intubation difficulty, combined with failure to maintain oxygen saturation, is analogous to the “can’t intubate, can’t oxygenate” situation. When oxygenation is adequate, however, the next consideration is whether RSI is appropriate, on the basis of the operator’s assessment of the likelihood of (1) successful ventilation with a bag and mask or an EGD in the event intubation is unsuccessful, and (2) the likelihood of successful intubation by laryngoscopy. If the operator judges laryngoscopy likely to succeed and is confident of the ability to oxygenate even if intubation fails, then RSI is performed. In such cases a double setup can be used in which RSI is performed and preparations simultaneously are undertaken for rescue cricothyrotomy or another rescue technique. If RSI is not advisable and time allows, an awake technique can be used. In this context, awake means that the patient continues to breathe and is able to cooperate with caregivers. Usually the technique involves sedation and topical anesthesia, ideally preceded by a drying agent such as glycopyrrolate. The awake technique often is direct or flexible or rigid videolaryngoscopy, assisted by topical anesthesia and sedation (comparable to that for a painful procedure). If the glottis is adequately visualized, the patient can be intubated at that time, or, in a stable difficult airway situation, the clinician may proceed with planned RSI, now assured of intubation success. If the awake laryngoscopy is unsuccessful, the patient is intubated with any of numerous techniques shown in the last box in Figure 1-8. For each of these methods, the patient is kept breathing but variably sedated and anesthetized, and each of the methods results in placement of a cuffed ETT in the trachea. The choice among these methods depends on clinician experience and preference, device availability, and patient attributes.
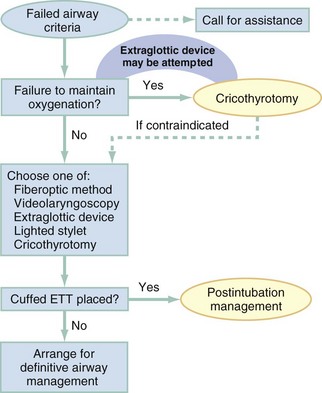
Failed Airway
Management of the failed airway is dictated by whether the patient can be oxygenated.10 If adequate oxygenation cannot be maintained, the rescue technique of first resort is cricothyrotomy (see Fig. 1-9). Multiple attempts at other methods in the context of failed oxygenation delay cricothyrotomy and place the patient at increased risk for hypoxic brain injury. If an alternative device (i.e., an EGD such as an LMA or Combitube) is immediately available, however, and the operator judges it to be an appropriate device for the patient’s anatomy, an attempt can be made to use it simultaneously with preparations for immediate cricothyrotomy, as long as initiation of cricothyrotomy is not delayed. Only a single attempt with the EGD is recommended in this circumstance.
Therapeutic Modalities
Although many techniques are available for intubation of the emergency patient, four methods are most common, with RSI being the most frequently used in nonarrested patients.7–9,40
Rapid Sequence Intubation
RSI is the cornerstone of modern emergency airway management and is defined as the virtually simultaneous administration of a potent sedative (induction) agent and an NMBA, traditionally succinylcholine, for the purpose of endotracheal intubation. This approach provides optimal intubating conditions and has long been believed to minimize the risk of aspiration of gastric contents. A systematic review of the literature in 2007 failed to prove that RSI results in a lower incidence of aspiration than other techniques, but the authors correctly noted that virtually no studies have ever been designed to measure this precise endpoint.41 RSI is nevertheless the most widely used technique for emergency intubation of patients without identifiable difficult airway attributes.7–9
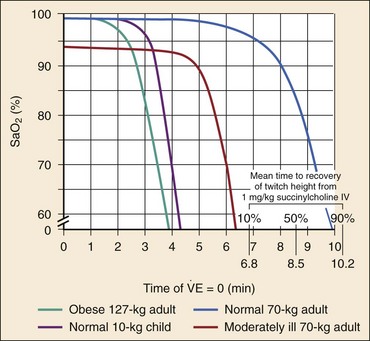
The central concept of RSI is to take the patient from the starting point (e.g., conscious, breathing spontaneously) to a state of unconsciousness with complete neuromuscular paralysis, then to achieve intubation without interposed assisted ventilation. The risk of aspiration of gastric contents is felt to be significantly higher for patients who have not fasted before induction. Application of positive-pressure ventilation can cause air to pass into the stomach, resulting in gastric distention and likely increasing the risk of regurgitation and aspiration.42 The purpose of RSI is to avoid positive-pressure ventilation until the ETT is placed correctly in the trachea with the cuff inflated. This requires a preoxygenation phase, during which the nitrogen reservoir in the functional residual capacity in the lungs is replaced with oxygen, permitting at least several minutes of apnea (see later discussion) in the normal adult before oxygen desaturation to 90% ensues (Fig. 1-10).36
Use of RSI also facilitates successful endotracheal intubation by causing complete relaxation of the patient’s musculature, allowing better access to the airway.37,38,43 Finally, RSI permits pharmacologic control of the physiologic responses to laryngoscopy and intubation, mitigating potential adverse effects. These effects include further intracranial pressure (ICP) increase in response to the procedure and to the sympathetic discharge resulting from laryngoscopy (Box 1-5).44 RSI is a series of discrete steps, and every step should be planned (Box 1-6).45
Preparation.: In the initial phase the patient is assessed for intubation difficulty (unless this has already been done), and the intubation is planned, including determining dosages and sequence of drugs, tube size, and laryngoscope type, blade, and size. Drugs are drawn up and labeled. All necessary equipment is assembled. All such patients require continuous cardiac monitoring and pulse oximetry. At least one and preferably two good-quality intravenous lines should be established. Redundancy is always desirable in case of equipment or intravenous access failure.
Preoxygenation.: Administration of 100% oxygen for 3 minutes of normal, tidal volume breathing in a normal, healthy adult establishes an adequate oxygen reservoir to permit 8 minutes of apnea before oxygen desaturation to less than 90% occurs (see Fig. 1-10).36 Additional preoxygenation does not improve arterial oxygen tension. The time to desaturation to less than 90% in children, obese adults, late-term pregnant women, and patients with significant comorbidity is considerably less.46 Desaturation time also is reduced if the patient does not inspire 100% oxygen.47 Nevertheless, adequate preoxygenation usually can be obtained, even in ED patients, to permit several minutes of apnea before oxygen desaturation to less than 90% occurs. In children and adults, preoxygenation is essential to the “no bagging” approach of RSI. If time is insufficient for a full 3-minute preoxygenation phase, eight vital capacity breaths with high-flow oxygen can achieve oxygen saturations and apnea times that match or exceed those obtained with traditional preoxygenation.48 Desaturation time in obese patients can be prolonged by preoxygenating in the head up position and by continuing supplemental oxygen (by nasal cannula) after motor paralysis.49,50 Oxygen saturation monitors permit earlier detection of desaturation during laryngoscopy, but preoxygenation remains an essential step in RSI.
Pretreatment.: During this phase, drugs are administered 3 minutes before administration of the succinylcholine and induction agent to mitigate the effects of laryngoscopy and intubation on the patient’s presenting or comorbid conditions. Intubation is intensely stimulating and results in sympathetic discharge (the reflex sympathetic response to laryngoscopy [RSRL]), elevation of ICP in patients with ICP disturbance, and reactive bronchospasm. Bradycardia can occur in children, particularly children younger than 1 year, but appears multifactorial, likely involving underlying parasympathetic tone, parasympathetic discharge in response to airway instrumentation, and perhaps some contributory effect of succinylcholine.
Pretreatment focuses on three main objectives in certain at-risk patients. The three groups of patients at risk are those with reactive airway disease, elevated ICP, or a cardiovascular or neurovascular condition for which an acute elevation in blood pressure and heart rate might be hazardous. Patients with reactive airway disease often experience a worsening of their bronchospasm when intubated. Controversy exists regarding whether albuterol alone, lidocaine alone, or both drugs together are effective in reducing this intubation-related bronchospasm.51,52 Asthmatic patients being intubated in the ED for status asthmaticus will have received albuterol before intubation, and there is conflicting evidence regarding whether lidocaine has any additive protective effect in these situations. Pending larger studies, it is reasonable to administer lidocaine (1.5 mg/kg) as a pretreatment drug. When an asthmatic patient is being intubated for a condition other than acute asthma (e.g., trauma), nebulized albuterol, intravenous lidocaine, or both should be given before intubation, if possible. Patients with significant cardiovascular disease (e.g., ischemic coronary disease) who are being intubated in the ED may benefit from the administration of the synthetic opioid fentanyl in a dose of 3 µg/kg to blunt the release of catecholamines in response to airway manipulation. Similarly, patients with intracranial hemorrhage, elevated ICP, or marked hypertension may benefit from fentanyl (3 µg/kg) to mitigate the sympathetic induced rises in blood pressure. Finally, lidocaine may have a role in intracranial hypertension; however, the extent to which it can attenuate rises in ICP is controversial. Although there is some evidence that patients with elevated ICP may experience less exacerbation of the ICP during intubation if they are pretreated with lidocaine (1.5 mg/kg), results are not conclusive.53,54 Nevertheless, there is evidence supporting the physiologic effects of these agents even though outcome data are lacking. Individualization is necessary, and critical time should not be lost administering pretreatment drugs if the patient requires immediate intubation. Despite the lack of outcome studies, considerable inferential evidence supports this approach, and these agents, particularly fentanyl, probably provide protection for vulnerable patients against the adverse hemodynamic and intracranial effects of laryngoscopy and intubation. Although many variations are possible for pretreatment regimens in various conditions, pretreatment can be simplified to these three basic indications (see Box 1-5).
Paralysis with Induction.: In this phase, a potent sedative agent is administered by rapid intravenous push in a dose capable of rapidly producing unconsciousness. This is immediately followed by rapid administration of an intubating dose of an NMBA, usually succinylcholine at a dose of 1.5 mg/kg intravenously (IV). Rocuronium is an acceptable alternative agent and is the drug of choice if succinylcholine is contraindicated (see later discussion on RSI pharmacology). Rocuronium may become the NMBA of choice for all patients if sugammadex (a nondepolarizing reversal agent) is available. The intubating dose of rocuronium is 1.0 mg/kg IV. It is usual to wait 45 seconds from the time the succinylcholine is given and 60 seconds from the time rocuronium is given to allow sufficient paralysis to occur.
Positioning.: The patient should be positioned for intubation as consciousness is lost. Usually, positioning involves head extension, often with flexion of the neck on the body, but there is evidence that simple extension of the head alone or extension of both the head and neck (the extension-extension position) are equivalent or superior.22,23 (See earlier discussion.) The Sellick maneuver (application of firm backward-directed pressure over the cricoid cartilage with the goal of obstructing the cervical esophagus) has long been recommended to minimize the risk of passive regurgitation and hence aspiration but is no longer recommended.55–57 There is evidence that the Sellick maneuver is incorrectly applied by a variety of operators, that it may make laryngoscopy or intubation more difficult in some patients, and that aspiration often occurs despite the Sellick maneuver’s having been used.56,58–61 Imaging studies suggest the cervical esophagus is positioned lateral to the cricoid ring in most patients, a relationship that is exaggerated by posterior pressure, rarely resulting in esophageal obstruction. Accordingly, the Sellick maneuver should be considered optional, applied selectively, and released or modified if laryngeal view is poor or tube passage is difficult.62 During this phase after administration of the induction agent and NMBA, although the patient becomes unconscious and apneic, BMV should not be initiated unless the oxygen saturation falls to 90%.
Placement of Tube.: Approximately 45 to 60 seconds after the administration of the NMBA, the patient is relaxed sufficiently to permit laryngoscopy; this is assessed most easily by moving the mandible to test for absence of muscle tone. Place the ETT during glottic visualization with the laryngoscope. Confirm placement as described earlier. If the first attempt is unsuccessful but oxygen saturation remains high, it is not necessary to ventilate the patient with a bag and mask between intubation attempts. If the oxygen saturation is approaching 90%, the patient may be ventilated briefly with a bag and mask between attempts to reestablish the oxygen reservoir. When BMV is performed, the Sellick maneuver is advisable to minimize passage of air into the stomach.42
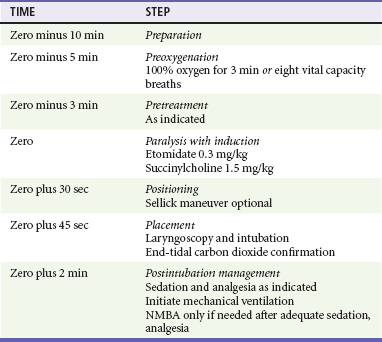
Postintubation Management.: Obtain a chest radiograph to confirm that mainstem intubation has not occurred and to assess the lungs. In general, long-acting NMBAs (e.g., pancuronium, vecuronium) are avoided and the focus is on optimal management using opioid analgesics and sedative agents to facilitate mechanical ventilation. An adequate dose of a benzodiazepine (e.g., midazolam 0.1-0.2 mg/kg IV) and an opioid analgesic (e.g., fentanyl 3-5 µg/kg IV, or morphine 0.2-0.3 mg/kg IV) is given to improve patient comfort and decrease sympathetic response to the ETT. Propofol infusion (5-50 µg/kg/min IV) with supplemental analgesia is an effective method for managing intubated patients without hypotension or ongoing bleeding and is especially helpful for management of neurologic emergencies, as its clinical duration of action is very short (<5 minutes), allowing frequent neurologic examinations. An NMBA is added only if appropriate use of sedation and analgesia fail to adequately control the patient. Table 1-1 presents a sample RSI protocol using etomidate and succinylcholine. “Zero” refers to the time at which the induction agent and succinylcholine are pushed.
Blind Nasotracheal Intubation
Historically, blind nasotracheal intubation (BNTI) was used extensively in the ED and out-of-hospital setting, but it has fallen out of favor largely because of the superiority of RSI.5,63 Prehospital intubation success between RSI and BNTI favors RSI, and ED studies have shown that RSI is clearly superior.5,40
BNTI remains a valid method of intubation in the out-of-hospital setting, where it occasionally is used. In the ED, BNTI rarely, if ever, should be used and is reserved for patients in whom the presence of a narrowly defined type of difficult airway makes RSI undesirable or contraindicated and alternatives (e.g., flexible endoscope) are not available. A recent review of nearly 9000 ED intubations showed that nasal intubation was used in only 5% of intubations performed from 1997 to 2002.7 The incidence likely is significantly lower today.
Awake Oral Intubation
Awake oral intubation is distinct from the practice of oral intubation with a sedative or opioid agent to obtund the patient for intubation without neuromuscular blockade. This latter technique can be referred to as “intubation with sedation alone” or, paradoxically, “nonparalytic RSI.” Intubating conditions achieved even with deep anesthesia are significantly inferior to the conditions achieved when neuromuscular blockade is used.37,38,64 The same superiority of neuromuscular blockade–assisted intubation over intubation with sedation alone has been observed in pediatric emergency medicine and in emergency medical services (EMS) care.65 In general, the technique of administering a potent sedative agent to obtund the patient’s responses and permit intubation in the absence of neuromuscular blockade is ill-advised and inappropriate for endotracheal intubation in the ED, unless it is performed as part of an “awake” intubation as described earlier.
Oral Intubation without Pharmacologic Agents
The arrested or near death patient may not require pharmacologic agents for intubation. Even an arrested patient may retain sufficient muscle tone to render intubation difficult, however. If the glottis is not adequately visualized, administration of a single dose of succinylcholine alone may facilitate laryngoscopy (see discussion of crash airway). Success rates for intubating unconscious, unresponsive patients are comparable to those achieved with RSI, presumably because the patient is in a similar physiologic state (i.e., muscle relaxation, no ability to react to laryngoscopy or tube insertion).7 This does not apply to patients who are unconscious from neurologic catastrophe or trauma and those who overdosed or had other medical causes of coma, who warrant an induction agent and are intubated with standard RSI procedures as described earlier.
Pharmacologic Agents
Succinylcholine.: Succinylcholine is a combination of two molecules of ACh. Succinylcholine is rapidly hydrolyzed by plasma pseudocholinesterase to succinylmonocholine, which is a weak NMBA, then to succinic acid and choline, which have no NMBA activity. Pseudocholinesterase is not present at the motor endplate and exerts its effects systemically before the succinylcholine reaches the ACh receptor.66 Only a small amount of the succinylcholine that is administered survives to reach the motor endplate. Succinylcholine is active at the motor endplate until it diffuses away. Decreased plasma pseudocholinesterase activity can increase the amount of succinylcholine reaching the motor endplate, prolonging succinylcholine block, but this is of little significance in the emergency setting because the prolongation of action rarely is significant, reaching only 23 minutes at the extreme.66
Uses and Dosing.: Succinylcholine is rapidly active, typically producing intubating conditions within 45 seconds of administration by rapid intravenous bolus injection.38,67 The clinical duration of action before spontaneous respiration is 6 to 10 minutes (see Fig. 1-10).36 Full recovery of normal neuromuscular function occurs within 15 minutes. The combination of rapid onset, complete reliability, short duration of action, and absence of common serious side effects maintains succinylcholine as the drug of choice for most ED intubations.7,8,65 The use of a competitive, or nondepolarizing, NMBA for RSI may be desirable when succinylcholine is contraindicated and in certain other settings. The appropriate dose of succinylcholine for emergency airway management is 1.5 mg/kg IV. Although the ED95 (dose at which paralysis is achieved in 95% of patients) for succinylcholine paralysis is much lower (0.3 mg/kg), onset of muscle paralysis is excessively long at these lower doses and not compatible with emergency intubation. Review of studies of succinylcholine pharmacokinetics supports the recommended dose of 1.5 mg/kg.38 Multiple studies confirm that the dose of succinylcholine is based on the patient’s total body weight (TBW) and is not adjusted (downward) regardless of the degree of obesity.68,69
Cardiovascular Effects.: As an ACh analogue, succinylcholine binds to ACh receptors throughout the body, not just at the motor endplate. It is difficult to separate the effects of succinylcholine on the heart that are caused by direct cardiac muscarinic stimulation from those caused by stimulation of autonomic ganglia by succinylcholine and from the effects induced by the autonomic responses to laryngoscopy and intubation. Succinylcholine can be a negative chronotrope, especially in children, and sinus bradycardia may ensue after succinylcholine administration. Sinus bradycardia is treated with atropine, if necessary, but is often self-limiting. Some pediatric practitioners recommend pretreatment with atropine for children younger than 1 year, but there is no evidence for benefit. Other cardiac dysrhythmias, including ventricular fibrillation and asystole, have been reported with succinylcholine, but it is impossible to distinguish the effects of the drug itself from those caused by the intense vagal stimulation and catecholamine release that accompany laryngoscopy and intubation. In addition, many of these catastrophic complications occur in critically ill patients, further confounding attempts to identify whether the illness or any particular drug or procedure is the cause.
Fasciculations.: The depolarizing action of succinylcholine results in fine, chaotic contractions of the muscles throughout the body for several seconds at the onset of paralysis in over 90% of patients. Muscle pain occurs in approximately 50% of patients who receive succinylcholine. Although it is widely believed that muscle pains are reduced or abolished by prior administration of a defasciculating dose of a competitive NMBA, the evidence is not conclusive.70 Use of 1.5 mg/kg of succinylcholine results in both less fasciculation and less myalgia than occur with 1 mg/kg.70
Hyperkalemia.: Succinylcholine has been associated with severe, fatal hyperkalemia when administered to patients with specific predisposing clinical conditions (Table 1-2).71 The mechanism by which severe hyperkalemia occurs is related to receptor upregulation on the postsynaptic muscle membrane. When a muscle is deprived of ACh stimulation for several days, receptor upregulation occurs, causing an increase in receptor density and a change of receptor subtypes on the muscle surface.72,73 ACh receptors are primarily K+ ion channels, and at-risk patients can have immediate, massive efflux of potassium as these newly recruited receptors are depolarized by succinylcholine. This occurs predominantly at the site of injury but may occur also in tissue remote from original insult.74 Although the hyperkalemia occurs within minutes after administration of succinylcholine and may be severe or fatal, the patient’s vulnerability to succinylcholine-induced hyperkalemia does not become significant until 5 days after the inciting injury or burn as receptor upregulation production of protein subunits. Succinylcholine remains the agent of choice for RSI in acute burn, trauma, stroke, and spinal cord injury if intubation occurs less than 5 days after onset of the condition. If doubt exists regarding the onset time, succinylcholine should be replaced with a competitive NMBA, usually rocuronium. Denervation syndromes or primary myopathies (e.g., multiple sclerosis, amyotrophic lateral sclerosis, Duchenne muscular dystrophy) can be particularly troubling, however, because the risk begins with the onset of the disease and continues indefinitely, regardless of the apparent stability of the symptoms. In patients with denervation caused by stroke or spinal cord injury, the up-regulated receptors eventually regress, and the patient can safely receive succinylcholine beginning six months after the original insult. Potassium release does not occur to any significant extent in the general population. Succinylcholine is not contraindicated in renal failure but probably should not be used in patients with known or presumed hyperkalemia sufficient to manifest on the electrocardiogram. Treatment for succinylcholine-induced hyperkalemia is the same as for any other hyperkalemic emergency. The only published series examining succinylcholine use in patients with preexisting hyperkalemia, many of whom had renal failure, failed to show a single adverse event related to succinylcholine administration.75
Table 1-2
Conditions Associated with Hyperkalemia after Succinylcholine Administration
| CONDITION | PERIOD OF CONCERN |
| Burns >10% BSA | >5 days until healed |
| Crush injury | >5 days until healed |
| Denervation (stroke, spinal cord injury) | >5 days until 6 months postinjury |
| Neuromuscular disease (ALS, MS, MD) | Indefinitely |
| Intra-abdominal sepsis | >5 days until resolution |
Increased Intraocular Pressure.: Succinylcholine may cause a modest increase in intraocular pressure and historically has been considered contraindicated in penetrating globe injury. There is no published evidence to support this view, however, and several large series show safety when succinylcholine is used in patients with open globes. The admonition to avoid succinylcholine in open globe injuries is unjustified and should be abandoned.76
Masseter Spasm.: Succinylcholine has been reported rarely to cause masseter spasm, primarily in children.66 The clinical significance of this phenomenon is unclear, but administration of a competitive NMBA terminates the spasm. Severe, persistent spasm should raise suspicion of malignant hyperthermia.
Malignant Hyperthermia.: Succinylcholine has been associated with malignant hyperthermia, a perplexing syndrome of rapid temperature rise and aggressive rhabdomyolysis. Malignant hyperthermia occurs in genetically predisposed individuals who receive certain volatile anesthetic agents or succinylcholine. The condition is extremely rare and has not been reported in the context of ED intubation. Treatment consists of cessation of any potential offending agents, administration of dantrolene (1-2.5 mg/kg IV every 5 min to a maximum dose of 10 mg/kg IV), and attempts to reduce body temperature by external means.77 A national malignant hyperthermia hotline is available for emergency consultation at 1-800-644-9737 (then dial zero).
Refrigeration.: The standard recommendation to keep succinylcholine refrigerated creates problems related to its storage, timely retrieval, and ready availability on intubation carts or kits in the ED. Succinylcholine undergoes degradation beginning at the time of manufacture, and the rate of this degradation is much lower when the drug is refrigerated. Succinylcholine retains more than 90% of its original activity when stored at room temperature for 3 months. Succinylcholine may be kept at room temperature in the ED or EMS setting, provided that a proper inventory control system ensures that all supplies are replaced not more than 3 months after introduction.
Competitive Agents.: Competitive NMBAs are classified according to their chemical structure. The aminosteroid agents include pancuronium, vecuronium, and rocuronium. Vecuronium neither releases histamine nor exhibits cardiac muscarinic blockade and is an excellent agent for maintenance of neuromuscular blockade when this is desirable. Rocuronium is the best agent for use in RSI when succinylcholine is contraindicated. In a recent study of ED intubations performed with either rocuronium or succinylcholine, first-pass intubation success was independent of the NMBA used.78
Rocuronium.: When a patient has a contraindication to succinylcholine, rocuronium bromide is the paralytic of choice. At a dose of 1.0 mg/kg IV, rocuronium achieves intubating conditions closely approaching those of succinylcholine, lasts approximately 50 minutes, and has been used in the ED with success.64,78,79 Intubating level paralysis may take 15 to 20 seconds longer than with succinylcholine, and it is reasonable to allow 60 seconds to elapse before attempting intubation when rocuronium is used, versus 45 seconds for succinylcholine. There are no absolute contraindications to rocuronium. In the subset of critically ill patients who require frequent, serial neurologic examinations, the more prolonged duration of paralysis with rocuronium may make it less desirable than succinylcholine for routine use.
Vecuronium.: Vecuronium was the first competitive NMBA to establish a role in RSI and is the only other NMBA that should be considered for emergency airway management. Pancuronium is vagolytic and has an unacceptably long clinical duration. For intubation, vecuronium is given as a split dose. First, 0.01 mg/kg is administered as a “priming” dose. Three minutes later, 0.15 mg/kg is given for paralysis, which is achieved in about 75 to 90 seconds.
Paralysis after Intubation.: After intubation, prolonged paralysis may be desired to optimize mechanical ventilation; however, current management trends are away from the use of prolonged paralysis in favor of deep sedation with analgesia. If neuromuscular blockade is desired, vecuronium (0.1 mg/kg IV) can be given, but longer-term neuromuscular blockade must not be undertaken without ensuring appropriate sedation and analgesia of the patient and a means to be certain that ongoing sedation and analgesia are adequate. A sedating dose of a benzodiazepine, such as midazolam (0.1 mg/kg IV), combined with an opioid analgesic, such as fentanyl (3-5 µg/kg IV) or morphine (0.2-0.3 mg/kg IV), is required to improve patient comfort and decrease sympathetic response to the ETT. With appropriate attention to achieving optimal sedation and analgesia, ongoing NMBA usually is not necessary.
Induction Agents
A patient with any degree of clinical responsiveness, including reactivity to noxious stimuli, requires a sedative or induction agent at the time of administration of any NMBA. Patients who are deeply unconscious and unresponsive may require only a reduced dose of an induction agent if the unconscious state is caused by drugs or alcohol (themselves general anesthetic agents.) Patients who are unconscious because of a central nervous system insult should receive a full induction dose of an appropriate agent to attenuate adverse responses to airway manipulation. Induction agents also enhance the effect of the NMBA and improve intubation conditions because the intubation is done at the earliest phase of neuromuscular blockade, and the relaxation effects of the induction agent are additive to those of the NMBA.80
Etomidate.: Etomidate is an imidazole derivative that has been in use since 1972. Its activity profile is similar to that of thiopental, with rapid onset, rapid peak activity, and brief duration, but it is remarkable in its lack of adverse hemodynamic effects.81 Etomidate has emerged as the agent of choice for ED RSI, and numerous reports attest to its effectiveness and safety.7,9 The induction dose is 0.3 mg/kg IV. Because etomidate is able to decrease ICP, cerebral blood flow, and cerebral metabolic rate without adversely affecting systemic mean arterial blood pressure and cerebral perfusion pressure (CPP), it is an excellent induction agent for patients with elevated ICP, even in cases of hemodynamic instability.82 Etomidate may cause brief myoclonus, but this is of no clinical significance. Etomidate by continuous infusion has been reported to cause suppression of endogenous cortisol production. Recently, controversy has emerged regarding the role of etomidate for intubation of patients with septic shock.83–85 Several retrospective studies have claimed to demonstrate that etomidate, used in a single dose for intubation, causes suppression of the adrenal response to exogenously administered adrenocorticotropic hormone and have attempted to link this to increased mortality.86–88 Other retrospective studies have shown the opposite.89–91 Recent prospective randomized trials looking at both undifferentiated ICU admissions and those specifically involving individuals with septic shock showed that single-dose etomidate has no effect on outcome.92,93 Ironically, much of the original criticism of etomidate arose from the belief that adrenocortical response to exogenous corticotropin predicts outcome in patients with septic shock, a belief that has since been abandoned.94 Also, the most comprehensive study of the role of exogenous corticosteroids in septic shock failed to show any benefit, casting further doubt about any possible mortality effect of a single dose of etomidate.95 Pending a properly constructed, prospective, randomized clinical trial, there is not sufficient evidence to support a recommendation that etomidate be avoided in patients with septic shock.85,96 In fact, etomidate’s superior hemodynamic profile makes it an excellent choice, as is ketamine, in these generally unstable patients.
Ketamine.: Ketamine, a phencyclidine derivative, has been widely used as a general anesthetic agent since 1970. After an intravenous dose of 1 to 2 mg/kg, ketamine produces loss of awareness within 30 seconds, peaks in approximately 1 minute, and has a clinical duration of 10 to 15 minutes. As a dissociative anesthetic agent, ketamine induces a cataleptic state rather than a true unconscious state. The patient has profound analgesia but may have open eyes. Many protective reflexes, including airway reflexes, are preserved.
Controversy exists regarding the use of ketamine in patients with elevated ICP because ketamine may increase cerebral metabolic rate, ICP, and cerebral blood flow.97 The evidence that ketamine can produce harm in this way is conflicting, however, and may be outweighed in trauma patients because of its overall favorable hemodynamic profile.98 Ketamine does not appear to be harmful in children when given in procedural doses to patients with known elevated ICP, and may actually lower ICP.99 Because it may cause release of catecholamines and increase blood pressure, ketamine should probably be avoided in traumatic brain injury (TBI) patients with normal or elevated blood pressure. However, we recommend use of ketamine or etomidate for induction of patients with TBI and hypotension. Ketamine may produce unpleasant emergence phenomena, especially disturbing or frightening dreams in the first 3 hours after awakening. These reactions, which are more prominent in adults than in children, in women than in men, in patients receiving larger doses, and in certain personality types, may be mitigated by benzodiazepine administration.100 Patients who undergo RSI with ketamine should receive a benzodiazepine (e.g., lorazepam 0.05 mg/kg or midazolam 0.1 mg/kg) as part of postintubation management.
Propofol.: Propofol is a highly lipophilic alkylphenol with GABAergic activity. Its primary use in the emergency setting has been for postintubation sedation in head-injured patients; however, it can be used for induction during RSI. It reduces intracranial pressure and cerebral oxygen usage and is indicated for patients with elevated ICP caused by medical or traumatic emergencies. Because of the propensity of propofol to cause hypotension, through both vasodilation and direct myocardial depression, the dosage is reduced or the drug is avoided altogether in hemodynamically compromised patients. The usual induction dose of propofol is 1.5 to 2.0 mg/kg IV, but reduced doses should be used in elderly patients or those with hemodynamic compromise or poor cardiovascular reserve. Propofol is delivered in a soybean oil and lecithin vehicle and should not be used in patients with allergies to these substances. Although propofol has traditionally been avoided in patients with egg allergy, it is likely safe unless a history of anaphylaxis to egg protein exists.101 Propofol causes pain at the site of administration in as many as 60% of patients. Using a proximal (antecubital) vein in lieu of distal venous injection is the most important preventative measure. Pretreatment with intravenous lidocaine, coadministration of lidocaine mixed with propofol, and pretreatment with opioids or ketamine have all been shown to limit this common adverse reaction.102
Other Induction Agents.: Given the widespread acceptance and familiarity with etomidate, propofol, and ketamine, other drug classes such as barbiturates and benzodiazepines are infrequently used as induction agents for RSI. Rapidly acting barbiturates, such as thiopental, are highly lipid soluble and readily cross the blood-brain barrier, acting on the γ-aminobutyric acid (GABA) receptor neuroinhibitory complex to rapidly depress central nervous system activity. A single dose of 3 mg/kg of thiopental produces loss of consciousness in less than 30 seconds, has a peak effect at 1 minute, and has a clinical duration of 5 to 8 minutes. Thiopental is a negative inotrope and a potent venodilator and should be used with caution in patients whose cardiovascular reserve is diminished. Thiopental releases histamine and should not be used in asthmatic patients. Of the benzodiazepines, only midazolam is used as an induction agent, a role for which it is inferior to other, more commonly used agents, such as etomidate and propofol. The usual induction dose is 0.2 to 0.3 mg/kg IV. At a dose of 0.3 mg/kg IV, midazolam produces loss of consciousness in about 30 seconds and has a clinical duration of 15 to 20 minutes. Midazolam is a negative inotrope and should be used with caution in hemodynamically compromised and elderly patients, for whom the dose can be reduced to 0.1 mg/kg or 0.05 mg/kg. Onset is slower at these reduced doses.
Special Clinical Circumstances
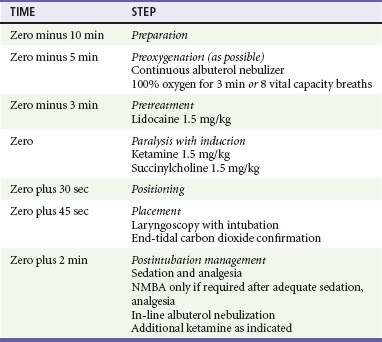
The asthmatic patient has highly reactive airways, and steps should be taken to minimize any additional bronchospasm that may occur during intubation. The bronchoconstriction that occurs with ETT placement is thought to be neurally mediated, and local anesthetics, particularly lidocaine, have been studied as a way to blunt this airway reflex. Lidocaine has been shown to suppress the coughing that occurs in response to airway manipulation and may improve ETT tolerance and reduce reactive bronchospasm in asthmatic patients.52 Although there is not clear proof of outcome benefit, we recommend lidocaine (1.5 mg/kg) as a pretreatment drug before intubation in status asthmaticus and in asthmatic patients being intubated for reasons other than their asthma, particularly if beta-agonists have not been administered. High-dose, inhaled beta-agonists may provide maximal protection against reactive bronchospasm during intubation in asthmatics without active bronchospasm, and lidocaine may provide little additional benefit in this setting.51 This approach has not been tested in patients in status asthmaticus, however. Ketamine has been shown to produce bronchodilation in humans and animal models and has been reported to mitigate bronchospasm in patients who are not intubated and in patients who are already intubated and who are not improving with mechanical ventilation. Although studies to date have been limited, there is a growing body of experience with ketamine that suggests that it may be the preferred induction agent for the emergency intubation of patients with status asthmaticus (Table 1-3).103
Hemodynamic Consequences of Intubation
Laryngoscopy and intubation are potent stimuli for the reflex release of catecholamines.104 This reflex sympatheitc response to laryngoscopy (RSRL) produces only modest increases in blood pressure and heart rate and is of little consequence in otherwise healthy patients. The RSRL is of potential clinical significance in two settings: acute elevation of ICP, and certain cardiovascular diseases (e.g., intracerebral hemorrhage, subarachnoid hemorrhage, aortic dissection or aneurysm, and ischemic heart disease). In these settings the reflexive release of catecholamines, increased myocardial oxygen demand, and attendant rise in mean arterial blood pressure and heart rate may produce deleterious effects. The synthetic opioids (e.g., fentanyl) and beta-adrenergic blocking agents (e.g., esmolol) are capable of blunting the RSRL and stabilizing heart rate and blood pressure during intubation.104 Lidocaine also has been studied, but the results are contradictory and inconclusive.105 In patients at risk from acute blood pressure elevation, administration of fentanyl (3 µg/kg) during the pretreatment phase of RSI attenuates the heart rate and blood pressure increase. The full sympatholytic dose of fentanyl is 5 to 9 µg/kg, but if this dose is administered as a single pretreatment bolus, hypoventilation or apnea can occur. The administration of 3 µg/kg is safer and can be supplemented with an additional 3 µg/kg immediately after intubation if greater sympathetic blockade is desired or if hypertension and tachycardia persist. Fentanyl should be given as the last pretreatment drug and is administered over 60 seconds to prevent hypoventilation or apnea.
Elevated Intracranial Pressure
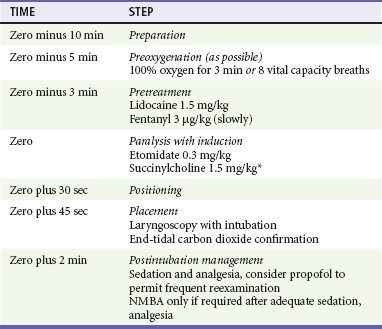
When ICP is elevated as a result of head injury or acute intracranial catastrophe, maintenance of CPP and avoidance of further increases in ICP are desirable.44 Significant reductions in mean arterial blood pressure decrease CPP with the reduction of the driving gradient between arterial pressure and ICP. If CPP falls below 50 to 60 mm Hg, cerebral ischemia may occur and cerebral autoregulation of blood flow may fail. Maintenance of the systemic mean arterial blood pressure at 100 mm Hg or greater supports CPP and reduces the likelihood of secondary injury. Therefore RSI induction agents that could lower MAP should be avoided in patients with brain injury. In patients with suspected or documented elevation of ICP, control of RSRL is desirable to avoid further elevation of ICP. Fentanyl (3 µg/kg) given as a pretreatment drug is the best choice for this purpose in the emergency setting.44,106
Evidence suggests a separate reflex that increases ICP in response to laryngoscopy and intubation, although the precise mechanism is not understood. Intravenous lidocaine has been shown to reduce ICP or blunt the ICP response to laryngoscopy and intubation, although study results are mixed.53,54 However, if time allows and in the absence of an obvious contraindication (e.g., known allergy to amide anesthetics), lidocaine (1.5 mg/kg IV), administered during the pretreatment phase of RSI, is desirable to blunt the ICP response to laryngoscopy and intubation. Similarly, RSRL and the ICP response to intubation make BNTI inadvisable in brain injury patients.
In emergency patients who may have elevated ICP, the physician should choose an induction agent that balances a favorable effect on cerebral dynamics and ICP with a stable systemic hemodynamic profile. At present, etomidate (0.3 mg/kg) appears to be the best choice in this situation, although propofol is also a good option when hemodynamic compromise is not present (Table 1-4).
Potential Cervical Spine Injury
Historically, most patients with suspected blunt cervical spine injury were intubated orally by direct laryngoscopy with in-line cervical spine immobilization, whether done as an awake procedure or with neuromuscular blockade. However, with this approach glottic views can be inadequate and excessive lifting force often is required.107–111 Videolaryngoscopes provide superior laryngeal views without excessive lifting force or cervical spine movement and have higher intubation success rates when compared with conventional direct laryngoscopy.112,113
The intubating laryngeal mask airway (ILMA) also may result in less cervical spine movement during intubation than direct laryngoscopy.114 Alternative devices to direct laryngoscopes also have shown promise for safe intubation of patients with cervical spine injury. A fluoroscopic study in which intubation with the Shikani optical stylet (SOS) was compared with direct laryngoscopy showed significantly less cervical spine movement with the SOS but a slightly longer intubation time (28 seconds vs. 17 seconds).115 The Airtraq and Pentax Airway Scope are curved intubation devices that integrate an ETT channel and either a viewing lens or a video screen to facilitate intubation. Both devices have shown high levels of intubation success and minimal cervical spine motion compared with direct laryngoscopy.116–118 The CTrach, a fiberoptically enhanced ILMA, also has demonstrated success both with and without in-line cervical spine stabilization.119
In the absence of a coexistent blunt traumatic mechanism or a neurologic examination indicating spinal cord injury, cervical spine immobilization for intubation of patients with penetrating head and neck trauma rarely is indicated.120 It is not proven whether patients with gunshot or shotgun injuries to the head or neck are at risk of exacerbation of cervical cord injury during intubation, and there is no report of such a patient, with or without clinical evidence of spinal cord injury, who was injured by intubation. In addition, cervical spine immobilization in patients with penetrating neck injuries may be harmful. A recent large retrospective review of more than 45,000 trauma patients with penetrating injuries found that those in whom prehospital cervical collars were applied were two to three times more likely to die. Delays in transport and patient assessment and added difficulty for airway procedures were postulated as potential contributors.121
Pediatric Intubation
For direct laryngoscopy, a straight laryngoscope blade is desirable to displace the floppy epiglottis, especially in young children, and positioning for intubation may be different. Although the product insert for succinylcholine now advises against its routine use in pediatric anesthesia because of the risk of hyperkalemia in children with undiagnosed congenital neuromuscular disorders (e.g., muscular dystrophy), it remains the drug of choice for emergency RSI of infants and children.65 Rocuronium has been used in children, but experience is too limited to recommend that it replace succinylcholine for pediatric RSI in the ED. RSI may be used in children in a manner similar to that in adults, with two important differences. Excessive bradycardia may be seen with succinylcholine in children younger than 1 year, but it is not known whether administration of atropine (0.02 mg/kg) during the pretreatment phase prevents any possible adverse outcome. The dose of succinylcholine in infants is 2 mg/kg. Induction agents may be selected using similar criteria as for adults. Particular attention should be paid to providing appropriate postintubation sedation and pain control, just as in adults. One recent study showed that less than 25% of pediatric patients received ongoing sedation within 15 minutes after intubation.122 The major difficulty in intubating children and infants is choosing the correct size of equipment and the correct drug doses for age or size. These obstacles can be overcome by use of a length-based system such as the Broselow-Luten color coding system, which provides dosing and equipment sizes based on the length of the child. Cricothyrotomy is impossible in small children, and alternative rescue airway devices (e.g., percutaneous oxygenation via the cricothyroid membrane) are required.
Evolution of Airway Devices
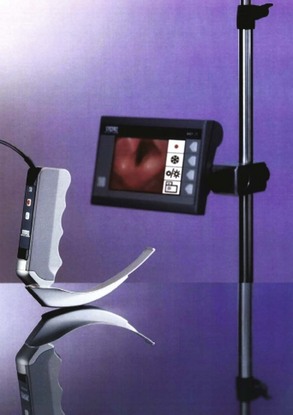
Videolaryngoscopes
Modern laryngoscopes incorporate video imaging into specially designed laryngoscope blades to provide glottic visualization superior to that of a direct laryngoscope without the need to create a straight-line visual axis through the mouth. The GlideScope videolaryngoscope system (GVL) uses a modified Macintosh blade with a straightened, angulated, and elongated tip enclosing a proximally placed camera to provide a wide-angle view of the glottis and the surrounding anatomy, even in patients with difficult airways (Fig. 1-11). Video images are transmitted to a high-resolution display that can record both still pictures and video clips. Handle and blade sizes range from neonate to obese adult. The GlideScope Ranger is an ultraportable version of the device, designed for use in the out-of-hospital environment. One large series of out-of-hospital intubations showed that the Ranger significantly reduced the number of attempts needed to intubate compared with direct laryngoscopy.123 The GlideScope Cobalt is a system designed for a single use, without the need for cleaning. It consists of a flexible video wand insert that fits inside a disposable, single-piece transparent blade or handle sheath, and comes in sizes comparable to those for the standard GlideScope. The placement of the camera distally along the blade to create a viewing field essentially negates the obstructive potential of the tongue, so GlideScope laryngoscopy, and most other videolaryngoscopy, is performed with the blade introduced in the midline of the mouth and rotated around the tongue with very little lifting. A proprietary rigid preformed stylet is available for use with the GlideScope, or a malleable stylet can be shaped to match the exaggerated curve of the GlideScope blade. Either stylet may be used, and there appears to be no advantage of one over the other, either for novice or experienced operators.124,125 When compared with direct laryngoscopy, the GlideScope provides an equivalent or superior glottic view and has a very high intubation success rate.126,127 Traditional predictors of difficult direct laryngoscopy likely will not apply to videolaryngoscopy, as most are based on limitations of creating a direct line of sight, which is not a part of videolaryngoscopy.128 The GlideScope causes less cervical spine movement than conventional direct laryngoscopy and provides better glottic exposure in patients with strict cervical spine precautions.112,129 The C-MAC videolaryngoscope (Fig. 1-12) incorporates a complementary metal oxide semiconductor (CMOS) video chip into a range of laryngoscope blades to enhance glottic view. Images are displayed on a high-resolution monitor, with image and video saving capabilities. The Macintosh blade version of the C-MAC can be used as a direct laryngoscope by a trainee while a supervisor observes the video output, providing an excellent tool for teaching direct laryngoscopy. Although head-to-head comparisons with other videolaryngoscopes are lacking, early clinical experience with the C-MAC is positive, and one study showed superior views obtained by videolaryngoscopy, with a forerunner of the C-MAC compared with direct laryngoscopy.130,131 There are several other models of videolaryngoscopes in various sizes and with varying features, such as disposable sheaths or blades, and at various price points.131–134 Individual evaluation of these devices is important in selecting the best videolaryngoscope for an individual practitioner or practice group. Overall, videolaryngoscopy offers the promise of transforming laryngoscopy and has the potential to render conventional, direct laryngoscopy obsolete.
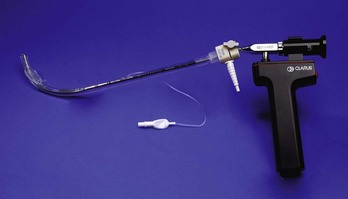
Fiberoptic and Video Intubating Stylets
Several semirigid fiberoptic and video intubating stylets also are available. The SOS (Clarus Medical, Minneapolis, Minn.) is the most studied of these, although a newer video device (Clarus Video System; Fig. 1-13) based on the same principles likely will perform as well as or better than its fiberoptic forerunner. The ETT is placed over a semirigid stylet, consisting of a metal sheath with a distally placed fiberoptic or video image acquisition system, then advanced through the mouth and over the tongue in the midline and into the trachea under fiberoptic or video visualization (Fig. 1-14). The SOS appears to cause less movement of the cervical spine than conventional laryngoscopy during intubation with in-line stabilization.115 A smaller version, the Levitan scope, uses a light-emitting diode (LED)–illuminated fiberoptic stylet to facilitate intubation by direct laryngoscopy. The device is recommended by the manufacturer to facilitate first-pass success when a limited glottic view is obtained by direct laryngoscopy. In the only study comparing the Levitan scope with the gum elastic bougie, however, the two devices achieved similar success.135
Flexible Intubating Scopes
Intubation using a flexible endoscope is an important option for certain difficult airways, particularly in those with distorted upper airway anatomy, such as angioedema or blunt anterior neck trauma. These scopes long relied on fiberoptic technology, but this largely has been supplanted by miniaturized high-quality video systems. After appropriate patient preparation, the endoscope is passed through the vocal cords under continuous visualization, serving as an introducer for an ETT, which is then placed through the glottis. Flexible endoscopic examination also is used for airway assessment to determine whether intubation is needed, such as in patients with smoke inhalation or supraglottitis. Intubation of morbidly obese patients, those with distorted airway anatomy (e.g., penetrating or blunt anterior neck injury), or those with fixed cervical spine deformity can be achieved with the flexible endoscope with topical anesthesia and judicious sedation, thus preserving the patient’s ability to breathe until intubation has been achieved. Scopes also have been used successfully to intubate through an ILMA, and video systems likely would work well in this application also.136
Extraglottic Devices
Laryngeal Mask Airways.: Laryngeal mask airways (LMAs) collectively include a number of commercially available ovoid, silicone mask devices designed to seal above the glottis and permit ventilation through a central channel with a standard bag. The most widely used is the original LMA, shown in Figure 1-15. It is available in both reusable and single-use configurations; single-use models in both conventional and intubating formats are offered by several manufacturers and are probably equivalent.137 The mask is inserted blindly into the pharynx, then inflated, providing a seal that permits ventilation of the trachea with minimal gastric insufflation. In elective anesthesia the LMA has an extremely high insertion success rate and low complication rate, including a low incidence of tracheal aspiration.138,139 Evaluations of LMA insertion by experienced and inexperienced personnel consistently have shown ease of insertion, high insertion success rates, and successful ventilation.140 Novice users appear to be able to both ventilate and intubate more easily and successfully with the ILMA than with BMV and direct laryngoscopy.140 The LMA may be a viable alternative to endotracheal intubation for in-hospital or out-of-hospital treatment of cardiac arrest, particularly when responders are inexperienced airway managers. At a minimum, the device may serve a temporizing role equal or superior to BMV until definitive airway management can be achieved. The LMA Supreme (LMAS) is a new robust LMA with a rigid angled tube, similar to an ILMA, and offers an orogastric tube channel and higher seal pressures than the standard LMA.141 A new form of noninflatable LMA, the i-gel, has a viscous gel cuff and does not require inflation (Fig. 1-16). It is available in a variety of sizes for adults and pediatric patients. The device is placed blindly, and insertion depths are marked on the side of the device. It has an integrated bite block and a channel for passage of an orogastric tube. Initial experience with the device, even with minimally trained novice users, is promising, with high insertion success rates and shorter insertion times when compared with the LMA or laryngeal tube airway.142,143
The ILMA is designed to facilitate intubation through the mask after correct placement (Fig. 1-17). It differs from the LMA in two main ways: The mask is attached to a rigid, stainless steel ventilation tube that is bent almost to a right angle, and the mask incorporates an epiglottic elevator at its distal end. Placement of the ILMA results in successful ventilation in almost 100% of cases and successful subsequent intubation in 95%.114,144–146 The ILMA can also be used for both ventilation and intubation in obese patients with similarly high success rates.147 The ILMA has a special ETT and a stabilizer rod to remove the mask over the ETT after intubation has been accomplished, but intubation can be comparably successful with a conventional polyvinylchloride (PVC) ETT.148
The ILMA is a better device than the standard LMA for use in the ED because it facilitates both rescue ventilation and intubation. Intubation through the ILMA has compared favorably in terms of success with direct laryngoscopy and is superior in the hands of novice intubators.140,144 When the ILMA is placed, intubation can be performed blindly or guided by a lighted stylet or a fiberoptic scope. The ILMA comes only in sizes 3, 4, and 5 and so is not suitable for use in patients weighing less than about 30 kg. For smaller patients, the standard LMA, which has sizes down to size 1 (infant), should be used. Intubation can be achieved through the standard LMA, but the success rate is significantly less than with the ILMA. As experience with both the LMA and ILMA grows, it is likely that there will be increasing adoption of the LMA as a primary airway management technique by nonhospital first responders, and the ILMA is gaining attention as a primary rescue device in the ED.
The CTrach ILMA incorporates fiberoptic bundles and a detachable viewing screen to provide a view of the glottis during intubation. The device performs better than the standard ILMA for first-attempt intubation, where it achieved almost 93% success versus approximately 80% for the ILMA in one well-conducted study.149 It can be placed equally as rapidly in patients with and without cervical spine immobilization.119 Ultimately, though, the ILMA’s intubation success rate is so high (on three or fewer attempts) that it is not clear that the CTrach provides additional benefit overall. The view can be obscured by secretions, but this is easily solved by removing and reinserting the device or cleaning it with a swab through the airway lumen.150 The greatest issue is cost, with the CTrach priced almost five times higher than the corresponding set of standard ILMAs. Whether the additional cost provides additional benefit for application in the ED is not clear.
In the ED the primary use of the LMA or ILMA is as a rescue technique to provide a temporary airway when intubation has failed, bag ventilation is satisfactory, and the patient has been paralyzed and may either require prolonged ventilation or be in need of immediate airway management. In such cases the LMA is one of numerous acceptable devices. In the “can’t intubate, can’t ventilate” situation, cricothyrotomy is indicated, but an ILMA may be placed rapidly in an attempt to achieve ventilation (converting the situation to “can’t intubate, can ventilate”) as long as this is done in parallel with preparations for cricothyrotomy and does not delay the initiation of a surgical airway.151 Availability of the LMA and adequate prior training of the clinician offer a legitimate option for the management of the failed airway, and the ILMA compares well with fiberoptic intubation in terms of successful intubation of difficult airways.146 The standard LMA may also offer advantages for providing ventilation in unconventional positions, such as when the patient is lying on his or her side.152 In the out-of-hospital setting, where concerns about esophageal placement of ETTs have focused interest on methods used for airway management, the LMA and Combitube offer excellent placement and ventilation characteristics and may be preferable to endotracheal intubation in this setting, especially when intubation is relatively infrequently performed.153 If the patient is in a difficult position in terms of intubation access, the LMA may facilitate more rapid ventilation.154
Other Extraglottic Devices.: In addition to LMAs, which sit above the glottis, there are several other types of EGDs, which are placed blindly posterior to the laryngeal inlet to provide oxygenation and ventilation through side ports, while inflatable cuffs occlude the pharynx above and the esophageal inlet below. Because of their positioning behind the larynx, these often are called retroglottic devices. The prototype for these devices is the esophagotracheal Combitube. The Combitube is a plastic double-lumen tube with one lumen functioning as an airway after esophageal insertion and the other lumen functioning as a tracheal airway (Fig. 1-18). The tube is placed blindly into the esophagus, and then proximal and distal balloons are inflated sequentially through different ports. The balloons prevent escape of ventilatory gases upward through the pharynx or downward through the esophagus. The tube is placed into the esophagus, as designed, almost 100% of the time, but both lumens are patent, so ventilation is still possible if the tube has been placed inadvertently into the trachea. It comes in two sizes and is used only in patients taller than 48 inches.
Newer types of retroglottic devices are smaller, are simpler, and can be easier to use. The King laryngeal tube airway (King LT) has a single port through which both distal and proximal low-pressure balloons are inflated as a single step (Fig. 1-19). The distal balloon, when seated correctly, obstructs the upper esophageal sphincter, and the larger proximal balloon obstructs the hypopharynx, preventing regurgitation of air. A new version of the King LT has a posterior channel that accepts a nasogastric tube, which can be passed through the device into the stomach for aspiration of gastric contents. The King LT is disposable, is rapidly placed, is easy to use by operators of various skill levels, and has seal pressures similar to those of standard LMAs. It can be safely left in place for 4 hours without mucosal pressure damage. Another device, the Rusch EasyTube, is similar in concept and appearance to the Combitube but is available in 41F and a smaller 28F size for smaller patients. All retroglottic devices are primarily a substitute for endotracheal intubation for non–ETT-trained personnel but are also used by advanced airway managers as a way to oxygenate and ventilate patients during crash and failed airway scenarios.155–159 These devices should be considered temporary measures, do not protect against aspiration, and should be exchanged for a definitive airway as soon as possible.
Surgical Airway Management
Cricothyrotomy
Cricothyrotomy is the creation of an opening in the cricothyroid membrane through which a cannula, usually a cuffed tracheostomy tube, is inserted to permit ventilation. The techniques and variations thereof are well described elsewhere.160 When surgical airway management is required, cricothyrotomy is the procedure of choice in the emergency setting, where it is faster, more straightforward, and more likely to be successful than tracheotomy.
Cricothyrotomy is indicated when oral or nasal intubation is impossible or fails and when BMV cannot maintain adequate oxygen saturation (the “can’t intubate, can’t ventilate” situation). Several large series have established that the incidence of cricothyrotomy is approximately 1% of all ED intubations, with the highest rates seen in trauma patients.7,40 Cricothyrotomy is relatively contraindicated by distorted neck anatomy, preexisting infection in the neck, and coagulopathy; these contraindications are relative, however, and the establishment of the airway takes precedence over all other considerations. The procedure should be avoided in children younger than 10 years, in whom anatomic limitations make it exceedingly difficult. Studies suggest that approximately five “practice” cricothyrotomies on a simulator or animal model are sufficient to achieve at least baseline capability with the procedure, although training intervals for skills maintenance have not been well defined.161
A number of commercial kits and devices are used to perform percutaneous cricothyrotomy. Percutaneous cricothyrotomy with the Seldinger technique appears comparable to formal open cricothyrotomy in terms of ease of learning and success rates.162 Patients with clear landmarks are the best candidates for this procedure because patient obesity or altered anatomy may lead to paratracheal tube placement. In patients with indistinct landmarks or for novice operators, standard open cricothyrotomy may be more successful.163 Bougie-assisted cricothyrotomy, during which a bougie is placed through the cricoid incision and used as a guidewire for ETT placement, may also improve surgical airway success for inexperienced practitioners.164 The safety and effectiveness of the many cricothyrotomy kits and devices are not clearly established. Only two percutaneous cricothyrotomy sets on the market currently have the ability to place a cuffed tracheostomy tube. One is a dedicated Seldinger cricothyrotomy set; the other is a combination set that has all necessary equipment for either a Seldinger percutaneous cricothyrotomy or a standard surgical cricothyrotomy (Melker universal cricothyrotomy kit; Cook Critical Care, Bloomington, Ind.) (Fig. 1-20).
Outcomes
Few studies of emergency airway management have characterized complications and outcomes. The largest single-institution series reported a success rate for ED RSI of 99% and a complication rate of 9.3%; most complications were minor.9 Phase II of the large National Emergency Airway Registry study (NEAR II) of almost 9000 ED intubations reported that most patients were intubated by emergency physicians using RSI with overall success rates of 96%. The NEAR classification system characterizes potentially adverse occurrences during intubation as “adverse events.”7,40,165 In the NEAR study the overall rate of adverse events was 12%, with recognized esophageal or mainstem intubation and hypotension being most common.7 No studies have evaluated the long-term outcome of intubated ED patients.
References
1. Davies, AE, Kidd, D, Stone, SP, MacMahon, J. Pharyngeal sensation and gag reflex in healthy subjects. Lancet. 1995;345:487–488.
2. Cross, AM, Cameron, P, Kierce, M, Ragg, M, Kelly, AM. Non-invasive ventilation in acute respiratory failure: A randomised comparison of continuous positive airway pressure and bi-level positive airway pressure. Emerg Med J. 2003;20:531–534.
3. Christensen, EF, Hoyer, CC. Prehospital tracheal intubation in severely injured patients: A Danish observational study. BMJ. 2003;327:533–534.
4. Ciesla, DJ, et al. Intubation alone does not mandate trauma surgeon presence on patient arrival to the emergency department. J Trauma. 2004;56:937–941.
5. Weitzel, N, Kendall, J, Pons, P. Blind nasotracheal intubation for patients with penetrating neck trauma. J Trauma. 2004;56:1097–1101.
6. Mandavia, DP, Qualls, S, Rokos, I. Emergency airway management in penetrating neck injury. Ann Emerg Med. 2000;35:221–225.
7. Walls, RM, Brown, CA, 3rd., Bair, AE, Pallin, DJ, NEAR II Investigators. Emergency Airway Management: A Multi-center Report of 8937 Emergency Department Intubations. J Emerg Med. 2011;41:347–354.
8. Sagarin, MJ, et al. Airway management by US and Canadian emergency medicine residents: A multicenter analysis of more than 6,000 endotracheal intubation attempts. Ann Emerg Med. 2005;46:328–336.
9. Sakles, JC, Laurin, EG, Rantapaa, AA, Panacek, EA. Airway management in the emergency department: A one-year study of 610 tracheal intubations. Ann Emerg Med. 1998;31:325–332.
10. Murphy, MF, Walls, RM. Identification of the difficult and failed airway. In: Walls RM, Murphy MF, Luten RC, eds. Manual of Emergency Airway Management. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health; 2008:81–93.
11. Langeron, O, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
12. Kheterpal, S, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891.
13. Klock, A, Jr., Benumof, JL. Definition and incidence of the difficult airway. In: Hagberg CA, ed. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby/Elsevier; 2007:215–220.
14. American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
15. Reed, MJ, Dunn, MJ, McKeown, DW. Can an airway assessment score predict difficulty at intubation in the emergency department? Emerg Med J. 2005;22:99–102.
16. Krobbuaban, B, Diregpoke, S, Kumkeaw, S, Tanomsat, M. The predictive value of the height ratio and thyromental distance: Four predictive tests for difficult laryngoscopy. Anesth Analg. 2005;101:1542–1545.
17. Mallampati, SR, et al. A clinical sign to predict difficult tracheal intubation: A prospective study. Can Anaesth Soc J. 1985;32:429–434.
18. Lee, A, et al. A systematic review (meta-analysis) of the accuracy of the Mallampati tests to predict the difficult airway. Anesth Analg. 2006;102:1867–1878.
19. Bair, AE, Caravelli, R, Tyler, K, Laurin, EG. Feasibility of the preoperative Mallampati airway assessment in emergency department patients. J Emerg Med. 2010;38:677–680.
20. Juvin, P, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth Analg. 2003;97:595–600.
21. Brodsky, JB, Lemmens, HJ, Brock-Utne, JG, Vierra, M, Saidman, LJ. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–736.
22. Adnet, F, et al. Randomized study comparing the “sniffing position” with simple head extension for laryngoscopic view in elective surgery patients. Anesthesiology. 2001;95:836–841.
23. Lee, L, Weightman, WM. Laryngoscopy force in the sniffing position compared to the extension-extension position. Anaesthesia. 2008;63:375–378.
24. Conlon, NP, Sullivan, RP, Herbison, PG, Zacharias, M, Buggy, DJ. The effect of leaving dentures in place on bag-mask ventilation at induction of general anesthesia. Anesth Analg. 2007;105:370–373.
25. Racine, SX, et al. Face mask ventilation in edentulous patients: A comparison of mandibular groove and lower lip placement. Anesthesiology. 2010;112:1190–1193.
26. Kheterpal, S, Martin, L, Shanks, AM, Tremper, KK. Prediction and outcomes of impossible mask ventilation: A review of 50,000 anesthetics. Anesthesiology. 2009;110:891–897.
26a. Cormack, RS, Lehane, J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111.
27. Yentis, SM, Lee, DJ. Evaluation of an improved scoring system for the grading of direct laryngoscopy. Anaesthesia. 1998;53:1041–1044.
28. Martin, LD, Mhyre, JM, Shanks, AM, Tremper, KK, Kheterpal, S. 3,423 emergency tracheal intubations at a university hospital: Airway outcomes and complications. Anesthesiology. 2011;114:42–48.
29. Wong, E, Ng, YY. The difficult airway in the emergency department. Int J Emerg Med. 2008;1:107–111.
30. Levitan, RM, Kinkle, WC, Levin, WJ, Everett, WW. Laryngeal view during laryngoscopy: A randomized trial comparing cricoid pressure, backward-upward-rightward pressure, and bimanual laryngoscopy. Ann Emerg Med. 2006;47:548–555.
31. Knapp, S, et al. The assessment of four different methods to verify tracheal tube placement in the critical care setting. Anesth Analg. 1999;88:766–770.
32. Takeda, T, et al. The assessment of three methods to verify tracheal tube placement in the emergency setting. Resuscitation. 2003;56:153–157.
33. Tanigawa, K, Takeda, T, Goto, E, Tanaka, K. The efficacy of esophageal detector devices in verifying tracheal tube placement: A randomized cross-over study of out-of-hospital cardiac arrest patients. Anesth Analg. 2001;92:375–378.
34. Ma, G, et al. The sensitivity and specificity of transcricothyroid ultrasonography to confirm endotracheal tube placement in a cadaver model. J Emerg Med. 2007;32:405–407.
35. Werner, SL, Smith, CE, Goldstein, JR, Jones, RA, Cydulka, RK. Pilot study to evaluate the accuracy of ultrasonography in confirming endotracheal tube placement. Ann Emerg Med. 2007;49:75–80.
36. Benumof, JL, Dagg, R, Benumof, R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87:979–982.
37. Bozeman, WP, Kleiner, DM, Huggett, V. A comparison of rapid-sequence intubation and etomidate-only intubation in the prehospital air medical setting. Prehosp Emerg Care. 2006;10:8–13.
38. Naguib, M, et al. The dose of succinylcholine required for excellent endotracheal intubating conditions. Anesth Analg. 2006;102:151–155.
39. Orebaugh, SL. Difficult airway management in the emergency department. J Emerg Med. 2002;22:31–48.
40. Bair, AE, Filbin, MR, Kulkarni, RG, Walls, RM. The failed intubation attempt in the emergency department: Analysis of prevalence, rescue techniques, and personnel. J Emerg Med. 2002;23:131–140.
41. Neilipovitz, DT, Crosby, ET. No evidence for decreased incidence of aspiration after rapid sequence induction. Can J Anaesth. 2007;54:748–764.
42. Lawes, EG, Campbell, I, Mercer, D. Inflation pressure, gastric insufflation and rapid sequence induction. Br J Anaesth. 1987;59:315–318.
43. Pace, SA, Fuller, FP. Out-of-hospital succinylcholine-assisted endotracheal intubation by paramedics. Ann Emerg Med. 2000;35:568–572.
44. Levitt, MA, Dresden, GM. The efficacy of esmolol versus lidocaine to attenuate the hemodynamic response to intubation in isolated head trauma patients. Acad Emerg Med. 2001;8:19–24.
45. Walls, RM. The decision to intubate. In: Walls RM, Murphy MF, Luten RC, eds. Manual of Emergency Airway Management. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health; 2008:1–8.
46. Mort, TC, Waberski, BH, Clive, J. Extending the preoxygenation period from 4 to 8 mins in critically ill patients undergoing emergency intubation. Crit Care Med. 2009;37:68–71.
47. Nimmagadda, U, et al. Efficacy of preoxygenation with tidal volume breathing. Comparison of breathing systems. Anesthesiology. 2000;93:693–698.
48. Baraka, AS, Taha, SK, Aouad, MT, El-Khatib, MF, Kawkabani, NI. Preoxygenation: Comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology. 1999;91:612–616.
49. Dixon, BJ, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: A randomized controlled study. Anesthesiology. 2005;102:1110–1115.
50. Ramachandran, SK, Cosnowski, A, Shanks, A, Turner, CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: A randomized, controlled trial of nasal oxygen administration. J Clin Anesth. 2010;22:164–168.
51. Maslow, AD, et al. Inhaled albuterol, but not intravenous lidocaine, protects against intubation-induced bronchoconstriction in asthma. Anesthesiology. 2000;93:1198–1204.
52. Groeben, H, Silvanus, MT, Beste, M, Peters, J. Both intravenous and inhaled lidocaine attenuate reflex bronchoconstriction but at different plasma concentrations. Am J Respir Crit Care Med. 1999;159:530–535.
53. Samaha, T, Ravussin, P, Claquin, C, Ecoffey, C. [Prevention of increase of blood pressure and intracranial pressure during endotracheal intubation in neurosurgery: Esmolol versus lidocaine]. Ann Fr Anesth Reanim. 1996;15:36–40.
54. Grover, VK, Reddy, GM, Kak, VK, Singh, S. Intracranial pressure changes with different doses of lignocaine under general anaesthesia. Neurol India. 1999;47:118–121.
55. Walls, RM. Rapid sequence intubation. In: Walls RM, Murphy MF, Luten RC, eds. Manual of Emergency Airway Management. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health; 2008:23–35.
56. Ellis, DY, Harris, T, Zideman, D. Cricoid pressure in emergency department rapid sequence tracheal intubations: A risk-benefit analysis. Ann Emerg Med. 2007;50:653–665.
57. Sellick, BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
58. Olsen, JC, Gurr, DE, Hughes, M. Video analysis of emergency medicine residents performing rapid-sequence intubations. J Emerg Med. 2000;18:469–472.
59. Allman, KG. The effect of cricoid pressure application on airway patency. J Clin Anesth. 1995;7:197–199.
60. Aoyama, K, Takenaka, I, Sata, T, Shigematsu, A. Cricoid pressure impedes positioning and ventilation through the laryngeal mask airway. Can J Anaesth. 1996;43:1035–1040.
61. Kopka, A, Crawford, J. Cricoid pressure: A simple, yet effective biofeedback trainer. Eur J Anaesthesiol. 2004;21:443–447.
62. Smith, KJ, Dobranowski, J, Yip, G, Dauphin, A, Choi, PT. Cricoid pressure displaces the esophagus: An observational study using magnetic resonance imaging. Anesthesiology. 2003;99:60–64.
63. Roppolo, LP, et al. Nasotracheal intubation in the emergency department, revisited. J Emerg Med. 1999;17:791–799.
64. Kirkegaard-Nielsen, H, Caldwell, JE, Berry, PD. Rapid tracheal intubation with rocuronium: A probability approach to determining dose. Anesthesiology. 1999;91:131–136.
65. Sagarin, MJ, et al. Rapid sequence intubation for pediatric emergency airway management. Pediatr Emerg Care. 2002;18:417–423.
66. Naguib, M, Lien, C, Pharmacology of muscle relaxants and their antagonists, Miller’s Anesthesia. 6th ed. Miller, RD, eds., Miller’s Anesthesia. Philadelphia:Elsevier/Churchill Livingstone; 2005;vol 2:481–572.
67. Naguib, M, Samarkandi, A, Riad, W, Alharby, SW. Optimal dose of succinylcholine revisited. Anesthesiology. 2003;99:1045–1049.
68. Rose, JB, Theroux, MC, Katz, MS. The potency of succinylcholine in obese adolescents. Anesth Analg. 2000;90:576–578.
69. Lemmens, HJ, Brodsky, JB. The dose of succinylcholine in morbid obesity. Anesth Analg. 2006;102:438–442.
70. Schreiber, JU, Lysakowski, C, Fuchs-Buder, T, Tramèr, MR. Prevention of succinylcholine-induced fasciculation and myalgia: A meta-analysis of randomized trials. Anesthesiology. 2005;103:877–884.
71. Gronert, GA. Cardiac arrest after succinylcholine: mortality greater with rhabdomyolysis than receptor upregulation. Anesthesiology. 2001;94:523–529.
72. Martyn, JA, Richtsfeld, M. Succinylcholine-induced hyperkalemia in acquired pathologic states: Etiologic factors and molecular mechanisms. Anesthesiology. 2006;104:158–169.
73. Pang, YL, Tseng, FL, Tsai, YC, Liu, YC. Suxamethonium-induced hyperkalaemia in a patient with a normal potassium level before rapid-sequence intubation. Crit Care Resusc. 2006;8:213–214.
74. Osta, WA, et al. Nicotinic acetylcholine receptor gene expression is altered in burn patients. Anesth Analg. 2010;110:1355–1359.
75. Schow, AJ, Lubarsky, DA, Olson, RP, Gan, TJ. Can succinylcholine be used safely in hyperkalemic patients? Anesth Analg. 2002;95:119–122.
76. Vachon, CA, Warner, DO, Bacon, DR. Succinylcholine and the open globe. Tracing the teaching. Anesthesiology. 2003;99:220–223.
77. Zhou, J, Allen, PD, Pessah, IN, Naguib, M. Neuromuscular disorders and malignant hyperthermia. In: Miller RD, ed. Miller’s Anesthesia. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:1171–1196.
78. Patanwala, AE, Stahle, SA, Sakles, JC, Erstad, BL. Comparison of succinylcholine and rocuronium for first-attempt intubation success in the emergency department. Acad Emerg Med. 2011;18:10–14.
79. Perry, JJ, Lee, J, Wells, G. Are intubation conditions using rocuronium equivalent to those using succinylcholine? Acad Emerg Med. 2002;9:813–823.
80. Sivilotti, ML, et al. Does the sedative agent facilitate emergency rapid sequence intubation? Acad Emerg Med. 2003;10:612–620.
81. Weiss-Bloom, LJ, Reich, DL. Haemodynamic responses to tracheal intubation following etomidate and fentanyl for anaesthetic induction. Can J Anaesth. 1992;39:780–785.
82. Caro, DA, Tyler, KR. Sedative induction agents. In: Walls RM, Murphy MF, Luten RC, eds. Manual of Emergency Airway Management. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health; 2008:234–247.
83. Annane, D. ICU physicians should abandon the use of etomidate!. Intensive Care Med. 2005;31:325–326.
84. Jackson, WL, Jr. Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock? A critical appraisal. Chest. 2005;127:1031–1038.
85. Walls, RM, Osborn, TM. Special report: Should etomidate be used as an induction agent for patients with sepsis? J Watch Emerg Med [online]. 12(2), 2008.
86. Albert, SG, Ariyan, S, Rather, A. The effect of etomidate on adrenal function in critical illness: A systematic review. Intensive Care Med. 2011;37:901–910.
87. Annane, D, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871.
88. Lipiner-Friedman, D, et al. Adrenal function in sepsis: The retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–1018.
89. Dmello, D, Taylor, S, O’Brien, J, Matuschak, GM. Outcomes of etomidate in severe sepsis and septic shock. Chest. 2010;138:1327–1332.
90. Riché, FC, et al. Adrenal response in patients with septic shock of abdominal origin: Relationship to survival. Intensive Care Med. 2007;33:1761–1766.
91. Ray, DC, McKeown, DW. Effect of induction agent on vasopressor and steroid use, and outcome in patients with septic shock. Crit Care. 2007;11:R56.
92. Tekwani, KL, et al. A comparison of the effects of etomidate and midazolam on hospital length of stay in patients with suspected sepsis: A prospective, randomized study. Ann Emerg Med. 2010;56:481–489.
93. Jabre, P, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: A multicentre randomised controlled trial. Lancet. 2009;374:293–300.
94. Dellinger, RP, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60.
95. Sprung, CL, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124.
96. Walls, RM, Murphy, MF. Clinical controversies: etomidate as an induction agent for endotracheal intubation in patients with sepsis: Continue to use etomidate for intubation of patients with septic shock. Ann Emerg Med. 2008;52:13–14.
97. Albanèse, J, et al. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology. 1997;87:1328–1334.
98. Walls, RM. Management of the difficult airway in the trauma patient. Emerg Med Clin North Am. 1998;16:45–61.
99. Bar-Joseph, G, Guilburd, Y, Tamir, A, Guilburd, JN. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J Neurosurg Pediatr. 2009;4:40–46.
100. Sener, S, Eken, C, Schultz, CH, Serinken, M, Ozsarac, M. Ketamine with and without midazolam for emergency department sedation in adults: A randomized controlled trial. Ann Emerg Med. 2011;57:109–114.
101. Murphy, A, Campbell, DE, Baines, D, Mehr, S. Allergic reactions to propofol in egg-allergic children. Anesth Analg. 2011;113:140–144.
102. Jalota, L, et al. Prevention of pain on injection of propofol: Systematic review and meta-analysis. BMJ. 2011;342:d1110.
103. Shlamovitz, GZ, Hawthorne, T. Intravenous ketamine in a dissociating dose as a temporizing measure to avoid mechanical ventilation in adult patient with severe asthma exacerbation. J Emerg Med. 2008;41:492–494.
104. Adachi, YU, Satomoto, M, Higuchi, H, Watanabe, K. Fentanyl attenuates the hemodynamic response to endotracheal intubation more than the response to laryngoscopy. Anesth Analg. 2002;95:233–237.
105. Helfman, SM, Gold, MI, DeLisser, EA, Herrington, CA. Which drug prevents tachycardia and hypertension associated with tracheal intubation: Lidocaine, fentanyl, or esmolol? Anesth Analg. 1991;72:482–486.
106. Jagoda, AS, Bruns, JJ, Jr. Elevated intracranial pressure. In Walls RM, Murphy MF, Luten RC, eds.: Manual of Emergency Airway Management, 4th ed, Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health, 2012. [395–366].
107. Crosby, E. Airway management after upper cervical spine injury: What have we learned? Can J Anaesth. 2002;49:733–744.
108. Criswell, JC, Parr, MJ, Nolan, JP. Emergency airway management in patients with cervical spine injuries. Anaesthesia. 1994;49:900–903.
109. Santoni, BG, et al. Manual in-line stabilization increases pressures applied by the laryngoscope blade during direct laryngoscopy and orotracheal intubation. Anesthesiology. 2009;110:24–31.
110. Manoach, S, Paladino, L. Laryngoscopy force, visualization, and intubation failure in acute trauma: Should we modify the practice of manual in-line stabilization? Anesthesiology. 2009;110:6–7.
111. Thiboutot, F, Nicole, PC, Trépanier, CA, Turgeon, AF, Lessard, MR. Effect of manual in-line stabilization of the cervical spine in adults on the rate of difficult orotracheal intubation by direct laryngoscopy: A randomized controlled trial. Can J Anaesth. 2009;56:412–418.
112. Robitaille, A, et al. Cervical spine motion during tracheal intubation with manual in-line stabilization: Direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg. 2008;106:935–941.
113. Turkstra, TP, Craen, RA, Pelz, DM, Gelb, AW. Cervical spine motion: A fluoroscopic comparison during intubation with lighted stylet, GlideScope, and Macintosh laryngoscope. Anesth Analg. 2005;101:910–915.
114. Walt, B, et al. Tracheal intubation and cervical spine excursion: Direct laryngoscopy vs. intubating laryngeal mask. Anaesthesia. 2001;56:221–226.
115. Turkstra, TP, Pelz, DM, Shaikh, AA, Craen, RA. Cervical spine motion: A fluoroscopic comparison of Shikani Optical Stylet vs Macintosh laryngoscope. Can J Anaesth. 2007;54:441–447.
116. Turkstra, TP, Pelz, DM, Jones, PM. Cervical spine motion: A fluoroscopic comparison of the Airtraq Laryngoscope versus the Macintosh laryngoscope. Anesthesiology. 2009;111:97–101.
117. Liu, EH, Goy, RW, Tan, BH, Asai, T. Tracheal intubation with videolaryngoscopes in patients with cervical spine immobilization: A randomized trial of the Airway Scope and the GlideScope. Br J Anaesth. 2009;103:446–451.
118. Maharaj, CH, Buckley, E, Harte, BH, Laffey, JG. Endotracheal intubation in patients with cervical spine immobilization: A comparison of Macintosh and Airtraq laryngoscopes. Anesthesiology. 2007;107:53–59.
119. Ng, BS, Goy, RW, Bain, JA, Chen, FG, Liu, EH. The impact of manual in-line stabilisation on ventilation and visualisation of the glottis with the LMA CTrach: A randomised crossover trial. Anaesthesia. 2009;64:894–898.
120. Chong, CL, Ware, DN, Harris, JH, Jr. Is cervical spine imaging indicated in gunshot wounds to the cranium? J Trauma. 1998;44:501–502.
121. Haut, ER, et al. Spine immobilization in penetrating trauma: More harm than good? J Trauma. 2010;68:115–120.
122. Kendrick, DB, Monroe, KW, Bernard, DW, Tofil, NM. Sedation after intubation using etomidate and a long-acting neuromuscular blocker. Pediatr Emerg Care. 2009;25:393–396.
123. Wane, MA, McDonnell, M. Comparison of traditional versus video laryngoscopy in out-of-hospital tracheal intubation. Prehosp Emerg Care. 2010;14:278–282.
124. Jones, PM, Loh, FL, Youssef, HN, Turkstra, TP. A randomized comparison of the GlideRite(®) Rigid Stylet to a malleable stylet for orotracheal intubation by novices using the GlideScope(®). Can J Anaesth. 2011;58:256–261.
125. Turkstra, TP, et al. The GlideScope-specific rigid stylet and standard malleable stylet are equally effective for GlideScope use. Can J Anaesth. 2007;54:891–896.
126. Cooper, RM, Pacey, JA, Bishop, MJ, McCluskey, SA. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Can J Anaesth. 2005;52:191–198.
127. Tremblay, MH, Williams, S, Robitaille, A, Drolet, P. Poor visualization during direct laryngoscopy and high upper lip bite test score are predictors of difficult intubation with the GlideScope videolaryngoscope. Anesth Analg. 2008;106:1495–1500.
128. Aziz, MF, et al. Routine clinical practice effectiveness of the GlideScope in difficult airway management: An analysis of 2,004 GlideScope intubations, complications, and failures from two institutions. Anesthesiology. 2011;114:34–41.
129. Bathory, I, Frascarolo, P, Kern, C, Schoettker, P. Evaluation of the GlideScope for tracheal intubation in patients with cervical spine immobilisation by a semi-rigid collar. Anaesthesia. 2009;64:1337–1341.
130. Cavus, E, et al. The C-MAC videolaryngoscope: First experiences with a new device for videolaryngoscopy-guided intubation. Anesth Analg. 2010;110:473–477.
131. Brown, CA, 3rd., et al. Improved glottic exposure with the Video Macintosh Laryngoscope in adult emergency department tracheal intubations. Ann Emerg Med. 2010;56:83–88.
132. Komatsu, R, Kamata, K, Sessler, DI, Ozaki, M. Airway scope and Macintosh laryngoscope for tracheal intubation in patients lying on the ground. Anesth Analg. 2010;111:427–431.
133. Noppens, RR, et al. Evaluation of the McGrath Series 5 videolaryngoscope after failed direct laryngoscopy. Anaesthesia. 2010;65:716–720.
134. Liu, L, et al. Tracheal intubation of a difficult airway using Airway Scope, Airtraq, and Macintosh laryngoscope: A comparative manikin study of inexperienced personnel. Anesth Analg. 2010;110:1049–1055.
135. Greenland, KB, Liu, G, Tan, H, Edwards, M, Irwin, MG. Comparison of the Levitan FPS scope and the single-use bougie for simulated difficult intubation in anaesthetised patients. Anaesthesia. 2007;62:509–515.
136. Asai, T, Murao, K, Tsutsumi, T, Shingu, K. Ease of tracheal intubation through the intubating laryngeal mask during manual in-line head and neck stabilisation. Anaesthesia. 2000;55:82–85.
137. Hagberg, CA, et al. A multicenter study of the Ambu laryngeal mask in nonparalyzed, anesthetized patients. Anesth Analg. 2005;101:1862–1866.
138. Brimacombe, JR, Berry, A. The incidence of aspiration associated with the laryngeal mask airway: A meta-analysis of published literature. J Clin Anesth. 1995;7:297–305.
139. Choyce, A, et al. A comparison of the intubating and standard laryngeal mask airways for airway management by inexperienced personnel. Anaesthesia. 2001;56:357–360.
140. Timmermann, A, et al. Novices ventilate and intubate quicker and safer via intubating laryngeal mask than by conventional bag-mask ventilation and laryngoscopy. Anesthesiology. 2007;107:570–576.
141. Timmermann, A, et al. Prospective clinical and fiberoptic evaluation of the Supreme laryngeal mask airway. Anesthesiology. 2009;110:262–265.
142. Castle, N, Owen, R, Hann, M, Naidoo, R, Reeves, D. Assessment of the speed and ease of insertion of three supraglottic airway devices by paramedics: A manikin study. Emerg Med J. 2010;27:860–863.
143. Wharton, NM, et al. I-gel insertion by novices in manikins and patients. Anaesthesia. 2008;63:991–995.
144. Joo, HS, Rose, DK. The intubating laryngeal mask airway with and without fiberoptic guidance. Anesth Analg. 1999;88:662–666.
145. Baskett, PJ, Parr, MJ, Nolan, JP. The intubating laryngeal mask. Results of a multicentre trial with experience of 500 cases. Anaesthesia. 1998;53:1174–1179.
146. Langeron, O, Semjen, F, Bourgain, JL, Marsac, A, Cros, AM. Comparison of the intubating laryngeal mask airway with the fiberoptic intubation in anticipated difficult airway management. Anesthesiology. 2001;94:968–972.
147. Combes, X, et al. Intubating laryngeal mask airway in morbidly obese and lean patients: A comparative study. Anesthesiology. 2005;102:1106–1109.
148. Kundra, P, Sujata, N, Ravishankar, M. Conventional tracheal tubes for intubation through the intubating laryngeal mask airway. Anesth Analg. 2005;100:284–288.
149. Liu, EH, Goy, RW, Lim, Y, Chen, FG. Success of tracheal intubation with intubating laryngeal mask airways: A randomized trial of the LMA Fastrach and LMA CTrach. Anesthesiology. 2008;108:621–626.
150. Maurtua, MA, Maurtua, DB, Zura, A, Doyle, DJ. Improving intubation success using the CTrach Laryngeal Mask Airway. Anesthesiology. 2007;106:640–641.
151. Parmet, JL, et al. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg. 1998;87:661–665.
152. McCaul, CL, et al. Airway management in the lateral position: A randomized controlled trial. Anesth Analg. 2005;101:1221–1225.
153. Katz, SH, Falk, JL. Misplaced endotracheal tubes by paramedics in an urban emergency medical services system. Ann Emerg Med. 2001;37:32–37.
154. Hoyle, JD, Jr., Jones, JS, Deibel, M, Lock, DT, Reischman, D. Comparative study of airway management techniques with restricted access to patient airway. Prehosp Emerg Care. 2007;11:330–336.
155. Lefrancois, DP, Dufour, DG. Use of the esophageal tracheal Combitube by basic emergency medical technicians. Resuscitation. 2002;52:77–83.
156. Mort, TC. Laryngeal mask airway and bougie intubation failures: The Combitube as a secondary rescue device for in-hospital emergency airway management. Anesth Analg. 2006;103:1264–1266.
157. Russi, CS, Miller, L, Hartley, MJ. A comparison of the King-LT to endotracheal intubation and Combitube in a simulated difficult airway. Prehosp Emerg Care. 2008;12:35–41.
158. Russi, CS, Wilcox, CL, House, HR. The laryngeal tube device: A simple and timely adjunct to airway management. Am J Emerg Med. 2007;25:263–267.
159. Burns, JB, Jr., Branson, R, Barnes, SL, Tsuei, BJ. Emergency airway placement by EMS providers: Comparison between the King LT supralaryngeal airway and endotracheal intubation. Prehosp Disaster Med. 2010;25:92–95.
160. Vissers, RJ, Bair, AE. Surgical airway techniques. In: Walls RM, Murphy MF, Luten RC, eds. Manual of Emergency Airway Management. 4th ed. Philadelphia: Lippincott, Williams & Wilkins/Wolters Kluwer Health; 2012:193–219.
161. Wong, DT, Prabhu, AJ, Coloma, M, Imasogie, N, Chung, FF. What is the minimum training required for successful cricothyroidotomy? A study in mannequins. Anesthesiology. 2003;98:349–353.
162. Schaumann, N, et al. Evaluation of Seldinger technique emergency cricothyroidotomy versus standard surgical cricothyroidotomy in 200 cadavers. Anesthesiology. 2005;102:7–11.
163. Schober, P, Hegemann, MC, Schwarte, LA, Loer, SA, Noetges, P. Emergency cricothyrotomy—a comparative study of different techniques in human cadavers. Resuscitation. 2009;80:204–209.
164. Hill, C, Reardon, R, Joing, S, Falvey, D, Miner, J. Cricothyrotomy technique using gum elastic bougie is faster than standard technique: A study of emergency medicine residents and medical students in an animal lab. Acad Emerg Med. 2010;17:666–669.
165. Barton, ED, et al. What is a complication? Classifying events during emergency intubations. Acad Emerg Med. 1999;6:506–507.