Ageing of the lumbar spine
Introduction
The human spine functions in a reverse way to that for which it was originally developed: instead of a hanging bridge connecting the anterior to the posterior limbs of a quadruped, it has become a vertical column of a biped, fundamentally changing the biomechanics and imposing larger loads on the lower lumbar discs. Humans’ upright posture has long been blamed as the main cause of the development of early disc degeneration: ‘intervertebral disc disease is the price we pay for the gift of the erect posture.’1
Disc degeneration in animals is very unusual.2 Neither have localized bulging and Schmorl’s nodules ever been observed in quadrupeds.3 However, a quadruped forced to adopt an erect position quickly develops structural changes in the annulus fibrosus and nucleus pulposus.4–6 Large disc herniations at the lumbosacral level also occur in 25% of bipedal rats.7 It seems obvious, therefore, that the upright position and the consequent increase in static loading of the disc8 are the main causes of the early and progressive development of degeneration in the human lumbar spine.
Degeneration of the lumbar discs starts very early in life. Clear microscopic signs of degeneration have been demonstrated in a 4-year-old.9 Several reports of prolapsed discs in children and adolescents show that major disc degeneration appears at or before puberty10–14 and it has been maintained that after the age of 30 years there is no lumbar disc which does not show some degenerative changes.15 There seems to be no way to escape this degeneration because it affects all, whatever their weight, physical build or athleticism.16
Ageing of the disc
Mechanism
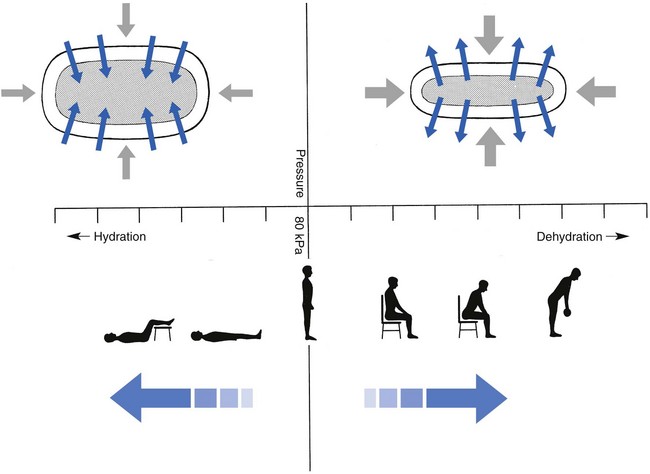
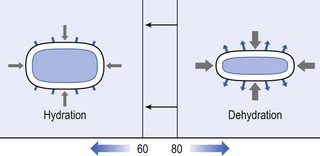
Both the erect posture, which imposes heavier mechanical stresses on the spine, and the decreased regenerative capacity of the disc make it very vulnerable. As has been described (see Ch. 31), the disc depends for its nutrition on diffusion through the endplates and annulus, which can only take place as an outcome of change in intradiscal pressure within a physical range (Fig. 32.1). Consequently, both excessive forces and immobilization will have deleterious effects on disc tissue.17 A modern, mostly sedentary lifestyle, for example, puts continuous increased pressure on the disc; it is then constantly kept low in water content, which prevents the transport of nutrients. As the cells are undernourished, the fibrils and ground substance become damaged and their chemical composition changes.18 Enzymatic depolymerization of collagen and mucopolysaccharide causes a decrease in weight and content that results in a drop in oncotic pressure, which leads to further loss of water.19 The drying out of the disc is the most characteristic sign of intervertebral degeneration. The loss of hydrostatic pressure in an ageing disc is also reflected in the change in daily variations in body height: in adolescence the difference in body height between morning and night is 2%, but this drops to 0.2% in adults.20 The mature disc is thus permanently dried out and lacks the osmotic elasticity of the young disc.
Loss of water-binding capacity is also responsible for the decrease of disc turgor and loss of endurance. The disc does not sustain the normal loads and the hydration–dehydration curve shifts in the direction of further dehydration (Fig. 32.2). A vicious circle of further degeneration, further decrease in molecular weight and further fall in water-binding capacity is set up (Fig. 32.3).21
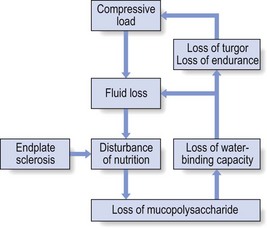
Fig 32.3 Vicious circle of disc degeneration.
With advancing age, the permeability of the vertebral endplates also decreases. The accompanying calcification compromises the nutrient supply to the adjacent nucleus, so enhancing further disc degeneration.22,23 The early stage of the changes in vertebral bone structure is first seen on magnetic resonance imaging (MRI).24 Vertebral endplate sclerosis later becomes radiographically visible.25
Structural changes
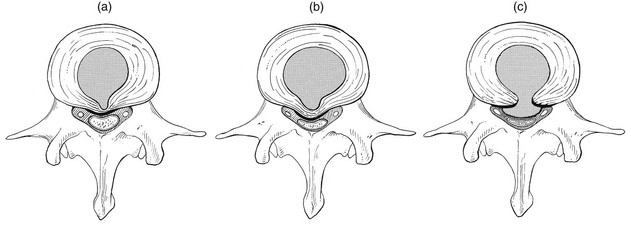
The intervertebral disc is characterized by a tension-resisting annulus fibrosus and a compression-resisting nucleus pulposus. As the nucleus pulposus dries out, it becomes less able to exert fluid pressure. The annular fibres, no longer under constant tension in relation to the nucleus, bear a greater share of vertical load and are subjected to bulk deformations and shear strains.26 This leads to tears in the annular fibres.27 The posterior annulus, especially the area between annulus and nucleus, is subjected to most of the mechanical strain.28 As this posterior boundary zone is also most exposed to nutrient deficiency,29 it will be the first to wear through, so that small radial fissures appear.30,31 Further softening and loosening of annular tissue leads to expansion of these fissures and the formation of cavities and cysts, which are radiographically seen as air pockets.32 Nuclear displacements through these fissures will lead to a further decrease in disc height. As a result, the intervertebral joint becomes unstable and is subjected to more bulk deformation and more shear strain. Ruptures permit further migration of the disc material in the direction of least resistance (mostly backwards). The result is intradiscal displacements, bulging beyond the vertebral borders and disc prolapses (see Ch. 33).
Ageing of the disc also involves structural changes in the endplate. Between the ages of 20 and 65, the endplate becomes thinner and cell death occurs in the superficial layer of the cartilage.33 The vascular channels in the subchondral bone of the endplate are gradually occluded, finally leading to complete endplate sclerosis. As the endplate plays an important role in the nutrition of the disc, changes in it will contribute to further degeneration of the whole intervertebral disc. Also, the strength of the vertebral endplate decreases with age, which renders it more liable to (micro)fractures. Microscopic defects may be followed by invasion of the disc by young blood vessels and connective tissue cells.34 The latter undergo metaplasia into cartilage cells and granulation tissue, and adult connective tissue also forms.35 This ‘invading’ connective tissue can spread out into the disc, create zones of decreased resistance and cause further weakness and instability. It has even been hypothesized that this ‘new’ proliferative tissue plays a part in disc herniations.36
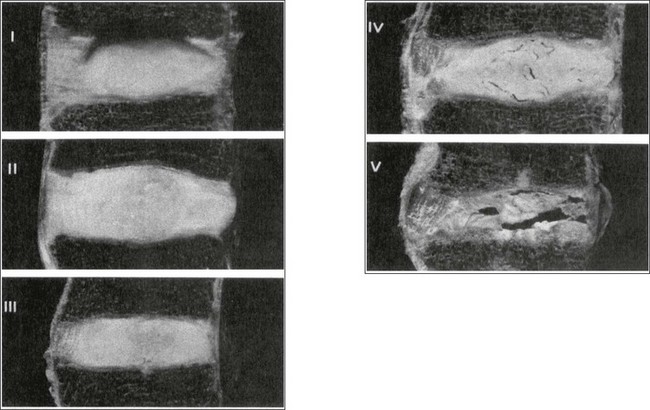
The macroscopic degeneration of the disc was divided into four grades by Friberg and Hirsch37 and later by Nachemson.38 This scheme for assessing the gross morphological changes in the lumbar intervertebral discs has recently been improved by Thompson et al,39 who consider five morphological grades (Table 32.1 and Fig. 32.4).
Table 32.1
Description of the morphological grades
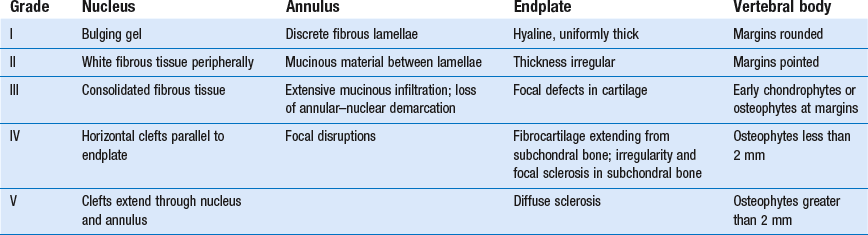
Reproduced with pernission from Thompson et al.39
Macroscopic grades of disc degeneration have been correlated with age, sex and spinal level for 600 lumbar intervertebral discs from 273 cadavers in a review by Miller et al.40 Their conclusion was that, by the age of 50 years, 97% of all lumbar discs exhibit degeneration and that the L3–L4 and L4–L5 levels usually contain the most severely degenerated discs. Another study demonstrated that there is disc degeneration in at least one lumbar level in 35% of subjects between 20 and 39 years of age, and that all subjects 60–80 years of age show disc degeneration.41
These figures establish that disc degeneration is a normal age-dependent pathophysiological process; it therefore cannot be regarded as a ‘disease’ but is a normal biological event from which no one will escape.42
Disc displacements
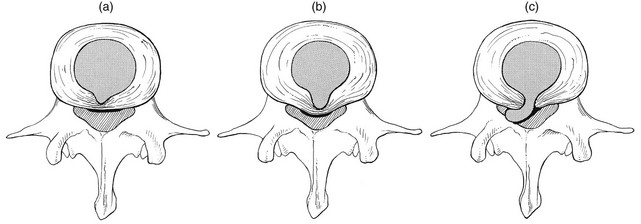
Fissures in the annulus fibrosus will inevitably lead to changes in the biomechanical behaviour of nucleus and annulus, which can then result in intradiscal nuclear displacements, protrusions and prolapses (Fig. 32.5).
The nucleus and inner annulus do not contain free nerve endings. Displacements of these structures are therefore painless, except when they irritate other more sensitive tissues, such as the outer annulus, the dura and the nerve root sleeves. Therefore it is not the size of the migration that is important but the degree of irritation of the pain-sensitive tissues. Because imaging techniques (computed tomography (CT), CT discogram and MRI) can only detect the size and localization of the bulges, and not always their effect on surrounding tissues, many more disc displacements are detected during imaging than actually cause symptoms.43,44 More than 50% of asymptomatic patients over 40 years of age have either alterations in or displacements of disc.44 Single or multilevel disc bulging is visible on the MRIs of between 28 and 85% of the adult population who do not have activity-limiting low back pain.45–48
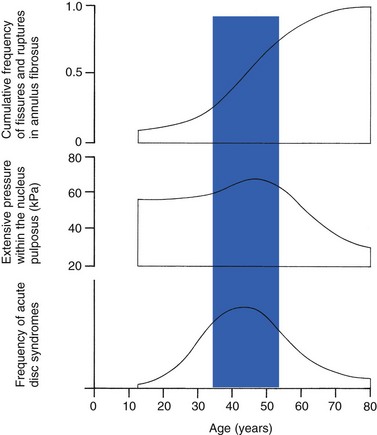
Cyriax49 listed five different directions in which discal tissue can move: posterior, posterolateral, anterior, vertical and circular. Each type of displacement occurs during a specific period in the degeneration of the disc. Posterior and posterolateral displacements are typical in the third, fourth and fifth decades. Anterior displacement resulting in osteochondrosis is a disorder of teenagers, whereas anterior displacement in combination with disc resorption is a lesion of the elderly and is seldom seen below the age of 50. It is surprising to find that low back syndromes (lumbago, backache, sciatica) are age-related,50,51 whereas the incidence of fissures, ruptures and degeneration of the disc increases linearly during the whole of life. Extensive pressure within the nucleus pulposus is higher in individuals between 30 and 50 years of age than in the elderly (Fig. 32.6).52 The conclusion is that posterior displacement of the disc will be more likely to appear when increased intradiscal pressure coincides with some damage to the posterior annular fibres. In other words, the combination of intradiscal pressure and lowered resistance of the annulus fibrosus is the main biomechanical factor contributing to protrusion–prolapse. When the expansive forces of the nucleus pulposus decrease and, because of the loss of water, the disc gradually deflates, the tendency to displacement diminishes.53,54 Other factors protecting the ageing disc against displacements are increasing stiffness of the posterior ligaments, which limit spinal mobility, and the formation of osteophytes, which distribute the load over a larger area.
Posterior displacement
The posterior boundary zone between the annulus and the nucleus is the first part to develop areas of degenerative change and fissures (see p. 438). In sitting and forward bending, the disc is asymmetrically loaded, with a compression strain anteriorly and a tensile stress dorsally. Because of the increase of intradiscal pressure, the nucleus pulposus is pushed dorsally and applies more stress to the weakened posterior fibres, which are already subjected to the strong tensile stress imposed by the flexion.55
Posterior migration of disc tissue beyond the posterior limits of the intervertebral joint space causes tension on the outer annulus and the posterior longitudinal ligament. Irritation of ligament, dura mater and the Hofmann complex combines to produce backache (Fig. 32.7a). The patient’s history usually suggests the type and localization of the displacement and the degree of dural irritation: progressively increasing backache usually stems from a nuclear displacement. A sudden shift indicates that the material that has moved is of harder, annular consistency. If the bulge irritates the dura centrally, the pain is central, probably with some bilateral gluteal radiation. If it lies more to one side, unilateral pain and pain in one buttock result.
Further irritation of the dura mater results in a clinical picture that is called acute lumbago (Fig. 32.7b). In a cartilaginous displacement, the patient states that bending, lifting or standing, after sitting for a while, resulted in acute and severe pain. Alternatively, the pain and disability have come on gradually, some hours after a period of stooping or sitting, which suggests protrusion of part of the nucleus. In both events, a constant ache is experienced which forces a pain-avoiding position, usually in flexion and/or lateral deviation. The pain is agonizing and radiates to one or both legs in an extrasegmental way. Dural symptoms (pain on coughing and sneezing) are positive, as are dural signs (limited straight leg raising and lumbar pain on full neck flexion), indicating that the dura is under constant and severe stress.
A massive posterior protrusion may cause a rupture of the posterior longitudinal ligament. The entire cauda equina will then be compressed between the protrusion and the laminae. This not only squeezes the nerve roots on both sides but also causes compression and atrophy of the centrally localized S3 and S4 roots, with disturbance of bladder and rectal function (the S4 syndrome or cauda equina syndrome) (Fig. 32.7c).56,57
Posterolateral displacement
Root pain results from pressure of the prolapsed or protruded disc against the dural sleeve of the nerve root and subsequent irritation of the latter (Fig. 32.8). This may be primary or secondary.
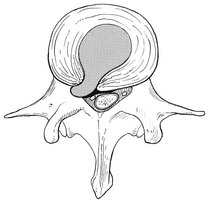
Fig 32.8 Posterolateral disc displacement.
Anterior displacement in adolescents
Because of its high oncotic and hydrostatic pressure, the young disc sometimes invades the vertebral endplates. This occurs particularly in the regions where the endplates have low resistance against increased anterior loading.58,59 The nucleus pulposus may then move forwards between the cartilaginous endplate and the bone of the vertebral body, causing injury to the growth zone of the vertebra (Fig. 32.9). The result is a developmental deformity of the anterior part of the vertebra. During the course of this, protrusion may separate a small triangle of bone at the anterior corner of the vertebral body (limbus vertebra).60 If the osteochondrosis involves several consecutive vertebrae, a kyphotic deformity results (Scheuermann’s disease).61
Such deformities occur only during the period of spinal growth and are asymptomatic. Care must therefore be taken not to overemphasize the significance of incidental radiological findings.62
Anterior displacement in the elderly
Tears at the anterior and anterolateral part of the disc lead to both anterior and anterolateral herniation. Anterior displacement of the disc causes dissection of the anterior longitudinal ligament from its bony attachments, which stimulates bony outgrowths along the anterior and anterolateral aspects of the vertebral body. These first appear in a horizontal direction and then vertically, following the course of the anterior longitudinal ligament (see p. 423). In advanced cases, the whole disc is displaced anteriorly. On a lateral radiograph this can be seen as two ‘beaks’ containing the disc, while the intervertebral space is so narrowed that the vertebral bodies come to lie in apposition.63 Cyriax called this situation the ‘mushroom’ phenomenon, because of the characteristic appearance of the osteophytes, surrounding a ‘mushroom’ – the anteriorly displaced disc (Fig. 32.10).64 The formation of the protrusion itself is completely painless but the gross narrowing of the intervertebral space, the folding of the posterior longitudinal ligament and the enlarged arthrotic posterior joints can cause considerable narrowing of the spinal canal and the lateral recess, which may result in compression of dura or nerve root when the spine is subjected to an axial load.
Vertical displacement
There are two types of vertical displacement: Schmorl’s node and biconvex disc.
A herniation of the nucleus through the cartilaginous endplate is known as Schmorl’s nodes (Fig. 32.11),65 although it was first described by von Luschka in 1858.66 After the nuclear material has invaded the cancellous bone, a reactive osseous shell forms, visible on a radiograph from the age of 17; this does not alter over the years. The disorder is very common at the lower thoracic and upper lumbar levels. Schmorl reported an incidence of 13% on radiographic examination of an adult and asymptomatic population but postmortem studies reported nodes in more than 75% of ordinary individuals.67
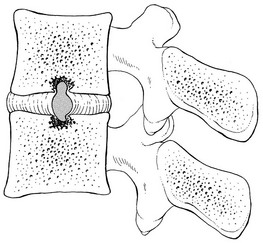
Fig 32.11 Vertical displacement: Schmorl’s nodes.
Biconvex discs appear as the result of traumatic or degenerative changes in the cartilaginous or bony endplates. Osteoporosis or osteomalacia causes a weakening of the bone and the disc balloons between two adjacent and slightly collapsed vertebrae.68
Circular displacement
As a result of degeneration, weakening and drying out, the disc may bulge out all the way round the joint. The outward pressure pulls on the ligaments and lifts the periosteum off the bone. Formation of osteophytes at the anterior and lateral aspects of the vertebra results. These bony outcrops encircle the damaged disc. Mechanically, the presence of osteophytes is beneficial because they increase the weight-bearing surface and decrease the load per unit area.69 They also decrease mobility. Both effects augment the stability of the joint.
Ageing of the surrounding tissues
Effects on the ligaments
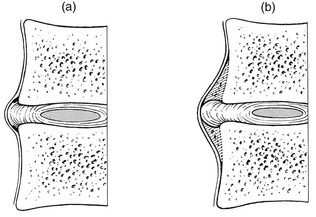
The adjacent vertebral endplates develop poorly demarcated sclerotic densities, probably as the result of changed load distribution on the disc.30 However, the most commonly observed radiographical manifestation of disc degeneration is osteophyte formation in the vertebral bodies.
The first sign of degeneration and instability of the disc is the formation of small traction spurs70 which project horizontally to about 2 mm above and below the vertebral body (Fig. 32.12a). Their formation is explained as follows: decrease in turgor in the disc and the increasing anteroposterior instability which results cause the outermost fibres of the annulus, firmly attached to the undersurface of the epiphyseal ring, to exert traction on the periosteum. A horizontal lifting of the periosteum results in the formation of traction spurs.71
Spondylitic spurs (Fig. 32.12b) or claw spurs are very common in the elderly and there is a positive correlation between osteophyte size and patient age.72 They have been reported to occur most frequently at L3–L4 in men who carry out heavy physical activity and in obese patients.73 The claws form as the result of subperiosteal ossification at the anterior and anterolateral aspect of the vertebrae and their aetiology is considered to be follows: a softened and dehydrated disc bulges easily under the influence of external load and lifts the longitudinal ligament and the periosteum from the vertebral margin. Osseous reaction takes place, provoking the formation of new bone in a beak-like protuberance.74 This process can be viewed as if the vertebral body were trying to expand its articular surface area. By distributing axial loads over a wider area, the vertebral body lessens the stress applied to the annulus fibrosus and the spinal column becomes more stabilized.75
Effects on the facet joints
The disc and facet joints form a ‘three-joint complex’. Degenerative changes affecting the disc are followed by increasing changes in the posterior joints. Arthrosis of a facet joint is thus always secondary to changes in mechanical load, induced by disc degeneration.76,77 Like disc degeneration, facet arthrosis is a universal finding in the human lumbar spine. Evidence of arthrosis begins early, with more than one-half of adults younger than 30 years demonstrating arthritic changes in the facets. The most common arthritic level appears to be L4–L5.78
Initially, alterations in the height and volume of the disc result in positional changes of the facet joints. Axial compression on a degenerated and narrowed disc causes subluxation of the posterior joints: they move telescopically in relation to each other and slide past the normal range of movement, sometimes to such a degree that the tip of the inferior articular facet impinges on the adjacent lamina.79
A height reduction in the functional unit results in changes in the orientation of capsular fibres: they become stretched during axial loading, which gives the capsule a load-bearing function in the upright position.80 The apophyseal joints resist about 16% of the intervertebral compressive forces in the erect standing posture if the disc is thinned.81
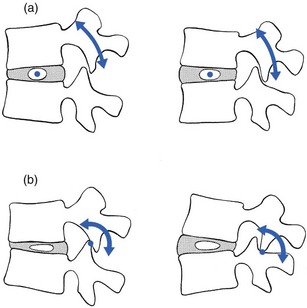
Another consequence of disc degeneration is that flexion–extension movement in the functional unit becomes disturbed. In a normal disc with adequate turgor, the instantaneous axis of rotation remains more or less in the region of the nucleus. In full flexion, it moves immediately anterior to the nucleus and in full extension it lies posterior to it.82 In a normal joint, the vertebral bodies thus rock over the nucleus and the facet joints serve only to guide the movement. When disc degeneration is present, the axis of movement becomes irregular and completely unpredictable: sliding and anteroposterior movements, or tilting of the upper vertebra during flexion, become possible. Alternatively, the axis of rotation remains constant in position through or behind the posterior joints.83 Then the facet joints not only guide and steady motion but are also subjected to a rocking movement, which results in compression of the articular surfaces and distraction of the capsule (Fig. 32.13).
These abnormal stresses cause laxity of the facet joints and the posterior ligaments. Together with the increasing laxity of the disc, the final result is instability of the functional unit, which in turn favours the development of internal derangement at the intervertebral disc.84 This instability of the three-joint complex most commonly appears during a particular period in the cycle of degeneration, which normally falls between the third and fifth decades, when backache is most likely to occur.50
At the facet joints, the repeated pressure results in erosion and thinning of the cartilage. Where cartilage is lost, fibro-fatty intra-articular inclusions may increase in size to fill the space vacated by the cartilage.85 Other regions may exhibit swelling, with subsequent formation of subperiosteal osteophytes and periarticular fibrosis.86 Osteoarthrotic changes in the apophyseal joints finally result in gross enlargements of both inferior and superior facets, giving them a bulbous aspect. All these degenerative changes cause a substantial decrease in motion of the facet joints and, together with the stabilizing changes in the disc, they provide, at the end of the degeneration cycle, the ultimate stabilization of the functional unit.
Effects on the spinal canal and intervertebral foramen
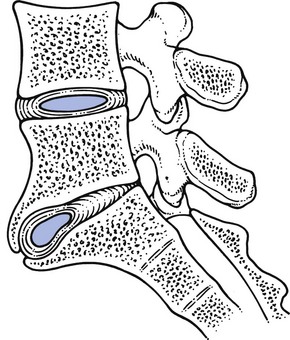
Because of the change in pressure and volume of the lumbar disc, the position of the intervertebral joints changes so that the articular processes override each other. As the vertebral bodies approach each other and because of the inclination of the joint surfaces, the upper vertebra moves backwards (retrospondylolisthesis) to the intervertebral foramen (Fig. 32.14). With increasing approximation of the vertebrae, both the ligamentum flavum and the posterior longitudinal ligament may buckle, so further narrowing the lumbar canal.87 Further, hypertrophied and bulbous arthrotic apophyseal joints commonly project into the intervertebral foramina, where they may add to compression on the dural sac and nerve roots.76
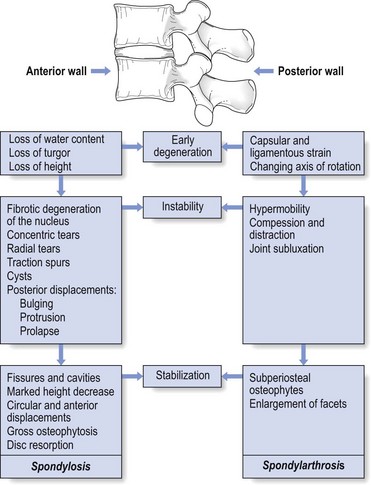
The natural history of degeneration of the lumbar spine is summarized in Figure 32.15.
Radiological changes
The presence of air pockets, located in discrete cavities within the nucleus, is evidence of long-standing disc degeneration.88 The mechanism by which the gas is produced has been ascribed to a vacuum phenomenon.89,90
Two types of osteophyte are commonly seen. Traction spurs, which project horizontally from the corner of the vertebral body, are located about 5 mm from the vertebral margin (see Fig. 32.12a). They are believed to be caused by degenerative disc disease, allowing abnormal motion between the vertebrae. Traction spurs are therefore thought to be associated with segmental instability. Claw osteophytes are larger and arise from the margin of the vertebral body. These bone formations are thought to be a response to increased mechanical loading which arises in deficient discs (see p. 443).
The radiological signs of degeneration in the apophyseal joints include joint space narrowing, sclerosis of the subchondral bone and osteophyte formation. Increasing narrowing of the intervertebral space also provokes some subluxation of the zygapophyseal joints. On a lateral view, the tip of the superior articular facet is then seen to extend more than a few millimetres beyond the posteroinferior border of the vertebral body above.91 Further damage to the posterior joints results in retrolisthesis. Oblique projections of the lumbar spine can detect more malalignment of the articular surfaces and the onset of arthrotic deformity.
The end result of disc degeneration is radiologically characterized by an almost complete loss of disc, which is now represented only by a thin intervertebral space between the dense and sclerotic vertebral endplates.92 Gross beak-like osteophytes are seen at the anterior aspect of the vertebrae. The intervertebral foramen is narrowed, the facet joints are subluxated and enlarged, and retrolisthesis may be visible (Table 32.2).
Relationship between degeneration and symptoms
Although disc degeneration and its radiographic appearances progressively increase with age, it cannot be too strongly emphasized that low back pain and disability do not. It is well known that backache peaks in the middle working years of life (40 years),93–96 which establishes that disc degeneration as such does not produce symptoms per se. It is therefore unwise to rely on radiographic appearances for the diagnosis of lumbar disorders. It has been shown repeatedly in controlled studies that there is absolutely no relationship between clinical symptoms and the radiographic changes of degeneration.97–104 To take but one example, a prospective study on 536 patients by Sanders et al compared the radiological findings in patients with backache and disablement (n = 270) with those in an asymptomatic control group (n = 266): there was not the slightest radiographic difference between the two. Their conclusion was that radiography afforded little or no help in the diagnosis of mechanical lumbar disorders105 and remains a very poor method to indicate past, present or future low back pain.106
Symptoms are produced by a more complex pathway than simple and uncomplicated arthrosis. Making a specific diagnosis and designing a specific treatment are only possible if symptoms and signs are meticulously assessed. The interpretation of the data obtained is based on knowledge of the mechanical behaviour of the tissues involved and the mechanism of pain production. In this way, it is possible to classify activity-related spinal disorders under three major concepts: the dural, the ligamentous and the stenotic (Chs 33–35). These three appear in a specific age group, have a certain correlation with the natural ageing of the lumbar spine and are clinically distinct. Classification of a particular patient’s backache into one of these types thus depends on the information obtained from the history and clinical examination, and not on interpretation of radiographs.
References
1. Reishauer, F. Untersuchungen über den lumbalen und cervikalen Wirbelbandscheibenvorfall. Stuttgart: Thieme; 1949.
2. Hansen, H-J, Comparative views on the pathology of disc degeneration in animals. Lab Invest 1959; 8:1242–1265. ![]()
3. Exner, G. Die Halswirbelsäule. Stuttgart: Thieme; 1959.
4. Ushikubo, S. Study of intervertebral disc herniation in bipedal rats Shikoku. Acta Med. 1959; 15:1759–1780.
5. Yamada, K, The dynamics of experimental posture: experimental study of intervertebral disc herniation in bipedal animals. Clin Orthop 1962; 25:20–31. ![]()
6. Wassilev, W, Dimova, R, Der Einfluss der mechanischen Faktoren auf die Struktur der Zwischenwirbelscheiben. Arch Orthop Unfall-Chir 1970; 68:273. ![]()
7. Cassidy, JD, Yong-Hing, K, Kirkaldy Willis, WH, Wilkinson, AA, A study on the effect of bipedism and upright posture on the lumbosacral spine and paravertebral muscles of the Wistar rat. Spine. 1988;13(3):301–308. ![]()
8. Lotz, JC, Colliou, OK, Chin, JR, et al, Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine 1998; 23:2493–2502. ![]()
9. Töndury, G. Der Wirbelsäulenrheumatismus. In: Rheumatismus in Forschung und Praxis. Bern: W. Belart Huber; 1968:115.
10. Key, JA, Intervertebral disc lesions in children and adolescence. J Bone Joint Surg 1950; 32:97. ![]()
11. O’Connell, JE, Intervertebral disc protrusions in children and adolescents. Br J Surg 1960; 47:611. ![]()
12. da Silva, U, Beyeler, F, Mumenthaler, M, et al, Die lumbale Diskushernie im Kindesalter. Ther Umsch 1977; 34:405. ![]()
13. Blaauw, G, Schaafsma, J, Blaauw-Van Dishoeck, M, Prolaps van de lumbale tussenwervelschijf bij kinderen en adolescenten. Ned Tijdschr Geneeskd. 1981;125(35):1404–1407. ![]()
14. Erkintalo, MO, Salminen, JJ, Alanen, AM, et al, Development of degenerative changes in the lumbar intervertebral disk: results of a prospective MR imaging study in adolescents with and without low back pain. Radiology 1995; 196:529–533. ![]()
15. Schmorl, G, Junghans, H. Die Gesunde und die kranke Wirbelsäule in Röntgenbild und Klinik, 5th ed. Stuttgart: Thieme; 1968.
16. Jochheim, K, Lowe, F, Rütt, A. Lumbaler Bandscheibenvorfall. Berlin: Springer; 1961.
17. Holm, S, Nachemson, A, Nutritional change in the canine intervertebral disc after spinal fusion. Clin Orthop 1982; 169:243–258. ![]()
18. Buckwater, JA, Spine update. Aging and degeneration of the human intervertebral disc. Spine 1995; 20:1307–1314. ![]()
19. Johnstone, B, Bayliss, MT, The late proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine 1995; 20:674–684. ![]()
20. Köller, W, Mühlhaus, S, Meier, W, Hartmann, F, Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech 1986; 19:807–816. ![]()
21. Krämer, J. Bandscheibenbedingte Erkrankungen. Stuttgart: Thieme; 1978.
22. Sobel, DF, Zyroff, J, Thorne, RP, Diskogenic vertebral sclerosis: MR imaging. J Comp Assist Tomogr 1987; 11:855–858. ![]()
23. Moore, RJ, The vertebral end-plate: what do we know? Eur Spine J. 2000;9(2):92–96. ![]()
24. Modic, MT, Steinberg, PM, Ross, JS, et al, Degenerative disc disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988; 166:193–199. ![]()
25. Bernick, S, Cailliet, R, Vertebral end-plate changes with aging of human vertebrae. Spine 1982; 7:97–102. ![]()
26. Acarpglu, ER, Iatridis, JC, Setton, LA, et al, Degeneration and aging affect the tensile behaviour of human lumbar annulus fibrosus. Spine 1995; 20:2690–2701. ![]()
27. Farfan, HF. Mechanical Disorders of the Low Back. Philadelphia: Lea & Febiger; 1973.
28. Belytschko, T, Kulak, RF, Schultz, A, Galante, J, Finite element stress analysis of an intervertebral disc. J Biomech 1974; 7:277–285. ![]()
29. Maroudas, A, Stockwell, RA, Nachemson, A, Urban, J, Factors involved in the nutrition of the human intervertebral disc; cellularity and diffusion of glucose in vitro. J Anat 1975; 120:113–130. ![]()
30. Vernon-Roberts, B. Pathology of degenerative spondylosis. In: Jayson M, ed. The Lumbar Spine and Back Pain. London: Pitman Medical; 1985:55–76.
31. Shirazi-Adl, A, Strain in fibers of a lumbar disc. Analysis of the role of lifting in producing disc prolapse. Spine 1989; 414:96–103. ![]()
32. Knutsson, F. The instability associated with disc degeneration in the lumbar spine. Acta Radiol (Stockh). 1944; 25:593.
33. Bernick, S, Cailliet, R, Vertebral end-plate changes with aging of human vertebrae. Spine 1982; 7:97–102. ![]()
34. Ritchie, JHW, Fahrni, WH, Age changes in lumbar intervertebral discs? Canad J Surg 1970; 13:65. ![]()
35. Bernick, S, Cailliet, R, Vertebral end-plate changes with aging of human vertebrae. Spine 1982; 7:97–102. ![]()
36. Lipson, SJ, Metaplastic proliferative fibrocartilage as an alternative concept to herniated intervertebral disc. Spine 1988; 13:1055–1060. ![]()
37. Friberg, S, Hirsch, C, Anatomical and clinical studies on lumbar disc degeneration. Acta Orthop Scand 1949; 419:222–242. ![]()
38. Nachemson, AL, Lumbar intervertebral pressure: experimental studies on post-mortem material. Acta Orthop Scand. 1960;(suppl 43):42–43. ![]()
39. Thompson, JP, Pearce, RH, Schechter, MT, et al, Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 1990; 15:411–415. ![]()
40. Miller, JA, Schmatz, C, Schultz, AB, Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine 1988; 13:173–178. ![]()
41. Boden, SD, Davis, DO, Dina, TS, et al, Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403–408. ![]()
42. Parisini, P, Di Silvestre, M, Greggi, T, et al, Lumbar disc excision in children and adolescents. Spine (Phila Pa 1976) 2001; 26:1997–2000. ![]()
43. Hitselberger, WE, Witten, RM, Abnormal myelograms in asymptomatic patients. J Neurosurg 1968; 28:204–206. ![]()
44. Wiesel, SW, Tsourmas, N, Feffer, HL, et al, A study of computer-assisted tomography: I. The incidence of positive CT scans in an asymptomatic group of patients. Spine 1984; 9:549–551. ![]()
45. Boden, SD, Davis, DO, Dina, TS, et al, Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg 1990; 72A:403–408. ![]()
46. Jensen, M, Brandt-Zawadzki, M, Obuchowski, N, Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994; 331:67–73. ![]()
47. Buirski, G, Silberstein, M, The symptomatic lumbar disc in patients with low-back pain: magnetic resonance imaging appearances in both symptomatic and control population. Spine 1993; 18:1801–1811. ![]()
48. Boos, N, Rieder, R, Schade, V, The diagnostic accuracy of magnetic resonance imaging, work place perception, and psychosocial factors in identifying symptomatic disc herniations. Spine. 1995;20(24):2613–2625. ![]()
49. Cyriax, JH. Textbook of Orthopaedic Medicine, Vol 1, Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
50. Kelsey, J, White, AA, Epidemiology and impact of low back pain. Spine 1980; 6:133–142. ![]()
51. Rowe, ML, Low back pain in industry. J Occup Med 1969; 11:161–169. ![]()
52. Krämer, J. Biomechanische Veränderungen im lumbale Bewegungssegment. In: Junghanns H, ed. Die Wirbelsäule in Forschung und Praxis. Stuttgart: Hippokrates, 1973.
53. Adams, MA, McNally, DS, Dolan, P, tress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78(6):965–972. ![]()
54. Adams, MA, McNally, DS, Wagstaff, J, Goodship, AE, Abnormal stress concentrations in lumbar intervertebral discs following damage to the vertebral bodies: a cause of disc failure? Eur Spine J. 1993;1(4):214–221. ![]()
55. McNally, DS, Adams, MA, Goodship, AE, Can intervertebral disc prolapse be predicted by disc mechanisms? Spine 1993; 18:1525–1530. ![]()
56. Kostuik, JP, Harrington, J, Alexander, D, et al, Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg 1986; 68A:386–391. ![]()
57. Olivero, WC, Wang, H, Hanigan, WC, et al, Cauda equina syndrome (CES) from lumbar disc herniations. J Spinal Disord Tech. 2009;22(3):202–206. ![]()
58. Mau, H, Verschaubung und dorsolaterale Versteifung im Lumbosakralabschnitt. Z Orthop 1974; 112:785. ![]()
59. Resnick, D, Niwayama, G, Intervertebral disc herniations; cartilaginous (Schmorl’s nodes). Radiology 1978; 126:57–65. ![]()
60. Ghelman, B, Freiberger, RH, The limbus vertebra: an anterior disc herniation demonstrated by discography. Am J Röntgenol 1976; 127:854. ![]()
61. Scheuermann, H. Kyphosis dorsalis juvenilis. Z Orthop Chir. 1921; 41:305.
62. Summers, BN, Singh, JP, Manns, RA, The radiological reporting of lumbar Scheuermann’s disease: an unnecessary source of confusion amongst clinicians and patients. Br J Radiol. 2008;81(965):383–385. ![]()
63. Crock, HV, A reappraisal of intervertebral disc lesions. Med J Aust 1970; 1:983–989. ![]()
64. Cyriax, JH, Treatment of lumbar disc lesions. BMJ 1950; ii:1343. ![]()
65. Schmorl, G. Über Verlagerungen von Bandscheibengewebe und ihre Folgen. Arch Klin Chir. 1932; 172:240.
66. Von Luschka, H. Die Halbgelenke des menschlichen Körpers. Berlin: Reimer; 1858.
67. Hilton, RC, Ball, J, Benn, RT, Vertebral end-plate lesions (Schmorl’s nodes). Ann Rheum Dis 1976; 35:127. ![]()
68. Vernon-Roberts, B, Price, CJ, Healing trabecular micro-fractures in the bodies of lumbar vertebrae. Ann Rheum Dis 1973; 32:406–412. ![]()
69. Frymoyer, JW, Moskowitz, RW. Spinal degeneration, pathogenesis and medical management. In: Frymoyer JW, ed. The Adult Spine. New York: Raven Press; 1991:629.
70. MacNab, I, The traction spur: an indication of segmental instability. J Bone Joint Surg 1971; 53A:891. ![]()
71. Leone, A, Guglielmi, G, Cassar-Pullicino, VN, Bonomo, L, Lumbar intervertebral instability: a review. Radiology 2007; 245:62–77. ![]()
72. Watanabe, S, Terazawa, K, Age estimation from the degree of osteophyte. Legal Medicine 2006; 8:156–160. ![]()
73. O’Neill, TW, McCloskey, EV, Kanis, JA, et al, The distribution, determinants, and clinical correlates of vertebral osteophytosis: a population based survey. J Rheumatol 1999; 26:842–848. ![]()
74. Collins, DH. The Pathology of Articular and Spinal Diseases. London: Edward Arnold; 1949.
75. Pye, SR, Reid, DM, Lunt, M, et al, Lumbar disc degeneration: association between osteophytes, end-plate sclerosis and disc space narrowing. Ann Rheum Dis 2007; 66:330–333. ![]()
76. Vernon-Roberts, B, Pirie, CJ, Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehabil 1977; 16:13–21. ![]()
77. Butler, D, Trafimow, JH, Anderson, GBJ, McNeill, TW, Discs degenerate before facets. Spine 1990; 15:111–113. ![]()
78. Eubanks, JD, Lee, MJ, Cassinelli, E, Ahn, NU, Prevalence of lumbar facet arthrosis and its relationship to age, sex, and race: an anatomic study of cadaveric specimens. Spine (Phila Pa 1976). 2007;32(19):2058–2062. ![]()
79. Kalichman, L, Hunter, DJ, Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37(2):69–80. ![]()
80. Hedtmann, A, Steffen, R, Methfessel, J, et al, Measurements of human lumbar spine ligaments during loaded and unloaded motion. Spine. 1989;14(2):175–185. ![]()
81. Adams, MA, Hutton, WC, The effect of posture on the role of the apophyseal joints in resisting intervertebral compressive forces. J Bone Joint Surg 1980; 62B:358–362. ![]()
82. Adams, MH, Hutton, WC, Stott, JRR, The resistance to flexion of the lumbar intervertebral joint. Spine 1980; 5:245. ![]()
83. Gianturco, C. A Röntgen analysis of the motion of the lower lumbar vertebrae in normal individuals and in patients with low back pain. AJR. 1944; 52:261–268.
84. Kirkaldy-Willis, WH, Wedge, JH, et al, Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978; 3:319. ![]()
85. Twomey, LT, Taylor, JR. Age changes in the lumbar articular triad. Aust J Physio. 1985; 31:106–112.
86. Taylor, JR, Twomey, LT, Age changes in lumbar zygapophyseal joints. Spine 1986; 11:739–745. ![]()
87. Vernon-Roberts, B. The pathology and interrelation of intervertebral disc lesions, osteoarthrosis of the apophyseal joint, lumbar spondylosis and low back pain. In Jayson MIV, ed.: The Lumbar Spine and Back Pain, 2nd ed, Bath: Pitman Press, 1985.
88. Armstrong, J. Lumbar Disc Lesions. Baltimore: Williams & Wilkins; 1965.
89. Edeiken, J, Pitt, MJ, The radiological diagnosis of disc disease – symposium on disease of the intervertebral disc. Orthop Clin North Am 1971; 2:405. ![]()
90. Ford, LT, Gilula, LA, Murphy, WA, Gado, M, Analysis of gas in vacuum lumbar disc. AJR 1977; 128:1056–1057. ![]()
91. Macnab, I, Disc degeneration and low back pain. Clin Orthop 1986; 208:3–14. ![]()
92. Crock, HV, Isolated lumbar disc resorption as a cause of nerve root canal stenosis. Clin Orthop 1976; 115:109. ![]()
93. Cochrane Report. Working Group on Back Pain. London: HMSO; 1979.
94. Horal, J, The clinical appearance of low back pain disorders in the city of Göthenborg, Sweden. Acta Orthop Scand. 1969;118(suppl):1–109. ![]()
95. Rowe, ML, Low back pain in industry. J Occup Med 1969; 11:161–169. ![]()
96. Biering-Sörensen, F, Low back trouble in a general population of 30- 40- 50- and 60-year-old men and women: study design, representativeness and basic results. Dan Med Bull 1982; 29:289–299. ![]()
97. Splithoff, CA, Lumbosacral junction: Röntgenographic comparison of patients with and without backache. JAMA 1953; 152:1610–1613. ![]()
98. Fullenlove, TM, Williams, AJ, Comparative röntgen findings in symptomatic and asymptomatic backs. JAMA 1957; 168:572–574. ![]()
99. La Rocca, H, MacNab, I, Value of pre-employment radiographic assessment of the lumbar spine. Can Med Assoc J 1969; 101:383–388. ![]()
100. Wiltse, LL, The effect of the common anomalies of the lumbar spine upon disc degeneration and low back pain. Orthop Clin North Am 1971; 2:569–582. ![]()
101. Magora, A, Schwartz, A. Relation between the low back pain syndrome and X-ray findings. Scand J Rehabil Med. 1976; 8:115–125.
102. Torgeson, WD, Dotler, WE, Comparative röntgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg 1976; 58A:850–853. ![]()
103. Park, WM. The place of the radiology in the investigation of low back pain. Clin Rheum Dis. 1980; 6:93–132.
104. Wiesel, SW, Bernini, P, Rothman, RH. Diagnostic studies in evaluating disease and aging in the lumbar spine. In: The Aging Lumbar Spine. Philadelphia: Saunders; 1982.
105. Sanders, HWA, et al, Klinische betekenis van degeneratieve afwijkingen van de lumbale wervelkolom en consequenties van het aantonen ervan. Ned Tijdschr Geneeskd 1983; 127:1374–1385. ![]()
106. Glover, JR. Prevention of back pain. In Jayson MIV, ed.: The Lumbar Spine and Back Pain, 1st ed, Tunbridge Wells: Pitman Medical, 1976.