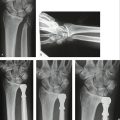
W5 Rehabilitation after Distal Radius Fractures
A methodological approach to the rehabilitation of distal radius fractures is proposed based on knowledge of fracture healing, tissue healing, biomechanics of fixation, and biomechanics of splinting. A distal radius fracture affects more than just the bone. Watson-Jones1 pointed out that a fracture is a soft tissue injury that happens to involve the bone. The soft tissue envelope greatly influences the final functional result, even though all of the initial attention may be focused on the fracture position. The inflammatory cascade that results in edema, pain, and joint stiffness must be treated aggressively and concomitantly with the bony injury. There are a multitude of types of distal radius fractures ranging from simple extra-articular fractures to daunting complex, multifragmented fracture-dislocations. Extra-articular and minimally displaced intra-articular fractures can often be treated with closed reduction and cast application. Comminuted extra-articular and displaced intra-articular fractures often require more rigid fixation. This chapter reviews the basic science behind fracture healing and the inflammatory response with a focus on the rehabilitation forces that can be applied during various stages of the healing process. Special considerations for each type of fixation are outlined to assist in maximizing therapeutic intervention and outcomes.
Basic Fracture Healing
When a bone fractures, the stored energy is released. At low loading speeds, the energy can dissipate through a single crack. At high loading speed, the energy cannot dissipate rapidly enough through a single crack. Comminution and extensive soft tissue damage occur.2 Fractures that exhibit multiple fracture lines are inherently more unstable because of the greater energy absorption at the time of injury. The difference in stability between an undisplaced fracture and a displaced fracture with comminution is significant and dictates a slower pace of fracture site loading during rehabilitation.
The major factors determining the mechanical environment of a healing fracture include the rigidity of the selected fixation device, the fracture configuration, the accuracy of fracture reduction, and the amount and type of loading at the fracture gap.3 The fracture site stability may be artificially enhanced by various external or internal means, including cast treatment, pins, external fixation, and plates. Fracture healing under unstable or flexible fixation typically occurs by callus formation. This applies to cast treatment with or without supplemental pin fixation and external fixation. The sequence of callus healing can be divided into four stages.4 The stages overlap and are determined arbitrarily.
Remodeling
This stage begins when the fracture has solidly united and may take a few months to several years. Four biomechanical stages of fracture healing have been defined: stage I, failure through original fracture site, with low stiffness; stage II, failure through original fracture site, with high stiffness; stage III, failure partially through original fracture site and partially through intact bone, with high stiffness; and stage IV, failure entirely through intact bone, with high stiffness. These data help to determine the level of activity that is safe for patients with a healing fracture.5
The distal radius is composed largely of cancellous metaphyseal bone. Bone healing in cortical and cancellous bone is qualitatively similar, but the speed and reliability of healing is generally better in cancellous bone because of the comparatively large fracture surface.6 Most extra-articular fractures heal by 3 to 5 weeks after injury.7
Fracture Site Forces
Movement of the bone fragments depends on the amount of external loading, the stiffness of the fixation device, and the stiffness of the tissue bridging the fracture. The initial mechanical stability of the bone fixation should be considered an important factor in clinical fracture treatment.8
The physiological forces with wrist motion have been estimated to be 88 N to 135 N.9,10 Eighty-two percent of the loads across the wrist are transmitted through the distal radius.11 Cadaver studies have shown that for every 10 N of grip force, 26 N is transmitted through the distal radius metaphysis. Given that the average male grip force is 463 N12 or 105 psi (1 lb of force = 4.48 N), this would imply that 2410 N of force could be applied to the distal radius during power gripping.13 Previous studies of radius osteotomies showed that plates fail at 830 N.14 External fixators compress 3 mm under a 729 N load.15 To prevent a failure of fixation, the grip forces during therapy should remain less than 159 N (36 psi) for plates and less than 140 N (31 psi) for external fixators during the initial 4 weeks.14,15 Either type of fixation provides enough stability to institute immediate wrist motion. Gripping and strengthening exercises during rehabilitation generally should be delayed until there is some fracture site healing.
Biochemical Response to Injury
The basic response to injury at the tissue level is well known. It consists of overlapping stages including an inflammatory phase (1 to 5 days), a fibroblastic phase (2 to 6 weeks), and a maturation phase (6 to 24 months).16 Following a fracture, there is bleeding from disrupted vessels, which leads to hematoma formation. Numerous chemical mediators, including histamine, prostaglandins, and various cytokines, are released from damaged cells at the injury site, inciting the inflammatory cascade.17,18 The resultant extravasation of fluid from intact vessels causes tissue swelling.19
Edema Fluid
Simple Hand Edema
Simple hand edema is a collection of water and electrolytes. It is precipitated by myriad events, such as limb immobilization or paralysis, axillary lymph node disorders, and thoracic outlet compression. Edema restricts finger motion by increasing the moment arms of skin on the extensor side and by direct obstruction on the flexor side. The work needed to effect a joint angle change depends on the product of the tissue pressure and the volumetric change during angulation. This requires an increase in the muscular force that is necessary to bend a swollen finger. Compression, repeated finger flexion, and dynamic splinting redistribute this fluid to areas with lower tissue pressure. This redistribution allows the skin to lie closer to the joint axis, which decreases the effort needed for finger flexion.20
Inflammatory Hand Edema
Inflammatory hand edema has the same mechanical effects as simple edema and is treated in a similar fashion. The consequences of neglect are dire, however. The swelling that occurs after wrist trauma as a part of the inflammatory response consists of a highly viscous, protein-laden exudate. This exudate leaks from capillaries and contains fibrinogen. In many instances, the fibrin network is reabsorbed by about 7 to 10 days. Other times, the fibrinogen is polymerized into fibrin, which becomes a lattice work for invading fibroblasts. The fibroblasts produce collagen, which, if the part is immobilized, forms a randomly oriented, dense interstitial scar that obliterates the normal gliding surfaces.21 The excessive fibrosis also impedes the flow of lymphatic fluid,22 which perpetuates the edema.
Management of Edema and Pain
Subacute and long-term management techniques include active and passive range of motion exercises, manual edema mobilization such as retrograde massage, compression dressings and garments, contrast baths, electrical stimulation, high-voltage galvanic stimulation, and the use of a Jobst intermittent compression unit as appropriate.23 The pressure generated by these techniques must be low to prevent obstruction of lymphatic flow, which plays a primary role in the absorption of larger plasma proteins associated with chronic edema and fibrosis.
Electrical stimulation has been shown to assist in the reduction of the amount of edema formed with an acute injury and inflammation.24 Explanatory theories include the idea that the negative charge of cathodal stimulation repels the negatively charged serum proteins, blocking their movement out of the blood vessels. It also is thought that the current decreases blood flow by reducing the microvessel diameter.25 A reduction in the pore size in the microvessel walls occurs, preventing large plasma protein leakage through the pores.26
Transcutaneous electrical nerve stimulation, based on the modified gate control theory, can be used for pain modulation by inhibiting the activation of pain and closing off the pain pathways.27 Electrical stimulation used on a conventional transcutaneous electrical nerve stimulator setting can interrupt the pain-spasm-pain cycle, resulting in some reduction of pain after the stimulation stops.26 Ice can be used in conjunction with the electrical stimulation to allow the patient to benefit from edema management and pain relief. Cold seems to increase the pain threshold by desensitizing the pain receptors and by reducing the chemical mediators of inflammation, which can stimulate the pain receptors.28 These modalities assist with the management of pain and edema, enabling the patient to participate more effectively in important therapeutic activities.
Tendon Gliding
Much of the work on tendon gliding has been applied to tendon repairs. The information gleaned from this work has therapeutic implications, however, with regards to distal radius fractures (Table W5-1). The dorsal connective tissue of the thumb and phalanges forms a peritendinous system of collagen lamellae that provide gliding spaces for the extensor apparatus.29–31 The extensor retinaculum is divided into six to eight separate osteofibrous gliding compartments. Within the tunnels and proximal and distal to it, the extensor tendons are surrounded by a synovial sheath.32 The flexor tendons are similarly surrounded by a synovial bursa and pass through a clearly defined pulley system. Hyaluronic acid is secreted from cells lining the inner gliding surfaces of the extensor retinaculum and the annular pulleys.33,34 The hyaluronate serves to decrease the friction force or gliding resistance at the tendon-pulley interface through boundary lubrication35; this influences the total work of finger flexion.36 Fracture hematoma can interfere with this boundary lubrication. Injury to the gliding surfaces by fracture fragments or surgical hardware can affect tendon excursion and lead to adhesions. Adhesions also can occur in nonsynovial regions, such as the flexor mass of the forearm, and restrict the muscle’s gliding and lengthening properties.37 Differential tendon gliding and active finger flexion are necessary to restore range of motion.
| Immobilized Wrist |
| Straight position (MCP, PIP, and DIP joints extended) |
| Hook fist (MCP joints extended, PIP and DIP joints flexed) |
| Full fist (MCP, PIP, and DIP joints flexed) |
| Straight fist (MCP and PIP joints flexed, DIP joints extended) |
| Platform position (MCP joints flexed, PIP and DIP joints extended) |
| Mobile Wrist |
| Synergistic wrist flexion and finger extension |
| Synergistic wrist extension and finger flexion |
| Active and passive finger extension with wrist extended >21 degrees |
| Active and passive thumb extension with wrist neutral in ulnar deviation |
DIP, distal interphalangeal; MCP, metacarpophalangeal; PIP, proximal interphalangeal.
Tendon Excursion
Wehbe and Hunter38,39 studied the in vivo flexor tendon excursion in the hand. With the wrist in neutral, the superficialis tendon achieved an excursion of 24 mm, and the profundus tendon achieved an excursion of 32 mm. The flexor pollicis longus excursion was 27 mm. When wrist motion was added, the amplitude of the superficialis became 49 mm; the profundus tendon, 50 mm; and the flexor pollicis longus tendon, 35 mm. Passive proximal interphalangeal (PIP) flexion results in more flexor tendon excursion than distal interphalangeal (DIP) flexion.40
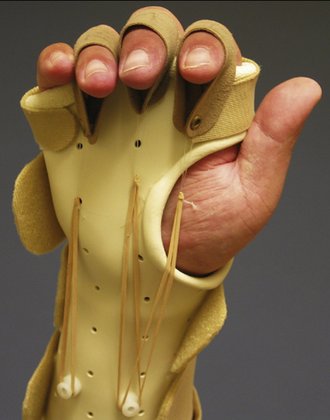
To allow flexor tendons to glide to their maximal potential, the three basic fist positions are performed as part of a tendon gliding exercise program: straight fist, full fist, and hook fist.41 The straight fist (metacarpophalangeal [MCP] and PIP joints flexed, DIP joints extended) elicits maximal flexor digitorum superficialis glide in relation to surrounding structures. The full fist (MCP, PIP, and DIP joints flexed) elicits maximal flexor digitorum profundus glide. The hook fist (MCP joints extended, PIP and DIP joints flexed) elicits maximal differential gliding between the two tendons.42 Synergistic wrist and finger motion increases passive flexor tendon excursion by generating forces that pull the tendon through the pulley system.43
Extensor tendon gliding can be facilitated by extending the wrist more than 21 degrees. This extension allows the extensor tendons to glide with little or no tension in zones 5 and 6.44 Similarly, positioning the wrist close to neutral with some ulnar deviation minimizes friction in the extensor pollicis longus sheath.45
Tissue Biomechanics
There is a constant turnover and remodeling of tissue components. Collagen in particular is being absorbed and then laid down again with updated length, strength, and new bonding patterns in response to stress. The periarticular tissue adaptively shortens if immobilized in a shortened position, leading to clinical joint stiffness.46 This tissue includes the skin, ligaments, and capsule as well as the neurovascular structures.47 To restore the length of the shortened tissue, one must hold the tissue in a moderately lengthened position for a significant time so that it grows. Growth takes days, and the stimulus (i.e., splinting) needs to be continuous for hours at a time to be most effective.
The following is an overview of specific mechanical properties and tissue composition that establish the foundation for splinting intervention in distal radius fracture management. Stress is the load per unit area that develops in a structure in response to an externally applied load. Strain is the deformation or change in length that occurs at a point in a structure under loading.48 Various materials have an elastic region whereby there is no permanent deformation of the material after the load is removed (e.g., a rubber band). When the point of no return is exceeded (the yield point), there is permanent deformation of the material (e.g., bending a paper clip until it deforms).
Collagen contributes 77% of the dry weight of connective tissue. The fibers are brittle and can elongate only 6% to 8% before rupturing.49 Elastin comprises only 5% of the soft tissue weight, but it can elongate 200% without deformity.50 Skin and connective tissues are a polymer of loosely woven strands of elastin and coiled collagen chains. With the initial application of tension, very little force is needed for skin elongation. The elastin and the collagen chains are unfolding and aligning with the direction of the stress, rather than stretching per se. When all of the fibers are lined up parallel to the line of pull, the tissue becomes quite stiff. Each fiber is uncoiled and can elongate only 6% to 8%. A much greater force now produces minimal additional gains in length.
Further attempts at rapid lengthening exceed the fiber’s elastic limit, causing microscopic tearing, bleeding, and inflammation. This situation leads to fibrin deposition with secondary interstitial fibrosis, which may result in further contracture.20 Knowledge of tissue biomechanics greatly enhances decision making and application of splinting during the rehabilitative process.
Types of Splints
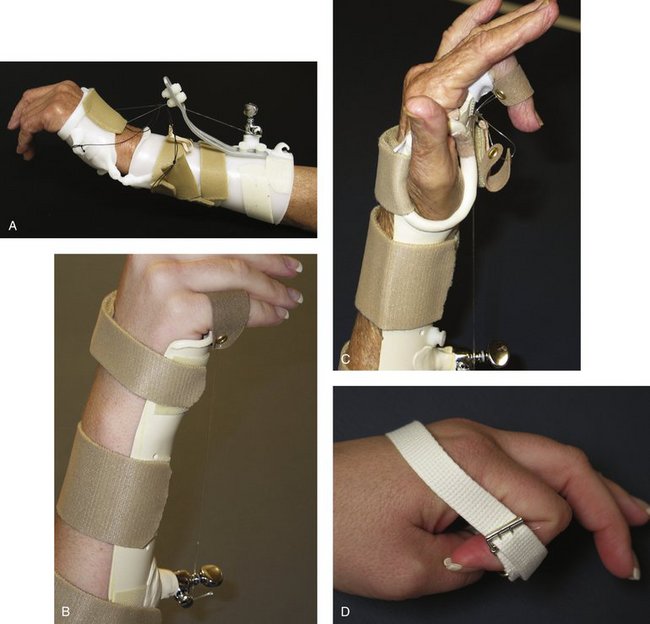
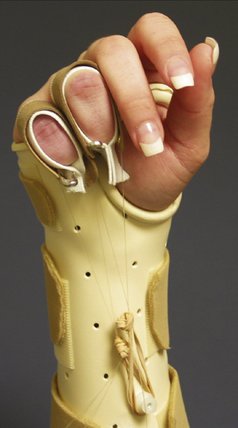
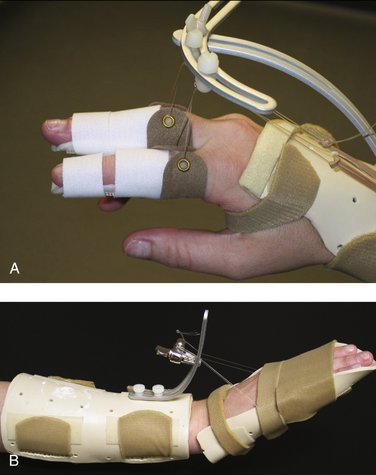
The principles of splinting exploit the biomechanical properties of tissue to overcome contracture and regain joint motion after injury. The types of splints may be grouped as follows: static, serial static, dynamic, and static progressive. Dynamic, static progressive, and serial static splinting are considered mobilization splints. The rationale of mobilization splinting is based on a physiological theory that controlled tension applied over a long time alters cell proliferation. The effectiveness is not based on the concept of stretching tissue, but relies on actual cell growth. The target tissue lengthens when the living cells of the contracted tissues are stimulated to grow. The stimulation occurs when consistent external tension is applied through the splint over time.51
According to Schultz-Johnson’s clinical experience,55 static progressive approaches to passive range of motion limitations for “soft end feel” joints offer faster results without additional tissue trauma. Some patients may tolerate static progressive splinting better than dynamic splinting, perhaps because the joint position is constant while the tissue readily accommodates to the tension and is less subject to the influences of gravity and motion.53,56
Schultz-Johnson55 also advocates wearing a static progressive splint during sleep, obtaining up to 8 hours of end range time that does not take away from function and movement during the day. She states that gains at a joint of 5 to 10 degrees per week indicate splint success. The more time the tissue spends at end range, the more quickly passive range of motion improves.57
Fracture Rehabilitation
For the purposes of rehabilitation, it is useful to consider the stability of the distal radius fracture site in three phases, which guides the therapist as to the loads that can be placed across the fracture site. When internal or external fixation is used, the loads placed on the fracture site may be adjusted accordingly. A rough knowledge of the intrinsic or augmented fracture site stability and the expected forces that are generated during therapy are necessary to minimize fracture site deformity (see section, Fracture Site Forces).
Phase I
Phase I is defined by low fracture site stiffness (stage I—see section, Basic Fracture Healing). The wrist splints used at this stage are static and used for immobilization to limit unwanted motion, prevent displacement at the fracture site, and prevent or correct joint contractures. Protected wrist motion is initiated in this phase.
South Bay Hand Surgery Center Protocol
Controlled and progressive joint mobilization following trauma has been shown to give superior results to immobilization.58 The biochemical and biomechanical events that occur during fracture healing provide the underlying foundation for the rehabilitation program after a distal radius fracture. The therapy protocol for regaining finger motion is tiered and instituted immediately in all patients (Table W5-2). Tendon gliding exercises and passive finger motion with the wrist neutral are started immediately because there are no biomechanical concerns regarding phalangeal stability. Dynamic and static progressive splinting are instituted early if necessary, based on the observation that the total active finger motion typically plateaus by 3 months.59 With resistant cases, dynamic splinting is initiated for 30- to 60-minute intervals two to three times per day, and then static progressive splinting is continued for 4 to 8 hours during sleep. Schultz-Johnson55 stresses that wearing the static progressive splint should be pain-free, and that too much tension would not increase the passive range of motion faster. Because an enthusiastic patient may apply excessive torque to an unstable fracture site, we prefer to delay static progressive splinting of the wrist until phase II.
| Days 1-7 |
| Individual passive and active finger and thumb motion |
| Gentle active finger flexion/extension |
| Gentle active finger PIP and DIP joint blocking |
| Thumb opposition and flexion/extension exercises |
| Intrinsic tightness stretching exercises |
| Aggressive edema management |
| Tendon gliding exercises |
| Weeks 2-4 |
| Dynamic MCP flexion splint if passive MCP flexion <40 degrees |
| Dynamic PIP flexion splint if passive PIP flexion <60 degrees |
| Switch to PIP flexion strap after >80 degrees of passive PIP flexion achieved |
| Dynamic PIP and DIP flexion strap if passive DIP flexion <40 degrees |
| Intrinsic tendon tightness: dynamic PIP flexion splint with MCP blocked in full extension |
| Intrinsic extensor tendon tightness: dynamic PIP/DIP flexion strap while in dynamic MCP flexion splint |
| Extrinsic flexor tendon tightness: add static PIP/DIP extension splints while in dynamic MCP extension splint; incrementally increase wrist extension if wrist is cleared for motion |
| Weeks 4-8 |
| Add static progressive PIP flexion splint at night if flexion is still <60 degrees |
| Add static progressive MCP flexion splint at night if flexion is still <40 degrees |
| Dynamic/static progressive PIP extension splint if PIP flexion contracture >30 degrees |
| Dynamic/static progressive MCP extension splint if MCP flexion contracture >30 degrees |
| Dynamic/static progressive thumb opposition splint if opposition >2 cm from fifth MCP joint |
| For resistant cases, consider dynamic splinting 30-60 min two to three times per day, combined with static progressive splinting 4-8 hours at night |
| After Week 8 |
| Home splinting until motion plateaus |
DIP, distal interphalangeal; MCP, metacarpophalangeal; PIP, proximal interphalangeal.
Wrist motion is initiated at different times depending on the fracture site stability and the type of splinting or fixation (Table W5-3). Patient factors such as age, bone density, pain tolerance, and systemic disease may significantly influence the pace of therapy, which should be adjusted accordingly. Synergistic wrist and finger motion for tendon excursion are started in tandem with wrist motion (see Table W5-1). Forceful gripping is delayed until there is some fracture site healing.
TABLE W5-3 Wrist Rehabilitation Protocol
| Phase I: Low Fracture Site Rigidity |
| Custom or noncustom below-elbow splint |
| Gentle active and passive wrist flexion/extension, radial/ulnar deviation, pronation/supination |
| Phase II: Intermediate Fracture Site Rigidity |
| Dynamic/static progressive wrist flexion splinting if passive wrist flexion <30 degrees |
| Dynamic/static progressive wrist extension splinting if passive wrist extension <30 degrees |
| Dynamic/static progressive supination splinting if passive supination <60 degrees |
| Dynamic/static progressive pronation splinting if passive pronation <60 degrees |
| Incorporate light functional activities and light strengthening |
| Phase III: High Fracture Site Rigidity |
| Progress strengthening exercises and functional activities as tolerated |
| Home splinting until motion plateaus |
Procedure-Specific Treatment
Cast Treatment
Rehabilitation
Special Considerations
Intrafocal Pinning
Intrafocal pinning is indicated in unstable extra-articular distal radius fractures. Intrafocal pinning does not provide rigid fixation, however. Supplemental cast or splint immobilization is necessary for 4 to 6 weeks; otherwise, early wrist motion may produce pain and dystrophy. The therapy protocol differs little from cast treatment alone, but there are added requirements for pin site care. Typically Kirschner wires (K-wires) are introduced through the snuffbox, where injury or irritation of the superficial radial nerve branches are common.63 K-wires introduced dorsally can interfere with normal extensor tendon gliding, whereas K-wires introduced through the first dorsal compartment can limit thumb MCP flexion and opposition. Pin site interference with thumb or finger extensors requires added emphasis on thumb opposition and extensor tendon gliding exercises (see Table W5-1). If comminution involves more than two cortices, or if the patient is older than age 55 years, there is a high likelihood of subsequent fracture collapse.64 In these cases, supplemental external fixation or a spanning bridge plate may be used. Wrist motion is delayed until after fixator/plate removal.
External Fixation
External fixation may provide improved wrist motion through less interference with the soft tissue envelope.65 External fixation is considered flexible fixation. Regardless of the type of external fixator, callus development is the overriding element providing the rigidity of the fixator-bone system.43 The stability of fixation can be significantly enhanced through the addition of 0.62-inch percutaneous K-wires, which approaches the rigidity of a 3.5-mm dorsal AO plate (Synthes, Paoli, PA).66,67 With intra-articular fractures, increasing the rigidity of the fixator does not appreciably increase the rigidity of fixation of the individual fragments.68 Augmentation with percutaneous K-wire fixation reduces the dependence on ligamentotaxis to position the fragment and significantly increases the stability of the construct, especially when the K-wire is attached to an outrigger.10
Pitfalls of Ligamentotaxis
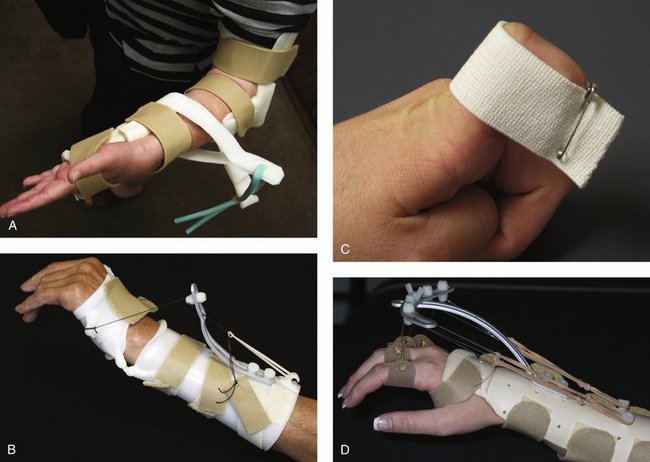
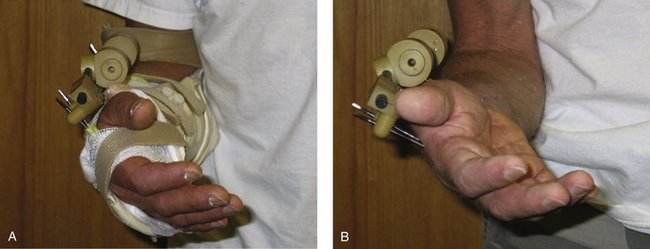
External fixators may be applied in a bridging or nonbridging manner. Bridging external fixation prevents wrist motion and relies on ligamentotaxis. Wrist distraction combined with hand swelling predisposes toward extensor tightness, which mandates an emphasis on MCP-to-DIP flexion exercises. If necessary, a dynamic MCP flexion splint is applied while the fixator is still in place. Added extensor tendon stretch is accomplished by strapping the PIP and DIP joints in full flexion while using the dynamic MCP flexion splint. Overdistraction of the wrist leads to intrinsic and extensor digitorum communis tightness and subsequent clawing of the fingers, necessitating intrinsic tightness stretching and splinting.69 The index finger extensor tendons are especially sensitive to this and act as an early sentinel warning device.
Patients with external fixators often keep their forearms in pronation, which may lead to contractures of the distal radioulnar joint even though the distal radioulnar joint is not immobilized.70 With the fixator in place, gentle supination/pronation should be initiated immediately. Additionally, splinting the patient in supination with an above-elbow splint in between exercises optimally decreases difficulty obtaining supination after fixator removal and decreases the likelihood of a pronation contracture. It may require the therapist to splint the forearm serially to obtain full supination.
Distraction, flexion, and locked ulnar deviation of the external fixator should be avoided because it encourages pronation contractures and may predispose to acute carpal tunnel syndrome. Ideally, the wrist should be positioned in mild extension, which relaxes the extensor tendons and facilitates finger flexion.69 This positioning often requires augmentation with percutaneous K-wire fixation of the fracture. In our clinical experience, it has been observed that if a fixator is left on too long (>7 weeks), intrinsic tightness, finger contractures, pronation/supination loss, and pin site problems multiply.
Because ligaments are viscoelastic, there is a gradual loss of the initial distraction force applied to the fracture site. The initial immediate improvements in radial height, inclination, and volar tilt are significantly decreased by the time of fixator removal.71 For this reason, light gripping exercises and using the hand for activities of daily living should not be encouraged in the initial 4 weeks, with fracture site loading limited to less than 31 psi.13
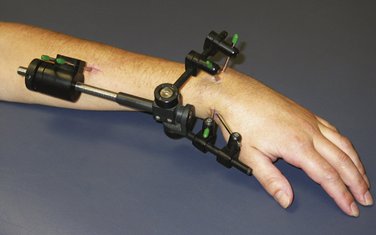
Nonbridging fixators allow the institution of early wrist motion (Fig. W5-6). In these cases, therapy includes the addition of early wrist flexion and extension in addition to the finger exercises. The fixator usually blocks radial deviation, and ulnar deviation exerts traction on the fixator pin sites, which is painful. Simple extra-articular fractures can tolerate the earlier onset of loading compared with comminuted extra-articular fractures. When nonbridging external fixation is used for complex intra-articular fractures, articular incongruity is common.72 This may be prevented by use of custom-designed fixators with dorsal outriggers.
Rehabilitation
Bridging External Fixator
Special Considerations
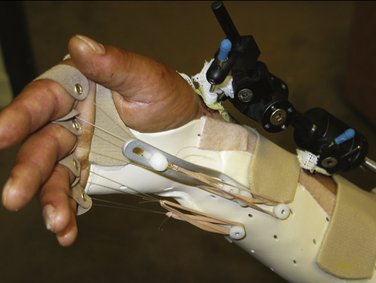
FIGURE W5-7 This dynamic metacarpophalangeal flexion splint is applied while the fixator is in place.
Plate Fixation
Rigid fixation of fractures by plating alters the biology of fracture healing. When motion is completely abolished between fracture fragments, no callus forms.73 This has been termed “direct healing,” whereby osteons directly bridge the fracture gap to regenerate bone, and the fracture heals by remodeling.74 Conventional plate fixation relies on friction between the plate-bone interface for stability and the dynamic compression properties of the plate, which preload the fracture site.75 Generally, plate fixation allows earlier loading of the fracture site.
Newer locking plates act by splinting the fracture site without compression, resulting in flexible elastic fixation and stimulation of callus formation. Locking plates do not require friction to secure the plate to the bone. Comminuted diaphyseal or metaphyseal fractures are particularly suited to bridging fixation using locked plates.76 Fragment-specific fixation relies on a combination of low-profile 2-mm plates. The radial column plates are usually applied dorsoradially underneath the first extensor compartment, whereas the intermediate-column plates may be applied dorsoulnarly between the finger extensors and the extensor digiti minimi. Plate fixation of the volar medial fragments is often necessary. Initially, the plate bears all the stress, and the rehabilitation forces must not exceed their tolerance. As healing progresses, the plate load shares until the fracture is healed and bears almost the entire stress.77 Feedback from the operating surgeon is necessary regarding the stability of fixation before instituting wrist motion and gripping, especially when there is significant intra-articular comminution. Most patients can be treated with a removable below-elbow splint and protected wrist range of motion exercises.
Dorsal Plating
Newer low-profile dorsal plate designs were greeted with much enthusiasm.78,79 Extensor tendon irritation is still a problem.80,81 Rapid tendon acceleration through preload has been proposed as one method to maximize extensor tendon excursion.82
Volar Plating
Volar fixed-angle plating is currently popular.83 Proponents of volar plating cite improved fracture stability and better soft tissue coverage of the implant. Normal wrist extension may be difficult to regain, which has led some authors to recommend splinting the patient’s wrist in 30 degrees of extension in between therapy.82 Flexor tendon tightness may occur and should be treated with dynamic MCP extension splinting. The MCP extension splint is combined with static extension splinting of the PIP and DIP joints, while the wrist is incrementally brought from neutral to extension.
Rehabilitation
Special Considerations
Combined Radius and Scaphoid Fixation
Simultaneous fractures of the distal radius and scaphoid represent a small subset of upper extremity injuries. Slade and colleagues84 recommend a three-step process for the fixation of combined radius and scaphoid fractures. The first step involves reduction and percutaneous K-wire fixation of the scaphoid. The second step is reduction and rigid fixation of the distal radius. The third step is the rigid fixation of the scaphoid. This step is performed last because distraction and reduction maneuvers to treat the distal radius fracture may result in loss of reduction, loss of fixation of the scaphoid fracture, or both. This approach of rigid fixation of both fractures has allowed for the early recovery of hand function with minimal complications.84
Causes of Treatment Failures
There are numerous extrinsic tendons crossing the fracture site. Dorsal angulation of greater than 30 degrees and radial angulation greater than 10 degrees greatly affect the moment arms and subsequently the excursion and strength of these tendons.85
If joint malalignment is the etiology of loss of forearm rotation, continued therapy is of no benefit. Biomechanical studies have shown that radial shortening of 10 mm or greater caused a 47% pronation loss and a 27% supination loss.86 More than 10 degrees of dorsal tilt leads to a dorsal carpal shift with compressive forces. This condition leads to pain and feelings of insecurity with gripping and difficulties with activities of daily living.87 More severe dorsal tilt leads to a dynamic dorsal intercalated segmental instability.88
1. Watson-Jones R. Fractures and Joint Injuries, 4th ed. Baltimore: Williams & Wilkins; 1955.
2. Weber E. A rational approach for the recognition and treatment of Colles’ fracture. Hand Clin. 1987;3:13-21.
3. Aro H, Chao E. Bone-healing patterns affected by loading, fracture fragment stability, fracture type, and fracture site compression. Clin Orthop. 1993;293:8-17.
4. McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60:150-162.
5. White AIII, Panjabi M, Southwick W. The four biomechanical stages of fracture repair. J Bone Joint Surg Am. 1977;59:188-192.
6. Perren L, editor. Biology and Biomechanics in Fracture Management. Stuttgart: Thieme, 2000.
7. Vang Hansen F, Staunstrup H, Mikkelsen S. A comparison of 3 and 5 weeks immobilization for older type 1 and 2 Colles’ fractures. J Hand Surg [Br]. 1998;23:400-401.
8. Klein P, Schell H, Streitparth F, et al. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J Orthop Res. 2003;21:662-669.
9. Trumble T, Glisson R, Seaber A, et al. Forearm force transmission after surgical treatment of distal radioulnar joint disorders. J Hand Surg [Am]. 1987;12:196-202.
10. Wolfe S, Swigart C, Grauer J, et al. Augmented external fixation of distal radius fractures: a biomechanical analysis. J Hand Surg [Am]. 1998;23:127-134.
11. Palmer A, Werner F. Biomechanics of the distal radioulnar joint. Clin Orthop. 1984;187:26-35.
12. Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69-74.
13. Putnam M, Meyer N, Nelson E, et al. Distal radial metaphyseal forces in an extrinsic grip model: implications for postfracture rehabilitation. J Hand Surg [Am]. 2000;25:469-475.
14. Gesensway D, Putnam M, Mente P, et al. Design and biomechanics of a plate for the distal radius. J Hand Surg [Am]. 1995;20:1021-1027.
15. Frykman G, Peckham R, Willard K, et al. External fixators for treatment of unstable wrist fractures: a biomechanical, design feature, and cost comparison. Hand Clin. 1993;9:555-565.
16. Peacock EJr. Biology of wound repair. Life Sci. 1973;13:I-IX.
17. Dekel S, Lenthall G, Francis M. Release of prostaglandins from bone and muscle after tibial fracture: an experimental study in rabbits. J Bone Joint Surg Br. 1981;63:185-189.
18. Rose S, Marzi I. Mediators in polytrauma: pathophysiological significance and clinical relevance. Langenbecks Arch Surg. 1998;383:199-208.
19. Bullough P, editor. Principles of Cell Injury, Inflammation and Repair. New York: Churchill Livingstone, 1984.
20. Brand P. Clinical Mechanics of the Hand, 3rd ed. St. Louis: Mosby; 1999.
21. Kottke F, Pauley D, Ptak R. The rationale for prolonged stretching for correction of shortening of connective tissue. Arch Phys Med Rehabil. 1966;47:345-352.
22. Gaffney R, Casley-Smith J. Excess plasma proteins as a cause of chronic inflammation and lymphoedema: biochemical estimations. J Pathol. 1981;133:229-242.
23. Stewart K. Therapist’s management of the complex injury. In: Hunter J, Callahan A, editors. Rehabilitation of the Hand: Surgery and Therapy. St. Louis: Mosby, 1995.
24. Reed B. Effect of high voltage pulsed electrical stimulation on microvascular permeability to plasma proteins: a possible mechanism in minimizing edema. Phys Ther. 1988;68:491-495.
25. Karnes J, Mendel F, Fish D, et al. High voltage pulsed current: its influences on diameters of histamine-dilated arterioles in hamster cheek pouches. Arch Phys Med Rehabil. 1995;76:381-386.
26. Shapiro S. Electrical currents. In: Cameron M, editor. Physical Agents in Rehabilitation: From Research to Practice. Philadelphia: WB Saunders, 2003.
27. Cameron M. Pain. In: Cameron M, editor. Physical Agents in Rehabilitation: From Research to Practice. Philadelphia: WB Saunders, 2003.
28. Lehmann J, Brunner G, Stow R. Pain threshold measurements after therapeutic application of ultrasound, microwaves, and infrared. Arch Phys Med. 1958;39:560.
29. Bade H, Krolak C, Koebke J. Fibrous architecture of the dorsal aponeurosis of the thumb. Anat Rec. 1995;243:524-530.
30. Bade H, Schubert M, Koebke J. Morphology of the dorsal phalangeal connective tissue body. Anat Rec. 1995;243:1-9.
31. Bade H, Schubert M, Koebke J. [Dorsal gliding and functional spaces of the metacarpophalangeal transition]. Handchir Mikrochir Plast Chir. 1994;26:251-257.
32. Schmidt H, Lahl J. [Studies on the tendinous compartments of the extensor muscles on the back of the human hand and their tendon sheaths]. Gegenbaurs Morphol Jahrb. 1988;134:155-173.
33. Amiel D, Ishizue K, Billings EJr, et al. Hyaluronan in flexor tendon repair. J Hand Surg [Am]. 1989;14:837-843.
34. Banes A, Link G, Bevin A, et al. Tendon synovial cells secrete fibronectin in vivo and in vitro. J Orthop Res. 1988;6:73-82.
35. Uchiyama S, Amadio P, Ishikawa J, et al. Boundary lubrication between the tendon and the pulley in the finger. J Bone Joint Surg Am. 1997;79:213-218.
36. Tanaka T, Amadio P, Zhao C, et al. Gliding resistance versus work of flexion: two methods to assess flexor tendon repair. J Orthop Res. 2003;21:813-818.
37. Alba C, LaStayo P. Postoperative management of functionally restrictive muscular adherence, a corollary to surgical tenolysis: a case report. J Hand Ther. 2001;14:43-50.
38. Wehbe M, Hunter J. Flexor tendon gliding in the hand, part I: in vivo excursions. J Hand Surg [Am]. 1985;10:570-574.
39. Wehbe M, Hunter J. Flexor tendon gliding in the hand, part II: differential gliding. J Hand Surg [Am]. 1985;10:575-579.
40. Horii E, Lin G, Cooney W, et al. Comparative flexor tendon excursion after passive mobilization: an in vitro study. J Hand Surg [Am]. 1992;17:559-566.
41. Wehbe M. Tendon gliding exercises. Am J Occup Ther. 1987;41:164-167.
42. Stewart K, Van Strien G. Postoperative management of flexor tendon injuries. In: Hunter J, Callahan A, editors. Rehabilitation of the Hand: Surgery and Therapy. St. Louis: Mosby, 1995.
43. Zhao C, Amadio PC, Zobitz ME, et al. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop Relat Res. 2002;396:223-230.
44. Minamikawa Y, Peimer C, Yamaguchi T, et al. Wrist position and extensor tendon amplitude following repair. J Hand Surg [Am]. 1992;17:268-271.
45. Kutsumi K, Amadio P, Zhao C, et al. Measurement of gliding resistance of the extensor pollicis longus and extensor digitorum communis II tendons within the extensor retinaculum. J Hand Surg [Am]. 2004;29:220-224.
46. Akeson W, Amiel D, Abel M, et al. Effects of immobilization on joints. Clin Orthop. 1987;219:28-37.
47. Wright V, Johns R. Physical factors concerned with the stiffness of normal and diseased joints. Bull Johns Hopkins Hosp. 1960;106:215-231.
48. Frankel V. Basic Biomechanics of the Skeletal System. Philadelphia: Lea & Febiger; 1980.
49. Abrahams M. Mechanical behaviour of tendon in vitro: a preliminary report. Med Biol Eng. 1967;5:433-443.
50. Carton R, Dainauskas J, Clark J. Elastic properties of single elastic fibers. J Appl Physiol. 1962;17:547-551.
51. Brand P, Thompson D. Mechanical resistance. In Brand P, Hollister A, editors: Clinical Mechanics of the Hand, 2nd ed, St. Louis: Mosby, 1993.
52. Jacobs M. Splint classifications. In: Jacobs M, Austin N, editors. Splinting the Hand and Upper Extremity: Principles and Process. Philadelphia: Lippincott: Williams & Wilkins, 2003.
53. Schultz-Johnson K. Splinting: a problem solving approach. In: Stanley B, Tribuzi S, editors. Concepts in Hand Rehabilitation. Philadelphia: FA Davis, 1992.
54. Bonutti P, Windau J, Ables B, et al. Static progressive stretch to reestablish elbow range of motion. Clin Orthop Relat Res. 1994;303:128-134.
55. Schultz-Johnson K. Static progressive splinting. J Hand Ther. 2002;15:163-178.
56. Schultz-Johnson K. Splinting the wrist: mobilization and protection. J Hand Ther. 1996;9:165-175.
57. Flowers K, LaStayo P. Effect of total end-range time on improving passive range of motion. J Hand Ther. 1994;7:150-257.
58. Kannus P, Parkkari J, Jarvinen T, et al. Basic science and clinical studies coincide: active treatment approach is needed after a sports injury. Scand J Med Sci Sports. 2003;13:150-154.
59. Slutsky D. The Minicondylar Blade Plate, 1996.
60. Weinstock T. Management of fractures of the distal radius: therapist’s commentary. J Hand Ther. 1999;23:99-102.
61. Laseter G, Carter P. Management of distal radius fractures. J Hand Ther. 1996;9:114-128.
62. Wakefield A, McQueen M. The role of physiotherapy and clinical predictors of outcome after fracture of the distal radius. J Bone Joint Surg Br. 2000;82:972-976.
63. Steinberg B, Plancher K, Idler R. Percutaneous Kirschner wire fixation through the snuff box: an anatomic study. J Hand Surg [Am]. 1995;20:57-62.
64. Trumble T, Wagner W, Hanel D, et al. Intrafocal (Kapandji) pinning of distal radius fractures with and without external fixation. J Hand Surg [Am]. 1998;23:381-394.
65. Slutsky D: A comparison of external vs. internal fixation of distal radius fractures. Presented at the American Association for Hand Surgery Annual Meeting, Boca Raton, FL, January 10, 1997.
66. Dunning C, Lindsay C, Bicknell R, et al. Supplemental pinning improves the stability of external fixation in distal radius fractures during simulated finger and forearm motion. J Hand Surg [Am]. 1999;24:992-1000.
67. Juan J, Prat J, Vera P, et al. Biomechanical consequences of callus development in Hoffmann, Wagner, Orthofix and Ilizarov external fixators. J Biomech. 1992;25:995-1006.
68. Wolfe S, Austin G, Lorenze M, et al. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg [Am]. 1999;24:516-524.
69. Agee J. Distal radius fractures: multiplanar ligamentotaxis. Hand Clin. 1993;9:577-585.
70. Kleinman W, Graham T. The distal radioulnar joint capsule: clinical anatomy and role in posttraumatic limitation of forearm rotation. J Hand Surg [Am]. 1998;23:588-599.
71. Sun J, Chang C, Wu C, et al. Extra-articular deformity in distal radial fractures treated by external fixation. Can J Surg. 2001;44:289-294.
72. Krishnan J, Chipchase L, Slavotinek J. Intraarticular fractures of the distal radius treated with metaphyseal external fixation: early clinical results. J Hand Surg [Br]. 1998;23:396-399.
73. Perren S. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop Relat Res. 1979;138:175-196.
74. Perren S, Cordey J, Rahn B, et al. Early temporary porosis of bone induced by internal fixation implants: a reaction to necrosis, not to stress protection? Clin Orthop Rel Res. 1988;232:139-151.
75. Uhthoff H, Dubuc F. Bone structure changes in the dog under rigid internal fixation. Clin Orthop. 1971;81:165-170.
76. Gardner M, Helfet D, Lorich D. Has locked plating completely replaced conventional plating? Am J Orthop. 2004;33:439-446.
77. Disegi J, Wyss H. Implant materials for fracture fixation: a clinical perspective. Orthopedics. 1989;12:75-79.
78. Ring D, Jupiter J, Brennwald J, et al. Prospective multicenter trial of a plate for dorsal fixation of distal radius fractures. J Hand Surg [Am]. 1997;22:777-784.
79. Carter P, Frederick H, Laseter G. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg [Am]. 1998;23:300-307.
80. Chiang P, Roach S, Baratz M. Failure of a retinacular flap to prevent dorsal wrist pain after titanium Pi plate fixation of distal radius fractures. J Hand Surg [Am]. 2002;27:724-728.
81. Schnur D, Chang B. Extensor tendon rupture after internal fixation of a distal radius fracture using a dorsally placed AO/ASIF titanium pi plate. Arbeitsgemeinschaft fur Osteosynthesefragen/Association for the Study of Internal Fixation. Ann Plast Surg. 2000;44:564-566.
82. Smith D, Brou K, Henry M. Early active rehabilitation for operatively stabilized distal radius fractures. J Hand Ther. 2004;17:43-49.
83. Orbay J, Fernandez D. Volar fixation for dorsally displaced fractures of the distal radius: a preliminary report. J Hand Surg [Am]. 2002;27:205-215.
84. Slade J, Taksali S, Safanda J. Combined fractures of the scaphoid and distal radius: a revised treatment rationale using percutaneous and arthroscopic techniques. Hand Clin. 2005;21:427-441.
85. Tang J, Ryu J, Omokawa S, et al. Biomechanical evaluation of wrist motor tendons after fractures of the distal radius. J Hand Surg [Am]. 1999;24:121-132.
86. Bronstein A, Trumble T, Tencer A. The effects of distal radius fracture malalignment on forearm rotation: a cadaveric study. J Hand Surg [Am]. 1997;22:258-262.
87. Gliatis J, Plessas S, Davis T. Outcome of distal radius fractures in young adults. J Hand Surg [Br]. 2000;25:535-543.
88. Taleisnik J, Watson H. Midcarpal instability caused by malunited fractures of the distal radius. J Hand Surg [Am]. 1984;9:350-357.