Aerosolized antiinfective agents
After reading this chapter, the reader will be able to:
1. Define terms that pertain to aerosolized antiinfective agents
2. Discuss the indications for inhaled antiinfective agents
3. List all available inhaled antiinfective agents used in respiratory therapy
4. Differentiate between specific antiinfective agent formulations
5. Discuss the route of administration available for the various antiinfective agents
6. Describe the mode of action for the various antiinfective agents
7. Recognize side effects for the various antiinfective agents
8. Discuss the use of each antiinfective agent in the treatment of lung disease
Inherited disease of the exocrine glands, affecting the pancreas, respiratory system, and apocrine glands. Symptoms usually begin in infancy and are characterized by increased electrolytes in the sweat, chronic respiratory infection, pancreatic insufficiency, and reduced fertility (females) and sterility (males).
Interstitial plasma cell pneumonia caused by the organism Pneumocystis carinii (now known as Pneumocystis jiroveci). This pneumonia is common among patients with lowered immune system response.
Respiratory syncytial virus (RSV)
Virus that causes formation of syncytial masses in infected cell structures.
Stopping a virus from replicating.
Killing a virus.
Obligate intracellular parasite, containing either DNA or RNA, that reproduces by synthesis of subunits within the host cell and causes disease as a consequence of this replication.
Identification of aerosolized antiinfective agents
The antiinfective agents available for inhalation are listed in Table 13-1 with details of formulation, usual recommended dosage, and clinical use. Each of these agents is discussed in more detail.
TABLE 13-1
Currently Available Inhaled Antiinfective Agents with Formulations, Usual Recommended Dosage, and Clinical Use*
| DRUG | BRAND NAME | FORMULATION AND DOSAGE | CLINICAL USE |
| Pentamidine isethionate | NebuPent | 300 mg of powder in 6 mL of sterile water; 300 mg once every 4 wk | PCP prophylaxis |
| Ribavirin | Virazole | 6 mg of powder in 300 mL of sterile water (20-mg/mL solution); given 12-18 hr/day for 3-7 days by SPAG nebulizer | RSV |
| Tobramycin | TOBI | 300 mg/5 mL ampoule | Pseudomonas aeruginosa in CF |
| Adults and children ≥6 yr: 300 mg bid, 28 days on/28 days off drug | |||
| Aztreonam | Cayston | 75 mg/1 mL | Pseudomonas aeruginosa in CF |
| Adults and children ≥7 yr: 75 mg tid, 28 days on/28 days off drug | |||
| Zanamivir | Relenza | DPI: 5 mg/inhalation | Influenza |
| Adults and children ≥5 yr: 2 inhalations (one 5-mg blister per inhalation) bid <12 hr apart for 5 days |
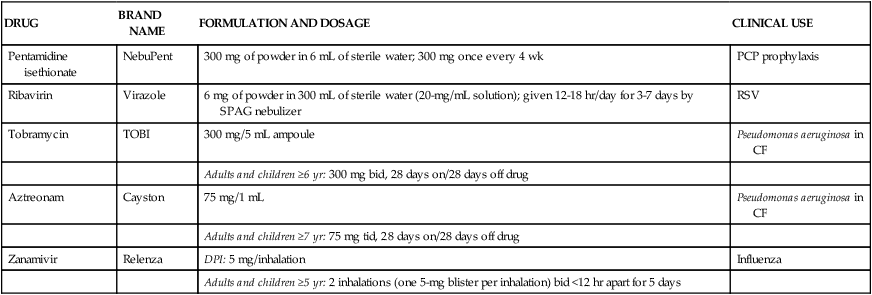
*Details on use and administration should be obtained from manufacturer’s drug insert material before use.
Aerosolized pentamidine (nebupent)
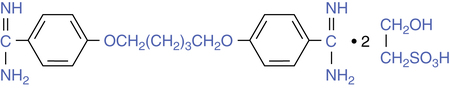
Pentamidine isethionate (NebuPent) is an antiprotozoal agent that is active against Pneumocystis carinii (now known as Pneumocystis jiroveci), the causative organism for PCP. Chemically, it is an aromatic diamidine; its structure is shown in Figure 13-1. Pentamidine can be given either parenterally or as an inhaled aerosol, but it is not absorbed with oral administration. When given parenterally, either intravenously or intramuscularly, the drug distributes quickly to the major organs (liver, kidneys, lung, and pancreas).
Introduction of aerosolized pentamidine
Both systemic administration and aerosol administration of pentamidine have been used for the treatment of Pneumocystis pneumonia (PCP), which occurs as a common opportunistic respiratory infection in patients with AIDS. In addition to the prophylactic use of aerosolized pentamidine, the aerosol form has been used for the treatment of acute episodes. The first report by Montgomery and associates1 was for therapy of acute episodes of PCP.
Rationale for aerosol administration
The rationale for aerosol administration of pentamidine to treat or prevent PCP is based on the same rationale for other inhaled aerosol drugs used to treat the pulmonary system: local targeted lung delivery, with fewer or less severe side effects compared with systemic administration. Aerosolized pentamidine produces significantly higher lung concentrations than intravenous administration.2 The San Francisco prophylaxis trial showed that 300 mg of aerosolized pentamidine every 4 weeks was effective in preventing PCP in patients with HIV infection.3 Subsequent clinical experience with aerosolized pentamidine did not show improved clinical efficacy compared with oral drugs such as trimethoprim-sulfamethoxazole (TMP-SMX; Septra and Bactrim), and toxic side effects still occurred.
Description of pneumocystis pneumonia
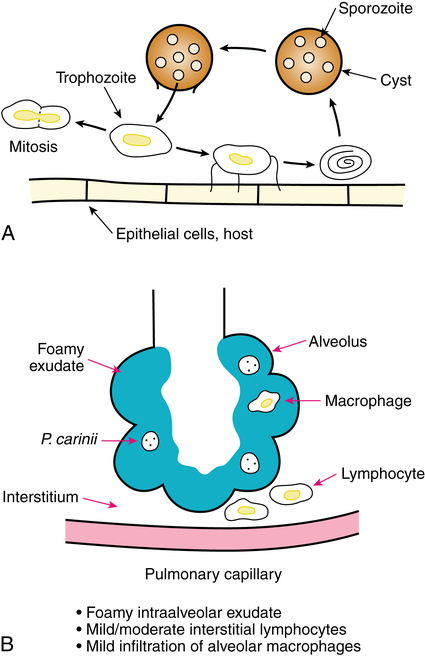
The organism P. carinii was first noted in the lungs of guinea pigs by Chagas in 1909 and Carini in 1910. It was named as a new organism by Delanöe and Delanöe in 1912 as Pneumocystis carinii, to describe the cystic form in the lungs and its earlier discoverer. Mammals are commonly infected with the organism at an early age, probably through an airborne vector. Disease occurs when there is suppression of the immune system. When not contained by a competent immune system, P. carinii causes PCP. Before the AIDS pandemic, PCP was reported in malnourished infants in the 1940s and 1950s and in the 1970s in premature infants able to survive.4 PCP produces a foamy intraalveolar exudate that contains cysts of P. carinii. The life cycle of P. carinii and the resulting pneumonia are illustrated in Figure 13-2. Both pentamidine and TMP-SMX are effective against PCP and are usually given parenterally to treat an acute episode.
Conflicting names for P. carinii may be found in scientific reading. Stringer and associates5 described the name change to Pneumocystis jiroveci in honor of Otto Jírovec, a Czech parasitologist. However, in a letter to the editor, Hughes6 pointed out that the name change is not valid or final because it has not been registered in the International Code of Botanical Nomenclature. Hughes6 also pointed out that the name change would cause confusion with discussion of P. carinii because many clinicians still use this terminology. In a letter, Gigliotti7 continued the stance taken by Hughes that no clear evidence exists that an official name change has occurred.
What is known is that P. carinii or P. jiroveci is a fungus. The acronym PCP for Pneumocystis pneumonia remains the same.5
Dosage and administration
Administration.
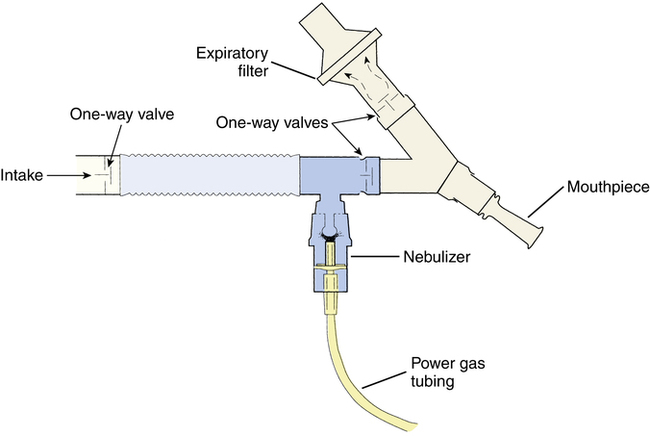
Approval of aerosolized pentamidine by the U.S. Food and Drug Administration (FDA) was for administration with the Respirgard II nebulizer (Vital Signs, Inc, Totowa, N.J.). This is a small volume nebulizer (SVN) system, powered by compressed gas, fitted with a series of one-way valves and an expiratory filter (Figure 13-3). This nebulizer system has been described by Montgomery and associates.2 The Respirgard II nebulizer should be powered with a flow rate of 5 to 7 L/min from a 50-psi source or, alternatively, by controlling the flow with a 22- to 25-psi pressure source connected to the small-bore tubing of the nebulizer. Pressures below 20 psi are insufficient to produce the desired particle size necessary for peripheral delivery of the drug. These requirements with Respirgard II are found in the manufacturer’s literature and discussed further by Corkery and colleagues.8 It may also be noted that Pentamidine may also be administered in a room or tent that acts as a “vacuum” to draw any particles that have escaped to a filter. Capturing of these particles results in less exposure to the respiratory therapist.
Nebulizer performance.
The general requirement for effective nebulization of pentamidine is a particle size or distribution of sizes with a mass median diameter (MMD) of 1 to 2 μm. This particle size is needed for the following two reasons:5
1. To achieve peripheral intraalveolar deposition targeted at the location of the microorganism
2. To reduce or prevent airway irritation seen with larger particle sizes, which deposit more in larger airways
Studies by Vinciguerra and Smaldone9 and by Smaldone and associates10 have examined nebulizer performance and compared treatment time and patient tolerance of aerosolized pentamidine with Respirgard II versus other nebulizers. Treatment times and efficiency in drug availability were greater with the AeroTech II (CIS-US, Bedford, Mass.) than the approved Respirgard II.
Mode of action
The exact mode of action of pentamidine is unknown. The toxic effect of the drug on P. carinii may be due to multiple actions. Pentamidine blocks RNA and DNA synthesis, inhibits oxidative phosphorylation, and interferes with folate transformation.4,11,12 Resistance to pentamidine by P. carinii has not been shown, and this may be due to the multiple effects of the drug on the metabolism of the organism.11
When given by inhaled aerosol, pentamidine reaches significantly higher concentrations in the lung than when given intravenously.2 The inhaled drug first binds to lung tissue. Although plasma levels are much less than with parenteral administration, the drug is slowly absorbed into the circulation and distributed to body tissues, as with parenteral administration. As a result, prolonged aerosol administration can result in systemic accumulation. Approximately 75% of the drug is excreted in urine and 25% in feces over the months after administration.
Side effects
Side effects with parenteral pentamidine.
Side effects with parenteral administration of pentamidine have been summarized in several reviews, with numerous references.8,11 Parenteral use of pentamidine has resulted in the following:
• Pain, swelling, and abscess formation at the site of injection with intramuscular administration
• Thrombophlebitis and urticarial eruptions with intravenous administration
• Hypoglycemia (up to 62% of patients), with a cumulative cytotoxic effect on pancreatic beta cells
Side effects with aerosol administration.
• Cough and bronchial irritation in 36% of patients in one study3
• Bad taste (bitter or burning) of the aerosol impacting in the oropharynx
In addition, the following systemic reactions have occurred with aerosolized pentamidine:
Preventing airway effects.
Use of a β-adrenergic bronchodilator before inhaling aerosolized pentamidine can reduce or prevent local airway reaction, including reduction of coughing or wheezing. Ipratropium has also been shown by Quieffin and colleagues16 to prevent bronchoconstriction. The airway reaction may be caused by the sulfite moiety in isethionate (see Figure 13-1), which is known to cause airway irritation, or by the drug itself.17,18 This effect can be reduced by use of a nebulizing system producing very small particle sizes, which lessen airway deposition and increase alveolar targeting.8
Environmental contamination by nebulized pentamidine
The following concerns exist regarding environmental contamination from nebulized pentamidine:
• Exposure to the drug itself from the exhaust aerosol
• Risk of infection with tuberculosis (TB), a disease associated with AIDS, from patients being treated with aerosolized pentamidine
Pentamidine is not known to be teratogenic, based on its use in pregnant women with African sleeping sickness (trypanosomiasis), although detailed clinical data were not kept. The drug is not mutagenic, and its carcinogenic potential is considered minimal.11 Studies have shown that low levels of pentamidine can be detected in health care workers exposed to the drug during treatments.19,20 The investigators concluded that exposure probably occurred during treatment interruptions, usually caused by coughing episodes. Health care workers have also complained of conjunctivitis and bronchospasm when aerosolizing the drug.11 On the basis of these reports and the long tissue half-life of pentamidine, contact with the drug should be kept to a minimum or prevented if possible.
Environmental precautions.
The following precautionary measures are suggested when administering the drug to reduce the risk of drug exposure and TB infection with aerosolized pentamidine:21–24
• Use a nebulizer system with one-way valves and expiratory filter.
• Stop nebulization if the patient takes the mouthpiece out of the mouth (a thumb control on the power gas tubing gives more control).
• Use nebulizers producing an MMD of 1 to 2 μm, to increase alveolar targeting and lessen large airway deposition and cough production.
• Always use a suitable expiratory filter and one-way valves with the nebulizer. Instruct patients to turn off the nebulizer when talking or when taking it out of the mouth.
• Screen patients for cough history and pretreat with a β agonist, with sufficient lead time for effect in reducing the bronchial reactivity.
• Administer aerosol in a negative-pressure room, with six air changes per hour, or consider using an isolation booth/hood assembly with an exhaust fan and air directed through a high-efficiency filter.
• Use barrier protection (gloves, mask, and eyewear) for health care workers.
• Screen patients with HIV infection for TB, and treat where evidence of infection exists.
• Do not allow treatment patients to mix with others until coughing subsides.
• Health care workers should periodically screen themselves for TB.
• Pregnant women and nursing mothers should avoid exposure to the drug, and all practitioners should limit exposure to the extent possible.
Although measures exist to radically limit environmental contamination with aerosolized pentamidine, many of these are expensive, such as negative-pressure rooms and improved ventilation exchange in older buildings. Other measures are difficult, such as the wearing of effective high-efficiency masks in a busy clinical setting for a prolonged period. The use of room disinfection with ultraviolet light has been reviewed25 but is debated.22
Aerosol therapy for prophylaxis of pneumocystis pneumonia: clinical application
Comparisons of the efficacy of aerosolized pentamidine with oral TMP-SMX, together with reports of serious adverse effects with aerosolized pentamidine, lead to a reevaluation of aerosol therapy with pentamidine for prophylaxis of PCP. General recommendations for prophylaxis of PCP have been published by the U.S. Centers for Disease Control and Prevention (CDC) in MMWR Recommendations and Reports for HIV-positive children26 and for adults.27 In the 2009 CDC recommendations, oral TMP-SMX was preferred for prophylaxis of PCP as long as adverse side effects from TMP-SMX were absent or acceptable.27 Aerosolized pentamidine is recommended as an alternative therapy for prophylaxis of PCP if TMP-SMX cannot be tolerated.
Ribavirin (virazole)
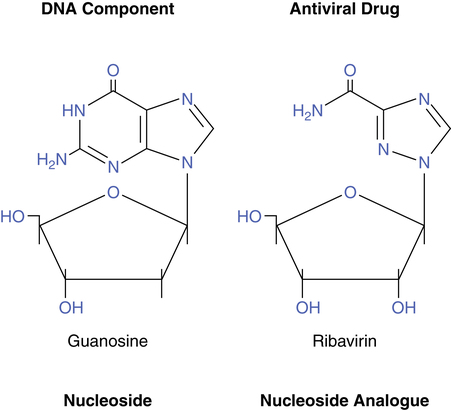
Ribavirin (Virazole) is classified as an antiviral drug; it is active against RSV, influenza viruses, and herpes simplex virus. Chemically, it is a nucleoside analogue and resembles guanosine and inosine.28 Ribavirin is virostatic, not virucidal, and inhibits DNA and RNA (retrovirus) viruses.
Ribavirin has been used throughout the world for various viral infections, including RSV and influenza types A and B. Clinical trials of aerosolized ribavirin for severe RSV infection were conducted by Hall and associates29,30 and showed significant improvement with ribavirin treatment compared with placebo; however, others have noted its ineffectiveness.
Clinical use
Infection with RSV in children results in either bronchiolitis or pneumonia. Guidelines concerning the use of ribavirin were published by the Committee on Infectious Diseases of the American Academy of Pediatrics in 2009. Generally, the drug is not recommended for routine RSV infection, but it may be considered for life-threatening infections.31 The Agency for Healthcare Research and Quality (AHRQ) has designated ribavirin as “possibly ineffective.”32
Ribavirin treatment by aerosol is expensive and risks environmental exposure to the drug by personnel. Studies have given conflicting results on whether the use of ribavirin significantly reduces outcomes such as ventilator days, oxygen needs, intensive care unit days, hospital days, or mortality.33,34
Nature of viral infection
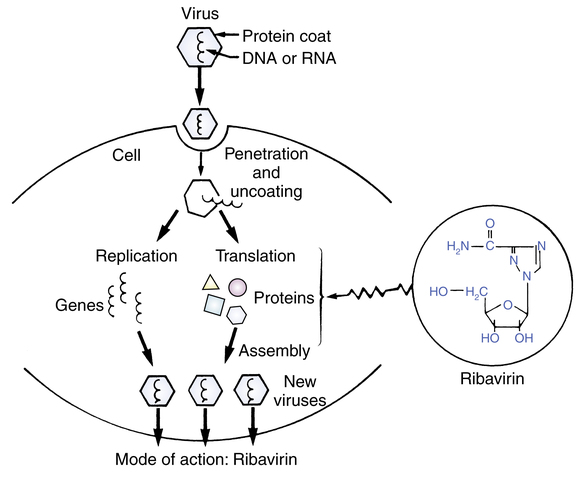
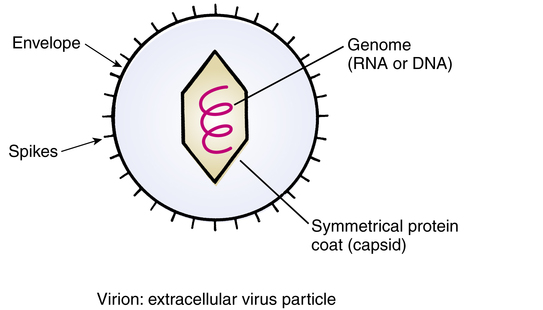
Figure 13-4 illustrates the simple structure of a virus. These are primitive members of the animal kingdom, submicronic in size, that consist of a strand of DNA or RNA that is surrounded by a protein coat. A virus may or may not be surrounded by an envelope, whose glycoprotein spikes are partially obtained from the host cell.
The concept and sequence of a viral infection are shown in Figure 13-5. A virus enters the body through various routes (oral, inhaled, mucous membranes) and invades a host cell. This is a multistep process consisting of phases in which the virus adsorbs to the cell; penetrates the cell; uncoats itself; goes through a process of recoding cell DNA (transcription, translation, synthesis); assembles itself; and sheds from the cell. The host cell usually dies in the process. Clinically, signs of a viral infection do not occur until after the initial latent period, when the virus leaves the cell (see Figure 13-5). At this point, infection is well established. The diagnosis of viral illness is usually based on clinical signs, including the symptoms, age of the patient, and time of year. Definitive diagnosis requires isolating the virus or showing an antibody titer increase. Diseases produced by viruses include chickenpox, smallpox, fever blisters (herpes simplex virus), genital herpes, poliomyelitis, the common cold, AIDS, influenza, mumps, and measles.
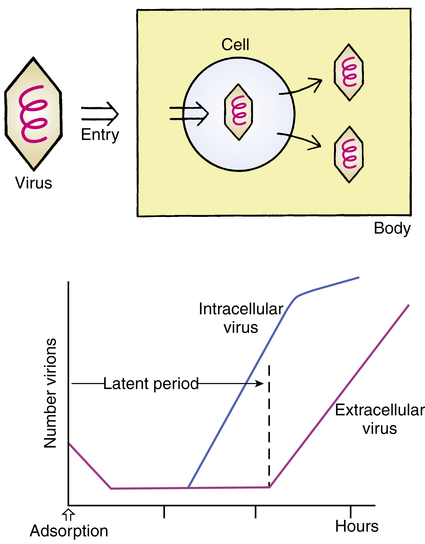
1. Attacking the intracellular virus may harm the host cell.
2. Viral replication is maximal before the appearance of symptoms.
3. Viruses have the property of antigenic mutability; that is, they change their appearance to the immune system.
Dosage and administration
Administration.
Clinical trials of ribavirin aerosol were carried out with a large volume nebulizing system, the SPAG. The drug was approved for general use with this aerosol generator. A diagram of the SPAG unit is shown in Figure 13-6. It is a large volume, pneumatically powered nebulizer operating on a jet shearing principle, with baffling of aerosol particles and a drying chamber to reduce particle size further to a level of approximately 1.3 μm, MMD. Solutions in the SPAG reservoir should be replaced after 24 hours. Residual solution in the reservoir should be discarded before adding newly reconstituted solution. The drug solution should always be visually inspected for particulate matter or discoloration before use.
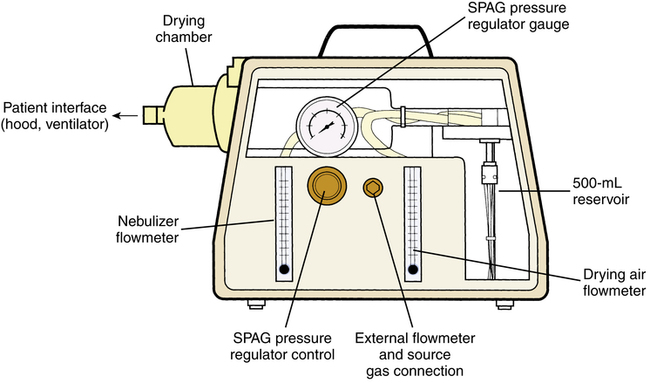
The nebulizer is connected to a hood as the patient interface. The manufacturer specifically warns against administration of the drug to infants requiring mechanical ventilation because of the risk of drug precipitation occluding expiratory valves and sensors or the endotracheal tube. The sickest infants with RSV are likely to need ventilatory support, however, and there are reports of drug use with mechanical ventilation. Demers and colleagues35 provided detailed information concerning precautions with ventilator use during administration of the drug. A clinical study of aerosol administration with mechanical ventilation of infants with severe RSV infection was reported by Smith and associates33; these authors showed that treatment reduced duration of ventilation, oxygen support, and hospital stay. Although labor-intensive, mechanical ventilatory administration of ribavirin actually simplifies environmental control.
Mode of action
The mechanism of action by which ribavirin exerts its virostatic effect is not completely understood. Viral inhibition is probably based on its structural resemblance to the nucleosides used to construct the DNA chain.28 Figure 13-7 shows the structures of the natural nucleoside guanosine with ribavirin, which is a synthetic nucleoside analogue. During the formation and assembly of new viral protein within the cell, ribavirin is most likely taken up instead of the natural nucleoside to form the DNA chain; this prevents construction of viable viral particles and subsequent shedding of virus into the bloodstream. Figure 13-8 is a conceptual illustration of the process. Ribavirin does not prevent the attachment or the penetration of RSV into the cell and does not induce interferon.28
When given by inhaled aerosol, ribavirin levels are much greater in respiratory secretions than in the bloodstream. Waskin11 provided a referenced summary of ribavirin kinetics. With 8 to 20 hours of aerosol treatment, peak plasma levels are 1 to 3 μg/mL, and respiratory secretion levels are greater than 1000 μg/mL. The minimal inhibitory concentration (MIC) for RSV is 4 to 16 μg/mL. The half-life of ribavirin is about 9 hours in plasma and about 1 to 2 hours in respiratory secretions, which is the rationale for almost continuous administration by aerosol.
Side effects
Side effects seen with aerosolized ribavirin are listed in the product literature and have been reviewed by Waskin.11 The following list summarizes adverse effects reported, including those seen with adults receiving the drug:
• Pulmonary: Deterioration of pulmonary function and worsening of asthma or chronic obstructive pulmonary disease (COPD) occur; pneumothorax, apnea, and bacterial pneumonia have been described.
• Cardiovascular: Cardiovascular instability, including hypotension, cardiac arrest, and digitalis toxicity, has been noted.
• Hematologic: Effects on blood cells have been reported with oral or parenteral administration but not with aerosol use. Reticulocytosis (excess of young erythrocytes in the circulation) has been reported with aerosol use.
• Dermatologic/topical: Rash, eyelid erythema, and conjunctivitis have been noted.
• Equipment-related: Equipment-related adverse effects with ribavirin treatment include occlusion and impairment of expiratory valves and sensors with ventilator use and endotracheal tube blockage from drug precipitate.
Environmental contamination with aerosolized ribavirin
There is concern among health care workers over exposure to ribavirin. The drug has potential for mutagenic and carcinogenic effects based on in vitro and animal studies.11 The effect on fertility is uncertain, but the drug has caused testicular lesions in rats. The effect on pregnancy is of particular concern because the drug is teratogenic or embryocidal in animal species. Acute effects from aerosolized ribavirin reported by health care workers have included precipitation on contact lens and conjunctivitis, headache (51%), rhinitis, nausea, rash, dizziness, pharyngitis, and lacrimation (10% to 20%). Several cases of bronchospasm or chest pain have been reported by individuals with reactive airways disease. The symptoms noted have resolved within hours after discontinuing exposure to the drug.36
Minimal levels of ribavirin exposure are difficult to specify because of the lack of dose-response data for humans.37 Corkery and others38 stated that the California Department of Health Services recommended an acceptable occupational airborne concentration for 8 hours of limited exposure to be 1/1000 of the lowest no-observed-effect level, which would be 2.5 μg/m3.
Although there are no reports to date of serious effects from drug exposure by aerosol, precautions to limit or avoid exposure to the drug are well indicated, as advocated by Kacmarek.39 Pregnant females, or those wishing to become pregnant should avoid exposure to the drug if possible. In addition, environmental containment is superior to personnel barrier protection alone. Standard surgical masks do not prevent inhalation of 1- to 2-μm particles. Dermal absorption of ribavirin seems to be negligible.40 It may be helpful to use a containment system when the drug is aerosolized to an oxygen hood; several systems have been proposed in the literature.41–43 All have common features of enclosure around the hood, with vacuum extraction and filtering of gas from the enclosure. Details needed for use can be found in the references given. It is recommended that the drug be administered in well-ventilated areas—that is, six or more air changes per hour.
Palivizumab (synagis)
Palivizumab (Synagis) represents the new drug class of therapeutic monoclonal antibodies.44 The drug was approved for the prevention and treatment of RSV in premature infants and infants with bronchopulmonary dysplasia (BPD).
Clinical use
Palivizumab is indicated for the prevention of serious lower respiratory tract disease caused by RSV in children and infants at high risk. Safety and efficacy were established for infants with BPD, infants born prematurely (less than 35 weeks), and children with congenital heart disease.45
Adverse reactions
The most serious adverse reaction is anaphylaxis; however, this occurs in less than 1 per 100,000 cases. Other reactions that occurred in treatment and placebo groups included fever, upper respiratory infection, otitis media, rhinitis, rash, pain, hernia, and coughing and wheezing.36
Clinical efficacy
In a large multicenter trial of infants at high risk of RSV infection, palivizumab given intravenously at 15 mg/kg reduced the rate of hospitalization resulting from RSV infection to 4.8% compared with 10.6% in placebo recipients.46 Adverse events were similar in placebo and treatment groups.
Feltes and colleagues47 found that palivizumab is safe and effective for RSV-positive children with congenital heart disease. In this study, 53% of the children had reduced hospital stays, and 73% had fewer days of supplemental oxygen use.
Aerosolized tobramycin (TOBI)
Clinical use
Baran and colleagues48 administered 40 mg of gentamicin by aerosol to eight children with CF and found high levels of drug (more than 20 μg/mL) in the bronchial secretions of seven of the children. Blood levels with the inhaled drug were low, supporting the case for minimal systemic toxicity by aerosol. Similar results with nebulized tobramycin (300 mg) were reported by Le Conte and associates.49 By contrast, intramuscular injection of 1.5 mg/kg gave low levels of less than 2 μg/mL in bronchial secretions and, in some cases, undetectable levels.48
Aerosol administration is attractive because of reduced cost potential and ease of use at home compared with intravenous therapy. Furthermore, fluoroquinolones such as ciprofloxacin and norfloxacin, which are active when taken orally, are not as suitable for prolonged maintenance or preventive therapy as the agents given by inhalation because of the risk of drug-resistant strains of bacteria.50 A report of the clinical trial establishing the safety and efficacy of inhaled tobramycin (TOBI) in managing P. aeruginosa in patients with CF was published by Ramsey and colleagues.51 TOBI is used to manage chronic infection with P. aeruginosa in CF, as follows:
• Treat or prevent early colonization with P. aeruginosa
• Maintain present lung function or reduce the rate of deterioration
Efficacy with Burkholderia cepacia has not been shown using the inhaled route of administration.
Side effects
Side effects for parenteral and inhaled administration of tobramycin are listed in Box 13-1. Adverse effects with nebulized delivery are based on the clinical trial of Ramsey and colleagues51 and on 2 years of experience after the approval of inhaled tobramycin.
Side effects with parenteral administration.
Neuromuscular blockade.
Neuromuscular blockade is another side effect resulting from the potential curarelike effect of aminoglycosides on the neuromuscular junction. Neuromuscular blockade can aggravate muscle weakness, can cause further worsening of neuromuscular disorders, or prolong and intensify neuromuscular blockade by curarelike paralyzing agents (see Chapter 18). Hypomagnesemia can occur in patients who have a poor diet or whose diet is restricted.
Side effects with nebulized tobramycin.
The only adverse effects reported after the 6-month clinical trial of Ramsey and colleagues51 were tinnitus and voice alteration. There was no hearing loss associated with nebulized use of tobramycin or changes in serum creatinine indicative of renal toxicity. There was a modest decrease in susceptibility of P. aeruginosa to tobramycin in the treatment group but not the placebo group in the study by Ramsey and colleagues.51 However, this was not associated with a lack of clinical response to inhaled therapy with tobramycin. Use of an alternating schedule of administration may reduce the risk of drug resistance. Ramsey and associates51,52 noted that their rationale for intermittent administration of tobramycin was the observation that “drug holidays” allow susceptible pathogens to repopulate the airway in patients with CF. Because tobramycin is delivered by inhalation, the airway concentration can be 100 times as high as systemic levels. Thresholds of pathogen susceptibility with parenteral administration do not apply well to direct inhalation doses.
Precautions in use of nebulized tobramycin
• Inhaled tobramycin should be administered with caution to patients with preexisting renal, auditory, vestibular, or neuromuscular dysfunction.
• Admixture incompatibility exists between β-lactam antibiotics (penicillins and cephalosporins) and aminoglycosides when mixed directly together; tobramycin solution should not be mixed with antibiotics in this group, and mixing with other drugs generally is discouraged.
• Factors that could increase the risk of hearing damage with prolonged tobramycin use are renal impairment; concomitant dosage of parenteral aminoglycosides; dehydration; and concomitant use of ethacrynic acid, furosemide, or other ototoxic drugs.
• Nebulization of antibiotics during hospitalization should be performed under conditions of containment, as previously described for pentamidine and ribavirin, to prevent environmental saturation and development of resistant organisms in the hospital.
• Aminoglycosides can cause fetal harm if administered to pregnant women; exposure to ambient aerosol drug should be avoided by women who are pregnant or trying to become pregnant.
• Local airway irritation resulting in cough and bronchospasm with decreased ventilatory flow rates is a possibility with inhaled antibiotics and seems to be related to the osmolality of the solution.53–56 Peak flow rates and chest auscultation should be used before and after treatments to evaluate airway changes. Pretreatment with a β agonist may be needed.
• Allergies in the patient, staff, or family should be considered, if exposure to the aerosolized drug is not controlled. The use of a nebulizing system with scavenging filter, one-way valves, and thumb control could reduce ambient contamination with the drug, as previously described.
Clinical efficacy
Clinical efficacy of inhaled tobramycin by nebulization was shown in the randomized controlled study by Ramsey and colleagues.51 In that study, which compared inhaled tobramycin with placebo in 521 patients with CF, 6 months of alternating inhaled tobramycin together with standard therapy for CF resulted in the following:
• Improved pulmonary function (Figure 13-9)
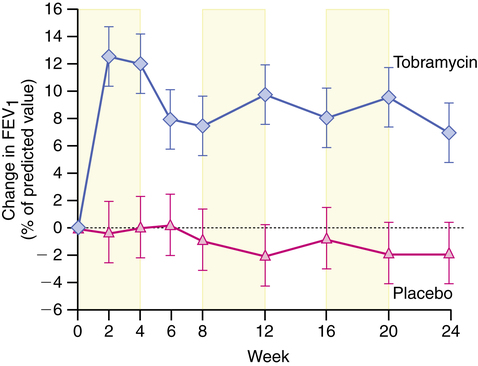
• Decreased density of P. aeruginosa in expectorated sputum
• Reduced need for intravenous antipseudomonal antibiotics and hospitalizations
Other studies, such as that by Gibson and colleagues,57 have produced similar results indicating that inhaled tobramycin is safe and effective in treating P. aeruginosa in patients with CF.
Because inhaled tobramycin is effective in patients with CF, is it effective in other patients with P. aeruginosa infection? LoBue58 reported that studies to date have been small or that the drug has not been used long-term. Inhaled tobramycin cannot be recommended for treatment other than for CF patients with P. aeruginosa infection.
Aerosolized aztreonam (cayston)
General considerations in aerosolizing antibiotics
• Antibiotic solutions such as gentamicin are more viscous than bronchodilator solutions, and this may affect nebulizer performance. Compressors must be suitably powerful; high-flow compressors are suggested. Flow rates of 10 to 12 L/min have also been suggested by Newman and associates59,60 for suitably small particle sizes with antibiotic solutions.
• Environmental contamination in health care agencies and practitioner exposure to aerosolized drug can be reduced by using expiratory filters with one-way valves and a thumb control, as with aerosolized pentamidine.
• Hata and Fick61 noted physical incompatibility between some antibiotics. Aminoglycosides such as gentamicin are chemically inactivated by carbenicillin and piperacillin when mixed together. These drugs should be given in separate nebulizer treatments, which has the disadvantage of requiring twice the patient treatment time. Any antibiotic combination and other drug combinations should at least be inspected for visible changes such as discoloration or precipitation and should not be used if such changes are observed. Ideally, drug mixtures for nebulization should be tested for chemical compatibility in addition to a visual inspection.
Inhaled zanamivir (relenza)
Clinical use
Zanamivir (Relenza) is an antiviral agent approved for use in the treatment of uncomplicated influenza illness in adults and children older than 5 years of age during the early onset (within the first 2 days) of infection. The agent has an off-label use for treatment and prophylaxis of H1N1 influenza A (“swine flu”). An oral antiinfluenza agent, oseltamivir phosphate (Tamiflu) is also available as 30-mg, 45-mg, and 75-mg capsules and oral liquid, 12 mg/mL. It is indicated in the treatment and prophylaxis of influenza in patients 1 year of age and older. Tamiflu has an off-label use in H1N1 influenza A. In addition, two older drugs, amantadine and rimantadine, have been used for prophylaxis and treatment of acute symptoms of influenza. Both of these agents are resistant to H1N1 influenza A. Table 13-2 summarizes information about these four agents, only one of which (zanamivir) is available by inhalation. Prophylactic vaccination against influenza, especially in high-risk patients with cardiovascular or respiratory disease, remains the unqualified recommendation, despite the availability of drugs to treat acute infection.
TABLE 13-2
Antiviral Agents Used to Treat or Prevent Influenza
| DRUG | BRAND NAME | FDA APPROVAL | ACTIVITY | CLINICAL USE | ROUTE OF ADMINISTRATION | ADULT DOSAGE |
| Amantadine | Symmetrel | 1966 | Influenza A | Prophylaxis, acute treatment | Oral: tablet, syrup | 200 mg/day |
| Rimantadine | Flumadine | 1993 | Influenza A | Prophylaxis, acute treatment | Oral: tablet, syrup | 100 mg bid |
| Oseltamivir | Tamiflu | 1999 | Influenza A and B; H1N1 (“swine flu”) | Prophylaxis, acute treatment | Oral: capsule, liquid suspension | 12 mg,* 75 mg bid, for 5 days |
| Zanamivir | Relenza | 1999 | Influenza A and B; H1N1 (“swine flu”) | Prophylaxis, acute treatment | DPI: Diskhaler | 10 mg (2 inhalations) bid, for 5 days |
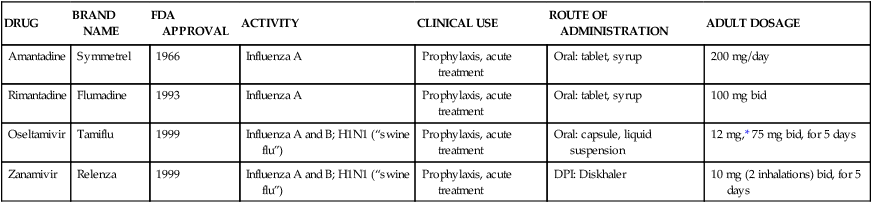
DPI, Dry powder inhaler; FDA, U.S. Food and Drug Administration.
*Depends on weight of child; see manufacturer’s dosing schedule.
Mode of action
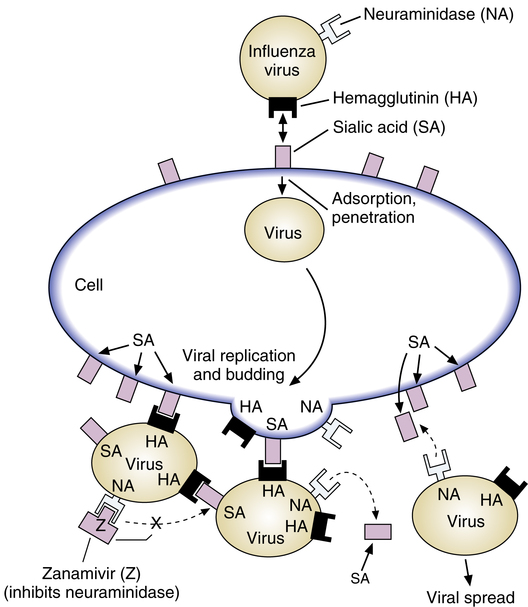
The general mechanism of viral infection was described previously with ribavirin (see discussion of the nature of viral infection). Zanamivir represents a new class of antiviral agents, termed neuraminidase inhibitors, which act by binding to the viral enzyme neuraminidase and blocking the action of the enzyme. The influenza virus has an envelope and a protein coat surrounding the viral RNA and targets the respiratory tract. Briefly, as illustrated in Figure 13-10, the virus envelope for both influenza A and influenza B has two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA).
HA binds to a sugary molecule, sialic acid (SA), on the surface of a cell to be infected. This binding leads to fusion of virus and cell membranes and allows adsorption and penetration of the virus into the cell. However, when the newly minted viral particles bud from the cell and are ready to be released, the viral envelope acquires sialic acid from the cell, along with its own HA and NA receptors. Without NA, the viral HA would combine with the sialic acid again, “sticking” the viral particles to each other and to the cell surface, preventing further infection. NA cleaves part of the sialic acid to prevent HA and SA combination and prevents viral aggregation (clumping). NA is essential for virus release from infected cells, prevents virus aggregation, and may decrease virus inactivation by respiratory mucus. Zanamivir is able to bind to NA and block the enzyme action. By inhibiting NA, zanamivir inhibits viral particle separation and cellular release needed for systemic infection to proceed.62
Adverse effects
Bronchospasm and deterioration of lung function.
Patients with underlying respiratory disease such as asthma or COPD may experience bronchospasm after inhaling zanamivir. Respiratory difficulty and wheezing have been reported in a patient with COPD63 and an asthma patient inhaling zanamivir.64 Apparently neither of these patients had influenza at the time of treatment. A clinical trial of zanamivir by Cass and colleagues65 in 11 patients with mild to moderate asthma with no influenza showed no symptoms of bronchospasm or airway responsiveness. However, such data do not establish the safety of zanamivir in asthmatics with influenza infection, which can cause mucosal damage and airway reactivity from the viral inflammation.68 In ongoing treatment studies of patients with COPD or asthma who had influenza-like illness, more patients receiving zanamivir compared with patients receiving placebo had a greater than 20% decline in FEV1 or peak expiratory flow rate.62 Zanamivir should be discontinued if bronchospasm or a decline in lung function occurs in any patient, and the managing physician should be consulted. The manufacturer recommends that any patient with underlying airway disease not take zanamivir.36
Undertreatment of bacterial infection.
Bacterial respiratory infections can manifest with influenza-like symptoms, and viral respiratory infections can progress to serious bacterial secondary infections.67 Treatment with an antiviral agent such as zanamivir is ineffective against bacterial infection and could possibly allow progression of the infection to serious illness such as pneumonia. Two deaths from bacterial infection in subjects taking zanamivir have been reported,68 although the reasons have not been determined.69 In a patient with COPD exacerbation who is treated inappropriately, risk of serious complications and the need for hospitalization can result.67
Clinical efficacy and safety
Clinical efficacy of zanamivir has been established in trials showing that inhaled zanamivir can shorten significantly the duration of influenza symptoms.66,69,70 With uncomplicated influenza-like illness, treatment with 10 mg of zanamivir twice daily resulted in approximately 1 day of shortening of the median time to improvement in symptoms compared with placebo.66 The time to improvement in major symptoms was defined as no fever and no or mild headache, myalgia, cough, and sore throat. Among patients who were febrile and began treatment 30 hours or less after onset of symptoms, treatment with zanamivir resulted in a shortening of 3 days in the median time to alleviation of symptoms.66 There are no data on efficacy when zanamivir is started after more than 2 days of symptoms of influenza.
Clinical trials of zanamivir were performed mainly with previously healthy subjects.66,69,70 The manufacturer’s literature states that safety and efficacy of zanamivir for treating influenza have not been shown in patients with COPD. Zanamivir may carry risk for patients with COPD or asthma, as indicated in the discussion of side effects. Revised labeling for zanamivir adds a warning that zanamivir is not generally recommended for patients with underlying airways disease because of the risk of serious adverse effects.66
Zanamivir is not approved for prophylaxis to prevent influenza, and it does not reduce the risk of transmission of the virus to others. However, some data suggest a prophylactic benefit with zanamivir in influenza A and influenza B in university and nursing home communities.71 Results of a controlled study of inhaled zanamivir for treatment and prevention of influenza in families in which one member developed influenza-like illness showed that zanamivir did reduce the rate of developing influenza in other family members. The proportion of families in which an initially healthy member developed influenza was 4% with zanamivir compared with 19% with placebo.72 In the trial, treatment of the index cases with zanamivir in families reduced the median duration of symptoms from 7.5 to 5.0 days, a significant reduction. Oseltamivir (Tamiflu) was approved for prevention of influenza A and influenza B in children 1 year of age or older who are in close contact with influenza. Adverse reactions were similar in both groups.36
The safety and efficacy of zanamivir have been tested in children. In a study by Hedrick and others,73 zanamivir was tested on children 5 to 12 years old. In the study of 471 children, 224 were given zanamivir, and the remaining children, the control group, were given placebo. The children taking zanamivir had reduced influenza symptoms 1.25 days before children in the placebo group. The zanamivir group returned to normal activities in less time and took fewer relief medications than placebo.
A final issue with the use of zanamivir or other antiinfluenza agents as acute treatment is the lack of a clinically easy and inexpensive diagnostic tool to confirm the presence of influenza infection. Zanamivir is not beneficial in patients with infections other than influenza. In the clinical trial by Hayden and colleagues,66 262 of 417 patients (63%) with influenza-like illness had confirmed influenza virus infection. As a result, symptoms alone can result in inappropriate use of antiinfluenza drugs, with attendant risks as outlined in the discussion of adverse effects. Inappropriate use contributes to increased cost.