Chapter 146 Adverse Reactions to Drugs
Epidemiology
The incidence of adverse drug reactions in the general as well as pediatric populations remains unknown, although data from hospitalized patients show it to be 6.7%, with a 0.32% incidence of fatal adverse drug reactions. Databases such as the FDA MedWatch program (www.fda.gov/medwatch/index.html) likely suffer from underreporting. Cutaneous reactions are the most common form of adverse drug reactions, with ampicillin, amoxicillin, penicillin, and trimethoprim-sulfamethoxazole being the most commonly implicated drugs. Although the majority of adverse drug reactions do not appear to be allergic in nature, 6-10% can be attributed to an allergic or immunologic mechanism. Importantly, given the high probability of recurrence of allergic reactions, these reactions should be preventable, and information technology–based interventions may be especially useful to reduce risk of reexposure.
Pathogenesis and Clinical Manifestations
Drug Metabolism and Adverse Reactions
Incomplete or delayed metabolism of some drugs can give rise to toxic metabolites. Hydroxylamine, a reactive metabolite produced by cytochrome P450 oxidative metabolism, may mediate adverse reactions to sulfonamides. Patients who are slow acetylators appear to be at increased risk (Chapter 56). In addition, cutaneous reactions in patients with AIDS treated with trimethoprim-sulfamethoxazole, rifampin, or other drugs may be due to glutathione deficiency resulting in toxic metabolites. Serum sickness–like reactions in which immune complexes have not been documented, which occur most commonly with cefaclor, may result from an inherited propensity for hepatic biotransformation of drugs into toxic or immunogenic metabolites.
Risk Factors for Hypersensitivity Reactions
Risk factors for adverse drug reactions include prior exposure, previous reactions, age (20-49 yr), route of administration (parenteral or topical), dose (high), and dosing schedule (intermittent) as well as genetic predisposition (slow acetylators). Atopy does not appear to predispose patients to allergic reactions to low molecular weight compounds, but atopic patients in whom an allergic reaction develops have a significantly increased risk of serious reaction. Atopic patients also appear to be at greater risk for pseudoallergic reactions induced by radiocontrast media. Pharmacogenomics has an important role in identifying individuals at risk for certain drug reactions (Chapter 56).
Treatment
β-Lactam Hypersensitivity
Penicillin is a frequent cause of anaphylaxis and is responsible for the majority of all drug-mediated anaphylactic deaths in the United States. Although IgE-mediated reactions may occur after administration of penicillin by any route, parenteral administration is more likely to cause anaphylaxis. If a patient requires penicillin and has a previous history suggestive of penicillin allergy, it is necessary perform skin tests on the patient for the presence of penicillin-specific IgE with both the major and minor determinants of penicillin. Skin tests for both major and minor determinants of penicillin are necessary because about 20% of patients with documented anaphylaxis do not demonstrate skin reactivity to the major determinant. Unfortunately, as mentioned earlier, the major determinant testing reagent, PrePen, was withdrawn from the market in the USA in 2004 owing to problems with manufacturing. The manufacturer, AllerQuest, LLC (West Hartford, CT), received approval from the FDA in January 2008 to manufacture Pre-Pen (www.allerquest.com/availability.html). The minor determinant mixture is currently not licensed and is synthesized as a nonstandardized testing reagent at select academic centers. Although penicillin G is often used as a substitute for the minor determinant mixture, there is a small but significant risk of false-negative skin test results with this approach. Thus, patients should be referred to an allergist capable of performing appropriate testing. If the skin test response is positive to either major or minor determinants of penicillin, the patient should receive an alternative non–cross reacting antibiotic. If administration of penicillin is deemed necessary, desensitization can be performed by an allergist in an appropriate medical setting. Skin testing for penicillin-specific IgE is not predictive for delayed-onset cutaneous, bullous, or immune complex reactions. In addition, penicillin skin testing does not appear to resensitize the patient.
Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
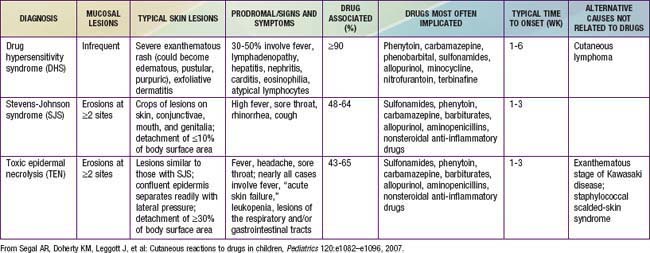
Blistering mucocutaneous disorders induced by drugs encompass a spectrum of reactions, including SJS and TEN (Chapter 646). Epidermal detachment of less than 10% is suggestive of SJS, 30% detachment suggests TEN, and 10-30% detachment suggests overlap of the two syndromes. The features of SJS include confluent purpuric macules on face and trunk and severe, explosive mucosal erosions, usually at more than one mucosal surface, accompanied by fever and constitutional symptoms. Ocular involvement may be particularly severe, and the liver, kidneys, and lungs may also be involved. TEN, which appears to be related to keratinocyte apoptosis, manifests as widespread areas of confluent erythema followed by epidermal necrosis and detachment with severe mucosal involvement. The risk of infection and mortality are high. Skin biopsy differentiates subepidermal cleavage characteristic of TEN from intraepidermal cleavage characteristic of the scalded-skin syndrome induced by staphylococcal toxins. TEN must be treated in a burn unit. Corticosteroids are contraindicated because they can significantly increase the risk of infection. High intravenous doses of immunoglobulin have been shown to be beneficial in patients with TEN, likely because of inhibition of Fas-mediated keratinocyte cell death by naturally occurring Fas-blocking antibodies in the intravenous immunoglobulin preparation.
Biologics
An increasing number of biologic agents has become available for the treatment of autoimmune, allergic, cardiovascular, infectious, and neoplastic diseases. Their use may be associated with a variety of adverse reactions, including hypersensitivity reactions. Given the occurrence of anaphylaxis, including cases with delayed onset and protracted progression in spontaneous postmarketing adverse event reports, the FDA issued a boxed warning regarding risk of anaphylaxis and need for patient monitoring with use of omalizumab (Chapter 138).
Drug-induced Hypersensitivity Syndrome
Drug-induced hypersensitivity syndrome, also referred to as DRESS (drug rash with eosinophilia and systemic symptoms) syndrome, is a potentially life-threatening syndrome that has been described primarily with anticonvulsants (Table 146-1). It is characterized by fever, maculopapular rash, generalized lymphadenopathy, and potentially life-threatening damage of one or more organs, including visceral organ involvement that resolves with discontinuation of the anticonvulsant. Drug-induced hypersensitivity syndrome/DRESS has also been described with minocycline, sulfonamides, aspirin, chlorambucil, and dapsone. Reactions are treated with discontinuation of the offending agent, systemic steroids, and supportive care.
Bernstein IL, Gruchalla RS, Lee RE, et al. Disease management of drug hypersensitivity: a practice parameter. Ann Allergy Asthma Immunol. 1999;83:678-679.
Blanca M, Romano A, Torres MJ, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64:183-193.
Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574-580.
Caubet JC, Kaiser L, Lemaitre B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222.
Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med. 2008;358:1109-1117.
Cresswell KM, Sheikh A. Information technology-based approaches to reducing repeat drug exposure in patients with known drug allergies. J Allergy Clin Immunol. 2008;121:1112-1117.
Davis CM, Shearer WT. Diagnosis and management of HIV drug hypersensitivity. J Allergy Clin Immunol. 2008;121:826-832.
Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann Pharmacother. 2009;43:304-315.
Gruchalla RS, Pirmohamed M. Antibiotic allergy. N Engl J Med. 2006;354:601-608.
Hughes AR, Brothers CH, Mosteller M, et al. Genetic association studies to detect adverse drug reactions: abacavir hypersensitivity as an example. Pharmacogenomics. 2009;10:225-233.
Lee SJ, Kavanaugh A. Adverse reactions to biologic agents: focus on autoimmune disease therapies. J Allergy Clin Immunol. 2005;116:900-905.
Limb SL, Starke PR, Lee CE, et al. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. 2007;120:1378-1381.
Pegler S, Healy B. In patients allergic to penicillin, consider second and third generation cephalosporins for life threatening infections. BMJ. 2007;335:991.
Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115:1048-1057.
Posadas SJ, Pichler WJ. Delayed drug hypersensitivity reactions: new concepts. Clin Exp Allergy. 2007;37:989-999.
Romano A, Gaeta F, Valluzzi RL, et al. Diagnosing hypersensitivity reactions to cephalosporins in children. Pediatrics. 2008;122:521-527.
Schafer JA, Mateo N, Parlier GL, et al. Penicillin allergy skin testing: what do we do now? Pharmacotherapy. 2007;27:542-545.
Schnyder B, Pichler WJ. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009;84:268-272.
Segal AR, Doherty KM, Leggott J, et al. Cutaneous reactions to drugs in children. Pediatrics. 2007;120:e1082-e1096.
Seth D, Kamat D, Montejo J. DRESS syndrome: a practical approach for primary care practitioners. Clin Pediatr. 2008;47:947-952.
Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
Yates AB. Management of patients with a history of allergy to beta-lactam antibiotics. Am J Med. 2008;121:572-576.
Weberschock TB, Muller SM, Boehncke S, et al. Tolerance to coxibs in patients with intolerance to non-steroidal anti-inflammatory drugs (NSAIDs): a systematic structured review of the literature. Arch Dermatol Res. 2007;299:169-175.