CHAPTER 30 Advantages and Limitations of Cranial Endoscopy
History of Endoscopy
The earliest use of the endoscope intracranially was reported by Lespinasse in 1910 for the treatment of hydrocephalus.1 Dandy2 used the technique more extensively and became known as the “father of neuroendoscopy”. Interest in cranial endoscopy was driven by the lack of alternative treatments of hydrocephalus in the first half of the 20th century. Endoscopic techniques evolved from fulguration or obliteration of the choroid plexus to fenestration techniques involving the ventricles and subarachnoid planes. The first endoscopic third ventriculostomy was reported by Mixter3 in 1923. Limited development in the technology for endoscopes combined with the placement of the first valved shunt by Nulsen and Spitz4 in 1949 decreased the interest in cranial endoscopy until the advent of rod lens endoscopes in 1960.5 Better illumination and resolution of the image enabled the application of the endoscope in the context of cranial neurosurgery. In 1973, Fukushima and colleagues6 introduced the modern endoscope, which could be used for the biopsy of intraventricular lesions, cyst fenestration, and treatment of hydrocephalus. The use of the endoscope in transsphenoidal approaches was introduced by Guiot, although he later abandoned the technique because it offered inadequate visualization.7–9 Bushe and Halves10 were the first to report the use of a modern endoscope in pituitary surgery in 1978. A few other reports emerged in the 1970s describing the use of an endoscope as an adjunct to the microscope in transsphenoidal approaches.10–12 Application of the endoscope to the sella turcica did not grow in popularity until the mid-1990s, when endoscopic sinus surgery had virtually replaced open techniques in use by otolaryngologists.9,13 Modern endoscopic cranial surgery developed from a combination of the growth in popularity of minimally invasive surgery through keyhole approaches14,15 and the development of purely endoscopic approaches to the skull base leading to functional endoscopic pituitary surgery.16
Endoscopic Instrumentation and General Principles
Flexible fiberscopes have the smallest diameter and can be used as a stylet within a ventricular catheter. They do not have a working channel but are appropriate for visualizing catheter placement and ensuring an intraventricular position. They have not been shown to improve the surgical outcome in hydrocephalus as no superiority of any specific location of catheter placement has been demonstrated.17,18
Rod lens endoscopes are used overwhelmingly in cranial endoscopy because of the superior quality of the image obtained. They are heavier because of the mandatory attachment of the camera and fiberoptic cable for the light source. An assortment of viewing angles is available (e.g., 0-, 30-, 70-, 120-degree angled endoscopes; Fig. 30-1). Up to two working channels are available on certain types of rod lens endoscopes through which instrumentation can be used. These working channels can also be used in addition to a trocar sheath, enabling the surgeon to use different viewing-angle endoscopes without the need to reinsert them through brain tissue. Different sheaths are available with one to multiple channels for inserting instruments, providing irrigation, or supplying suction.19 Instrumentation in the form of varying forceps, scissors, suction, or coagulation has been developed. The 0- and 30-degree endoscopes are the most widely used. The 0-degree scope minimizes the risk of disorientation, but the instruments inserted through the working channel remain in the periphery of the field of vision.18 The 30-degree endoscope allows better control of the instruments and, with simple rotation, provides an angle of view with a surface area twice as large as that of a 0-degree endoscope.
The optimal use of the endoscope requires a light source combined with a camera and monitor. Halogen, mercury vapor, and xenon light sources are available. The light source is connected to the endoscope through a fiberoptic cable, and its intensity can be modulated. Xenon light sources provide the best illumination for neuroendoscopy.18 The camera is connected to the endoscope by an adapter and transmits the image to a video monitor for viewing by the rest of the surgical team. Cameras are available as a single-chip or a three-chip charge-coupled device. Most systems use a single charge-coupled device because of its lower cost and lighter weight despite a lower quality image.18 A monitor with the highest possible quality should be selected, but its resolution should not exceed that of the camera. The size of the screen should not be excessive; a larger screen limits the quality of the image obtained. The loss of image quality is most pronounced in screens exceeding 13 inches and in cases in which a fiberscope is used.18 Irrigation is useful to optimize visualization and should be used while avoiding entrapment of fluid. Lactated Ringer’s solution is the method of choice for irrigation.20
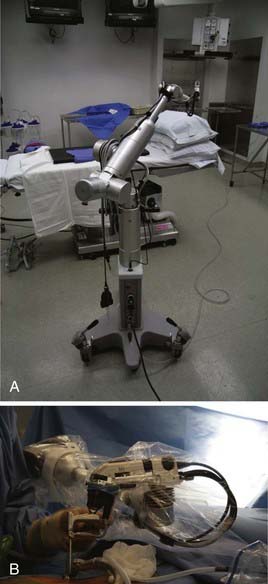
Rigid endoscope holders may increase the comfort of the surgeon during lengthy procedures but restrict the surgeon’s freedom of movement. Holders have been developed with a combination of pneumatic and electromagnetic brakes, combining the advantages of freehand movements with the possibility of very secure and firm positioning.21,22 Most endoscope holders need continuous manual adjustment at each of their joints, which limits their usefulness. They are usually mounted directly to the side of the operating room table, limiting the range of movement. We have used the Olympus EndoArm (Olympus Europa, Hamburg, Germany), a pneumatic endoscope holder mounted on its own base, extensively (Fig. 30-2). The EndoArm consists of an arm with several ball-and-socket joints that permit movement in all planes. Movement of the joints in either direction is controlled by a single button, giving the surgeon more fluidity in moving the endoscope.23
Endoscopy and Hydrocephalus
Cranial endoscopy was first used in the setting of hydrocephalus before the advent of shunt systems, when the condition was commonly fatal. A renewed interest in the use of endoscopy has arisen because of the high long-term morbidity associated with the use of shunts, most commonly shunt malfunctions and infections. In some cases, to avoid placement of a shunt system, the endoscope can be used to perform a third ventriculostomy or an obliteration of the choroid plexus. Third ventriculostomy has become an important part of the treatment of hydrocephalus, and its long-term success has varied greatly, depending on the cause of the hydrocephalus. Most long-term studies cite success rates of 65% to 75% for third ventriculostomies in the treatment of hydrocephalus.24–41
The endoscope can also be used as an adjunct to a diversion shunt technique when a fiberscope is placed in the ventricular catheter. Adequate placement of the ventricular catheter into the ventricular system is confirmed intraoperatively. Its cost-effectiveness remains questionable, but the technique can be useful in some situations. The endoscope has also been used extensively in cases of multiloculated hydrocephalus to prevent the high rate of shunt infections and revisions encountered in the setting of multiple shunts.42–44 The goal of endoscopic surgery in multiloculated hydrocephalus should be to convert the condition to uniloculated hydrocephalus requiring one or no shunt.42,45 Fenestration of the septum pellucidum by an endoscope may treat an isolated lateral ventricle46,47; in some cases, fenestration of multiple intraventricular membranes may be required,42,45 as well as aqueductoplasty or stenting in cases of fourth ventricular outlet obstruction.48,49
Image guidance is helpful in keeping the surgeon oriented, and a third ventriculostomy can be performed in the same setting if the hydrocephalus is thought to be obstructive. Endoscopic control permits placement of the catheter in an optimal location, and control rates of 62% to 100% can be accomplished in loculated hydrocephalus with one or no shunt.42,43,45,50
Endoscopy and Tumor Resection
Gross tumor resection has been accomplished endoscopically for intraventricular tumors. Favorable factors leading to complete tumor resection include soft tumor consistency, tumor less than 2 cm in diameter, moderate to low vascularity, associated hydrocephalus, low-grade tumor histologically, and tumor situated in the third or lateral ventricle.51 Tumor resection can be time-consuming because it is performed in a piecemeal fashion through the endoscope’s working channel,20 which in most endoscopes is limited to 2.4 mm. Complete tumor resections have most commonly been described in cases of colloid cysts.52–56 Colloid cysts are successfully treated in 60% to 90% of cases51,52,56–69 and seem to carry lower morbidity rates than does open craniotomy57–59; however, few endoscopic series report any long-term follow-up past 5 years.65 Other tumors that have been described as suitable for successful endoscopic resection include subependymal giant cell astrocytoma, exophytic low-grade gliomas into the ventricles, central neurocytoma, small choroid plexus tumors, and intraventricular craniopharyngiomas.32,51,53,70–73
The endoscope can be used effectively in treating intraventricular mass lesions, especially when they cause hydrocephalus.32–34,41,53,74–77 A prime example of the role of an endoscope in tumor resection is illustrated by cases of pineal tumors. In these tumors, the endoscope can be used to obtain CSF for tumor markers and cytologic studies, to inspect the intraventricular cavities to detect gross intraventricular nodules not visible on magnetic resonance imaging, to sample the tumor, and to perform a third ventriculostomy to address the hydrocephalus. Good results in controlling the hydrocephalus have been obtained.33,78,79 Advantages of the endoscopic approach over a stereotactic needle biopsy include the direct visualization of the tumor, a larger biopsy specimen, and the ability to stop the bleeding.51
Endoscopy can also be used as a palliative measure to treat the cystic components of certain inoperable tumors and to implant an Ommaya reservoir in locations more remote from the ventricular system.73
Endoscopy and Arachnoid Cysts
Endoscopy can be used to successfully treat symptomatic arachnoid cysts. Endoscopic fenestration of the cyst into the subarachnoid or intraventricular system enables the procedure to be performed through a smaller opening than is necessary with a craniotomy and alleviates the need for any long-term morbidity of shunt placement. Optimal candidates for endoscopic fenestration should have an area of continuity between the cyst wall and the ependyma or subarachnoid space. A large opening into the cyst wall should be made to prevent reclosure of the stoma. With stable cyst sizes, relief of symptoms without the need for other procedures can be obtained in 71% to 81% of cases.53,73,80–82 Fenestration of the cyst in conjunction with hydrocephalus increases the ease of the procedure and enables the surgeon to perform a concomitant third ventriculostomy.30,73,80,81,83 Fenestration of arachnoid cysts of the middle fossa is possible into the basal cisterns but carries some risk to the vessels of the sylvian fissure and may be best approached through a microsurgical technique.
Endoscopy and the Skull Base
The endoscope has increasingly been used in skull base surgery since the 1970s.9–13 First introduced in 1978 by Bushe and Halves,10 the endoscope was used to better visualize and reach the suprasellar extension of sellar lesions.84–91 The speculum used in the microscopic transsphenoidal approaches limits the working space of the endoscope and instruments, especially in reaching far superiorly, laterally, or posteriorly into the suprasellar space.84–89,91–94 Several variations involving the use of the endoscope in transsphenoidal surgery have been reported. The endoscope has been used to perform the initial approach in the sphenoid sinus, with decreased nasal morbidity to the approach.95 Other purely endoscopic endonasal transsphenoidal approaches have also been developed as an alternative to the microscopic techniques, alleviating the use of a nasal speculum.16,87,96–101
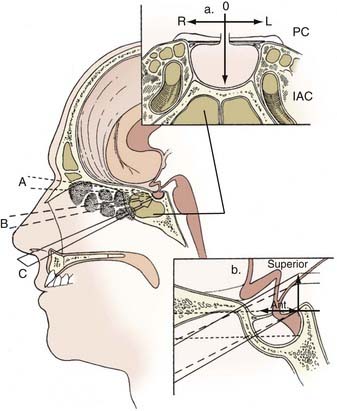
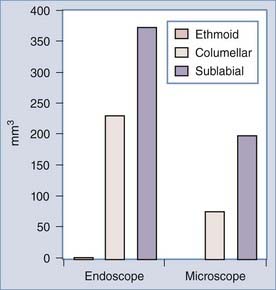
Among the advantages of the endoscope over the microscopic technique in transsphenoidal approaches are a panoramic view with better illumination.102,103 The addition of the endoscope consistently increases the area of visualization of the sellar and parasellar areas when a transethmoidal, transcolumellar, or sublabial approach is compared with its corresponding microscopic approach (Table 30-1; Fig. 30-3).102 In the microscopic approaches, the advantage of the sublabial approach over the transcolumellar approach, which, in turn, improves on the transethmoidal approach, is a better anterior and superior view to address the suprasellar area.104 The endoscope offers wider visualization in all axes with a maximal benefit in the superior and anterior aspects (Fig. 30-4). It is of note that a larger volume of visualization has been reported with an endoscopic transcolumellar approach than with a microscopic sublabial microscopic approach (see Fig. 30-3). Better detail in the image can be obtained with an endoscope than with a microscope because the camera source is closer to the structures of interest. Visualization in the microscopic technique is restricted by the “fixed tunnel” views offered through the openings of the nasal speculum. The microinstruments have to be used in a coaxial fashion, and their reach is limited by the nasal speculum. In addition, the microscopic technique often uses a sublabial or transseptal route, which may cause more trauma to the nasal mucosa,105 and vigorous opening of the speculum may cause optic nerve damage, facial pain, and swelling.106 A disadvantage of the endoscopic technique is the lack of depth perception compared with the binocular stereoscopic vision of the microscope. The endoscope’s image is also distorted, with maximal magnification at its center and contraction at the periphery (barrel effect). Continuous in-and-out movement of the endoscope can accommodate for the barrel effect and lack of depth perception.102
| Advantages |
| Wide-angle view |
| Placement of the surgeon’s eye at the site of surgery |
| Angled scopes look around corners |
| Increased volume of visualization at the site of surgery |
| Disadvantages |
| Lack of binocular vision with current technology |
| Endoscope occupies space in surgical corridor |
| Assistant or holder for scope is required for bimanual surgery to be performed |
| Lack of instrumentation to work through operative channel |
The endoscope’s initial use in transsphenoidal approaches to the sella was quickly expanded to address other midline skull base lesions because of their accessibility endonasally.107–123 The initial experience with these procedures was mixed because of high rates of postoperative CSF leaks, which remain the biggest limitation to the use of an endonasal technique compared with other transcranial neurosurgical approaches.94,121 The incidence of CSF leaks varies greatly with the nature of the condition treated and the degree of disruption of the arachnoid or ventricular system involved.121 Improvement in closure techniques has resulted in decreased rates of CSF leaks over time,121 as the use of fat, fascia lata, mucoperiosteum,124 and vascularized nasal septal flaps125 has been combined with the lumbar drainage, suturing,126 and balloon buttress techniques and fibrin glues.
The increasing familiarity of neurosurgeons with endoscopes combined with the use of angled scopes and more refined endonasal approaches has extended access to the skull base from the crista galli to the upper cervical vertebrae.127,128 Lateral extension of the approaches has also been accomplished to access the cavernous sinus129,130 and cranial middle and posterior fossae. The different pathologic conditions approached through an endoscopic technique to the skull base have expanded from tumor resections to the treatment of vascular lesions in selected cases.131 Optimization of the working space through both nostrils with a three- or four-hand technique has been essential in accessing and treating lesions in some of these locations. The instruments are inserted into the nostril blindly to prevent frequent obstruction of the lens. Two surgeons can work simultaneously, and the working space between the endoscope and instruments is optimized.132 All endoscopic approaches performed through both nostrils offer the surgeon better maneuverability of the instruments.127 The surgeon is then always able to access the lesion through the contralateral nostril, providing a more lateral reach to the lesion.103 The use of a bilateral nostril approach may require an additional mucosal incision for endonasal approaches accessing lesions anterior to the upper clivus. Lesions in the inferior part of the clivus or at the craniocervical junction can be accessed through an endonasal transchoanal approach.127 If a continuous view is desired from the sellar area to the craniocervical junction, a binostril approach with bilateral mucosal incisions is preferable.133
The extension of endonasal endoscopic approaches in this era is not limited to an alternative to the microscopic transsphenoidal and extended transsphenoidal approaches. An endonasal endoscopic approach can replace transoral approaches and can spare the splitting of the soft palate.133 It can also replace the need for some craniotomy skull base approaches.
Increased familiarity with endonasal endoscopic approaches to the skull base has increased the role of the endoscope in treatment of craniofacial disease while minimizing the need for disfiguring facial incisions or extensive facial osteotomies. Transsphenoidal endoscopic and transmaxillary endoscopic approaches can be combined with a bifrontal transbasal or frontotemporal craniotomy. A frontotemporal approach can be used to explore the middle fossa, pterygopalatine fossa, sella turcica, and cavernous sinus. A bifrontal transbasal approach can access lesions involving the frontal, ethmoid, and sphenoid sinuses all the way to the cervical-clival junction inferiorly. Blind spots in this approach consist of the superolateral corner of the maxillary sinus, which is impeded by the orbits, and the anteriormost portion of the nasal cavity, which may require a transmaxillary approach.134 An extended transsphenoidal approach may also be necessary to access lesions medial to the lateral nasal walls and intracavernous carotid arteries. A combined transmaxillary and extended transsphenoidal approach can be performed by bilateral Caldwell-Luc maxillotomies through a sublabial incision.134 Such surgical strategies have been successfully used in the treatment of allergic fungal sinusitis,135 esthesioneuroblastomas,136–138 and recurrent craniofacial meningiomas.139 Similar principles could be extended to the treatment of squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma, chondrosarcoma, angiofibroma, and inverted papilloma.
The decision to use an endoscopic approach rather than a microscopic transcranial approach to the skull base depends on the patient’s lesion as well as on local anatomic factors. The tumor size, vascularity, relationship to major vascular structures, and degree of lateral extension affect the surgeon’s decision to perform the operation through an endonasal endoscopic approach. The thickness of bone at the skull base, the degree of sphenoid sinus pneumatization, and the size of the sella will also influence the surgeon’s level of comfort.140 Presellar and conchal-type sinuses limit the effectiveness of an extended endoscopic transsphenoidal approach.118 Endoscopic approaches can limit the need for brain retraction to access certain deep subchiasmatic, retrosellar, and intraventricular lesions when compared with a transcranial approach. A straight trajectory can be achieved with a wider access of those areas at the price of a higher risk of a postoperative CSF leak.140
The endoscope is also used endonasally as a first-line therapy for CSF leaks in the anterior skull base, regardless of their cause.124 The addition of fluorescein has increased the surgeon’s ability to localize the source of the leak accurately, even at the submucosal level, and to improve the effectiveness of the repair. CSF rhinorrhea from an anterior skull base location was effectively treated in 85% to 100% of cases at the time of first surgery in published series.141–146 A repeated endoscopic endonasal approach may still appropriately treat the leak in a significant number of cases. The graft material and technique used do not seem to independently affect the results.147–151 Limitations to the endoscopic technique occur when the size of the defect is greater than 2.5 cm or when areas of the defect are not approachable endonasally, such as in cases involving the frontal sinus.143,152 Increasing experience in the treatment of some encephaloceles is developing.141
Endoscopy and Craniosynostosis
The endoscope has been used in the surgical treatment of craniosynostosis. Sagittal, lambdoid, coronal, and metopic craniosynostoses have been successfully treated endoscopically.153 The endoscope permits subgaleal and epidural dissection to be performed safely with less blood loss than in an open technique. The surgical treatment may be done in children at a younger age.
Endoscope-Assisted Microneurosurgery
Endoscope-assisted microsurgery offers the surgeon the advantages of both the endoscope and the microscope. The addition of the endoscope to the microscope provides better illumination and detail in the image14,15,82,154 while also providing wider angles of visualization.15 The craniotomy can be smaller, and the endoscope still provides a wide enough view for the surgery to be performed by use of the keyhole concept.15
Applications of endoscope-assisted microsurgery have been described in aneurysm surgery.14,155–160 The endoscope provides illumination in deep, dark corners as well as a clearer detail of structures. Angled endoscopes can provide the surgeon with better knowledge of the location of perforating arteries or cranial nerves around corners and behind the aneurysm sac. The endoscope can also better verify the relationship of those structures to the clip to determine whether optimal clip placement was performed.159
![]() The endoscope has similar advantages in tumor surgery.14,161,162 The endoscope can offer a view of the relationship of the tumor to adjacent structures behind the tumor and can ensure that manipulation or further tumor removal is safe. Visualization of the internal acoustic meatus before resection of intracanalicular schwannomas has been accomplished with the endoscope.161 An endoscope has also been used to complete tumor resection in cases of intracranial dermoid cysts (Video 30-1)
The endoscope has similar advantages in tumor surgery.14,161,162 The endoscope can offer a view of the relationship of the tumor to adjacent structures behind the tumor and can ensure that manipulation or further tumor removal is safe. Visualization of the internal acoustic meatus before resection of intracanalicular schwannomas has been accomplished with the endoscope.161 An endoscope has also been used to complete tumor resection in cases of intracranial dermoid cysts (Video 30-1)![]() .163
.163
Different methods have been described to integrate the images coming from the endoscope and microscope.15 The images can be kept separate on different monitors or integrated side by side through the microscope eyepieces or through high-definition LCD (liquid crystal display) visors on the forehead. Most surgeons choose to place the endoscope in a holder to keep it stable in the surgical field and to prevent it from causing injury to adjacent neural structures.15,154-157,162,164 Both of the surgeon’s hands become free to operate instruments, but a static endoscopic vision is obtained. Some surgeons have argued, however, that it is better to use the endoscope freehand to maintain a dynamic view, especially in aneurysm surgery.155 Endoscope holders with pneumatic arms are the most versatile and permit the surgeon to lock or to unlock all joints of the arm with one button.23
Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66-73.
Couldwell WT, Weiss MH, Rabb C, et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539-547.
Dandy W. An operative approach for hydrocephalus. Bull John Hopkins Hosp. 1922;33:189-190.
Das K, Spencer W, Nwagwu CI, et al. Approaches to the sellar and parasellar region: anatomic comparison of endonasal-transsphenoidal, sublabial-transsphenoidal, and transethmoidal approaches. Neurol Res. 2001;23:51-54.
de Divitiis E, Cappabianca P. Microscopic and endoscopic transsphenoidal surgery. Neurosurgery. 2002;51:1527-1529.
Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-231.
Guiot J, Rougerie J, Fourestier M, et al. [Intracranial endoscopic explorations.]. Presse Med. 1963;71:1225-1228.
Iantosca MR, Hader WJ, Drake JM. Results of endoscopic third ventriculostomy. Neurosurg Clin North Am. 2004;15:67-75.
Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3.
Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4.
Kehler U, Brunori A, Gliemroth J, et al. Twenty colloid cysts—comparison of endoscopic and microsurgical management. Minim Invasive Neurosurg. 2001;44:121-127.
Liu JK, Das K, Weiss MH, et al. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083-1096.
Liu JK, Decker D, Schaefer SD, et al. Zones of approach for craniofacial resection: minimizing facial incisions for resection of anterior cranial base and paranasal sinus tumors. Neurosurgery. 2003;53:1126-1135.
Mixter W. Ventriculoscopy and puncture of the floor of the third ventricle: preliminary report of a case. Boston Med Surg J. 1923;188:277-278.
Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1952;2:399-402.
Oi S, Abbott R. Loculated ventricles and isolated compartments in hydrocephalus: their pathophysiology and the efficacy of neuroendoscopic surgery. Neurosurg Clin North Am. 2004;15:77-87.
Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219-224.
Rao G, Klimo PJr, Jensen RL, et al. Surgical strategies for recurrent craniofacial meningiomas. Neurosurgery. 2006;58:874-879.
Spencer WR, Das K, Nwagu C, et al. Approaches to the sellar and parasellar region: anatomic comparison of the microscope versus endoscope. Laryngoscope. 1999;109:791-794.
Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg. 2000;123:195-201.
1 Davis L. Neurological Surgery. Philadelphia: Lea & Febiger; 1936.
2 Dandy W. An operative approach for hydrocephalus. Bull John Hopkins Hosp. 1922;33:189-190.
3 Mixter W. Ventriculoscopy and puncture of the floor of the third ventricle: preliminary report of a case. Boston Med Surg J. 1923;188:277-278.
4 Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1952;2:399-402.
5 Geiger M, Cohen A. The history of neuroendoscopy. In: Cohen AR, Haines SJ, editors. Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:1-13. Concepts in Neurosurgery, vol 7
6 Fukushima T, Ishijima B, Hirakawa K, et al. Ventriculofiberscope: a new technique for endoscopic diagnosis and operation. Technical note. J Neurosurg. 1973;38:251-256.
7 Doglietto F, Prevedello DM, Jane JAJr, et al. Brief history of endoscopic transsphenoidal surgery—from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19(1):E3.
8 Guiot J, Rougerie J, Fourestier M, et al. [Intracranial endoscopic explorations.]. Presse Med. 1963;71:1225-1228.
9 Liu JK, Das K, Weiss MH, et al. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083-1096.
10 Bushe KA, Halves E. [Modified technique in transsphenoidal operations of pituitary adenomas. Technical note (author’s transl).]. Acta Neurochir (Wien). 1978;41:163-175.
11 Apuzzo ML, Heifetz MD, Weiss MH, et al. Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg. 1977;46:398-400.
12 Halves E, Bushe KA. Transsphenoidal operation on craniopharyngiomas with extrasellar extensions. The advantage of the operating endoscope [proceedings]. Acta Neurochir Suppl (Wien). 1979;28:362.
13 Couldwell WT. Transsphenoidal and transcranial surgery for pituitary adenomas. J Neurooncol. 2004;69:237-256.
14 Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-231.
15 Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219-224.
16 Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66-73.
17 Levy ML, Lavine SD, Mendel E, et al. The endoscopic stylet: technical notes. Neurosurgery. 1994;35:335.
18 Siomin V, Constantini S. Basic principles and equipment in neuroendoscopy. Neurosurg Clin North Am. 2004;15:19-31.
19 Kehler U, Regelsberger J, Gliemroth J. Pro and cons of different designs of rigid endoscopes. Minim Invasive Neurosurg. 2003;46:205-207.
20 Schroeder HW, Gaab MR. Intracranial endoscopy. Neurosurg Focus. 1999;6(4):E3.
21 Nathan CO, Chakradeo V, Malhotra K, et al. The voice-controlled robotic assist scope holder AESOP for the endoscopic approach to the sella. Skull Base. 2006;16:123-131.
22 Arnholt JL, Mair EA. A “third hand” for endoscopic skull base surgery. Laryngoscope. 2002;112:2244-2249.
23 Eskandari R, Amini A, Yonemura KS, et al. The use of the Olympus EndoArm for spinal and skull-based transsphenoidal neurosurgery. Minim Invasive Neurosurg. 2008;51:370-372.
24 Fukuhara T, Vorster SJ, Luciano MG. Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery. 2000;46:1100-1109.
25 Iantosca MR, Hader WJ, Drake JM. Results of endoscopic third ventriculostomy. Neurosurg Clin North Am. 2004;15:67-75.
26 Choi JU, Kim DS, Kim SH. Endoscopic surgery for obstructive hydrocephalus. Yonsei Med J. 1999;40:600-607.
27 Cinalli G, Sainte-Rose C, Chumas P, et al. Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. J Neurosurg. 1999;90:448-454.
28 Cinalli G, Spennato P, Ruggiero C, et al. Aqueductal stenosis 9 years after mumps meningoencephalitis: treatment by endoscopic third ventriculostomy. Childs Nerv Syst. 2004;20:61-64.
29 Figaji AA, Fieggen AG, Peter JC. Endoscopic third ventriculostomy in tuberculous meningitis. Childs Nerv Syst. 2003;19:217-225.
30 Hellwig D, Grotenhuis JA, Tirakotai W, et al. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev. 2005;28:1-34.
31 Kelly PJ. Stereotactic third ventriculostomy in patients with nontumoral adolescent/adult onset aqueductal stenosis and symptomatic hydrocephalus. J Neurosurg. 1991;75:865-873.
32 Macarthur DC, Buxton N, Vloeberghs M, et al. The effectiveness of neuroendoscopic interventions in children with brain tumours. Childs Nerv Syst. 2001;17:589-594.
33 Oi S, Shibata M, Tominaga J, et al. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: a prospective study. J Neurosurg. 2000;93:245-253.
34 Oka K, Yamamoto M, Nagasaka S, et al. Endoneurosurgical treatment for hydrocephalus caused by intraventricular tumors. Childs Nerv Syst. 1994;10:162-166.
35 Ruggiero C, Cinalli G, Spennato P, et al. Endoscopic third ventriculostomy in the treatment of hydrocephalus in posterior fossa tumors in children. Childs Nerv Syst. 2004;20:828-833.
36 Schijman E, Peter JC, Rekate HL, et al. Management of hydrocephalus in posterior fossa tumors: how, what, when? Childs Nerv Syst. 2004;20:192-194.
37 Pople IK, Edwards RJ, Aquilina K. Endoscopic methods of hydrocephalus treatment. Neurosurg Clin North Am. 2001;12:719-735. viii
38 Cinalli G, Salazar C, Mallucci C, et al. The role of endoscopic third ventriculostomy in the management of shunt malfunction. Neurosurgery. 1998;43:1323-1327.
39 Jones RF, Kwok BC, Stening WA, et al. The current status of endoscopic third ventriculostomy in the management of non-communicating hydrocephalus. Minim Invasive Neurosurg. 1994;37:28-36.
40 Teo C, Jones R. Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg. 1996;25:57-63.
41 Kunwar S. Endoscopic adjuncts to intraventricular surgery. Neurosurg Clin North Am. 2003;14:547-557.
42 Lewis AI, Keiper GLJr, Crone KR. Endoscopic treatment of loculated hydrocephalus. J Neurosurg. 1995;82:780-785.
43 Oi S, Abbott R. Loculated ventricles and isolated compartments in hydrocephalus: their pathophysiology and the efficacy of neuroendoscopic surgery. Neurosurg Clin North Am. 2004;15:77-87.
44 Jamjoom AB, Mohammed AA, al-Boukai A, et al. Multiloculated hydrocephalus related to cerebrospinal fluid shunt infection. Acta Neurochir (Wien). 1996;138:714-719.
45 Nowoslawska E, Polis L, Kaniewska D, et al. Effectiveness of neuroendoscopic procedures in the treatment of complex compartmentalized hydrocephalus in children. Childs Nerv Syst. 2003;19:659-665.
46 Aldana PR, Kestle JR, Brockmeyer DL, et al. Results of endoscopic septal fenestration in the treatment of isolated ventricular hydrocephalus. Pediatr Neurosurg. 2003;38:286-294.
47 Gangemi M, Maiuri F, Donati PA, et al. Endoscopic surgery for monoventricular hydrocephalus. Surg Neurol. 1999;52:246-250.
48 Fritsch MJ, Mehdorn M. Endoscopic intraventricular surgery for treatment of hydrocephalus and loculated CSF space in children less than one year of age. Pediatr Neurosurg. 2002;36:183-188.
49 Teo C, Burson T, Misra S. Endoscopic treatment of the trapped fourth ventricle. Neurosurgery. 1999;44:1257-1261.
50 Heilman CB, Cohen AR. Endoscopic ventricular fenestration using a “saline torch. ”. J Neurosurg. 1991;74;:224-229.
51 Teo C, Nakaji P. Neuro-oncologic applications of endoscopy. Neurosurg Clin North Am. 2004;15:89-103.
52 Decq P, Le Guerinel C, Brugieres P, et al. Endoscopic management of colloid cysts. Neurosurgery. 1998;42:1288-1294.
53 Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998;88:496-505.
54 Hellwig D, Bauer BL, Schulte M, et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52:525-532.
55 Rodziewicz GS, Smith MV, Hodge CJJr. Endoscopic colloid cyst surgery. Neurosurgery. 2000;46:655-660.
56 Acerbi F, Rampini P, Egidi M, et al. Endoscopic treatment of colloid cysts of the third ventricle: long-term results in a series of 6 consecutive cases. J Neurosurg Sci. 2007;51:53-60.
57 King WA, Ullman JS, Frazee JG, et al. Endoscopic resection of colloid cysts: surgical considerations using the rigid endoscope. Neurosurgery. 1999;44:1103-1109.
58 Kehler U, Brunori A, Gliemroth J, et al. Twenty colloid cysts—comparison of endoscopic and microsurgical management. Minim Invasive Neurosurg. 2001;44:121-127.
59 Lewis AI, Crone KR, Taha J, et al. Surgical resection of third ventricle colloid cysts. Preliminary results comparing transcallosal microsurgery with endoscopy. J Neurosurg. 1994;81:174-178.
60 Longatti P, Godano U, Gangemi M, et al. Cooperative study by the Italian neuroendoscopy group on the treatment of 61 colloid cysts. Childs Nerv Syst. 2006;22:1263-1267.
61 Longatti P, Martinuzzi A, Moro M, et al. Endoscopic treatment of colloid cysts of the third ventricle: 9 consecutive cases. Minim Invasive Neurosurg. 2000;43:118-123.
62 Powell MP, Torrens MJ, Thomson JL, et al. Isodense colloid cysts of the third ventricle: a diagnostic and therapeutic problem resolved by ventriculoscopy. Neurosurgery. 1983;13:234-237.
63 Schroeder HW, Oertel J, Gaab MR. Incidence of complications in neuroendoscopic surgery. Childs Nerv Syst. 2004;20:878-883.
64 Charalampaki P, Filippi R, Welschehold S, et al. Endoscope-assisted removal of colloid cysts of the third ventricle. Neurosurg Rev. 2006;29:72-79.
65 Greenlee JD, Teo C, Ghahreman A, et al. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008;62:51-55.
66 Abdou MS, Cohen AR. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89:1062-1068.
67 Gonzalez-Martinez JA, Zamorano L, Li QH, et al. Interactive image-guided management of colloid cysts of the third ventricle. Minim Invasive Neurosurg. 2003;46:193-197.
68 Harris AE, Hadjipanayis CG, Lunsford LD, et al. Microsurgical removal of intraventricular lesions using endoscopic visualization and stereotactic guidance. Neurosurgery. 2005;56:125-132.
69 Souweidane MM. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2005;57:312-318.
70 Abdullah J, Caemaert J. Endoscopic management of craniopharyngiomas: a review of 3 cases. Minim Invasive Neurosurg. 1995;38:79-84.
71 Delitala A, Brunori A, Chiappetta F. Purely neuroendoscopic transventricular management of cystic craniopharyngiomas. Childs Nerv Syst. 2004;20:858-862.
72 Locatelli D, Levi D, Rampa F, et al. Endoscopic approach for the treatment of relapses in cystic craniopharyngiomas. Childs Nerv Syst. 2004;20:863-867.
73 Tirakotai W, Schulte DM, Bauer BL, et al. Neuroendoscopic surgery of intracranial cysts in adults. Childs Nerv Syst. 2004;20:842-851.
74 Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. Neurosurg Focus. 1999;6(4):E7.
75 Macarthur DC, Buxton N, Punt J, et al. The role of neuroendoscopy in the management of brain tumours. Br J Neurosurg. 2002;16:465-470.
76 Selvapandian S. Endoscopic management of thalamic gliomas. Minim Invasive Neurosurg. 2006;49:194-196.
77 Yurtseven T, Ersahin Y, Demirtas E, et al. Neuroendoscopic biopsy for intraventricular tumors. Minim Invasive Neurosurg. 2003;46:293-299.
78 Pople IK, Athanasiou TC, Sandeman DR, et al. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg. 2001;15:305-311.
79 Rieger A, Rainov NG, Brucke M, et al. Endoscopic third ventriculostomy is the treatment of choice for obstructive hydrocephalus due to pediatric pineal tumors. Minim Invasive Neurosurg. 2000;43:83-86.
80 Caemaert J, Abdullah J, Calliauw L, et al. Endoscopic treatment of suprasellar arachnoid cysts. Acta Neurochir (Wien). 1992;119:68-73.
81 Decq P, Brugieres P, Le Guerinel C, et al. Percutaneous endoscopic treatment of suprasellar arachnoid cysts: ventriculocystostomy or ventriculocystocisternostomy? Technical note. J Neurosurg. 1996;84:696-701.
82 Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery. 1998;43:1330-1336.
83 Kirollos RW, Javadpour M, May P, et al. Endoscopic treatment of suprasellar and third ventricle–related arachnoid cysts. Childs Nerv Syst. 2001;17:713-718.
84 Couldwell WT, Weiss MH, Rabb C, et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539-547.
85 Dumont AS, Kanter AS, Jane JAJr, et al. Extended transsphenoidal approach. Front Horm Res. 2006;34:29-45.
86 Dusick JR, Esposito F, Kelly DF, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832-841.
87 Frank G, Pasquini E, Mazzatenta D. Extended transsphenoidal approach. J Neurosurg. 2001;95:917-918.
88 Kaptain GJ, Vincent DA, Sheehan JP, et al. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94-100.
89 Kim J, Choe I, Bak K, et al. Transsphenoidal supradiaphragmatic intradural approach: technical note. Minim Invasive Neurosurg. 2000;43:33-37.
90 Kitano M, Taneda M. Extended transsphenoidal approach with submucosal posterior ethmoidectomy for parasellar tumors. Technical note. J Neurosurg. 2001;94:999-1004.
91 Laws ER, Kanter AS, Jane JAJr, et al. Extended transsphenoidal approach. J Neurosurg. 2005;102:825-827.
92 de Divitiis E. Endoscopic transsphenoidal surgery: stone-in-the-pond effect. Neurosurgery. 2006;59:512-520.
93 de Divitiis E, Cappabianca P. Microscopic and endoscopic transsphenoidal surgery. Neurosurgery. 2002;51:1527-1529.
94 Frank G, Pasquini E, Doglietto F, et al. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery. 2006;59(1 suppl 1):ONS75-ONS82.
95 Yaniv E, Rappaport ZH. Endoscopic transseptal transsphenoidal surgery for pituitary tumors. Neurosurgery. 1997;40:944-946.
96 Cappabianca P, Cavallo LM, Colao A, et al. Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002;45:193-200.
97 Jho HD. Endoscopic transsphenoidal surgery. J Neurooncol. 2001;54:187-195.
98 Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44-51.
99 de Divitiis E, Cappabianca P. Endoscopic endonasal transsphenoidal surgery. Adv Tech Stand Neurosurg. 2002;27:137-177.
100 Carrau RL, Jho HD, Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
101 Cavallo LM, Dal Fabbro M, Jalalod’din H, et al. Endoscopic endonasal transsphenoidal surgery. Before scrubbing in: tips and tricks. Surg Neurol. 2007;67:342-347.
102 Spencer WR, Das K, Nwagu C, et al. Approaches to the sellar and parasellar region: anatomic comparison of the microscope versus endoscope. Laryngoscope. 1999;109:791-794.
103 Catapano D, Sloffer CA, Frank G, et al. Comparison between the microscope and endoscope in the direct endonasal extended transsphenoidal approach: anatomical study. J Neurosurg. 2006;104:419-425.
104 Das K, Spencer W, Nwagwu CI, et al. Approaches to the sellar and parasellar region: anatomic comparison of endonasal-transsphenoidal, sublabial-transsphenoidal, and transethmoidal approaches. Neurol Res. 2001;23:51-54.
105 Cappabianca P, Cavallo LM, Colao A, et al. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97:293-298.
106 Garcia AS, Rhoton ALJr. Speculum opening in transsphenoidal surgery. Neurosurgery. 2006;59(1 suppl 1):ONS35-ONS39.
107 Cavallo LM, Messina A, Cappabianca P, et al. Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus. 2005;19(1):E2.
108 de Divitiis O, Conti A, Angileri FF, et al. Endoscopic transoral-transclival approach to the brainstem and surrounding cisternal space: anatomic study. Neurosurgery. 2004;54:125-130.
109 Esposito F, Becker DP, Villablanca JP, et al. Endonasal transsphenoidal transclival removal of prepontine epidermoid tumors: technical note. Neurosurgery. 2005;56:E443.
110 Jho HD, Carrau RL, McLaughlin MR, et al. Endoscopic transsphenoidal resection of a large chordoma in the posterior fossa. Acta Neurochir (Wien). 1997;139:343-347.
111 Jho HD, Ha HG. Endoscopic endonasal skull base surgery: part 3—the clivus and posterior fossa. Minim Invasive Neurosurg. 2004;47:16-23.
112 Jho HD, Ha HG. Endoscopic endonasal skull base surgery: part 2—the cavernous sinus. Minim Invasive Neurosurg. 2004;47:9-15.
113 Jho HD, Ha HG. Endoscopic endonasal skull base surgery: part 1—the midline anterior fossa skull base. Minim Invasive Neurosurg. 2004;47:1-8.
114 Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4.
115 Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3.
116 Kassam AB, Gardner P, Snyderman C, et al. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(1):E6.
117 Cook SW, Smith Z, Kelly DF. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery. 2004;55:239-244.
118 de Divitiis E, Cappabianca P, Cavallo LM, et al. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery. 2007;61:219-227.
119 de Divitiis E, Cavallo LM, Esposito F, et al. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery. 2007;61:229-237.
120 Duz B, Secer HI, Tosun F, et al. Endoscopic endonasal resection of a midline intradural frontobasal dermoid tumour. Minim Invasive Neurosurg. 2007;50:363-366.
121 Gardner PA, Kassam AB, Snyderman CH, et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg. 2008;109:6-16.
122 Gardner PA, Prevedello DM, Kassam AB, et al. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg. 2008;108:1043-1047.
123 Kassam AB, Gardner PA, Snyderman CH, et al. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108:715-728.
124 Silva LR, Santos RP, Zymberg ST. Endoscopic endonasal approach for cerebrospinal fluid fistulae. Minim Invasive Neurosurg. 2006;49:88-92.
125 Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882-1886.
126 Gardner P, Kassam A, Snyderman C, et al. Endoscopic endonasal suturing of dural reconstruction grafts: a novel application of the U-Clip technology. Technical note. J Neurosurg. 2008;108:395-400.
127 Alfieri A, Jho HD, Tschabitscher M. Endoscopic endonasal approach to the ventral cranio-cervical junction: anatomical study. Acta Neurochir (Wien). 2002;144:219-225.
128 Ragel BT, Bishop FS, Couldwell WT. Recurrent infrasellar clival craniopharyngioma. Acta Neurochir (Wien). 2007;149:729-730.
129 Alfieri A, Jho HD. Endoscopic endonasal approaches to the cavernous sinus: surgical approaches. Neurosurgery. 2001;49:354-360.
130 Alfieri A, Jho HD. Endoscopic endonasal cavernous sinus surgery: an anatomic study. Neurosurgery. 2001;48:827-836.
131 Kan P, Stevens EA, Orlandi RR, et al. Carotid artery–sparing repair of a cavernous carotid artery pseudoaneurysm. Case illustration. J Neurosurg. 2006;104:978.
132 Castelnuovo P, Pistochini A, Locatelli D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique.”. Rhinology. 2006;44:2-7.
133 Cavallo LM, Cappabianca P, Messina A, et al. The extended endoscopic endonasal approach to the clivus and cranio-vertebral junction: anatomical study. Childs Nerv Syst. 2007;23:665-671.
134 Liu JK, Decker D, Schaefer SD, et al. Zones of approach for craniofacial resection: minimizing facial incisions for resection of anterior cranial base and paranasal sinus tumors. Neurosurgery. 2003;53:1126-1135.
135 Liu JK, Schaefer SD, Moscatello AL, et al. Neurosurgical implications of allergic fungal sinusitis. J Neurosurg. 2004;100:883-890.
136 Casiano RR, Numa WA, Falquez AM. Endoscopic resection of esthesioneuroblastoma. Am J Rhinol. 2001;15:271-279.
137 Walch C, Stammberger H, Anderhuber W, et al. The minimally invasive approach to olfactory neuroblastoma: combined endoscopic and stereotactic treatment. Laryngoscope. 2000;110:635-640.
138 Liu JK, O’Neill B, Orlandi RR, et al. Endoscopic-assisted craniofacial resection of esthesioneuroblastoma: minimizing facial incisions—technical note and report of 3 cases. Minim Invasive Neurosurg. 2003;46:310-315.
139 Rao G, Klimo PJr, Jensen RL, et al. Surgical strategies for recurrent craniofacial meningiomas. Neurosurgery. 2006;58:874-879.
140 Cavallo LM, de Divitiis O, Aydin S, et al. Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: anatomic considerations—part 1. Neurosurgery. 2007;61:24-33.
141 Marton E, Billeci D, Schiesari E, et al. Transnasal endoscopic repair of cerebrospinal fluid fistulas and encephaloceles: surgical indications and complications. Minim Invasive Neurosurg. 2005;48:175-181.
142 Calcaterra TC. Extracranial surgical repair of cerebrospinal rhinorrhea. Ann Otol Rhinol Laryngol. 1980;89:108-116.
143 Dodson EE, Gross CW, Swerdloff JL, et al. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea and skull base defects: a review of twenty-nine cases. Otolaryngol Head Neck Surg. 1994;111:600-605.
144 Mattox DE, Kennedy DW. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100:857-862.
145 Persky MS, Rothstein SG, Breda SD, et al. Extracranial repair of cerebrospinal fluid otorhinorrhea. Laryngoscope. 1991;101:134-136.
146 Wigand ME. Transnasal ethmoidectomy under endoscopical control. Rhinology. 1981;19:7-15.
147 Burns JA, Dodson EE, Gross CW. Transnasal endoscopic repair of cranionasal fistulae: a refined technique with long-term follow-up. Laryngoscope. 1996;106:1080-1083.
148 Lanza DC, O’Brien DA, Kennedy DW. Endoscopic repair of cerebrospinal fluid fistulae and encephaloceles. Laryngoscope. 1996;106:1119-1125.
149 Mao VH, Keane WM, Atkins JP, et al. Endoscopic repair of cerebrospinal fluid rhinorrhea. Otolaryngol Head Neck Surg. 2000;122:56-60.
150 Schick B, Ibing R, Brors D, et al. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001;110:142-147.
151 Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg. 2000;123:195-201.
152 Hughes RG, Jones NS, Robertson IJ. The endoscopic treatment of cerebrospinal fluid rhinorrhoea: the Nottingham experience. J Laryngol Otol. 1997;111:125-128.
153 Barone CM, Jimenez DF. Endoscopic craniectomy for early correction of craniosynostosis. Plast Reconstr Surg. 1999;104:1965-1973.
154 Hayashi N, Cohen AR. Endoscope-assisted far-lateral transcondylar approach to the skull base. Minim Invasive Neurosurg. 2002;45:132-135.
155 Kalavakonda C, Sekhar LN, Ramachandran P, et al. Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery. 2002;51:1119-1126.
156 Ramos-Zuniga R, Velazquez H, Barajas MA, et al. Trans-supraorbital approach to supratentorial aneurysms. Neurosurgery. 2002;51:125-130.
157 Wang E, Yong NP, Ng I. Endoscopic assisted microneurosurgery for cerebral aneurysms. J Clin Neurosci. 2003;10:174-176.
158 Kato Y, Sano H, Nagahisa S, et al. Endoscope-assisted microsurgery for cerebral aneurysms. Minim Invasive Neurosurg. 2000;43:91-97.
159 Takaishi Y, Yamashita H, Tamaki N. Cadaveric and clinical study of endoscope-assisted microneurosurgery for cerebral aneurysms using angle-type rigid endoscope. Kobe J Med Sci. 2002;48:1-11.
160 Zhao J, Wang Y, Zhao Y, et al. Neuroendoscope-assisted minimally invasive microsurgery for clipping intracranial aneurysms. Minim Invasive Neurosurg. 2006;49:335-341.
161 Fukushima T. Endoscopy of Meckel’s cave, cisterna magna, and cerebellopontine angle. Technical note. J Neurosurg. 1978;48:302-306.
162 Menovsky T, Grotenhuis JA, de Vries J, et al. Endoscope-assisted supraorbital craniotomy for lesions of the interpeduncular fossa. Neurosurgery. 1999;44:106-110.
163 Liu JK, Gottfried ON, Salzman KL, et al. Ruptured intracranial dermoid cysts: clinical, radiographic, and surgical features. Neurosurgery. 2008;62:377-384.
164 Cohen AR, Perneczky A, Rodziewicz GS, et al. Endoscope-assisted craniotomy: approach to the rostral brain stem. Neurosurgery. 1995;36:1128-1129.