Chapter 181 Advances in Anesthesia for Spine Surgery and the Prevention of Complications
Anesthesia for Cervical Spine Surgery
Movement of Cervical Spine with Intubation
The primary force applied by the laryngoscopist is upward lift with a little bit of angular force. This force can be as high as 50 to 70 N (40 N is enough to lift 10 pounds). The more difficult the exposure, the greater the force usually applied. Intubation needs extension at occiput-C1, combined with flexion at lower vertebrae (the fulcrum is probably at C7-T1).1 Direct laryngoscopy with a Macintosh size 3 blade results in near-maximal extension at occiput-C1 (with the posterior arch of C1 touching the skull).1,2
To identify an ideal method for intubation,3 Sahin et al. studied upper cervical vertebral motion with three intubation devices. Comparisons were made between direct laryngoscopy, the intubating laryngeal mask airway (ILMA), and fiberoptic intubation. In their study, the mean motion at the C1-2 level was 10.2 ± 7.3, 5.0 ± 6.3, and 1.6 ± 3.2 degrees, respectively. The fiberoptic method was found to produce the least motion in the upper cervical spine. The authors concluded that fiberoptic laryngoscopy is the most suitable intubation technique when cervical spine movement is not desired.
There are a few degrees less extension at C1-2 with use of a straight blade.4 It is unlikely that this difference is clinically meaningful. During intubation under general anesthesia with neuromuscular blockade and manual in-line stabilization, the use of the GlideScope video laryngoscope (Verathon, Inc., Bothell, WA) produced better glottic visualization, but did not significantly decrease movement of the nonpathologic cervical spine compared with direct laryngoscopy.5
Cervical Spine Movement and Laryngeal Mask Airways
Keller et al.6 implanted microchip sensors into the pharyngeal surfaces of C2 and C3 in 20 cadavers to determine the pressures exerted against the cervical spine by the laryngeal mask airway (LMA) and ILMA. The authors concluded that these devices exert high pressures against the upper cervical vertebrae during insertion, during inflation, and in situ. These pressures could produce posterior displacement of the upper cervical spine.
Manual In-line Stabilization and Cricoid Pressure
The goal of manual in-line stabilization is to apply forces to the head and neck equal in magnitude and opposite in direction to those generated by the laryngoscopist so as to limit the movement that might result during airway management; traction forces should be avoided. Manual in-line stabilization failed to reduce movement at the site of instability in cadaver models.7,8 Cricoid pressure (as long as it is not excessive) did not result in movement in a cadaver model of an injured upper cervical spine.9
Maintaining the head in neutral or near-neutral position can be very important in maintaining proper cervical cord blood supply. Flexion of the spine causes elongation of the cord with narrowing of the diameter of the longitudinal vessels.10 Extension causes an increase in diameter of the cervical cord and folding of the ligamentum flavum, which may exert pressure on the cord and posterior longitudinal vessels.11 Rau et al.12 described a case of quadriplegia in a patient who underwent posterior fossa surgery in the prone position. The authors state that during a prolonged period in which the neck was in hyperflexion, overstretching of the cervical spinal cord and compromise to its blood supply likely caused this devastating complication.
Practical Points to Remember
• Awake fiberoptic intubation is the gold standard for patients with unstable neck injuries.
• Surveys indicate that the majority of U.S. anesthesiologists would prefer to use a fiberoptic bronchoscope to intubate at-risk patients and to do so with the patient awake.13
• Induction of anesthesia diminishes the protective stabilization of the neck musculature. Neck motion during this phase can be substantial, sometimes producing dynamic cord compression that could result in cervical cord injury.
• If the cervical spine is grossly unstable, consideration should be given to both intubating the trachea and positioning the patient while he or she is still awake. If new neurologic symptoms developed during positioning, repositioning should be attempted. During that period, tight control of the patient’s blood pressure and even inducing hypertension can help resolve these new symptoms.
• Airway complications are common after anterior cervical spine surgery and may range from acute airway obstruction to chronic vocal cord dysfunction. Recurrent laryngeal nerve injury after anterior cervical spine surgery could be due to direct nerve injury at the time of neck dissection, surgical retractor placement, and endotracheal balloon insufflation pressure.14,15
• Cervical spine surgery in the prone position could result in laryngeal edema and macroglossia.7,16
• The use of fiberoptic intubation was shown to result in fewer airway complications after cervical spine surgery, thought to be due to a reduction in soft tissue trauma.17
• For patients with subaxial spondylotic myelopathy, neck extension can narrow the diameter of the spinal cord, whereas in patients with atlantoaxial subluxation, such as those with rheumatoid arthritis or Down syndrome, neck flexion will widen the atlantodental interval, narrowing the spinal canal.
• Maintaining adequate spinal cord perfusion is crucial during cervical spine surgery in patients with spondylitic myelopathy. Chronic mechanical compression inhibiting the spinal cord blood supply leads to gradual, intermittent microinfarction of the cord. In this setting, invasive blood pressure monitoring using an arterial line and close attention to maintaining adequate perfusion pressure are very important.14
Physiologic Changes in the Prone Position
Cardiovascular Changes
Using a noninvasive cardiac output monitor, both cardiac index (CI) and venous return18 decreased in unanesthetized, healthy volunteers in the prone position. CI decreased compared with the supine position as follows: knee-chest position (20%); on pelvic props from a modified Relton-Hall frame under the anterior superior iliac spines and padded support under the chest (17%); on an evacuatable mattress (11%); and on pillows (3%; one pillow under the thorax and one under the abdomen, leaving the abdomen free to move). Toyota and Amaki19 studied transesophageal echocardiograms in 15 healthy patients undergoing prone-position lumbar laminectomy. The prone position caused left ventricular volume and compliance to decrease. These changes were attributed to a decrease in the venous return due to inferior vena caval compression, and decreased left ventricular compliance due to increased intrathoracic pressure in the prone position. These results had been confirmed by other studies using thermodilution pulmonary artery catheters to measure the cardiac index when transferring from the supine to the prone position. Cardiac output in these studies decreased by 17% to 24%.14 The reduction in cardiac output in the prone position also leads to a decrease in the metabolism of propofol.20 A reduction in propofol metabolism while in the prone position could also explain the results of Sudheer et al.,21 who showed a significant reduction in cardiac output in the prone position during maintenance of anesthesia using propofol compared with isoflurane. Pearce22 observed vena caval pressures to be 0 to 40 mm H2O in patients in the prone position with the abdomen hanging free. In contrast, patients with abdominal compression had vena caval pressures greater than 300 mm H2O. Increased venous pressure not only increases bleeding during spine surgery owing to congestion of vertebral veins but can impair spinal cord perfusion.
The use of the prone position with abdominal compression was identified as a plausible cause of spinal cord ischemia leading to neurologic deficits after cervical laminectomy. The authors of this case series recommended the avoidance of abdominal compression and hypotension, especially in myelopathic patients for whom maintenance of spinal cord perfusion pressure is of paramount importance.23
Changes in Respiratory Physiology
In an elegant study, Nyren et al.24 studied the regional distribution of pulmonary blood flow in 10 healthy volunteers. The subjects were studied in both prone and supine positions with and without lung distention caused by 10 cm H2O of continuous positive airway pressure. The results demonstrated that ventilation-perfusion matching during both normal breathing and positive pressure is more favorable in the prone than in the supine position. Because perfusion is more evenly distributed in the prone position, the recruitment of dorsal airways results in an increase in lung units and consequently increased functional residual capacity, with near-normal ventilation-perfusion matching and a reduction in shunt.25 By turning the patient prone and recruiting airways in the dorsal lung, prone positioning achieves similar beneficial effects as positive end-expiratory pressure ventilation but without the risks of barotrauma or interference with cardiac function. Of note, the prone position is sometimes used in patients with acute respiratory distress syndrome to improve oxygenation and decrease shunt.25 Similar findings were confirmed by Pelosi et al.26 during general anesthesia. Prone positioning during general anesthesia did not negatively affect respiratory mechanisms, and it improved lung volumes and oxygenation.
Estimating Intravascular Volume Status and Predicting Fluid Responsiveness
Although unquestionably useful, the traditional clinical findings in hypovolemia often lack sensitivity and specificity. This fact has led to a decades-long program of ongoing research to improve the clinical monitoring of volume status. Earlier expectations that cardiac filling pressure data (e.g., central venous pressure, pulmonary capillary wedge pressure) would be helpful in guiding fluid therapy were not fully realized when it became apparent that cardiac filling pressures are often influenced by factors other than blood volume. Later experience with transesophageal echocardiography showed that this technique can be especially helpful in assessing right- and left-sided ventricular filling, but the technique is intermittent in nature, requires extensive training to use and interpret, and entails a very high equipment cost. Consequently, the search for practical and reliable means of monitoring blood volume and related parameters continues. Approaches to this search fall into one of three broad areas: identifying occult hypoperfusion (e.g., by tissue oxygen and carbon dioxide levels), assessing preload responsiveness (e.g., by systolic pressure variation, pulse pressure variation, or stroke volume variation), and identifying end points for fluid resuscitation (e.g., seeking a specific cardiac output or aortic flow velocity value rather than a specific blood pressure). The following is a brief description of some of the methods that show particular promise in this regard.
Arterial pressure variation as a consequence of respiration has been advocated as a relatively simple technique that requires only an arterial line. Clinically, one can “eyeball” the degree of pressure variation with mechanical ventilation to get a rough indication of the degree of hypovolemia. However, more quantitative measures such as measurements of pulse pressure (systolic−diastolic) variation, systolic blood pressure variation, and delta down (decrease in systolic blood pressure from the value during apnea) have all been advocated as more appropriate alternatives to simple visual inspection.27–29
In a similar manner, respiratory variation of stroke volume has been demonstrated to predict fluid responsiveness in mechanically ventilated patients.28,30,31 Methods using either pulse pressure variation or aortic blood flow variation have been advocated. The mechanisms involved in producing this variation have been explained by Mahjoub et al.32
Developments in Pulse Oximetry Technology
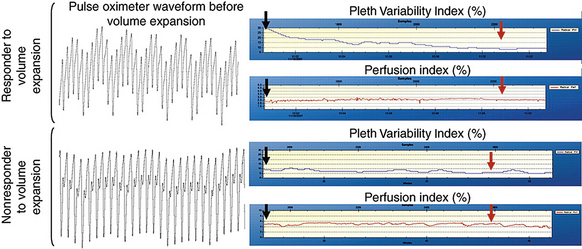
Recent advances in pulse oximetry technology have led to developments that should be of special interest to clinicians dealing with complex surgical cases such as major spine surgery. For example, existing and pending developments from Masimo Corporation (Irvine, CA), a major vendor of pulse oximeters, will allow clinicians continuously to measure oxyhemoglobin, oxygen content, carboxyhemoglobin, methemoglobin, and total hemoglobin by simple, noninvasive means. Perhaps even more important, respiratory variations in the pulse oximeter waveform can be used to predict fluid responsiveness in mechanically ventilated patients. As noted earlier, this can be helpful in discerning which hypotensive patients will respond to a fluid challenge. Masimo makes a pulse oximeter that provides a measurement known as the Pleth Variability Index (PVI) that has been shown to be useful in predicting fluid responsiveness. In a 25-patient study by Cannesson et al.,33 a ventilated patient was defined as a “responder” if his or her cardiac output increased by 15% or more after administration of 500 mL of hetastarch 6%; otherwise, the patient was considered to be a “nonresponder.” Of the patients evaluated, 16 were responders and 9 were nonresponders. In this study, a PVI greater than 14% before volume expansion discriminated between responders and nonresponders with 81% sensitivity and 100% specificity. In addition, the noninvasive PVI was as accurate at predicting fluid responsiveness as was analysis of pulse pressure variation from an invasive arterial catheter. Finally, the PVI had superior predictive accuracy compared with traditional central venous pressure and pulmonary capillary wedge pressure indicators of intravascular volume. Figure 181-1 shows sample waveforms from a responder and a nonresponder.
Automating the Delivery of Intravenous Fluids in Hypovolemic Patients
For some time there has been interest in developing methods to automate the delivery of intravenous fluids in hypovolemic patients, such as patients undergoing major spine surgery or those with severe trauma. Although a number of hemodynamic parameters have been suggested as end points to guide plasma volume expansion, simple measurements of systemic blood pressure (e.g., data obtained from an arterial line) are used most commonly for the initial assessment of hypovolemic shock and the need for plasma volume expansion. However, many other hemodynamic parameters (e.g., central venous pressure, pulmonary artery pressures, systolic pressure variation, cardiac output) may also be useful in such a setting. Although this technology is still very much in its infancy, closed-loop systems are slowly undergoing the transition from experimental animal studies to clinical application.34,35 An example of the state of the art is a series of systems described by Kramer et al.,36 who studied algorithms based on proportional-integral, fuzzy logic, and nonlinear decision table methods. They studied three sets of sheep that were subjected to a 25 mL/kg hemorrhage. In each case, closed-loop resuscitation with a target mean arterial pressure of 80 mm Hg began 30 minutes from the beginning of the hemorrhage, with two additional hemorrhages of 5 mL/kg being carried out during the resuscitation. The authors noted that all three algorithms were equally effective in restoring mean arterial pressure and cardiac output. Future systems of this genre may benefit from new noninvasive technologies for monitoring volume status and from continuing developments in computer-controlled infusion pumps.
Voluven
A recent important entry in the colloid scene in the United States is Voluven (6% hydroxyethyl starch, 130/0.4), which is produced by Fresenius Kabi (Bad Homburg, Germany). This new colloid was approved by the U.S. Food and Drug Administration in late 2007. Voluven has characteristics such as a smaller molecular weight that suggest it may have improved pharmacokinetic properties and better effects on coagulation parameters compared with older hydroxyethyl starch (HES) products. Because Voluven is more rapidly degraded than older HESs with higher molecular weights, it is eliminated more quickly by the kidneys, has a shorter volume effect, and (perhaps most importantly) has fewer adverse effects on coagulation. Experience in the settings of orthopaedic and other forms of surgery has been favorable.37–39
Postoperative Visual Loss after Spine Surgery
Vision loss or impairment after spine surgery is a devastating problem with an incidence of 0.1% to 1%.40 The causes remain poorly understood, but appear to be multifactorial and may include impaired perfusion of the eye or occlusion of retinal vessels from improper positioning.
Retinal Perfusion Pressure
The main source of blood supply to the optic nerve head is the posterior ciliary circulation by way of the pericapillary choroids and the short posterior ciliary arteries. The blood supply in the optic nerve head has a sectorial distribution, which helps to explain why visual loss is usually segmental in anterior ischemic optic neuropathy. The blood supply to the optic nerve head shows marked interindividual variation, and even varies within an individual from eye to eye. This anatomic variability may explain why vision loss or impairment develops in some people after spine surgery but not in others despite exposure to similar conditions. Posterior ciliary arteries in vivo behave as end arteries. They also have watershed zones between their distributions (i.e., no anastomoses between the arteries in these areas). These areas are most vulnerable to ischemia when perfusion pressure is inadequate.41
Perfusion pressure of the eye is defined as the difference between mean systemic arterial pressure and intraocular pressure (IOP).41 Either decreased mean arterial pressure or elevated IOP will thus decrease ocular perfusion pressure. Most commonly, perfusion pressure is impaired by a combination of decreased mean arterial pressure and increased IOP.41
Arterial Blood Pressure
Arterial hypertension, as well as arterial hypotension, may influence blood flow in the optic nerve head. In hypertensive individuals, autoregulation of ocular blood vessels usually shifts to higher levels to maintain constant blood flow. Although this improves the patients’ tolerance to high blood pressure, this upward shift in the autoregulation range makes hypertensive patients less tolerant to low blood pressures. In the optic nerve head, reduction in blood pressure below the critical autoregulatory level decreases blood flow.41 Hence, hypotension is associated with perioperative vision loss.42–44
Intraocular Pressure
Intraocular pressure is the other factor that determines ocular perfusion pressure. IOP usually increases over time in the prone position. Cheng et al.45 showed that IOP can reach up to 40 mm Hg (normal is 8–20 mm Hg) after 6 hours in the prone position. Hunt et al.46 confirmed that IOP increases in the prone position. However, they could not find a relationship between an increase in IOP and duration of the procedure, as did Cheng et al.45 The causes for increased IOP during prone positioning might include increased episcleral venous pressure,47 but also might be related to positive intraoperative fluid balance. In healthy volunteers, acute water loading (14 mL/kg) increased IOP,48 whereas exercise-induced dehydration reduced IOP.49
Types of Visual Loss and Impairment Associated with Spine Surgery
Ischemic Optic Neuropathy
Ischemic optic neuropathy occurs either in the anterior part of the optic nerve where the nerve enters the globe or the posterior part where the nerve lies within the orbit. Blood flow to the optic nerve is autoregulated41 to maintain a nearly constant supply despite changes in perfusion pressure. However, autoregulation operates effectively only over a particular range of perfusion pressures. Above and below this range, blood flow depends directly on perfusion pressure, so ischemic damage can result. Anterior ischemic optic neuropathy can also result from a low hematocrit because choroidal blood flow decreases with hemodilution, whereas the blood flow to the retina increases.50 A small increase in IOP can lead to a decrease in perfusion pressure and in anterior ischemic optic neuropathy, especially in the presence of arterial hypotension and defective autoregulation. Anterior ischemic optic neuropathy is frequently first noticed immediately on awakening from normal sleep, which corresponds to a diurnal peak of IOP.51 The true incidence of postoperative anterior ischemic optic neuropathy may be underestimated because small areas of anterior optic nerve infarction may produce only small visual defects that pass unnoticed during the postoperative period. These patients present later with low-pressure glaucoma with multiple areas of optic atrophy.52
Diagnostic criteria for posterior ischemic optic neuropathy include the following:
• An acute deficit in visual acuity, visual field, or both
• An ipsilateral relative afferent papillary defect with unilateral disease and sluggish or nonreactive pupils with bilateral symmetric involvement
• Visual deficit in the presence of a normal optic disc and funduscopic examination
• Abnormal visual evoked response
• Development of optic atrophy within 4 to 8 weeks of the onset of visual loss
The main causes of posterior ischemic optic neuropathy are hypotension, anemia, and facial edema. Facial edema can lead to increased IOP and accumulation of fluid in the orbit, which jeopardizes perfusion of the optic nerve. Posterior ischemic optic neuropathy has been described after bilateral neck dissection due to bilateral ligation of the internal jugular veins, which led to facial edema, venous congestion, and increased intraorbital pressure.44,53,54
Cortical Blindness
Cortical blindness results from damage to the occipital cortex or optic radiation; the main causes for cortical blindness are ischemic or traumatic. Clinically, loss of visual sensation is accompanied by retention of pupillary reaction to light and a normal funduscopic examination.55 Cortical blindness is usually best diagnosed by CT or MRI, which helps identify infarcted areas in the occipital lobe. Cortical blindness has been described after cardiopulmonary bypass due to generalized hypoperfusion or emboli. It also has been described after craniotomy and laryngectomy surgery due to hypoperfusion caused by hemorrhagic hypotension.55,56 Cortical blindness has been reported during spine surgery due to hypotension, anemia, and abnormal head position jeopardizing the vertebrobasilar circulation.57,58
Central Retinal Artery Occlusion
Central retinal artery occlusion is often caused by an embolic ulcerated plaque from the ipsilateral carotid artery.56 The main cause of central retinal artery occlusion after spine surgery is external ocular pressure from a head rest combined with arterial hypotension, resulting in obstruction to flow in the retinal artery.59 Central retinal artery occlusion typically presents as a complete loss of vision in one eye that usually improves with time. Funduscopic examination reveals pallor and edema of the retina, with a cherry-red spot at the fovea.
Central Retinal Vein Occlusion
Central retinal vein occlusion has been reported after spine surgery due to external pressure on the globe from a head rest in the prone position.60 Funduscopic findings usually include retinal hemorrhages in all quadrants, cotton-wool spots, and dilated, tortuous retinal veins.61
Factors Contributing to Vision Loss and Impairment after Spine Surgery
Case reports and studies of postoperative vision loss after spine surgery suggest that hypotension and anemia are major culprits in the development of ischemic vision loss. This results from the end-arterial nature of the posterior ciliary arteries, with no collaterals to compensate for low perfusion pressure.42,43,62
This is consistent with findings by Lee et al.,63 who used a porcine optic nerve model to show that the optic nerve has a limited compensatory mechanism to maintain blood flow and oxygen delivery in the presence of anemia and hypotension. Also, in the presence of severe anemia and hypotension, there is a “steal” from the ophthalmic artery to the brain to maintain perfusion of the brain in preference to the eye.63
Increased resistance to blood flow can also decrease ocular perfusion pressure. As mentioned previously, increased IOP during spine surgery in the prone position can lead to decreased ocular perfusion pressure.40,64 The other interesting finding is that blindness has been observed after bilateral ligation of the internal jugular veins. Blindness in these patients was attributed to increased IOP due to impaired drainage of the orbital venous plexus into the ophthalmic veins.54,65
During extensive spine surgery, the excessive use of crystalloids to maintain the intravascular compartment and replace blood loss has been suggested as a cause of vision loss after surgery.43 Roughly two thirds of administered crystalloid volume distributes to the extravascular compartment; the eye socket is part of this compartment. Accumulation of fluid in the eye socket can thus lead to increased IOP, along with facial edema, and therefore development of eye compartment syndrome, which compromises the retinal blood supply.66 It has been shown that a decrease in plasma oncotic pressure results from using crystalloid as the primary solution for cardiopulmonary bypass rather than colloid.67 The American Society of Anesthesiologists practice advisory for perioperative vision loss associated with spine surgery thus recommends the increased use of colloids to maintain intravascular volume in patients who have substantial blood loss.68
Effect of the Type of Fluid Replacement on Facial Edema, Chemosis, and Intraocular Pressure
Fluid management during spine surgery in the prone position plays a crucial role in determining the degree of facial edema, accumulation of fluid in the eye socket, chemosis, and IOP. It has been shown that large amounts of intravenous fluids and prolonged duration of the prone position can result in fluid collection in the face, and especially the globe, during the prone position because of venous stasis in the dependent soft tissues.53 Furthermore, a recent report of vision loss after spine surgery attributed the defect to excessive use of crystalloids leading to massive facial edema with severe chemosis, which could have increased the IOP to such a degree that eye perfusion was jeopardized despite the patient’s blood pressure and hematocrit being kept within normal limits.69 Jeon et al. have demonstrated that patient position, intraoperative fluid balance, and duration of surgery all influence the severity of postoperative chemosis.70
The type of fluid used in prone-position spine surgery is likely to determine the extent to which facial edema and chemosis develop. After crystalloid infusion, only one third remains in the intravascular compartment, whereas two thirds distributes to the extravascular compartment and to soft tissues. This leads to tissue edema and aggravates facial swelling, accumulation of the fluid in the globe, and chemosis, and increases the IOP. The potential for edema is aggravated by the fact that blood loss is usually replaced at a three-to-one ratio with crystalloid. In contrast, a major advantage of using colloids is that they stay mainly in the intravascular compartment, thus potentially decreasing facial edema, chemosis, and accumulation of fluid in the eye socket that can lead to an increased IOP. Consistent with this theory, use of crystalloid for priming bypass machines increases IOP by decreasing the colloid oncotic pressure, whereas priming with colloid solutions does not increase the colloid oncotic pressure.67
Head Position and Compartment Syndrome
Ocular chemosis (i.e., conjunctival edema) can cause short-term decreased visual acuity, patient discomfort, and an increased risk for bacterial keratitis.71 The risk factors for chemosis after prone spine surgery are the following70:
In a recent case report, a patient underwent a 4-hour L3-4 decompression with fusion in the prone position.72 His head was positioned on a C-shaped head rest on a soft bed with the left side of the face up. After surgery. the patient developed ischemic orbital compartment syndrome, manifested in his right eye with proptosis, ptosis, loss of light perception and visual acuity, and complete ophthalmoplegia. The right eye IOP was 33 mm Hg, which reached 40 mm Hg 24 hours after surgery despite emergency administration of mannitol and acetazolamide. The apparent reason for the development of compartment syndrome in this case was that the patient’s face was turned to one side, which occluded the right internal jugular vein and resulted in impaired venous drainage and increased IOP in the right eye.72
This case highlights the importance of keeping the head in the neutral position and elevated to avoid the development of an ocular compartment syndrome. In a French survey of ophthalmic complications after spine surgery, the authors proposed two preventive measures to avoid vision loss after spine surgery in the prone position. The first was to avoid eye compression when using a horseshoe-shaped head rest, and the second was to avoid lateral rotation of the head in patients with suspected carotid atheroma.73
Effect of α2-Adrenergic Agonists on the Eye during Spine Surgery
α2-Adrenergic agonists decrease IOP. After several hours of use, the topical α2 agonist brimonidine decreased aqueous production by 29%. After days of use, uveoscleral outflow was increased by 60%.74 As might thus be expected, brimonidine has been used successfully as monotherapy for glaucoma. α2-Adrenergic agonists have also been shown to have a neuroprotective effect on retinal ganglion cells, the mechanisms of which can be summarized as follows:
• α2-Adrenergic agonists increase basic fibroblast growth factor in retinal photoreceptors but not in the brain.75
• α2-Adrenergic receptor stimulation activates the antiapoptotic phosphatidyl inositol-3 kinase and protein kinase/AKt pathways. These are major pathways in the promotion of cell survival, and they block apoptosis by phosphorylation-dependent inhibition of proapoptotic signaling molecules, including BAD and capsase-9, and activation of antiapoptotic molecules such NF-κB.72 α2-Adrenergic stimulation also leads to activation of extracellular signal-regulated kinase and increased synthesis of survival factors, such as basic fibroblast growth factor and Bcl-2.76,77
• α2-Adrenergic receptor stimulation may reduce ischemic retinal injury by preventing accumulation of extracellular glutamate and asparate.78
• Brimonidine 0.2% is a commonly used ocular α2-adrenergic agonist. It has a peak effect 2 hours after administration and its effect lasts at least 8 hours.75
Cannesson M., Desebbe O., Rosamel P., et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101:200-206.
Cheng M.A., Sigurdson W., Tempelhoff R., et al. Visual loss after spine surgery: a survey. Neurosurgery. 2000;46:625-630.
Crosby E.T. Airway management in adults after cervical spine trauma. Anesthesiology. 2006;104:1293-1318.
Hayreh S.S. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4:251-263.
Toyota S., Amaki Y. Hemodynamic evaluation of the prone position by transesophageal echocardiography. J Clin Anesth. 1998;10:32-35.
1. Sawin P.D., Todd M.M., Traynelis V.C., et al. Cervical spine motion with direct laryngoscopy and orotracheal intubation: an in vivo cinefluoroscopic study of subjects without cervical abnormality. Anesthesiology. 1996;85:26-36.
2. Watts A.D., Gelb A.W., Bach D.B., et al. Comparison of the Bullard and Macintosh laryngoscopes for endotracheal intubation of patients with a potential cervical spine injury. Anesthesiology. 1997;87:1335-1342.
3. Sahin A., Salman M.A., Erden I.A., et al. Upper cervical vertebrae movement during intubating laryngeal mask, fibreoptic and direct laryngoscopy: a video-fluoroscopic study. Eur J Anaesthesiol. 2004;21:819-823.
4. LeGrand S.A., Hindman B.J., Dexter F., et al. Craniocervical motion during direct laryngoscopy and orotracheal intubation with the Macintosh and Miller blades: an in vivo cinefluoroscopic study. Anesthesiology. 2007;107:884-891.
5. Robitaille A., Williams S.R., Tremblay M.H., et al. Cervical spine motion during tracheal intubation with manual in-line stabilization: direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg. 2008;106:935-941.
6. Keller C., Brimacombe J., Keller K. Pressures exerted against the cervical vertebrae by the standard and intubating laryngeal mask airways: a randomized, controlled, cross-over study in fresh cadavers. Anesth Analg. 1999;89:1296-1300.
7. Santoni B.G., Hindman B.J., Puttlitz C.M., et al. Manual in-line stabilization increases pressures applied by the laryngoscope blade during direct laryngoscopy and orotracheal intubation. Anesthesiology. 2009;110:24-31.
8. Lennarson P.J., Smith D., Todd M.M., et al. Segmental cervical spine motion during orotracheal intubation of the intact and injured spine with and without external stabilization. J Neurosurg. 2000;92:201-206.
9. Donaldson W.F.III, Heil B.V., Donaldson V.P., et al. The effect of airway maneuvers on the unstable C1-C2 segment: a cadaver study. Spine (Phila Pa 1976). 1997;22:1215-1218.
10. Breig A., Turnbull I., Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis: a study on fresh cadaver material. J Neurosurg. 1966;25:45-56.
11. McLeod A.D., Calder I. Spinal cord injury and direct laryngoscopy: the legend lives on. Br J Anaesth. 2000;84:705-709.
12. Rau C.S., Liang C.L., Lui C.C., et al. Quadriplegia in a patient who underwent posterior fossa surgery in the prone position: case report. J Neurosurg. 2002;96:101-103.
13. Crosby E.T. Airway management in adults after cervical spine trauma. Anesthesiology. 2006;104:1293-1318.
14. Kim K.A., Wang M.Y. Anesthetic considerations in the treatment of cervical myelopathy. Spine J. 2006;6:207S-211S.
15. Sagi H.C., Beutler W., Carroll E., et al. Airway complications associated with surgery on the anterior cervical spine. Spine (Phila Pa 1976). 2002;27:949-953.
16. Sinha A., Agarwal A., Gaur A., et al. Oropharyngeal swelling and macroglossia after cervical spine surgery in the prone position. J Neurosurg Anesthesiol. 2001;13:237-239.
17. Wattenmaker I., Concepcion M., Hibberd P., et al. Upper-airway obstruction and perioperative management of the airway in patients managed with posterior operations on the cervical spine for rheumatoid arthritis. J Bone Joint Surg [Am]. 1994;76:360-365.
18. Wadsworth R., Anderton J.M., Vohra A. The effect of four different surgical prone positions on cardiovascular parameters in healthy volunteers. Anaesthesia. 1996;51:819-822.
19. Toyota S., Amaki Y. Hemodynamic evaluation of the prone position by transesophageal echocardiography. J Clin Anesth. 1998;10:32-35.
20. Takizawa D., Hiraoka H., Nakamura K., et al. Influence of the prone position on propofol pharmacokinetics. Anaesthesia. 2004;59:1250-1251.
21. Sudheer P.S., Logan S.W., Ateleanu B., et al. Haemodynamic effects of the prone position: a comparison of propofol total intravenous and inhalation anaesthesia. Anaesthesia. 2006;61:138-141.
22. Pearce D.J. The role of posture in laminectomy. Proc R Soc Med. 1957;50:109-112.
23. Bhardwaj A., Long D.M., Ducker T.B., et al. Neurologic deficits after cervical laminectomy in the prone position. J Neurosurg Anesthesiol. 2001;13:314-319.
24. Nyren S., Mure M., Jacobsson H., et al. Pulmonary perfusion is more uniform in the prone than in the supine position: scintigraphy in healthy humans. J Appl Physiol. 1999;86:1135-1141.
25. Tobin A., Kelly W. Prone ventilation: it’s time. Anaesth Intensive Care. 1999;27:194-201.
26. Pelosi P., Croci M., Calappi E., et al. The prone positioning during general anesthesia minimally affects respiratory mechanics while improving functional residual capacity and increasing oxygen tension. Anesth Analg. 1995;80:955-960.
27. Tavernier B., Makhotine O., Lebuffe G., et al. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313-1321.
28. Michard F., Boussat S., Chemla D., et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134-138.
29. Rooke G.A., Schwid H.A., Shapira Y. The effect of graded hemorrhage and intravascular volume replacement on systolic pressure variation in humans during mechanical and spontaneous ventilation. Anesth Analg. 1995;80:925-932.
30. Feissel M., Michard F., Mangin I., et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867-873.
31. Slama M., Masson H., Teboul J.L., et al. Respiratory variations of aortic VTI: a new index of hypovolemia and fluid responsiveness. Am J Physiol Heart Circ Physiol. 2002;283:H1729-H1733.
32. Mahjoub Y., Pila C., Friggeri A., et al. Assessing fluid responsiveness in critically ill patients: false-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med. 2009;37:2570-2575.
33. Cannesson M., Desebbe O., Rosamel P., et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101:200-206.
34. Chaisson N.F., Kirschner R.A., Deyo D.J., et al. Near-infrared spectroscopy-guided closed-loop resuscitation of hemorrhage. J Trauma. 2003;54:S183-S192.
35. Hoskins S.L., Elgjo G.I., Lu J., et al. Closed-loop resuscitation of burn shock. J Burn Care Res. 2006;27:377-385.
36. Kramer G.C., Kinsky M.P., Prough D.S., et al. Closed-loop control of fluid therapy for treatment of hypovolemia. J Trauma. 2008;64:S333-S341.
37. Treib J., Baron J.F., Grauer M.T., et al. An international view of hydroxyethyl starches. Intensive Care Med. 1999;25:258-268.
38. Langeron O., Doelberg M., Ang E.T., et al. Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg. 2001;92:855-862.
39. Gandhi S.D., Weiskopf R.B., Jungheinrich C., et al. Volume replacement therapy during major orthopedic surgery using Voluven (hydroxyethyl starch 130/0.4) or hetastarch. Anesthesiology. 2007;106:1120-1127.
40. Cheng M.A., Sigurdson W., Tempelhoff R., et al. Visual loss after spine surgery: a survey. Neurosurgery. 2000;46:625-630.
41. Hayreh S.S. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4:251-263.
42. Brown R.H., Schauble J.F., Miller N.R. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994;80:222-226.
43. Chang S.H., Miller N.R. The incidence of vision loss due to perioperative ischemic optic neuropathy associated with spine surgery: the Johns Hopkins Hospital experience. Spine (Phila Pa 1976). 2005;30:1299-1302.
44. Dunker S., Hsu H.Y., Sebag J., et al. Perioperative risk factors for posterior ischemic optic neuropathy. J Am Coll Surg. 2002;194:705-710.
45. Cheng M.A., Todorov A., Tempelhoff R., et al. The effect of prone positioning on intraocular pressure in anesthetized patients. Anesthesiology. 2001;95:1351-1355.
46. Hunt K., Bajekal R., Calder I., et al. Changes in intraocular pressure in anesthetized prone patients. J Neurosurg Anesthesiol. 2004;16:287-290.
47. Lam A.K., Douthwaite W.A. Does the change of anterior chamber depth or/and episcleral venous pressure cause intraocular pressure change in postural variation? Optom Vis Sci. 1997;74:664-667.
48. Brucculeri M., Hammel T., Harris A., et al. Regulation of intraocular pressure after water drinking. J Glaucoma. 1999;8:111-116.
49. Martin B., Harris A., Hammel T., et al. Mechanism of exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci. 1999;40:1011-1015.
50. Roth S. The effects of isovolumic hemodilution on ocular blood flow. Exp Eye Res. 1992;55:59-63.
51. James C.B., Smith S.E. The effect of posture on the intraocular pressure and pulsatile ocular blood flow in patients with non-arteritic anterior ischaemic optic neuropathy. Eye. 1991;5:309-314.
52. Yamamoto T., Kitazawa Y. Vascular pathogenesis of normal-tension glaucoma: a possible pathogenetic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Prog Retin Eye Res. 1998;17:127-143.
53. Buono L.M., Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50:15-26.
54. Schobel G.A., Schmidbauer M., Millesi W., et al. Posterior ischemic optic neuropathy following bilateral radical neck dissection. Int J Oral Maxillofac Surg. 1995;24:283-287.
55. Aldrich M.S., Alessi A.G., Beck R.W., et al. Cortical blindness: etiology, diagnosis, and prognosis. Ann Neurol. 1987;21:149-158.
56. Kollarits C.R., Lubow M., Hissong S.L. Retinal strokes: I. Incidence of carotid atheromata. JAMA. 1972;222:1273-1275.
57. Huber J.F., Grob D. Bilateral cortical blindness after lumbar spine surgery: a case report. Spine (Phila Pa 1976). 1998;23:1807-1809.
58. Myers M.A., Hamilton S.R., Bogosian A.J., et al. Visual loss as a complication of spine surgery: a review of 37 cases. Spine (Phila Pa 1976). 1997;22:1325-1329.
59. Grossman W., Ward W.T. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest: case report and literature review. Spine (Phila Pa 1976). 1993;18:1226-1228.
60. Hollenhorst R.W., Svien H.J., Benoit C.F. Unilateral blindness occurring during anesthesia for neurosurgical operations. AMA Arch Ophthalmol. 1954;52:819-830.
61. Robinson M.K., Halpern J.I. Retinal vein occlusion. Am Fam Physician. 1992;45:2661-2666.
62. Stevens W.R., Glazer P.A., Kelley S.D., et al. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22:1319-1324.
63. Lee L.A., Deem S., Glenny R.W., et al. Effects of anemia and hypotension on porcine optic nerve blood flow and oxygen delivery. Anesthesiology. 2008;108:864-872.
64. Abraham M., Sakhuja N., Sinha S., et al. Unilateral visual loss after cervical spine surgery. J Neurosurg Anesthesiol. 2003;15:319-322.
65. Wilson J.F., Freeman S.B., Breene D.P. Anterior ischemic optic neuropathy causing blindness in the head and neck surgery patient. Arch Otolaryngol Head Neck Surg. 1991;117:1304-1306.
66. Farag E., Doyle D.J. Vision loss after spine surgery: a new hypothesis. Can J Anaesth. 2006;53:420.
67. Abbott M.A., McLaren A.D., Algie T. Intra-ocular pressure during cardiopulmonary bypass: a comparison of crystalloid and colloid priming solutions. Anaesthesia. 1994;49:343-346.
68. American Society of Anesthesiologists Task Force on Perioperative Blindness. Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006;104:1319-1328.
69. Kamming D., Clarke S. Postoperative visual loss following prone spinal surgery. Br J Anaesth. 2005;95:257-260.
70. Jeon Y.T., Park Y.O., won Hwang H.J., et al. Effect of head position on postoperative chemosis after prone spinal surgery. J Neurosurg Anesthesiol. 2007;19:1-4.
71. Parkin B., Turner A., Moore E., et al. Bacterial keratitis in the critically ill. Br J Ophthalmol. 1997;81:1060-1063.
72. Yu Y.H., Chen W.J., Chen L.H., et al. Ischemic orbital compartment syndrome after posterior spinal surgery. Spine (Phila Pa 1976). 2008;33:E569-E572.
73. Delattre O., Thoreux P., Liverneaux P., et al. Spinal surgery and ophthalmic complications: a French survey with review of 17 cases. J Spinal Disord Tech. 2007;20:302-307.
74. Cantor L.B. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother. 2000;1:815-834.
75. Wen R., Cheng T., Li Y., et al. Alpha 2-adrenergic agonists induce basic fibroblast growth factor expression in photoreceptors in vivo and ameliorate light damage. J Neurosci. 1996;16:5986-5992.
76. Ballif B.A., Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397-408.
77. Xia Z., Dickens M., Raingeaud J., et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326-1331.
78. Donello J.E., Padillo E.U., Webster M.L., et al. α2-Adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J Pharmacol Exp Ther. 2001;296:216-223.