CHAPTER 87 ADVANCED TECHNIQUES IN MECHANICAL VENTILATION
Since the introduction of mechanical ventilation using a bicycle tire and bellows about 50 years ago, the science and art of respiratory therapy has advanced dramatically—allowing the clinician to ventilate and oxygenate patients who would have died in the past due to limitations of man and machine. This chapter focuses on recent advances and future considerations in ventilatory support to allow further improvements in respiratory care and survival from acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and chronic respiratory failure.
ALTERNATIVES TO CONVENTIONAL MECHANICAL VENTILATION
Pressure-Controlled Ventilation
Pressure-controlled ventilation (PCV) is pressure-limited timecycled breathing that is completely controlled by the ventilator, with no participation by the patient. Inspiratory airway pressure increases early in the respiratory cycle, and is maintained at that specified pressure throughout the remainder of the delivery phase. The major benefit of PCV relates to the inspiratory flow pattern and its benefit in gas delivery for patients with suppressed respiratory efforts. In pressure-cycled breathing, the inspiratory flow decreases exponentially during lung inflation in order to keep the airway pressure at the preselected value. Thus, this type of flow pattern can improve gas exchange. The primary disadvantage of PCV is the tendency for inflation volumes to vary with changes in the mechanical properties of the lungs. There is a proportional relationship between the lung inflation and the peak inflation pressure. When a constant peak inflation pressure is reached, the inflation volume decreases as the airway resistance increases or lung compliance decreases. The result is fluctuation in inflation volumes due to reliance on a specific pressure target.
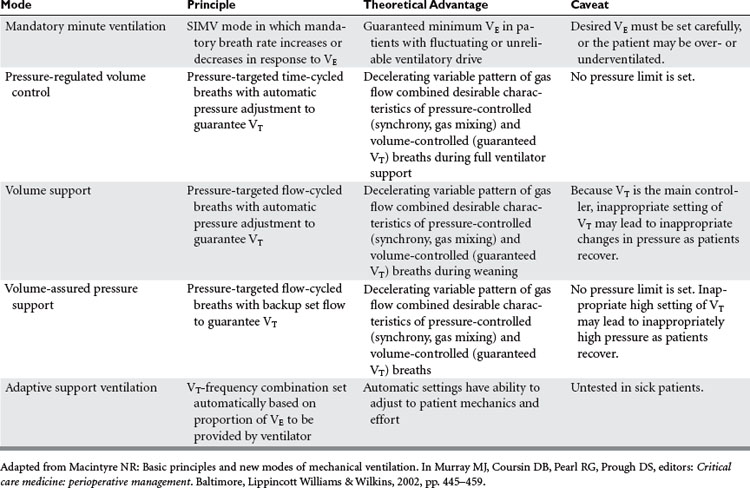
Mandatory Minute Ventilation
Mandatory minute ventilation (MMV) is a mode of mechanical ventilation in which the minimum level of VE needed by the patient is provided. If the patient’s spontaneous ventilation is insufficient to meet the predetermined VE, the ventilator provides the difference. Conversely, if the patient’s spontaneous breathing exceeds the target VE no ventilator support is provided. This mode is one of the so-called “closed-loop” ventilation modes (Table 1) because the ventilator varies its parameters in response to the patient’s own intrinsic ventilatory requirements. The major advantage of MMV is the capability to vary ventilatory support according to the response of the patient. This mode of mechanical ventilation is best suited for patients with severe neuromuscular disease or drug overdose, or patients heavily sedated. One of the main disadvantages with MMV is that alveolar ventilation may not be matched equally with exhaled VE, thus diminishing closing volumes and leading to atelectasis. None of the closed-loop modes, MMV included, has been tested sufficiently on critically ill patients to recommend widespread incorporation into practice.
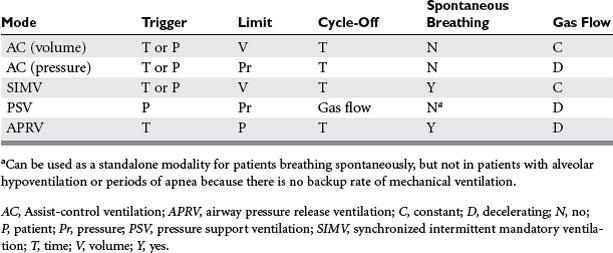
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) (Table 2) has been used as an alternative mode of mechanical ventilation in patients with acute respiratory failure. APRV, which has been available in some ventilator models since the mid-1990s, allows for the unloading during exhalation of any positive pressure provided during inhalation in order to facilitate the egress of the tidal breath. Release of airway pressure from an elevated baseline simulates exhalation. Technically, APRV is time-triggered, pressure-limited, time-cycled mechanical ventilation. Conceptualizing APRV as continuous positive airway pressure (CPAP) with regular, brief, intermittent releases of airway pressure may facilitate understanding. It can augment alveolar ventilation in the patient breathing spontaneously, or provide full support to the apneic patient.
Permissive Hypercapnia
Permissive hypercapnia is an adjunctive protective ventilatory strategy. Permissive hypercapnia defines a ventilatory strategy for acute respiratory failure in which the lungs are ventilated with a low VT, permitting PaCO2 levels to increase. Permissive hypercapnia aims to avoid hyperinflation-induced lung trauma, as described initially by limiting the plateau airway pressure (as a surrogate of static alveolar pressure) to approximately 30–35 cm H2O while allowing PaCO2 to increase absent any contraindications (such as increased intracranial pressure).
PHARMACOTHERAPY
Inhaled Nitric Oxide
Inhaled nitric oxide (NO) is a selective pulmonary vasodilator that acts on the alveolar endothelium to produce regional vasodilation in well-ventilated lung units where it is distributed. NO at a dose of 40 parts per million (PPM) has been demonstrated to improve ventilation-perfusion mismatching, hypoxemia, and pulmonary hypertension in patients who have ALI/ARDS. In contrast, systemic vasodilators may actually cause pulmonary vasodilation in nonventilated lung, thereby abrogating hypoxic vasoconstriction and leading to hypoxemia and exacerbated ventilation-perfusion inequality. NO is also a bronchodilator and has anti-inflammatory properties that have been helpful in lung transplant patients. When inhaled, it diffuses into the blood stream—where it is metabolized rapidly and excreted via the urine, minimizing systemic effects. Three randomized controlled trials suggest that NO may improve oxygenation for up to 72 hours, but neither survival nor a shorter duration of mechanical ventilation was observed. In addition, rebound pulmonary hypertension has been reported with cessation of therapy. Therefore, inhaled NO is not recommended for therapy of ALI/ARDS even as rescue therapy.
UNCONVENTIONAL METHODS OF PULMONARY SUPPORT
Prone Positioning
Prone positioning (PP)—in which the patient is positioned prone, most commonly using a specialty bed—improves oxygenation by decreasing ventilation-perfusion mismatching. Ventilated lung segments tend to be in nondependent portions of lung, whereas perfused lung segments (and higher pulmonary vascular pressures and hence lung edema) tend to be in dependent portions of lung. Positive-pressure ventilation exacerbates the mismatching. PP reverses the dependency of the lung, and newly dependent wellventilated lung segments are well perfused for several hours until the effects of gravity and positive airway pressures restore the previous conditions over a period of several hours. Drainage of secretions may also be facilitated in the prone position. The large multicenter randomized controlled trial by Gattinoni et al. showed significant improvement in PaO2:FIO2 and in 10-day mortality, but this modest advantage did not persist beyond ICU discharge. Complications with PP include pressure sores, need for increased sedation, facial edema, and difficulty maintaining airway patency or restoring it when the patient is inverted. Recently, facilitated PP using a specialized bed has shown promise. Thus, this intervention may yet prove useful for management of severely hypoxic patients with ARDS.
Betensley AD, Kakkar R. Noninvasive positive pressure ventilation. Hosp Physician. 2005;8:3-12.
Branson RD, Johannigman JA, Campbell RS. Closed-loop mechanical ventilation. Respir Care. 2002;47:427-451.
Burns KE, Sinuff T, Adhikari NK, et al. Bilevel non-invasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477-1483.
Carlucci A, Richard J, Wysocki M, et al. Noninvasive versus conventional mechanical ventilation. An epidemiological survey. Am J Respir Crit Care Med. 2001;163:874-880.
Cheung TM, Yam LY, So LK, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845-850.
Cinnella G, Dambrosio M, Brienza N, et al. Compliance and capnography monitoring during independent lung ventilation: report of two cases. Anesthesiology. 2000;93:275-278.
Dellinger RP. Inhaled nitric oxide in acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 1999;25:881-883.
Fan E, Mehta S. High-frequency oscillatory ventilation and adjunctive therapies: inhaled nitric oxide and prone positioning. Crit Care Med. 2005;33(Suppl 3):S182-S187.
Frawley PM, Habashi NM. Airway pressure release ventilation: theory and practice. AACN Clin Issues. 2001;12:234-246.
Gattinoni L, Tognoni G, Presenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568-573.
Grasso S, Ranieri MV. Proportional assist ventilation. Semin Respir Crit Care Med. 2000;21:161-166.
Hemmila MR, Rowe SA, Boules TN. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595-607.
Imai Y, Parodo J, Kajakawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104-2112.
Kacmarek RM, Slutsky AS, editors. Mechanical Ventilation: Current Trends and Future Directions. Des Plaines, IL: Society of Critical Care Medicine, 2005.
MacIntyre NR. Basic principles and new modes of mechanical ventilation. In: Murray MJ, Coursin DB, Pearl RG, Prough DS, editors. Critical Care Medicine. Perioperative Management. Baltimore, Lippincott: Williams & Wilkins; 2002:445-459.
MacIntyre NR, Ho L. Effects of initial flow rate and breath termination criteria on pressure support ventilation. Chest. 1991;99:134-138.
Matthay MA, editor. Acute Respiratory Distress Syndrome. New York: Marcel Dekker, 2003.
Needham DM, Bronskill SE, Calinawan JR, et al. Projected incidence of mechanical ventilation in Ontario to 2026: preparing for the aging baby boomers. Crit Care Med. 2005;33:574-579.
Sevransky JE, Levy MM, Marini JJ. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Crit Care Med. 2004;32:S548-S553. (Suppl)
Siegel JH, Stoklosa JC, Borg U, et al. Quantification of asymmetric lung pathophysiology as a guide to the use of simultaneous independent lung ventilation in post-traumatic and septic ARDS. Ann Surg. 1985;202:425-439.
Sydow M, Burchardi H, Ephraim E, et al. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury: comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med. 1994;149:1550-1556.
The Acute Respiratory Distress Network Investigators. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. New York: McGraw-Hill, 1994.
Uhlig S, Ranieri M, Slutsky AS. Biotrauma hypothesis of ventilator-associated lung injury. Am J Respir Crit Care Med. 2004;169:314-315.
Varelmann D, Wrigge H, Zinserling J, et al. Proportional assist versus pressure support ventilation in patients with acute respiratory failure: cardiorespiratory responses to artificially increased ventilatory demand. Crit Care Med. 2005;33:1968-1975.