CHAPTER 15 Advanced MRI and PET Imaging of Meningiomas
INTRODUCTION
Meningiomas are typically diagnosed by their characteristic appearance on conventional imaging. However, 10% to 15% of meningiomas demonstrate an atypical appearance, with rimlike enhancement, cystic components, hemorrhage, marked peritumoral edema, or parenchymal invasion.1 These meningiomas may resemble malignant intra-axial neoplasms with cystic or necrotic components. Further, other extra-axial pathology, such as metastatic disease or lymphoma, may mimic the appearance of meningiomas. After complete surgical excision, 5-year recurrence rates for benign, atypical, and malignant meningiomas have been reported as 3%, 38%, and 78%, respectively.2 For these reasons, advanced noninvasive imaging techniques may provide useful diagnostic information to aid in surgical and treatment planning and in predicting prognosis.3
DIFFUSION-WEIGHTED MAGNETIC RESONANCE IMAGING
Diffusion describes the thermally induced behavior of molecules moving in a random pattern. Diffusion-weighted magnetic resonance imaging (DWI) measures the diffusivity of hydrogen atoms in biologic tissue. The diffusivity is quantified by means of an apparent diffusion coefficient (ADC). Water diffusion is highly dependent on the ratio of extracellular to intracellular space in biologic tissue. DWI has proven utility in acute stroke management, and has been used to evaluate primary brain neoplasms. Studies have shown a correlation between ADC values, tumor cellularity, and tumor grade. Primary brain tumors with higher cellularity or higher grades typically have lower ADC values when compared with lower grade neoplasms.4–6 Low ADC values in malignant tumors likely reflect an underlying histologic pattern of densely packed tumor cells, thus decreasing the relative fraction of extracellular space and inhibiting the motion, or diffusivity, of water molecules.7,8
Delineation of meningioma from surrounding tissue on DWI and ADC maps is inferior to that by contrast-enhanced T1-weighted MR imaging.9 Meningiomas typically appear isointense to normal white matter on DWI, with ADC values only slightly higher than those of white matter.4,8 The ADC values of solid gliomas, metastases, and meningiomas are all within a similar range.9
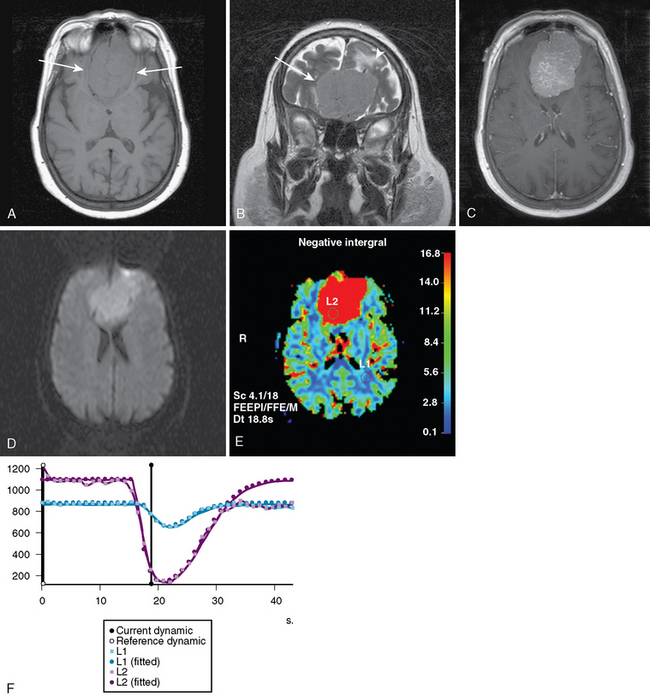
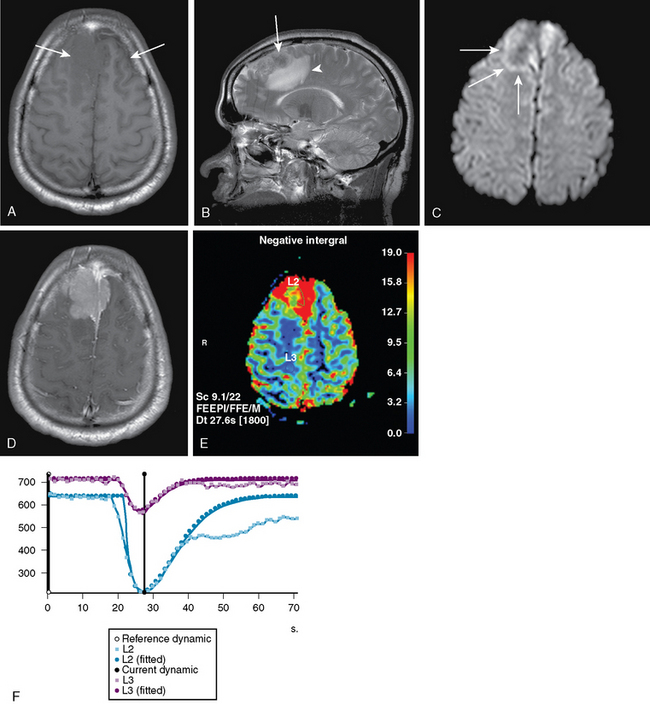
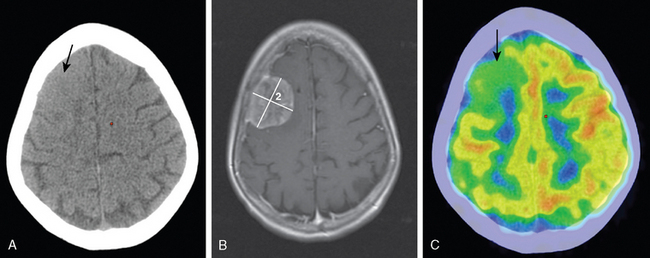
Studies analyzing the potential role of DWI in distinguishing typical from atypical/malignant meningiomas have yielded varying results10–13 (Figs. 15-1 and 15-2). These studies have in common relatively small sample sizes, which may underlie their differing results. A study examining the potential role of DWI in the evaluation of peritumoral edema adjacent to meningiomas found that mean ADC values of peritumoral edema did not differ significantly between typical and atypical meningiomas.11 In addition, ADC values of peritumoral edema do not differ significantly in meningiomas, astrocytomas, or metastatic disease.12 Thus the role of DWI in the imaging workup of meningiomas is still evolving and should be studied in larger series.
PERFUSION-WEIGHTED MRI
The overall principle of MR perfusion in oncologic imaging is that tumor growth occurs in the setting of increased metabolic demands required by rapid cell growth and increased cell turnover. This, in turn, leads to new blood vessel formation, or angiogenesis, which results in higher blood volume and blood flow in the tumor bed.14 Given the correlation between microvascular density and cerebral blood volume (rCBV), and the further correlation of microvascular density and tumor grade, higher grade tumors would be expected to have higher rCBV.
The best established method of evaluating tissue perfusion using MR imaging is dynamic-susceptibility imaging, which exploits the T2* susceptibility effects of a paramagnetic contrast agent during its first pass through tissue. Using this method, the loss of T2* signal is proportional to the concentration of gadolinium within a particular tissue. Measurement of rCBV using dynamic contrast-enhanced MR has shown good correlation with tumor grade according to the World Health Organization (WHO) tumor grading scheme.15
Meningiomas are highly vascular tumors that demonstrate intense contrast enhancement on conventional imaging. Accordingly, meningiomas have elevated rCBV values, which exceed those of high-grade gliomas.16–18 Attempts to prospectively assess the grade of meningiomas using rCBV values have been unsuccessful19 (see Figs. 15-1 and 15-2).
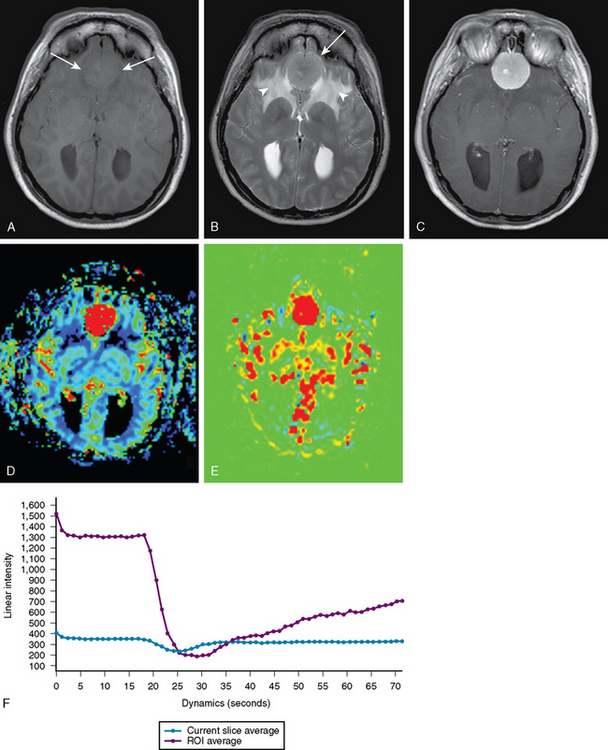
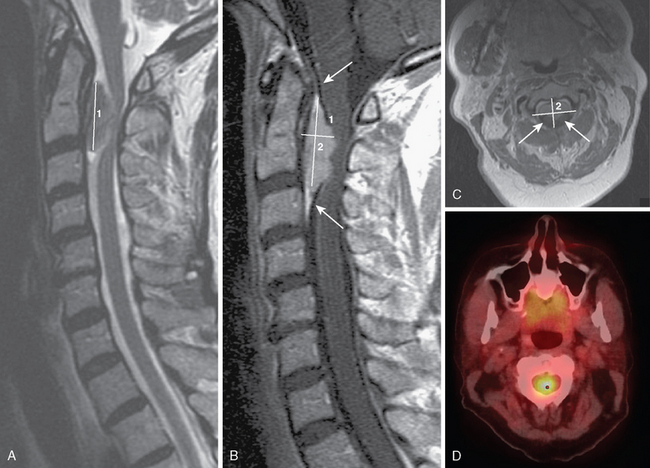
Another approach to MR perfusion imaging, arterial spin labeling (ASL), is an endogenous contrast approach in which the patient’s own blood is magnetically labeled with an MR prepulse before the blood’s entry into the imaging volume.20 Because of the use of a purely diffusible tracer, ASL techniques may allow absolute quantification of blood flow unaffected by disruption of the blood–brain barrier (BBB), a common problem using contrast reagent-based techniques for assessing tumor perfusion (Fig. 15-3).
Assessment of endothelial permeability using perfusion-weighted magnetic resonance imaging has proven useful in distinguishing meningioma tumor grade. Specifically, atypical meningiomas were found to have a significantly higher degree of degree of permeability than typical meningiomas. It has been hypothesized that the elevated permeability seen in atypical meningiomas may be related to capillary leakiness and the larger size of endothelial gap junctions when compared with typical meningiomas.19
Occasionally, meningiomas cannot be distinguished from an isolated dural metastasis by conventional imaging. Because meningiomas have high rCBV, a relatively low rCBV in a dural lesion should suggest metastasis rather than meningioma.21 On the other hand, hypervascular metastases, such as those from renal cell carcinoma, melanoma, or Merkel carcinoma, may present with elevated rCBV, which may be indistinguishable from that of meningioma.21,22
PROTON MAGNETIC RESONANCE SPECTROSCOPY
Proton MR spectroscopy (MRS) is a noninvasive imaging technique that gives additional information about the biochemical content of living tissue, which is often a useful adjunct to anatomic imaging findings.23–25 Spectroscopy data are analyzed as a spectrum. Each spectral peak is characterized by its resonance frequency, height, width, and area. Height or area under the peak may be calculated to give a relative measure of the concentrations of protons. The major brain metabolites detected by proton MRS are choline, creatine, N-acetyl aspartate (NAA), lactate, myoinositol, glutamine and glutamate, lipid, and the amino acids leucine and alanine.26
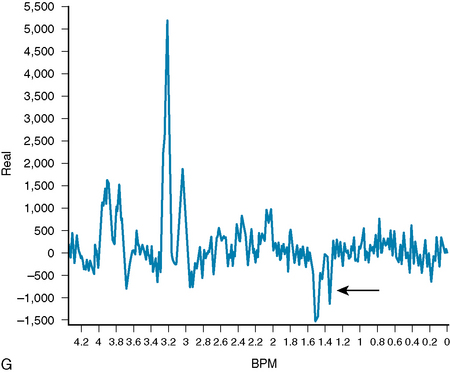
Using MRS, the majority of meningiomas can be confidently distinguished from other intracranial tumors on the basis of an increased amount of alanine (see Fig. 15-3). In meningiomas, pyruvate kinase is inhibited by L-alanine, resulting in an increased pool of pyruvate, which may then be converted to alanine.27 The alanine peak, defined as an inverted doublet centered at 1.47 ppm, has been found to be relatively specific for meningioma.28–30 However, in some series, alanine was not found; necrotic areas may show less alanine.27,31,32 An absence or low quantity of lipid at 0.9 and 1.30 ppm in meningiomas is useful in distinguishing them from gliomas, which characteristically have higher lipid content.33,34
MRS may also be useful in distinguishing typical from atypical/malignant meningiomas. In a study comparing MRS characteristics of 25 benign meningiomas, WHO grade I, with five meningiomas of WHO grade II and III,35 the mean choline-to-creatine ratio was significantly higher in the nonbenign meningiomas (7.85 ± 3.23) compared with the benign group (2.56 ± 1.26). The same study35 reported a methylene peak at 1.3 ppm, correlating highly with intratumoral necrosis and high tumor grade. An increase in choline concentration, reflected by a large choline peak on MRS, likely reflects increased membrane synthesis or increased cellularity. In gliomas, a correlation between high concentrations of choline and malignant grade has been well documented.36,37 The presence of necrosis in meningiomas, characterized by the methylene peak, is highly suggestive of malignancy in the absence of prior embolization.38
Increased levels of lactate and lipid, indicating areas of infarction, characterize the MRS of meningiomas treated with embolization.39 In patients undergoing embolization without surgery, long-term follow-up with MRS indicated subsequent fatty degeneration within the tumor.40
POSITRON EMISSION TOMOGRAPHY OF MENINGIOMAS
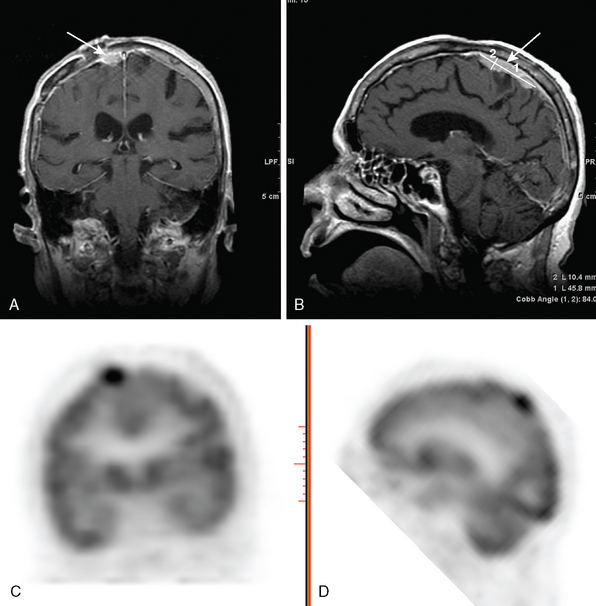
The use of positron emission tomography (PET) in the diagnosis of extracranial malignancies is well described.41 The high rate of glucose metabolism in many tumors relative to that of surrounding tissue makes them readily detectable by PET imaging. In the brain, which accounts for 25% of total body glucose metabolism, the metabolically active background of cortical activity results in a lower sensitivity for radiointense tumors. In lesions that anatomically approximate the cerebral cortex, such as classic meningiomas, tumor detection is even more difficult (Fig. 15-4). Consequently, the discovery of a meningioma by FDG-PET is usually incidental (Fig. 15-5).
Currently, [18F]fluorodeoxyglucose (FDG) is the only FDA-approved central nervous system (CNS) radiotracer. With the exception of high-grade neoplasms, the majority of intracranial tumors tend to show less FDG PET activity than the normal brain gray matter. Meningiomas demonstrate similar trends. The higher-grade meningioma cell types tend to display more intense uptake of [18F]FDG. One study42 demonstrated that FDG PET was helpful in distinguishing between low-grade and high-grade or malignant tumors (Fig. 15-6).
Ongoing research involves the development of radiotracers that take advantage of tumor protein metabolism by labeling amino acids with positron emitters. Numerous studies suggest that positron-labeled amino acids are more sensitive than FDG PET in detecting tumor amid the background of gray matter activity.43–46 The radiotracers relevant to primary intracranial tumors, including meningioma, include [11C]methionine (11C[MET]),42 [18F]fluorothymidine ([18F]FLT),47 and [18F]fluoro-L-tyrosine [18F]TYR PET.45 The relatively short half-life of [11C]MET limits its use to institutions with cyclotrons in close proximity.
The uptake of radiolabeled amino acids in tumors depends on many factors, including blood flow to the brain, the integrity of the BBB, and tissue binding factors. It has been suggested that protein transport across membranes and protein synthesis within tumors can be detected using different radiolabeled amino acids.48 Both [11C]MET and [18F]TYR seem to correlate best with amino acid transport within tumors and 14C-radiolabeled leucine ([14C]Leu) correlates best with protein synthesis rates. Further reports suggest that l-[1-11C]tyrosine PET could be used to approximate the rate of protein synthesis.49 Others have investigated the contribution of a disrupted BBB in high-grade tumors versus passive transport and “trapping” of [11C]MET within the tumor, acting as a metabolic compartment.50
The uptake of radiotracer into necrotic tumor through a disrupted BBB is common across all tumor types and does not yield additional information about tumor characterization. It does, however, imply a generally aggressive process. Further, rates of proliferation may differ between tumors of similar histopathology as well as between different regions in the same tumor.51
Interferon treatment of postoperative residual or inoperable meningiomas has been evaluated with [11C]MET PET.52 This study demonstrated that PET might complement MRI in the follow-up of such meningiomas. Using PET, imagers were better able to identify smaller tumors not clearly detected by MRI or CT imaging.
Similarly, [11C]MET PET has proven useful in delineating the extent of meningiomas difficult to assess on conventional imaging due to their proximity to the skull base, cavernous sinus, or the orbit. One study incorporated MRI, CT, and MET PET fused data to delineate these meningiomas accurately.43 In 40% of cases, smaller tumor volumes were predicted by [11C]MET PET imaging compared with MRI/CT imaging, therefore altering the treatment plan. Conversely, in 20% of cases, increased tumor volumes were predicted. Future work with larger patient series may provide clinical relevance for treatment planning with respect to actual tumor size and sparing of vital tissues.
FDG PET has been used as a noninvasive method of differentiating recurrent tumor from radiation necrosis,53 but hypermetabolism associated with radiation necrosis may enhance on MRI and take up FDG in a manner that mimics tumor recurrence.53 In several studies, extracranial sites of inflammation mimicking recurrent tumor have been reported.54–56
It is known that meningiomas contain a somatostatin receptor.57 In a series of 28 patients,58 the utility of DOTA-D-Phe1-Tyr3-octreotide (DOTA-TOC), a somatostatin analog radiolabeled with 68Ga, in detection of meningiomas was demonstrated. Meningiomas as small as 7 mm were identified by the relatively high uptake of [68Ga]DOTA-TOC. This work suggests that PET imaging with this somatostatin analog results in much higher uptake within meningiomas when compared to the background brain, bone, and soft tissues. This may impact treatment planning for meningiomas in close proximity to the skull base.59 In addition, this method may also be useful in differentiating meningeal infiltration from meningeal reaction.59
In a recent study of 13 patients with meningiomas who underwent irradiation,45 researchers reported that PET/CT and [2-18F]fluoro-L-tyrosine ([18F]TYR), a marker of amino acid transport and substrate of the L-type amino acid transporter 1 (LAT1), were complementary. In 38% of cases, MRI underestimated the tumor size while it overestimated tumor size in 8%. The results of this study confirm those of earlier studies43 in that PET, MRI, and CT should be complementary in the evaluation of meningioma.
Although CT is the gold standard in the evaluation of bone, MET PET and [18F]TYR may provide additional information. Evaluation of hyperostotic bone taken from skull base meningioma resections has shown that in 51% the neoplasm infiltrates bone.45 No current work makes reference to invasion of bone directly, but given the known affinity of emerging radiotracers for meningioma, the high tumor to local soft tissue signal and the improving resolution of PET/CT may allow for accurate assessment of bone involvement in surgical or radiotherapy planning.
PET has also been shown to demonstrate early, post-treatment functional change in the behavior of tumors. Such change is not detectable by MR or CT imaging. This information may impact the interval between subsequent surgeries or radiation treatments. It has been shown that after proton beam therapy, meningiomas take up less [11C]MET compared to their pretreatment status, although the meningioma may not change immediately in size.51 The mechanism for this post-treatment decrease in uptake is not well understood. Possible theories include postradiation decrease in the tumor’s capacity for cellular reproduction, apoptosis, or the effect of radiotherapy on small vessels with resulting thrombosis.
[1] Buetow M.P., Buetow P.C., Smirniotopoulos J.G. Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11:1087-1106.
[2] Jaaskelainen J., Haltia M., Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233-242.
[3] Ayerbe J., Lobato R.D., de la Cruz J., et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien). 1999;141:921-932.
[4] Eis M., Els T., Hoehn-Berlage M., Hossmann K.A. Quantitative diffusion MR imaging of cerebral tumor and edema. Acta Neurochir Suppl (Wien). 1994;60:344-346.
[5] Sugahara T., Korogi Y., Kochi M., et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53-60.
[6] Tien R.D., Felsberg G.J., Friedman H., Brown M., MacFall J. MR imaging of high-grade cerebral gliomas: value of diffusion-weighted echoplanar pulse sequences. AJR Am J Roentgenol. 1994;162:671-677.
[7] Szafer A., Zhong J., Anderson A.W., Gore J.C. Diffusion-weighted imaging in tissues: theoretical models. NMR Biomed. 1995;8:289-296.
[8] Vorisek I., Hajek M., Tintera J., Nicolay K., Sykova E. Water ADC, extracellular space volume, and tortuosity in the rat cortex after traumatic injury. Magn Reson Med. 2002;48:994-1003.
[9] Stadnik T.W., Chaskis C., Michotte A., et al. Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol. 2001;22:969-976.
[10] Filippi C.G., Edgar M.A., Ulug A.M., Prowda J.C., Heier L.A., Zimmerman R.D. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol. 2001;22:65-72.
[11] Hakyemez B., Yildirim N., Gokalp G., Erdogan C., Parlak M. The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology. 2006;48:513-520.
[12] Kono K., Inoue Y., Nakayama K., et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001;22:1081-1088.
[13] Yamasaki F., Kurisu K., Satoh K., et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985-991.
[14] Jackson A., Kassner A., Annesley-Williams D., Reid H., Zhu X.P., Li K.L. Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tumor grade. AJNR Am J Neuroradiol. 2002;23:7-14.
[15] Ludemann L., Grieger W., Wurm R., Budzisch M., Hamm B., Zimmer C. Comparison of dynamic contrast-enhanced MRI with WHO tumor grading for gliomas. Eur Radiol. 2001;11:1231-1241.
[16] Cha S., Knopp E.A., Johnson G., Wetzel S.G., Litt A.W., Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11-29.
[17] Hakyemez B., Erdogan C., Bolca N., Yildirim N., Gokalp G., Parlak M. Evaluation of different cerebral mass lesions by perfusion-weighted MR imaging. J Magn Reson Imaging. 2006;24:817-824.
[18] Kremer S., Grand S., Remy C., et al. Cerebral blood volume mapping by MR imaging in the initial evaluation of brain tumors. J Neuroradiol. 2002;29:105-113.
[19] Yang S., Law M., Zagzag D., et al. Dynamic contrast-enhanced perfusion MR imaging measurements of endothelial permeability: differentiation between atypical and typical meningiomas. AJNR Am J Neuroradiol. 2003;24:1554-1559.
[20] Silva A.C., Kim S.G., Garwood M. Imaging blood flow in brain tumors using arterial spin labeling. Magn Reson Med. 2000;44:169-173.
[21] Kremer S., Grand S., Remy C., et al. Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningioma. Neuroradiology. 2004;46:642-648.
[22] Kremer S., Grand S., Berger F., et al. Dynamic contrast-enhanced MRI: differentiating melanoma and renal carcinoma metastases from high-grade astrocytomas and other metastases. Neuroradiology. 2003;45:44-49.
[23] Dowling C., Bollen A.W., Noworolski S.M., et al. Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. AJNR Am J Neuroradiol. 2001;22:604-612.
[24] Majos C., Cucurella G., Aguilera C., Coll S., Pons L.C. Intraventricular meningiomas: MR imaging and MR spectroscopic findings in two cases. AJNR Am J Neuroradiol. 1999;20:882-885.
[25] Usenius J.P., Kauppinen R.A., Vainio P.A., et al. Quantitative metabolite patterns of human brain tumors: detection by 1H NMR spectroscopy in vivo and in vitro. J Comput Assist Tomogr. 1994;18:705-713.
[26] Castillo M., Kwock L., Mukherji S.K. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol. 1996;17:1-15.
[27] Castillo M., Kwock L. Clinical applications of proton magnetic resonance spectroscopy in the evaluation of common intracranial tumors. Top Magn Reson Imaging. 1999;10:104-113.
[28] Kinoshita Y., Yokota A. Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed. 1997;10:2-12.
[29] Majos C., Alonso J., Aguilera C., et al. Proton magnetic resonance spectroscopy ((1)H MRS) of human brain tumours: assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol. 2003;13:582-591.
[30] Shimizu H., Kumabe T., Tominaga T., et al. Noninvasive evaluation of malignancy of brain tumors with proton MR spectroscopy. AJNR Am J Neuroradiol. 1996;17:737-747.
[31] Gill S.S., Thomas D.G., Van Bruggen N., et al. Proton MR spectroscopy of intracranial tumours: in vivo and in vitro studies. J Comput Assist Tomogr. 1990;14:497-504.
[32] Harting I., Hartmann M., Bonsanto M.M., Sommer C., Sartor K. Characterization of necrotic meningioma using diffusion MRI, perfusion MRI, and MR spectroscopy: case report and review of the literature. Neuroradiology. 2004;46:189-193.
[33] Majos C., Alonso J., Aguilera C., et al. Utility of proton MR spectroscopy in the diagnosis of radiologically atypical intracranial meningiomas. Neuroradiology. 2003;45:129-136.
[34] Negendank W.G., Sauter R., Brown T.R., et al. Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg. 1996;84:449-458.
[35] Shino A., Nakasu S., Matsuda M., Handa J., Morikawa S., Inubushi T. Noninvasive evaluation of the malignant potential of intracranial meningiomas performed using proton magnetic resonance spectroscopy. J Neurosurg. 1999;91:928-934.
[36] Poptani H., Gupta R.K., Roy R., Pandey R., Jain V.K., Chhabra D.K. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am J Neuroradiol. 1995;16:1593-1603.
[37] Tedeschi G., Lundbom N., Raman R., et al. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg. 1997;87:516-524.
[38] Perry A., Stafford S.L., Scheithauer B.W., Suman V.J., Lohse C.M. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455-1465.
[39] Wakhloo A.K., Juengling F.D., Van Velthoven V., Schumacher M., Hennig J., Schwechheimer K. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993;14:571-582.
[40] Bendszus M., Martin-Schrader I., Warmuth-Metz M., Hofmann E., Solymosi L. MR imaging- and MR spectroscopy-revealed changes in meningiomas for which embolization was performed without subsequent surgery. AJNR Am J Neuroradiol. 2000;21:666-669.
[41] Schulthess V., Gustav K. Molecular anatomic imaging: PET-CT and SPECT-CT integrated modality imaging, 2nd ed. Lippincott: Williams and Wilkins, 2006.
[42] Ogawa T., Inugami A., Hatazawa J., et al. Clinical positron emission tomography for brain tumors: comparison of fludeoxyglucose F 18 and L-methyl-11C-methionine. AJNR Am J Neuroradiol. 1996;17:345-353.
[43] Grosu A.L., Weber W.A., Astner S.T., et al. 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:339-344.
[44] Grosu A.L., Weber W.A., Riedel E., et al. L-(Methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:64-74.
[45] Rutten I., Cabay J.E., Withofs N., et al. PET/CT of skull base meningiomas using 2–18F-fluoro-L-tyrosine: initial report. J Nucl Med. 2007;48:720-725.
[46] Voges J., Herholz K., Holzer T., et al. 11C-methionine and 18F-2–fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg. 1997;69:129-135.
[47] Chen W., Cloughesy T., Kamdar N., et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46:945-952.
[48] Ishiwata K., Kubota K., Murakami M., Kubota R., Senda M. A comparative study on protein incorporation of L-[methyl-3H]methionine, L-[1–14C]leucine and L-2–[18F]fluorotyrosine in tumor bearing mice. Nucl Med Biol. 1993;20:895-899.
[49] Pruim J., Willemsen A.T., Molenaar W.M., et al. Brain tumors: L-[1–C-11]tyrosine PET for visualization and quantification of protein synthesis rate. Radiology. 1995;197:221-226.
[50] Roelcke U., Radu E., Ametamey S., Pellikka R., Steinbrich W., Leenders K.L. Association of rubidium and C-methionine uptake in brain tumors measured by positron emission tomography. J Neurooncol. 1996;27:163-171.
[51] Iuchi T., Iwadate Y., Namba H., et al. Glucose and methionine uptake and proliferative activity in meningiomas. Neurol Res. 1999;21:640-644.
[52] Muhr C., Gudjonsson O., Lilja A., Hartman M., Zhang Z.J., Langstrom B. Meningioma treated with interferon-alpha, evaluated with [(11)C]-L-methionine positron emission tomography. Clin Cancer Res. 2001;7:2269-2276.
[53] Fischman A.J., Thornton A.F., Frosch M.P., Swearinger B., Gonzalez R.G., Alpert N.M. FDG hypermetabolism associated with inflammatory necrotic changes following radiation of meningioma. J Nucl Med. 1997;38:1027-1029.
[54] Jones H.A., Clark R.J., Rhodes C.G., Schofield J.B., Krausz T., Haslett C. Positron emission tomography of 18FDG uptake in localized pulmonary inflammation. Acta Radiol Suppl. 1991;376:148.
[55] Palmer W.E., Rosenthal D.I., Schoenberg O.I., et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2–[F-18]-fluoro-2–deoxy-D-glucose. Radiology. 1995;196:647-655.
[56] Yamada S., Kubota K., Kubota R., Ido T., Tamahashi N. High accumulation of fluorine-18–fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995;36:1301-1306.
[57] Schmidt M., Scheidhauer K., Luyken C., et al. Somatostatin receptor imaging in intracranial tumours. Eur J Nucl Med. 1998;25:675-686.
[58] Henze M., Dimitrakopoulou-Strauss A., Milker-Zabel S., et al. Characterization of 68Ga-DOTA-D-Phe1–Tyr3–octreotide kinetics in patients with meningiomas. J Nucl Med. 2005;46:763-769.
[59] Henze M., Schuhmacher J., Hipp P., et al. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1–Tyr3–octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42:1053-1056.