CHAPTER 356 Adult Moyamoya Disease
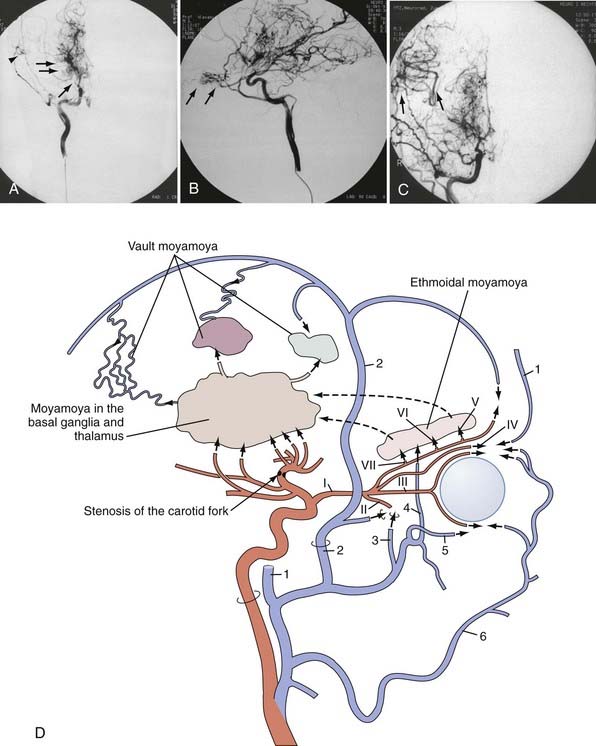
MMD was first described in the Japanese medical literature in 1957 by Takeuchi and Shimizu.1 They reported a 29-year-old man who had suffered from visual disturbances since 10 years of age and hemiconvulsive seizures since 13 years of age and later became blind on pneumocephalogram at the age of 24. His blindness resolved only partially. Bilateral ICA occlusion was confirmed on angiography. Biopsy of the superior laryngeal artery revealed a slight proliferative change in the intima and media. The authors considered the occlusion to be due to congenital hypoplasia causing insufficient collateral circulation to the brain. The term moyamoya (Japanese for “puff of smoke”) was coined by Suzuki and Takaku in 1969 to describe the peculiar appearance of the abnormal vasculature at the base of the brain on cerebral angiography (Fig. 356-1).2 Kudo called this disease “spontaneous occlusion of the circle of Willis” in 1968 from a pathologic point of view,3 and this name was officially accepted later by the Research Committee of the Ministry of Welfare and Health, Japan (RCMWHJ), which was founded in 1977. Since its initial discovery some 50 years ago, the clinical features of the disease have become clearer. It has been hypothesized that in the setting of arterial stenosis-occlusion, hypoxic regions of the brain induce deep collateral flow by the dilation of tortuous perforating arteries, namely the moyamoya vasculature. This revascularization phenomenon is thought to be orchestrated by the expression of various angiogenettic signaling cascades.4,5
Epidemiology
MMD was initially thought to be confined to the Japanese population,3 but cases in other Asian populations were subsequently confirmed.6,7 Moreover, its presence in non-Asian populations has increasingly been recognized, although at a much lower incidence,6,8 and ethnicity seems to play a decisive role in the United States.9–12 In 1992, Goto and Yonekawa reviewed 1063 patients with MMD from countries other than Japan and found 625 patients in Asia, 201 in Europe, 176 in North and South America, 52 in Africa, and 9 in Australia as published in the literature.6 The incidence and prevalence of the disease in Europe are believed to be around a 10th that in Japan.13
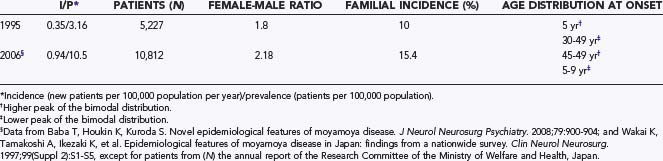
In Japan in 1995, an annual incidence of 0.35 per 100,000 population and a prevalence of 3.16 per 100,000 population were reported. The female-to-male ratio was 1.8. The age at onset had two peaks: a higher peak at 5 years and a lower one around 30 to 49 years.14–16 These figures, however, seem to have changed (Table 356-1), as indicated by recent surveys.17,18 The incidence and prevalence are more than twice as high as in the previous surveys. As for the age distribution, the higher incidence was observed in adults 45 to 49 years of age and the second in children 5 to 9 years of age.17 One of the reasons for the higher annual incidence and prevalence in the recent survey is thought to be the fact that MMD has been diagnosed in asymptomatic patients (up to 18%) on the basis of MRI and MRA findings.19 This situation is similar to that with cerebral aneurysms, in which unruptured or incidental aneurysms have been detected by MRI and MRA. The tendency for a decrease in the number of children affected in the population might be another reason for these changes. This tendency also affects the incidence of familial MMD. Previously, about 10% of patients had a familial form of the disease, but the recent report indicates an increased incidence of up to 15%. In the United States and Europe, however, familial occurrence has been reported to be less common (less than 6%).10,20
Pathophysiology and Etiology
Since presumably the first autopsy report by Maki and Nakata in 1965 of a 9-year-old child who died of a subdural hematoma after a 7-year history of relapsing episodes of choreatic movements associated with progressive deterioration of vision and hearing,21 postmortem analyses have been performed mostly on adults with intracranial hemorrhage. The characteristic findings of intimal thickening and subsequent stenosis-occlusion at the terminal portion of the ICA along with pathologic changes in neighboring arteries have been enumerated in the guidelines for the diagnosis of MMD15,22–24; fibrocellular thickening of the intima, irregular disruption of the internal elastic lamina, and attenuation of the media are the main findings. These changes have been observed not only in the carotid fork but also in cortical branches of the middle cerebral artery (MCA). In perforating arteries, microaneurysm formation and fragmented elastic lamina have been detected and are considered to be one of the reasons for intracerebral hemorrhage, as discussed later.
Sometimes, extracranial arteries such as the superficial temporal arteries (STAs) and renal arteries have also been shown to be affected by the same stenotic changes, so MMD can be considered to be a type of systemic disease.14,23,25,26
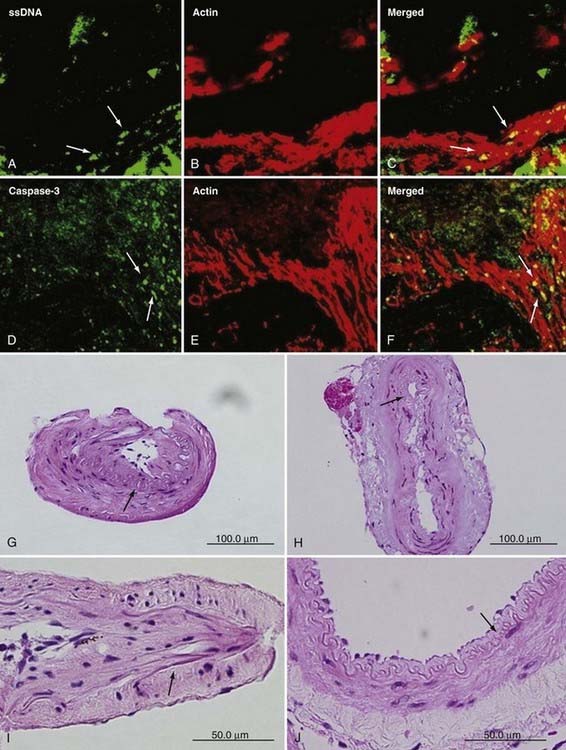
Pluripotent peptides and their receptors, such as basic fibroblast growth factor, transforming growth factor-β, and hepatocyte growth factor, have been detected in increased amounts in the STA and the diseased wall of the ICA.27–29 These growth factors can possibly affect the extent of angiogenesis and intimal hyperplasia in the intracranial and extracranial arteries. In addition, elevated serum levels of soluble vascular cell adhesion molecule type 1, intracellular adhesion molecule type 1, and E-selectin and elevated cerebrospinal fluid levels of nitric oxide metabolites or some specific polypeptides have been reported as well.30 Presumably, endothelial activation by these molecular factors plays a cardinal role in inducing MMD. Recently, apoptosis of smooth muscle cells in the tunica media has been discovered, and elevated levels of caspase-3, an important molecule in the process of apoptosis, have been demonstrated in the tunica media of the MCA, along with elevated expression of hypoxia-inducing factor-1α in its endothelial layer (Fig. 356-2).31,32 These data confirm that not only the ICA carotid forks but also the neighboring MCAs are affected.
Since founding of the RCMHWJ in 1977, a genetic approach to investigating cases of familial MMD has been conducted to clarify its pathogenesis. According to recent studies, the mode of inheritance of familial MMD, after having been thought to be multifactorial,33,34 is presumed to be autosomal dominant with incomplete penetrance.35 In addition, genomic imprinting may be associated with the disease. Furthermore, based on data from genome-wide parametric linkage analysis of MMD in 15 extended Japanese families, significant evidence of linkage has been observed on chromosome 17q25.3, on which a major gene locus for autosomal dominant MMD is considered to lie.35,36
Clinical Findings
The clinical features of MMD differ considerably between pediatric and adult patients. According to reports from the RCMWHJ, cerebral ischemia, including transient ischemic attacks (TIAs), has been the most common finding (70% to 80%) in children, whereas intracranial bleeding is the typical finding in adults, especially women (up to 66%) (Table 356-2).37 Cerebral ischemia (either TIA or infarction) is the next most common manifestation in adults. In line with our observation and that of other authors, however, cerebral ischemia and not bleeding seems to be the usual manifestation in Europe and the United States.8–12,38,39 A sudden drop in performance because of low perfusion has been the only clinical finding in about 10% of our adult patients. Infarctions are observed in the cortical and subcortical regions, mainly in the watershed or posterior cerebral artery (PCA) territories, in about 40% of ischemic cases, but the basal ganglia and thalamus are usually spared.13,40,41
TABLE 356-2 Differences in Clinical Features between Children and Adults with Moyamoya Disease*
| CHILDREN (0-9 YEARS OLD) N = 431 |
ADULTS (30-39 YEARS OLD) N = 235 |
|
|---|---|---|
| Hemorrhage | 21 | 161 |
| Epileptic seizures | 107 | 11 |
| Infarction | 194 | 40 |
| Transient ischemic attacks | 194 | 23 |
| Others | 23 | 8 |
* In Europe and the United States, ischemia (and not bleeding) has been reported to be predominant in adults.8–12,20,38
Data from Handa H, Yonekawa Y. Analysis of filing data bank of 1500 cases of spontaneous occlusion of the circle of Willis and follow-up study of 200 cases for more than 5 years. Stroke (Tokyo). 1985;7:477-480.
The majority of bleeding in adults is intraventricular or periventricular in location and not subarachnoid. Such hemorrhages often recur, with an annual rebleeding rate of 7%, and a third of patients eventually suffer further hemorrhage after a variable interval (days to years)42–44; the morbidity and mortality associated with these hemorrhages have been reported to be considerable, with only 45% of patients having good neurological recovery and 7% dying. Rebleeding, which often occurs at a location different from the original bleeding site, carries an even graver prognosis: only 20% of patients have a good recovery and nearly 30% die. There are three main causes of intracranial bleeding in patients with MMD45–49: (1) rupture of dilated and stressed perforating arteries containing microaneurysms, (2) fibrinoid necrosis of the arterial wall in the basal ganglia, and (3) rupture of microaneurysms in the periventricular region, especially around the superolateral wall of the lateral ventricles. These peripheral “false” aneurysms located within moyamoya and peripheral arteries can be identified on cerebral angiography and may be the origin of the bleeding. A special type of subarachnoid hemorrhage over the cerebral cortex without any evidence of aneurysm and a fair prognosis has been sporadically but repeatedly reported in adult patients, although its pathophysiology still remains to be clarified.50,51
Saccular cerebral aneurysms, a possible cause of a rather rare subarachnoid hemorrhage in this disease, are detected in 4% to 14% of patients, and 16% of these patients have been reported to have multiple aneurysms. These aneurysms occur in three locations52–56: (1) 60% around the circle of Willis, mainly at the vertebrobasilar territory; (2) 20% in peripheral arteries, such as the posterior and anterior choroidal arteries; and (3) 20% in the abnormal moyamoya vasculature as mentioned earlier. The false aneurysms may disappear spontaneously or after revascularization procedures,52 but they might need to be removed surgically because of repeated bleeding.45,49
Pregnancy and delivery may increase the risk for ischemic or hemorrhagic stroke in female patients.57 Hemorrhagic stroke during pregnancy often leads to poor functional outcome.
Mortality in the acute stage has been reported to be low: 2.4% with the infarction type and 16.4% with the hemorrhagic type.20
Neuroimaging
Cerebral angiography has been the most common method of diagnosing MMD, as has been emphasized in the diagnostic criteria for MMD by the RCMWHJ.14,15 Known as the six-stage classification of Suzuki and Takaku,2 angiographic progression follows the sequence of (1) narrowing of the carotid fork, (2) initiation of the moyamoya, (3) intensification of the moyamoya, (4) minimization of the moyamoya, (5) reduction of the moyamoya, and (6) disappearance of the moyamoya. Accordingly, narrowing of the ICA proceed to occlusion of the ICA so that finally collateral maintenance of the cerebrum via only the external carotid and vertebrobasilar systems. Progression from stage 1 to stage 6 has been observed in only a limited number of cases. Stage 4 is encountered most frequently. The proximal portion of the PCA is also involved in around half of affected patients,41 although the posterior circulation has not usually been thought to be affected and contributes as a main source of collateral circulation to the insufficient anterior circulation; steno-occlusive lesions of the posterior circulation were encountered in our European series in 11 patients (16%).20 Attempts at staging on the basis of MRI or cerebral blood flow (CBF) findings or their combination have been made but they still need to be investigated and checked for general acceptance.38,58 MMD is also characterized by an extensive peculiar development of collateral pathways (see Fig. 356-1)2,59,60: (1) “basal moyamoya” in the basal ganglia and thalamus, namely abnormally dilated collaterals via the lenticulostriate arteries, the anterior choroidal artery, the posterior choroidal artery, and the posterior communicating artery, (2) “ethmoidal moyamoya” via the anterior and posterior ethmoidal arteries originating from the ophthalmic artery; and (3) “vault moyamoya” via the dural arteries, also called transdural anastomosis.
According to recent guidelines by the RCMWHJ, cerebral angiography is presently not necessary for definitive diagnosis if MRI and MRA (>1 T is desirable) clearly fulfill the criteria.15 Cerebral angiography still, however, has a solid position in the management of MMD, especially in the planning of surgical treatment and postoperative evaluation, although its inherent risk for ischemic complications should not be underestimated.
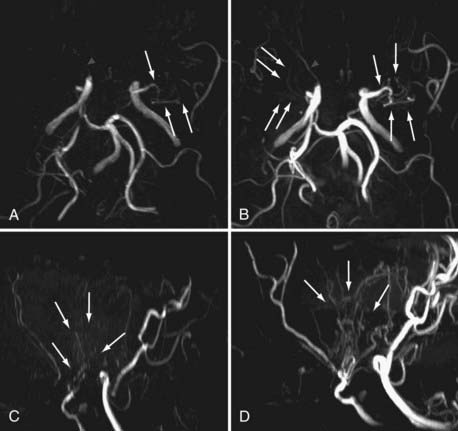
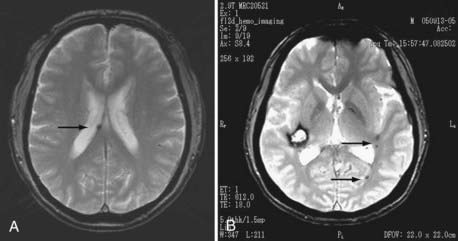
Noninvasive imaging techniques can now be effectively used for the diagnosis of MMD. In particular, MRI and MRA demonstrate the characteristic findings of stenotic-occlusive carotid lesions and basal moyamoya, especially with the use of high-tesla settings (Fig. 356-3). T1-weighted MRI is sensitive in detecting basal moyamoya.58,61 According to recent reports, microbleeding has been detected in about 15% to 44% of adult patients on T2-weighted images (Fig. 356-4). Microbleeding has been proposed as a predictor of subsequent hemorrhagic stroke.62
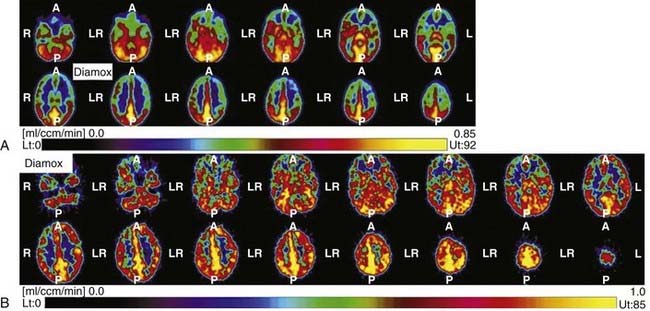
Once the diagnosis is made, other imaging modalities can provide additional decisive information for management of this disease. Xenon-enhanced computed tomography, single-photon emission computed tomography, and positron emission tomography (PET) can be used to measure regional CBF and metabolic distribution. Reduced CBF and cerebrovascular reactivity to CO2 or acetazolamide (or to both) are characteristically detected in the ICA territory (Fig. 356-5).63–67 Such reduction in cerebral perfusion pressure (cerebral blood volume [CBV]/CBF) is compensated by an increase in CBV and the oxygen extraction fraction. Perfusion instability detected by measurement of these parameters is supposed to forecast progression of the disease. These parameters can also be used to confirm the effectiveness of surgical revascularization.
Treatment
Because of its unknown etiology, no specific treatment of MMD is available. Acetylsalicylic acid or other antiplatelet drugs are given because studies have revealed that they may have an influence on the progression of vascular stenosis. Relatively fresh thrombus has been seen at the stenotic site of the ICA on autopsy.22,23 These agents might also be effective in the presence of atherosclerosis in adult MMD. Calcium antagonists have been administered empirically for headache and steroids for involuntary movement or at the time of frequent TIAs.14
Surgical Treatment
Surgical revascularization procedures are used to augment CBF and improve impaired hemodynamic situations that cannot be resolved by nonsurgical treatment. Neurosurgical techniques for the treatment of MMD have been grouped into two main categories: direct revascularization with microvascular extracranial-to-intracranial (EC-IC) bypass and indirect revascularization without microvascular anastomotic procedures. Currently, there are no clear data indicating definite superiority of either of the methods. The indirect revascularization method is aimed at stimulating the development of new vascular networks and is thought to lead to delayed collateralization, but the extent of revascularization is considered unpredictable, whereas direct revascularization can selectively perfuse ischemic areas immediately but, in so doing, may cause hyperperfusion syndrome as a complication.68,69 Many surgeons are combining STA-MCA bypass and indirect revascularization, although the latter is done inadvertently more or less at the time of the STA-MCA bypass procedure and during closure of the craniotomy.
Direct Revascularization Procedure Using a Microvascular Technique for Superior Temporal Artery–Middle Cerebral Artery Bypass
The STA-MCA bypass technique pioneered in 1967 by Donaghy and Yasargil70,71 was applied several years later by Reichman and colleagues, Karasawa and associates, and Yonekawa and Yasargil to the treatment of MMD.72–74 The parietal or, less often, the frontal branch of the STA (donor vessels; ≈1 mm in diameter) is first located by Doppler sonography and then dissected along its course via a linear incision (8 to 10 cm of dissection and free preparation). This is followed by a small craniotomy (2.5 to 3 cm in diameter) with its center about 6 cm cranial to the external acoustic meatus, which corresponds to the end of the sylvian fissure. After locating a suitable branch of the MCA, direct anastomosis between the STA branch and the cortical MCA branch is achieved with 8 to 10 interrupted stitches of 10-0 or 11-0 suture. Common branches used in this technique are the angular, posterior temporal, and posterior parietal arteries. The rolandic or prerolandic arteries (or both) and in some cases the operculofrontal or candelabra group of branches can also be used by placing the craniotomy at another location according to findings on angiography or PET. Direct revascularization thus has the advantage of selectively supplying territories of ischemia detected by PET or other methods (STA–anterior cerebral artery [ACA] or STA-PCA bypass) (Fig. 356-6). As with mental retardation in children, adults with a remarkable drop in performance because of low perfusion in the ACA territory could benefit from an STA-ACA bypass in combination with a standard STA-MCA bypass (see Fig. 356-5).8,75,76 The frontal branch of the STA is anastomosed to the medial internal frontal artery, a branch of the ACA (approximately 1 mm in diameter) at the medial convexity of the frontal lobe. It can usually be found a few centimeters anterior to the coronal suture. When the entire extent of the frontal branch is dissected, its length is long enough to reach the midline for completion of the anastomotic procedure.75 Otherwise, an interposition graft in which either a portion of the parietal branch of the STA or a portion of the saphenous vein is placed between the frontal branch and the medial internal frontal artery will also make the procedure possible.77 In patients with low perfusion of the PCA territory and corresponding symptomatology of visual disturbances, an STA-PCA or occipital artery–PCA bypass may be considered. Performance of the latter bypass via the supracerebellar transtentorial approach in the sitting position has the advantage that the trunk of the PCA does not need to be temporarily occluded for the anastomotic procedure; the posterior temporal artery, which courses over the parahippocampal gyrus at its posterior portion, is used as a recipient artery.76
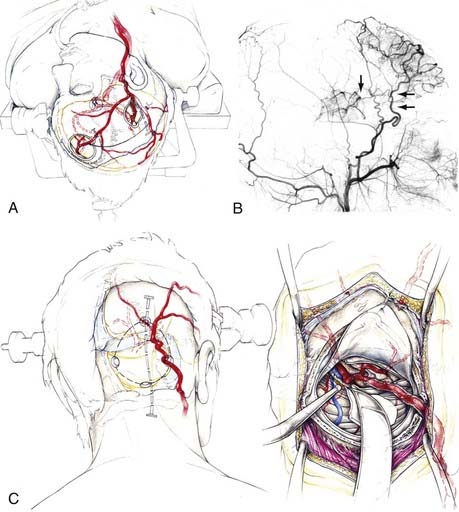
FIGURE 356-6 Artist’s drawing of a superior temporal artery–middle cerebral artery (STA-MCA) bypass along with an STA–anterior cerebral artery (ACA) bypass and an occipital artery–posterior cerebral artery (OA-PCA) bypass. A, Standard combination of an STA-MCA bypass with an STA-ACA bypass. The latter can be done by stretching the dissected frontal branch of the STA (as illustrated) or by an interposition graft with the use of a portion of the STA parietal branch or a portion of the saphenous vein. B, Lateral view of a postoperative angiogram indicating (arrows) the stretched frontal branch anastomosed to a branch of the distal ACA. The MCA branches are filled by the standard STA-MCA bypass, which was combined with aforementioned STA-MCA bypass. C, Operative sequence for an OA-PCA bypass via the paramedian supracerebellar transtentorial approach.75
Indirect Bypass Techniques
Encephalomyosynangiosis (EMS), reported by Henschen in 1950,78 was first applied to the treatment of MMD by Karasawa and colleagues in the late 1970s79 and has now been widely used as a surgical intervention. One reason for its current use may be the infrequent existence of an appropriate cortical branch of the MCA on the surface of the brain in patients with MMD, especially children, which makes direct revascularization difficult or impossible. Another reason is to facilitate gradual revascularization according to the needs of the ischemic brain so that hyperperfusion secondary to direct revascularization68,69 does not need to be taken into account. This technique involves implanting the temporalis muscle on the lateral brain surface and securing it to the dural edges. The temporalis muscle is considered to be a potential source of collateral circulation over the ischemic brain, and it is appropriately situated anatomically.
Encephaloduroarteriosynangiosis (EDAS) was developed by Matsushima and coworkers80 in 1979 and is now the preferred technique for indirect revascularization. This technique requires exposure and dissection of the parietal branch of the STA with preservation of vascular flow. After a craniotomy over the sylvian fissure, the dissected STA is laid onto the cortical surface after having opened the arachnoidea extensively beforehand.
Invasive free graft transplantation of the autogenic omentum majus on the ischemic brain surface81,82 might have only historical value as an indirect revascularization method, although selective application of this method for ischemia in the ACA or PCA territory could be kept in mind.
Selection of these surgical procedures is now dependent on the surgeon’s personal experience. Direct revascularization techniques or combining them with an indirect procedure is considered to be the therapy of choice in adults because indirect methods alone have been reported to be unpredictable or ineffective in achieving good revascularization.14,38
Perioperative Management
Without these careful measures, serious ischemic complications have been reported to occur at rates of up to 10% or greater84,85; such complications have been experienced by a number of bypass neurosurgeons in the early stages of applying bypass surgery to MMD patients.14 It should be noted that serious ischemic complication can occur with any intervention, even during diagnostic angiography, as a result of the compromised hemodynamic situation of patients with diminished reserve.
Prognosis
The natural history of MMD must still be clarified. Seventy-five percent to 80% of cases are thought to have a benign course in terms of life expectancy, with or without surgical treatment.14 These patients perform well in independently carrying out activities of daily life. However, limited adaptability to social and school life or impairment of neurological soft signs has been reported.
After revascularization procedures, the majority of adult patients with MMD have been reported to be free of TIAs and ischemic strokes.9,10,14,38,86 Rebleeding has been reported to occur in about 30% to 65% of patients during follow-up.42,44 The effectiveness of revascularization procedures in preventing rebleeding must still be settled, although a 12.5% to 20% reduction in rebleeding risk14,43 has been reported. At present, the Japanese Adult Moyamoya (JAM) trial, a multicenter randomized clinical trial, is now in progress to evaluate whether direct or combined bypass surgery can reduce the risk for rebleeding in adult patients with MMD.87
Patients with unilateral MMD should be monitored carefully because 7% to 27% of such patients, including children, have been reported to progress to bilateral disease within a few years of follow-up.88 Recently, 15 of 63 adult patients were reported to have shown progression during a follow-up period of approximately 6 years.89 A patient may have more than one manifestation, first ischemia and then bleeding, or vice versa. In a group of adult patients with MMD since childhood, about 44% are expected to have experienced bleeding.42
MRI and MRA can now detect asymptomatic patients with MMD more often than before, but their natural course is still unclear. The annual risk for any stroke in these patients has been reported to be 3.2%.19
Acknowledgment
Most of the information in this chapter originated from annual reports of the research committee on MMD sponsored by the Ministry of Health, Labor, and Welfare, Japan, founded in 1977 (the first principal investigator was Prof. Fumio Goto). The previous achievements were reported in 1992.14 The authors are indebted to the committee members. We thank Mr. Peter Roth for his artistic illustrations in this chapter.
Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900-904.
Fujimura M, Kaneta T, Mugikura S, et al. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery–middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol. 2007;67:273-282.
Fukui M, Members of Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (“moyamoya” disease). Clin Neurol Neurosurg. 1997;99(suppl 2):S238-S240.
Goto Y, Yonekawa Y. Worldwide distribution of moyamoya disease. Neurol Med Chir (Tokyo). 1992;32:883-886.
Hoshimaru M, Takahashi JA, Kikuchi H, et al. Possible role of fibroblastic growth factor in the pathogenesis of moyamoya disease. An immunohistochemical study. J Neurosurg. 1991;75:267-270.
Houkin K, Aoki T, Takahashi A, et al. Diagnosis of moyamoya disease with magnetic resonance angiography. Stroke. 1994;25:2159-2164.
Iwama T, Hashimoto N, Yonekawa Y. The relevance of hemodynamic factors in perioperative complications in childhood moyamoya disease. Clinical studies. Neurosurgery. 1996;38:1120-1126.
Karasawa J, Touho H, Ohnishi H, et al. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg. 1992;77:84-89.
Kawaguchi S, Sakaki T, Kakizaki T, et al. Clinical features of the haemorrhage type moyamoya disease based on 31 cases. Acta Neurochir (Wien). 1996;138:1200-1210.
Kawano T, Fukui M, Hashimoto N, et al. Follow-up study of patients with “unilateral” moyamoya disease. Neurol Med Chir (Tokyo). 1994;34:744-747.
Khan N, Schuknecht B, Boltshauser E, et al. Moyamoya disease and moyamoya syndrome: Experience in Europe: choice of revascularisation procedures. Acta Neurochir (Wien). 2003;145:1061-1071.
Kikuta K, Takagi Y, Nozaki K, et al. The presence of multiple microbleeds as a predictor of subsequent cerebral hemorrhage in patients with moyamoya disease. Neurosurgery. 2008;62:104-112.
Komiyama M, Yasui T, Kitano S, et al. Moyamoya disease and pregnancy: case report and review of the literature. Neurosurgery. 1998;43:360-368.
Kuroda S, Hashimoto N, Yoshimoto T, et al. for the Research Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 2007;38:1430-1435.
Kuroda S, Ishikawa T, Houkin K, et al. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005;36:2148-2153.
Mauro AJ, Johnson ES, Chikos PM, et al. Lipohyalinosis and miliary microaneurysms causing hemorrhage in a patient with moyamoya disease: a clincopathological study. Stroke. 1980;11:405-412.
Mineharu Y, Liu W, Inoue K, et al. Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology. 2008;70:2357-2363.
Miyamoto S, Kikuchi H, Karasawa J, et al. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J Neurosurg. 1984;61:1032-1037.
Muroi C, Yonekawa Y, Khan N, et al. Case report. Metabolic changes after H215O-positron emission tomography with acetazolamide in a patient with moyamoya disease: case report and review of previous cases. J Neurosurg Anesth. 2003;15:131-139.
Oka K, Yamashita M, Sadoshima S, et al. Cerebral hemorrhage in moyamoya disease at autopsy. Virchows Arch. 1981;392:247-261.
Piao R, Oku N, Kitagawa K, et al. Cerebral hemodynamics and metabolism in adult moyamoya disease: comparison of angiographic collateral circulation. Ann Nucl Med. 2004;18:115-121.
Scott RM, Smith ER. Moyamoya disease and Moyamoya syndrome. N Eng J Med. 2009;360:1226-1237.
Takagi Y, Kikuta K, Sadamasa N, et al. Caspase-3–dependent apoptosis in middle cerebral arteries in patients with moyamoya disease. Neurosurgery. 2006;59:894-901.
Yonekawa Y, Goto Y, Ogata N. Moyamoya disease: diagnosis, treatment, and recent achievement. Part IV. Specific medical disease and stroke. In: Barnett HJM, Mohr JP, Stein BM, et al, editors. Stroke, Pathophysiology, Diagnosis and Management. New York: Churchill Livingstone; 1992:721-747.
Yonekawa Y, Fandino J, Hug M, et al. Moyamoya angiopathy in Europe. In: Cho BK, Tominaga T, editors. Moyamoya disease update. Wien: Springer; 2010:337-342.
1 Takeuchi K, Shimuzu K. Hypoplasia of the bilateral internal carotid arteries. No To Shinkei. 1957;9:37-43.
2 Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288-299.
3 Kudo T. Spontaneous occlusion of the circle of Willis: a disease apparently confined to Japanese. Neurology. 1968;18:485-496.
4 Lim M, Cheshier S, Steinberg GK. New vessel formation in the central nervous system during tumor growth, vascular malformations, and moyamoya. Curr Neurovasc Res. 2006;3:237-245.
5 Sakamoto S, Kiura Y, Yamasaki F, et al. Expression of vascular endothelial growth factor in dura mater of patients with moyamoya disease. Neurosurg Rev. 2007;31:77-81.
6 Goto Y, Yonekawa Y. Worldwide distribution of moyamoya disease. Neurol Med Chir (Tokyo). 1992;32:883-886.
7 Han DH, Kwon OK, Byun BJ, et al. A co-operative study: clinical characteristic of 334 Korean patients with moyamoya disease treated at neurosurgical institutes (1976-1994). The Korean Society of Cerebrovascular Disease. Acta Neurochir (Wien). 2000;42:1263-1273.
8 Khan N, Schuknecht B, Boltshauser E, et al. Moyamoya disease and moyamoya syndrome: Experience in Europe: choice of revascularisation procedures. Acta Neurochir (Wien). 2003;145:1061-1071.
9 Chiu D, Shedden P, Bratina P, et al. Clinical features of moyamoya disease in the United States. Stroke. 1998;29:1347-1351.
10 Scott RM, Smith ER. Moyamoya disease and Moyamoya syndrome. N Eng J Med. 2009;360:1226-1237.
11 Peerless SJ. Risk factors of moyamoya disease in Canada and the USA. Clin Neurol Neurosurg. 1997;99(suppl 2):45-48.
12 Uchino K, Johnston SC, Becker KJ, et al. Moyamoya disease in Washington State and California. Neurology. 2005;65:956-958.
13 Yonekawa Y, Ogata N, Kaku Y, et al. Moyamoya disease in Europe, past and present status. Clin Neurol Neurosurg. 1997;99(suppl 2):S58-S60.
14 Yonekawa Y, Goto Y, Ogata N. Moyamoya disease: diagnosis, treatment, and recent achievement. Part IV. Specific medical disease and stroke. In: Barnett HJM, Mohr JP, Stein BM, et al, editors. Stroke, Pathophysiology, Diagnosis and Management. New York: Churchill Livingstone; 1992:721-747.
15 Fukui M, Members of Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (“moyamoya” disease). Clin Neurol Neurosurg. 1997;99(suppl 2):S238-S240.
16 Wakai K, Tamakoshi A, Ikezaki K, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997;99(suppl 2):S1-S5.
17 Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900-904.
18 Kuriyama S, Kusaka Y, Fujimura M, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42-47.
19 Kuroda S, Hashimoto N, Yoshimoto T, et al. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. for the Research Committee on Moyamoya Disease in Japan. Stroke. 2007;38;:1430-1435.
20 Yonekawa Y, Khan N. Moyamoya disease. Adv Neurol. 2003;92:113-118.
21 Maki Y, Nakata Y. Autopsy of a case with an anomalous hemangioma of the internal carotid artery at the skull base. Brain Nerve (Tokyo). 1965;17:764-766.
22 Hosoda Y. Pathology of so-called “spontaneous occlusion of the circle of Willis.”. Pathol Annu. 1984;19:221-224.
23 Ikeda E, Hosoda Y. Distribution of thrombotic lesions in the cerebral arteries in spontaneous occlusion of the circle of Willis: cerebrovascular moyamoya disease. Clin Neuropathol. 1993;12:44-48.
24 Masuda J, Ogata J, Yutani C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in moyamoya disease. Stroke. 1993;24:1960-1967.
25 Hoshimaru M, Kikuchi H. Involvement of the external carotid arteries in moyamoya disease: neuroradiological evaluation of 66 patients. Neurosurgery. 1992;31:398-400.
26 Ellison PH, Largent JA, Popp AJ. Moya-moya disease associated with renal artery stenosis. Arch Neurol. 1981;38:467.
27 Hojo M, Hoshimaru M, Miyamoto S, et al. Role of transforming growth factor-beta1 in the pathogenesis of moyamoya disease. J Neurosurg. 1998;89:623-629.
28 Hoshimaru M, Takahashi JA, Kikuchi H, et al. Possible role of fibroblastic growth factor in the pathogenesis of moyamoya disease. An immunohistochemical study. J Neurosurg. 1991;75:267-270.
29 Nanba R, Kuroda S, Ishikawa T, et al. Increased expression of hepatocyte growth factor in cerebrospinal fluid and intracranial artery in moyamoya disease. Stroke. 2004;35:2837-2842.
30 Soriano SG, Cowan DB, Proctor MR, et al. Levels of soluble adhesion molecules are elevated in the cerebrospinal fluid of children with moyamoya syndrome. Neurosurgery. 2002;50:544-549.
31 Takagi Y, Kikuta K, Nozaki K, et al. Expression of hypoxia-inducing factor-1 alpha and endoglin in intimal hyperplasia of the middle cerebral artery of patients with moyamoya disease. Neurosurgery. 2007;60:338-345.
32 Takagi Y, Kikuta K, Sadamasa N, et al. Caspase-3–dependent apoptosis in middle cerebral arteries in patients with moyamoya disease. Neurosurgery. 2006;59:894-901.
33 Fukuyama Y, Sugawara N, Osawa M. A genetic study of idiopathic spontaneous occlusion of the circle of Willis. In: Yonekawa Y, editor. Annual Report of the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) 1990 (in Japanese, English abstract). Tokyo: Japanese Ministry of Health and Welfare; 1991:139-144.
34 Ikeda H, Sasaki T, Yoshimoto T, et al. Mapping of a familial moyamoya disease gene to chromosome 3p24.2-p26. Am J Hum Genet. 1999;64:533-537.
35 Mineharu Y, Liu W, Inoue K, et al. Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology. 2008;70:2357-2363.
36 Mineharu Y, Takenaka K, Yamakawa H, et al. Inheritance pattern of familial moyamoya disease: autosomal dominant mode and genomic imprinting. J Neurol Neurosurg Psychiatry. 2006;77:1025-1029.
37 Handa H, Yonekawa Y. Analysis of filing data bank of 1500 cases of spontaneous occlusion of the circle of Willis and follow-up study of 200 cases for more than 5 years. Stroke (Tokyo). 1985;7:477-480.
38 Schmiedek P. Diagnosis and treatment of non-Japanese patients with moyamoya disease. Paper presented at the 4th European-Japanese Joint Conference on Stroke Surgery, 2008, Helsinki.
39 Yonekawa Y, Fandino J, Hug M, et al. Moyamoya angiopathy in Europe. In: Cho BK, Tominaga T, editors. Moyamoya disease update. Wien: Springer; 2010:337-342.
40 Handa J, Nakano Y, Okuno T, et al. Computerized tomography in moyamoya syndrome. Surg Neurol. 1977;7:315-319.
41 Miyamoto S, Kikuchi H, Karasawa J, et al. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J Neurosurg. 1984;61:1032-1037.
42 Hashimoto N, Iwama T, Tsukahara T, et al. Potential hazard of intracranial bleeding and cerebral ischemia in patients with moyamoya disease. In: Fukui M, editor. Annual Report of the Research Committee on Spontaneous Occlusion of the Circe of Willis (Moyamoya Disease) 1995. Tokyo: Japanese Ministry of Health and Welfare; 1996:55-60.
43 Kawaguchi S, Sakaki T, Kakizaki T, et al. Clinical features of the haemorrhage type moyamoya disease based on 31 cases. Acta Neurochir (Wien). 1996;138:1200-1210.
44 Kobayashi E, Saeki N, Oishi H, et al. Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J Neurosurg. 2000;93:976-980.
45 Furuse S, Matsumoto S, Tanaka Y, et al. Moyamoya disease associated with a false aneurysm. Case report and review of the literature. No Shinkei Geka (Tokyo). 1982;10:1005-1012.
46 Irikura K, Miyasaka Y, Kurata A, et al. A source of haemorrhage in adult patients with moyamoya disease: the significance of tributaries from the choroidal artery. Acta Neurochir (Wien). 1996;138:1282-1286.
47 Mauro AJ, Johnson ES, Chikos PM, et al. Lipohyalinosis and miliary microaneurysms causing hemorrhage in a patient with moyamoya disease: a clincopathological study. Stroke. 1980;11:405-412.
48 Oka K, Yamashita M, Sadoshima S, et al. Cerebral hemorrhage in moyamoya disease at autopsy. Virchows Arch. 1981;392:247-261.
49 Sadato A, Yonekawa Y, Morooka Y, et al. A case of moyamoya disease with repeated intraventricular hemorrhage due to ruptured pseudoaneurysm. Neurol Surg (Tokyo). 1988;17:755-758.
50 Marushima A, Yanaka K, Matsuki T, et al. Subarachnoid hemorrhage not due to ruptured aneurysm in moyamoya disease. J Clin Neurosci. 2006;13:146-149.
51 Osanai T, Kuroda S, Nakayama N, et al. Moyamoya disease presenting with subarachnoid hemorrhage localized over the frontal cortex: case report. Surg Neurol. 2008;69:197-200.
52 Kawaguchi S, Sakaki T, Morimoto T, et al. Characteristics of intracranial aneurysms associated with moyamoya disease. A review of 111 cases. Acta Neurochir (Wien). 1996;138:1287-1294.
53 Kodama N, Suzuki J. Moyamoya disease associated with aneurysms. J Neurosurg. 1978;48:565-569.
54 Yasargil MG, Smith RD. Association of middle cerebral anomalies with saccular aneurysms and moyamoya disease. Surg Neurol. 1976;6:37-43.
55 Kwak R, Ito S, Yamamoto N, et al. Significance of intracranial aneurysms associated with moyamoya disease. Differences between intracranial aneurysms associated with moyamoya disease and usual saccular aneurysms (Part 1). Review of the literature. Neurol Med Chir (Tokyo). 1984;24:97-103.
56 Kwak R, Emori T, Nakamura T, et al. Significance of intracranial aneurysms associated with moyamoya disease (Part II). Cause and site of hemorrhage. Review of the literature. Neurol Med Chir (Tokyo). 1984;24:104-109.
57 Komiyama M, Yasui T, Kitano S, et al. Moyamoya disease and pregnancy: case report and review of the literature. Neurosurgery. 1998;43:360-368.
58 Houkin K, Nakayama N, Kuroda S, et al. Novel magnetic resonance angiography stage grading for moyamoya disease. Cerebrovasc Dis. 2005;20:347-354.
59 Kodama N, Fujiwara S, Hone Y, et al. Transdural anastomosis in moyamoya disease: vault moyamoya. No Shinkei Geka. 1980;8:729-739.
60 Suzuki J, Kodama N. Cerebrovascular “moyamoya” disease. Second report—collateral routes to forebrain via ethmoidal sinus and superior nasal meatus. Angiology. 1971;23:233-236.
61 Houkin K, Aoki T, Takahashi A, et al. Diagnosis of moyamoya disease with magnetic resonance angiography. Stroke. 1994;25:2159-2164.
62 Kikuta K, Takagi Y, Nozaki K, et al. The presence of multiple microbleeds as a predictor of subsequent cerebral hemorrhage in patients with moyamoya disease. Neurosurgery. 2008;62:104-112.
63 Dietrichs E, Dahl A, Nyberg-Hansen R, et al. Cerebral blood flow findings in moyamoya disease in adults. Acta Neurol Scand. 1992;85:318-322.
64 Horowitz M, Yonas H, Albright AL. Evaluation of cerebral blood flow and hemodynamic reserve in symptomatic moyamoya disease using stable xenon CT blood flow. Surg Neurol. 1995;44:251-262.
65 Nariai T, Matsushima Y, Imae S, et al. Severe haemodynamic stress in selected subtypes of patients with moyamoya disease: a positron emission tomography study. J Neurol Neurosurg Psychiatry. 2005;76:663-669.
66 Piao R, Oku N, Kitagawa K, et al. Cerebral hemodynamics and metabolism in adult moyamoya disease: comparison of angiographic collateral circulation. Ann Nucl Med. 2004;18:115-121.
67 Taki Y, Yonekawa Y, Kobayashi A, et al. Cerebral circulation and oxygen metabolism in moyamoya disease of ischemic type in children. Childs Nerv Syst. 1988;4:259-262.
68 Fujimura M, Kaneta T, Mugikura S, et al. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery–middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol. 2007;67:273-282.
69 Yamane K, Shima T, Okada Y, et al. Effects of STA-MCA anastomosis on the hemodynamics of adult moyamoya disease evaluated by reflectance spectrophotometer. Jpn J Neurosurg (Tokyo). 1993;2:104-109.
70 Donaghy RM, Yasargil G. Extracranial blood flow diversions in microvascular surgery. Paper presented at the 36th Annual Meeting of the American Association of Neurological Surgeons, 1968, Chicago.
71 Yasargil MG. Microsurgery Applied to Neurosurgery. Stuttgart, Germany: Thieme; 1969.
72 Reichman OH, Anderson RE, Roberts TC, et al. The treatment of intracranial occlusive cerebrovascular disease by STA-cortical MCA anastomosis. In: Handa H, editor. Microneurosurgery. Tokyo: Igaku Shoin; 1975:31-46.
73 Karasawa J, Kikuchi H, Furuse S, et al. Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg. 1978;49:679-688.
74 Yonekawa Y, Yasargil MG. Arterial extracranial intracranial anastomosis: Technical and clinical aspects: results. In: Krayenbühl H, editor. Advances and Technical Standards of Neurosurgery, Vol 3. Vienna: Springer; 1976:47-78.
75 Iwama T, Hashimoto N, Miyake H, et al. Direct revascularization to the anterior cerebral artery territory in patients with moyamoya disease. Report of five cases. Neurosurgery. 1998;42:1157-1161.
76 Yonekawa Y, Imhof HG, Taub E, et al. Supracerebellar transtentorial approach to posterior temporomedial structures. J Neurosurg. 2001;94:339-345.
77 Tanaka K, Yonekawa Y, Satou K, et al. STA-ACA anastomosis with interposed vein graft. A case report. No Shinkei Geka (Tokyo). 1992;20:171-176.
78 Henschen C. Operative Revascularization des zirkulatorisch geschädigten Gehirns durch Auflage gestielten Muskellappen (Encephalo-myo-synangiose). Langenbecks Arch Klein Chir. 1950;264:392-401.
79 Karasawa J, Kikuchi H, Furuse S. A surgical treatment of moyamoya disease. Encephalomyosynangiosis. Neurol Med Chir (Tokyo). 1977;17:29-37.
80 Matsushima Y, Fukai N, Tanaka K, et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol. 1981;15:313-320.
81 Yonekawa Y, Yasargil MG. Brain vascularization by transplanted omentum. A possible treatment of cerebral ischemia. Neurosurgery. 1977;1:256-259.
82 Karasawa J, Touho H, Ohnishi H, et al. Cerebral vascularization using omental transplantation for childhood moyamoya disease. J Neurosurg. 1993;79:192-196.
83 Muroi C, Yonekawa Y, Khan N, et al. Case report. Metabolic changes after H215O-positron emission tomography with acetazolamide in a patient with moyamoya disease: case report and review of previous cases. J Neurosurg Anesth. 2003;15:131-139.
84 Iwama T, Hashimoto N, Yonekawa Y. The relevance of hemodynamic factors in perioperative complications in childhood moyamoya disease. Clinical studies. Neurosurgery. 1996;38:1120-1126.
85 Sato K, Shirane R, Yoshimoto T. Perioperative factors related to the development of ischemic complications in patients with moyamoya disease. Childs Nerv Syst. 1997;13:68-72.
86 Karasawa J, Touho H, Ohnishi H, et al. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg. 1992;77:84-89.
87 Miyamoto S, Japan Adult Moyamoya Trial Group. Study design for a prospective randomized trial of extracranial-intracranial bypass surgery for adults with moyamoya disease and hemorrhagic onset—the Japan Adult Moyamoya Trial Group. Neurol Med Chir (Tokyo). 2004;44:218-219.
88 Kawano T, Fukui M, Hashimoto N, et al. Follow-up study of patients with “unilateral” moyamoya disease. Neurol Med Chir (Tokyo). 1994;34:744-747.
89 Kuroda S, Ishikawa T, Houkin K, et al. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005;36:2148-2153.