Chapter 17 Adult cardiopulmonary resuscitation
The incidence and outcomes of cardiac arrests appeared not to have changed dramatically over a number of decades. However, a number of recent advances offer significant promise in our attempts to increase neurologically intact survival.
PREVALENCE AND OUTCOMES OF CARDIAC ARRESTS
Approximately 75% of deaths from cardiac arrests occur in the pre-hospital setting.1 Cardiac arrests in the community occur at approximately 50–150/100 000 person-years.2–4 This incidence (and the outcome) is dramatically affected by the definition of the denominator (e.g. all cardiac arrests (89/100 000 person-years) versus those with a presumed cardiac cause and where resuscitation was attempted (31/100 000 person-years)3).
In-hospital cardiac arrests occur at approximately 1–5/1000 admissions,5,6 with a similar denominator effect (as the majority of in-hospital cardiac deaths are expected and occur without attempts at resuscitation7).
The majority of cardiac arrests in both pre- and in-hospital settings appear to be of cardiac origin, but the underlying causes, comorbidities and presenting rhythms vary significantly between studies.4,6,8,9
Outcomes of cardiac arrests are variable depending on the origin of the report, and are also critically dependent on the denominator.2,3,10 The best outcomes from a cardiac arrest (near 100%) occur in the electrophysiology laboratory (where ventricular fibrillation [VF] is often deliberately induced). The outcomes from in-hospital cardiac arrest are surprisingly good (hospital discharge as high as 42%) despite significant comorbidities, and are probably related to their early detection and the early arrival of the advanced life support (ALS) team.6
INTERNATIONAL REVIEW PROCESS
Since the formation of the International Liaison Committee on Resuscitation in 1992, a cooperative international evaluation of the resuscitation science has resulted in the publication of international guidelines in 200011 and an international consensus on resuscitation science in 2005.12 The published guidelines of the major resuscitation councils throughout the world (including the American Heart Association,13 the Australian Resuscitation Council (www.resus.org.au) and the European Resuscitation Council14) are based on this document. The process for the 2005 consensus on resuscitation science involved the review of 276 topics by 281 international contributors, with the completion of 403 worksheets15 (completed worksheets available at www.c2005.org). This science review process continues to be refined and a new consensus on resuscitation science document is planned for publication in 2010.
IMPORTANCE OF CHAIN OF SURVIVAL
The term ‘chain of survival’ has been used to define the important links in the chain for the resuscitation process.10 The key links, which apply to both in- and out-of-hospital cardiac arrests, are: early recognition and the summoning of help, early basic life support (BLS), early access to defibrillation and early ALS, including postresuscitation care.10
RECENT CHANGES TO GUIDELINES
As a result of the science review published in 2005,12 a number of important changes have been made to the guidelines for both basic and ALS.
In summary, the BLS changes include:
These changes are discussed in more detail in the sections that follow.
BASIC LIFE SUPPORT
The general flow of BLS management is provided in the Australian Resuscitation Council BLS flowchart (www.resus.org.au; Figure 17.1).

Figure 17.1 Basic life support flowchart.
(Reproduced from the Australian Resuscitation Council (www.resus.org.au), with permission.)
COMMENCEMENT OF CPR
The use of a pulse check as a means of determining the need for external cardiac compressions was downplayed in the 2000 guidelines, largely as a result of the review of a number of papers suggesting that even experienced providers may have difficulty in accurately assessing the presence or absence of a pulse.18 This was again confirmed in the current guidelines, where it has been recommended that CPR be commenced if the victim has no signs of life (unconscious/unresponsive, not breathing normally and not moving).16 An appropriately trained ALS provider can check for a central pulse (e.g. carotid) for up to 10 seconds during this period of assessment for signs of life.
EXTERNAL CARDIAC COMPRESSION
SITE OF COMPRESSION
The desired compression point for CPR in adults is over the lower half of the sternum. Compressions that are provided higher than this become less effective, and compressions lower than this are also less effective and have an increased risk of damage to intra-abdominal organs. Previous techniques that were widely taught to find this compression point may have introduced delays in commencing and recommencing chest compressions. To minimise pauses between ventilations and compressions, it is reasonable for laypeople and health care professionals to be taught to position the heel of their dominant hand in the centre of the chest of an adult victim (with the non-dominant hand on top).2
RATE OF COMPRESSION
The optimal rate of cardiac compression during cardiac arrest in adults has not yet been determined.2 In a recent human study,19 lower rates (e.g. < 80/min) were associated with worse outcomes and higher rates (> 120/min) with more fatigue and no benefits. It is recommended that chest compressions should be performed at a rate of approximately 100 compressions/minute.
DEPTH OF COMPRESSION
The ideal depth of compression is unknown. Compression depth is usually inadequate when it is measured in either manikin studies or actual cardiac arrests, and increasing depth of compression appears to be associated with higher defibrillation success.20 It is currently recommended that, when performing chest compressions in adults, the chest should be compressed by at least 4–5 cm (or approximately one-third of its depth).
MINIMISE INTERRUPTIONS TO COMPRESSIONS
Interruptions in chest compressions (‘hands-off time’) are common, often prolonged, and are associated with a decrease in coronary perfusion pressure and a deceased likelihood of defibrillation success.20–22 These adverse effects commence within 10 seconds, but appear to be at least partially reversible with the recommencement of chest compressions.23 It is recommended that CPR (initial breaths, then chest compressions) be commenced as soon as the victim is confirmed to have no signs of life. Pauses in compressions for rhythm recognition or specific interventions (such as ventilations, defibrillation or intubation) should be minimised.
COMPRESSION–VENTILATION RATIO
The minute ventilation requirements during cardiac arrest are less than that in the non-arrested state, so the respiratory rate can be decreased. To increase the number of compressions given per minute, minimise interruptions to chest compressions and simplify instruction for teaching and skills retention, a single compression-to-ventilation ratio of 30:2 is recommended for adult BLS before the airway is secured (irrespective of the number of rescuers).2 The tidal breath should be delivered within 1 second, and the desired tidal volume to be delivered is one that results in a visible chest rise.2
MONITORING THE QUALITY OF CPR
A number of different techniques are available to monitor the quality of CPR, some of which are more applicable toALS. Simple monitoring techniques include observation of the rate, depth and positioning of chest compressions, the rate and depth of ventilation and palpation of central pulses. Additional monitoring techniques that can be used include end-tidal carbon dioxide (Table 17.1), mechanical devices (e.g. for monitoring the depth of compressions) and new monitor/defibrillators (e.g. for monitoring the depth and rate of compressions and ventilation). Feedback from these devices can improve the quality of CPR and should result in improved outcomes.24
Table 17.1 Utility of end-tidal carbon dioxide (ETCO2) monitoring during cardiac arrest24
| Cardiovascular (absolute value of ETCO2) |
| Falls immediately at the onset of cardiac arrest |
| Increases immediately with chest compressions |
| Provides a linear correlation with cardiac index |
| Allows early detection of return of spontaneous circulation (sudden increase) |
| Respiratory (ETCO2 waveform) |
| Allows assessment of endotracheal tube placement |
| Allows assessment of expiratory flow limitation |
| Prognosis (absolute value of ETCO2) |
| Predicts successful resuscitation |
‘COMPRESSION-ONLY’ CPR
Increasing anxiety about the performance of mouth-to-mouth ventilation has required the consideration of an alternative approach to traditional bystander CPR. A number of animal studies have suggested that ventilation may not be necessary during the initial phase of resuscitation from an arrest of a cardiac cause (e.g. where VF was electrically induced).2 Recent research in human out-of-hospital cardiac arrests suggests that outcomes are not worse and may actually be better if ventilation is not initially attempted,25,26 although no studies have compared ‘compression-only’ CPR with the BLS protocols that are currently recommended. It is recommended that if rescuers are unable, not trained, or unwilling to perform mouth-to-mouth ventilation (rescue breathing) then they should perform ‘compression-only’ CPR.
DEFIBRILLATION
EARLY DEFIBRILLATION VERSUS CPR BEFORE DEFIBRILLATION
The timing of defibrillation with regard to other interventions appears crucial. The traditional approach to the treatment of a shockable rhythm during cardiac arrest has been to perform defibrillation as soon as possible. In the scenario of recent-onset VF, this still holds, and the best outcomes are associated with defibrillation within 3 minutes (e.g. in electrophysiology labs or coronary care units). However, in situations where the VF has persisted for more than a few minutes, an initial period of CPR may actually improve the likelihood of shock success, and result in better outcomes.23,27,28
WAVEFORM FOR DEFIBRILLATION
No specific defibrillator waveform (either monophasic or biphasic) is consistently associated with a greater incidence of return of spontaneous circulation (ROSC) or increased hospital discharge rates from cardiac arrest due to VF.29 Defibrillation with biphasic waveforms (either truncated exponential or rectilinear), using equal or lower energy levels, appears at least as effective for termination of VF as monophasic waveforms.30,31
ENERGY LEVELS
Recommendations for energy levels to be used for defibrillation vary according to the type of defibrillator (and the specific waveform) that the rescuers are using. Previous recommendations regarding energy levels were based on studies using monophasic waveforms that demonstrated increased heart block with higher energy levels. Current recommendations are based on maximising the likelihood of the success of each shock. The recommended energy level for defibrillation in adults where monophasic defibrillators are used is 360 J for all shocks. When using biphasic waveforms, the energy level should be set at 200 J for all shocks, unless there are relevant clinical data for the specific defibrillator that suggest that an alternative energy level provides adequate shock success (e.g. > 90%). There is no consistent evidence (e.g. survival benefit) to suggest that an escalation of energy levels is required for subsequent shocks.29,32
SINGLE-SHOCK TECHNIQUE
The use of a single-shock strategy for defibrillation is now recommended (i.e. deliver a single shock and then immediately commence CPR, rather than deliver up to three shocks in a sequence). This strategy has been proposed to decrease the interruptions to chest compressions which occur as a result of the repeated assessment of rhythm and signs of life that were inherent in the stacked-shock approach.29 This protocol would be of particular benefit in scenarios where there is a significant time required for rhythm recognition and recharging of the defibrillator (i.e. > 10 seconds), such as with AEDs. The benefits of this strategy are clearly dependent on the quality of CPR, as the next shock will be delayed for at least 2 minutes while CPR is performed.
The Australian Resuscitation Council does however recommend the retention of a stacked-shock strategy (up to three shocks as necessary) in a specific circumstance: for the first defibrillation attempt in a witnessed arrest, where a manual defibrillator is immediately available, and the time required for rhythm recognition and charging of the defibrillator is short (e.g. < 10 seconds), resulting in delivery of the (up to) three shocks within 30 seconds. All subsequent shocks should be given using a single-shock strategy (see www.resus.org.au).
ADVANCED LIFE SUPPORT
ADVANCED LIFE SUPPORT FLOWCHART
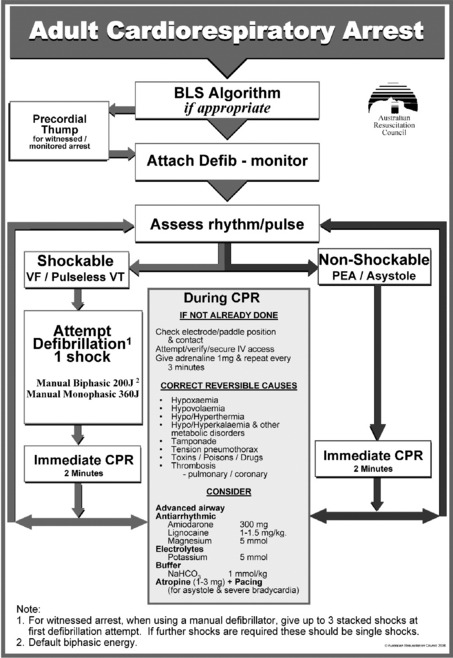
The recommended sequence of treatment to be followed is designated on the ALS flowchart of the Australian Resuscitation Council (www.resus.org.au; Figure 17.2). This is designed to be used as an aide-mémoire and a teaching tool.
PRECORDIAL THUMP
The provision of a precordial thump in a witnessed and monitored arrest due to a shockable rhythm may be of value if a defibrillator is not immediately available.29 However the technique is not without risks, and should not delay defibrillation.
CHEST COMPRESSIONS
The provision of good BLS is an essential part of the ALS management of both shockable and non-shockable rhythms. Interruptions to chest compressions for definitive procedures or interventions should be kept as brief as possible. Chest compressions should be continued up until defibrillation, and should be commenced again immediately following defibrillation (without checking the rhythm) and continued for at least 2 minutes unless signs of life return. Even if defibrillation has successfully reverted the rhythm into one that could generate a pulse, in the vast majority of cases this is not initially associated with an output.33 Immediate compressions in these situations avoid the detrimental effects of prolonged interruptions in compressions, maintain the coronary perfusion pressure and are not associated with an increased risk of refibrillation.34 After each 2 minutes of CPR (or if signs of life return), the underlying rhythm should be checked, and if a rhythm compatible with a return of spontaneous circulation is observed at this stage, then the pulse should also be checked.
AIRWAY MANAGEMENT DURING CPR
There are no data to support the routine use of any specific approach to airway management during cardiac arrest.35 Despite this, endotracheal intubation remains the gold standard for airway maintenance and airway protection in CPR. If the victim is unconscious and has no gag reflex, and a trained operator is available, endotracheal intubation should be performed at the first appropriate opportunity, and the patient ventilated with 100% oxygen. The endotracheal tube provides optimal isolation and patency of the airway, allows suctioning of the airway and also provides access for the delivery of some drugs (e.g. adrenaline (epinephrine), lidocaine and atropine). However, attempts at endotracheal intubation should not interrupt cardiac compressions for more than 20 seconds. The routine use of an endotracheal tube during cardiac arrest management has not been shown to improve outcomes, and without adequate training and experience the incidence of complications, such as unrecognised oesophageal intubation, is unacceptably high. Alternatives to the endotracheal tube that have been studied during CPR include the bag-valve mask and other advanced airway devices such as the laryngeal mask airway and oesophageal–tracheal combitube.35 The training and experience of the resuscitation team members and availability of such devices will determine the appropriate choice for airway adjunct.
VENTILATION DURING CPR
The minute ventilation requirements during cardiac arrest are less than those required in the non-arrested state. Hyperventilation during cardiac arrest is associated with increased intrathoracic pressure, decreased coronary and cerebral perfusion and, at least in animals, a decreased rate of return of spontaneous circulation.36 A compression-to-ventilation ratio of 30:2 is recommended before the airway is secured, and after the airway is secured the recommended ventilation rate is 8–10/min.2 One way to provide this, and to minimise interruptions to compressions, is to use a compression-to-ventilation ratio of 15:1 once the airway is secured (www.resus.com.au). If there is a concern about potential gas trapping, a period of disconnection from the ventilation circuit may be beneficial.35 The tidal volume recommended is one that results in a visible chest rise.2
IDENTIFICATION OF REVERSIBLE CAUSES
Irrespective of the initial or subsequent rhythms, cardiac arrests can be precipitated or perpetuated by a number of conditions, which, if not detected and corrected, may prevent successful resuscitation. These ‘reversible causes’ are categorised in the ALS algorithm as the ‘4Hs and 4Ts’ (www.resus.org.au; see Figure 17.2). A number of techniques are available to assist in the diagnosis and exclusion of these conditions (ranging from a good history, through careful clinical examination to investigations and interventions).24 Echocardiography can potentially diagnose (or help exclude) a number of cardiac and non-cardiac reversible causes (Table 17.2). Transoesophageal echocardiography requires a more skilled technician, but useful information can be obtained after minimal training with the transthoracic approach.24
Table 17.2 Potentially useful diagnoses detectable by echocardiography24
| Hypovolaemia* |
| Tamponade* (pericardial) |
| Tension pneumothorax* |
| Thrombosis – pulmonary* (thromboembolism) |
| Thrombosis – coronary* (regional or global wall motion abnormalities, including lack of cardiac motion) |
| Pacemaker capture |
| Unexpected ventricular fibrillation |
| Acute valvular insufficiency (e.g. papillary muscle rupture) |
| Ventricular rupture |
| Aortic dissection |
| Massive pleural effusion |
* Reversible causes listed in the ‘4Hs and 4Ts’ (www.resus.org.au).
DRUGS DURING CPR
Although various drugs are recommended for use during the management of cardiac arrests, there are no placebo-controlled studies that show that the routine use of any drugs at any stage during human cardiac arrest increase survival to hospital discharge.35
VASOPRESSORS
The putative beneficial effects of vasopressors during cardiac arrest are to increase the perfusion pressure to heart and brain. No vasopressor has been shown to improve long-term survival when compared with placebo for the management of cardiac arrests,35 but despite this lack of confirmatory evidence, it is reasonable to continue to use a vasopressor routinely in the management of cardiac arrests. There are insufficient data to support any particular drug or combination of drugs.35 Adrenaline remains the vasopressor of choice during the management of cardiac arrest (1 mg every 3 min). Vasopressin is an alternative drug, but studies have been unable to demonstrate any consistent benefit.35,37
ANTIARRHYTHMICS
No antiarrhythmic drug has been shown to improve long-term survival when compared with placebo for the management of cardiac arrests.35 However, administration of amiodarone (300 mg or 5 mg/kg) for shock-refractory VF has been associated with an increased survival to hospital when compared with either placebo38 or lidocaine.39 Either amiodarone or lidocaine (but not both) should be considered in those patients still in VF after repeated attempts at defibrillation (including attempted defibrillation after the administration of adrenaline) have failed.
OTHER DRUGS
Other drugs that are listed in the ALS flowchart to be considered during cardiac arrest include electrolytes (such as magnesium or potassium), atropine and sodium bicarbonate (www.resus.org.au; see Figure 17.2). Additional specific drugs may be indicated depending on the specific circumstances of the arrest (see summary in Table 17.3).40–42
Table 17.3 Cardiac arrest medications in specific circumstances40–42
| Medication | Potential indications |
|---|---|
| Adrenaline (epinephrine) | Beta-blocker/calcium channel blocker toxicity |
| Atropine | Cholinergic/cardiac glycoside toxicity |
| Benzodiazepines | Sympathomimetic toxicity |
| Calcium | Hypocalcaemia, hypermagnesaemia, hyperkalaemia, beta-blocker/calcium channel blocker toxicity |
| Digoxin-specific antibodies | Cardiac glycoside toxicity |
| Flumazenil | Benzodiazepine toxicity |
| Glucagon | Beta-blocker/calcium channel blocker toxicity |
| Magnesium | Hypomagnesaemia, hypokalaemia, hypercalcaemia, tricyclic antidepressant/cardiac glycoside toxicity, torsade de pointes |
| Naloxone | Opioid toxicity |
| Potassium | Hypokalaemia |
| Pyridoxine | Isoniazid toxicity |
| Sodium bicarbonate | Hyperkalaemia, tricyclic antidepressant/sodium channel blocker toxicity |
ADJUNCTS TO CPR
Many technologies and techniques have been evaluated as adjuncts to CPR in an attempt to improve survival in the management of cardiac arrests, but none have been consistently associated with improved outcomes.35 Active-compression decompression (ACD) CPR is the most widely evaluated technique, but it has not been associated with improved long-term survival.35 An automated version of ACD CPR has been developed (LUCAS) and is currently being evaluated. A modification of vest CPR (the load-distributing band) has had recent conflicting results.43,44 The impedance threshold valve appears promising (especially in combination with ACD CPR), and there has also been a resurgence of interest in extracorporeal techniques. At this stage there is insufficient supportive evidence to recommend the routine use of any of these adjunctive techniques.35
POSTRESUSCITATION CARE
INDUCED HYPOTHERMIA
Induced hypothermia has been used for postcardiac arrest management since the late 1950s, but it was brought to the attention of the wider medical community with the publication of two randomised controlled trials in 2002.45,46 Both of these trials, in unconscious but haemodynamically stable survivors of out-of-hospital cardiac arrests due to VF, demonstrated improved neurologically intact survival with a 12–24-hour period of induced hypothermia (32–34°C).
Mild hypothermia is associated with a number of potential beneficial effects in the postarrest patient, but also a number of potential adverse effects (Table 17.4).47 During hypothermia, the sedation and/or paralysis that may be needed to prevent the adverse effects of shivering may in turn mask seizure activity. Several techniques are available to cool patients, ranging from cold intravenous fluids through to commercial devices,45–47 and these continue to be evaluated.
Table 17.4 Potential risks and benefits of induced hypothermia after cardiac arrest47
| Potential neurological benefits |
| Decreased cerebral oxygen consumption (6–7%/°C) |
| Decreased excitatory amino acids (especially glutamate) |
| Decreased free radical formation/oxidative stress |
| Decreased neuron-specific enolase |
| Decreased cerebral lactate |
| Decreased cerebral oedema |
| Decreased intracranial pressure |
| Decreased cell-destructive enzymes |
| Decreased expression of intercellular adhesion molecule-1 (ICAM-1) |
| Decreased neutrophil migration to ischaemic tissue |
| Downregulates ongoing inflammatory response |
| Anticonvulsant effects |
| Better redistribution of blood to ischaemic areas |
| Increased neurotrophic factors |
| Potential risks |
| Cardiovascular |
| Bradycardia |
| Vasoconstriction |
| Arrhythmias (uncommon at 33°C) |
| Haematological |
| Decreased numbers/function of white blood cells |
| Decreased numbers/function of platelets |
| Prolonged clotting times |
| Gastrointestinal |
| Decreased gut motility |
| Hyperglycaemia |
| Renal |
| Renal dysfunction |
| Diuresis |
| Metabolic |
| Hypokalaemia |
| Hypophosphataemia |
| Musculoskeletal |
| Shivering (with associated lactic acidosis)* |
* Sedation and/or paralysis to control shivering may mask ongoing seizure activity.
It is recommended that unconscious but haemodynamically stable survivors of out-of-hospital cardiac arrests due to VF should be cooled to 32–34°C for 12–24 hours. A period of induced hypothermia should also be considered for cardiac arrests due to other rhythms, as well as in-hospital arrests.35,48
OTHER FACTORS IN POSTRESUSCITATION CARE
Clearly other factors are important in the postarrest period, but studies are limited.35 It is likely that hyperventilation and the resultant cerebral vasoconstriction are potentially harmful. Tight blood glucose control may be beneficial but its use remains controversial. Maintenance of cerebral perfusion, adequate oxygenation, treatment of seizures and good supportive care are likely to be beneficial. Norwegian investigators were able to double their survival to hospital discharge (with a favourable neurological outcome) for out-of-hospital cardiac arrests by introducing a standardised postresuscitation protocol. This protocol focused on vital organ function, including the use of therapeutic hypothermia, percutaneous coronary interventions (PCI) and the control of haemodynamics (mean arterial pressure (MAP) > 65 mmHg), blood glucose (5–8 mmol/l), ventilation (normocapnia) and seizures.49
BLOOD PRESSURE CONTROL
There are limited human data to guide hemodynamic management after cardiac arrest. Reported successful blood pressure goals have varied from a period of relative hypertension (MAP 90–100 mmHg45) to more standard goals (MAP > 65–70 mmHg49). It is recommended to aim for a blood pressure equal to the patient’s usual blood pressure or a systolic pressure greater than 100 mmHg.
GLUCOSE CONTROL
Tight blood glucose control (to normoglycaemia) in one study involving critically ill surgical patients improved survival,50 but this has not been replicated in subsequent studies.51 There is a large amount of circumstantial evidence to suggest that hyperglycaemia should be avoided but the optimal target for control is unknown.
PERCUTANEOUS CORONARY INTERVENTION
Thrombolysis and PCI are the mainstay of management after acute coronary syndromes. PCI has been used successfully when indicated in the early period after recovery of spontaneous circulation, and should be considered as part of routine postarrest care.49,52
MEDICAL EMERGENCY TEAMS (MET)
It has long been recognised that in-hospital cardiac arrests are usually preceded by some deterioration in physiological criteria.7,53 A number of different mechanisms have been proposed to respond to these early signs.35 The most common of these are variations of an MET (usually involving a multidisciplinary response to a single abnormality5) and early-warning systems (a response to an accumulated score). Commonly used criteria to initiate a MET call include:
OUTCOMES
Promising data from studies using historical controls (or before-and-after methodology) suggested that the introduction of an MET response resulted in a number of benefits, including reductions in hospital deaths and cardiac arrest rates, and improved outcomes following cardiac arrest.35 A large prospective cluster randomised study was performed in an attempt to confirm these benefits,5 but it was unable to demonstrate any statistically significant improvement in outcomes (see Chapter 2).
PRE-MET
Recent observations have confirmed that minor derangements in vital signs predict adverse clinical outcomes.54,55 Many of these derangements occur at a level that would not elicit either an MET call or an early-warning system response,55 therefore ward-based systems are required to respond to these factors.
PROGNOSTICATION
It is impossible to predict accurately the degree of neurological recovery during or immediately after a cardiac arrest.35,56 Relying on the neurologic examination during cardiac arrest to predict outcome is not recommended and should not be used as it has insufficient negative predictive value.35 After cessation of sedation (and/or induced hypothermia), the probability of awakening decreases with each day of coma. Clinical examination (absence of pupillary response or motor response to pain on day 3), somatosensory evoked potentials and electroencephalography offer the best prognostic estimates35,56 (www.resus.org.au).
1 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 3: Overview of CPR. Circulation. 2005;112(Suppl.):IV12-18.
2 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 2: Adult basic life support. Resuscitation. 2005;67:187-201.
3 Finn JC, Jacobs IG, Holman CD, et al. Outcomes of out-of-hospital cardiac arrest patients in Perth, Western Australia, 1996–1999. Resuscitation. 2001;51:247-255.
4 Jennings PA, Cameron P, Walker T, et al. Out-of-hospital cardiac arrest in Victoria: rural and urban outcomes. Med J Aust. 2006;185:135-139.
5 Hillman K, Chen J, Cretikos M, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365:2091-2097.
6 Sandroni C, Nolan J, Cavallaro F, et al. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intens Care Med. 2007;33:237-245.
7 Kause J, Smith G, Prytherch D, et al. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom – the ACADEMIA study. Resuscitation. 2004;62:275-282.
8 Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14 720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297-308.
9 Cohn AC, Wilson WM, Yan B, et al. Analysis of clinical outcomes following in-hospital adult cardiac arrest. Intern Med J. 2004;34:398-402.
10 Perkins GD, Soar J. In hospital cardiac arrest: missing links in the chain of survival. Resuscitation. 2005;66:253-255.
11 American Heart Association in collaboration with the International Liaison Committee on Resuscitation (ILCOR). Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2000;102(suppl. I):13-403. I
12 Proceedings of the 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2005;67:157-341.
13 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(Suppl.):IV1-203.
14 European Resuscitation Council guidelines for resuscitation 2005. Resuscitation. 2005;67(Suppl. 1):S1-189.
15 Morley PT, Zaritsky A. The evidence evaluation process for the 2005 International Consensus Conference on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2005;67:167-170.
16 Jacobs IG, Morley PT. The Australian Resuscitation Council: new guidelines for 2006. Crit Care Resusc. 2006;8:87-88.
17 Morley PT, Walker T. Australian Resuscitation Council: adult advanced life support (ALS) guidelines 2006. Crit Care Resusc. 2006;8:129-131.
18 Cummins RO, Hazinski MF. Guidelines based on fear of type II (false-negative) errors. Why we dropped the pulse check for lay rescuers. Resuscitation. 2000;46:439-442.
19 Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111:428-434.
20 Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71:137-145.
21 Eftestol T, Sunde K, Steen PA. Effects of interrupting precordial compressions on the calculated probability of defibrillation success during out-of-hospital cardiac arrest. Circulation. 2002;105:2270-2273.
22 Ewy GA. Cardiac arrest – guideline changes urgently needed. Lancet. 2007;369:882-884.
23 Eftestol T, Wik L, Sunde K, et al. Effects of cardiopulmonary resuscitation on predictors of ventricular fibrillation defibrillation success during out-of-hospital cardiac arrest. Circulation. 2004;110:10-15.
24 Morley PT. Monitoring the quality of CPR. Curr Opin Crit Care. 2007;13:261-267.
25 Kellum MJ, Kennedy KW, Ewy GA. Cardiocerebral resuscitation improves survival of patients with out-of-hospital cardiac arrest. Am J Med. 2006;119:335-340.
26 Cardiopulmonary resuscitation by bystanders with chest compression only (SOS-KANTO): an observational study. Lancet. 2007;369:920-926.
27 Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182-1188.
28 Wik L, Hansen TB, Fylling F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389-1395.
29 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 3: defibrillation. Resuscitation. 2005;67:203-211.
30 Morrison LJ, Dorian P, Long J, et al. Out-of-hospital cardiac arrest rectilinear biphasic to monophasic damped sine defibrillation waveforms with advanced life support intervention trial (ORBIT). Resuscitation. 2005;66:149-157.
31 Kudenchuk PJ, Cobb LA, Copass MK, et al. Transthoracic incremental monophasic versus biphasic defibrillation by emergency responders (TIMBER): a randomized comparison of monophasic with biphasic waveform ascending energy defibrillation for the resuscitation of out-of-hospital cardiac arrest due to ventricular fibrillation. Circulation. 2006;114:2010-2018.
32 Stiell IG, Walker RG, Nesbitt LP, et al. BIPHASIC trial: a randomized comparison of fixed lower versus escalating higher energy levels for defibrillation in out-of-hospital cardiac arrest. Circulation. 2007;115:1511-1517.
33 Rea TD, Shah S, Kudenchuk PJ, et al. Automated external defibrillators: to what extent does the algorithm delay CPR? Ann Emerg Med. 2005;46:132-141.
34 Hess EP, White RD. Ventricular fibrillation is not provoked by chest compression during post-shock organized rhythms in out-of-hospital cardiac arrest. Resuscitation. 2005;66:7-11.
35 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 4: advanced life support. Resuscitation. 2005;67:213-247.
36 Aufderheide TP. The problem with and benefit of ventilations: should our approach be the same in cardiac and respiratory arrest? Curr Opin Crit Care. 2006;12:207-212.
37 Aung K, Htay T. Vasopressin for cardiac arrest: a systematic review and meta-analysis. Arch Intern Med. 2005;165:17-24.
38 Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341:871-878.
39 Dorian P, Cass D, Schwartz B, et al. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346:884-890.
40 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 7.4: monitoring and medications. Circulation. 2005;112(Suppl.):IV78-83.
41 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10.1: life-threatening electrolyte abnormalities. Circulation. 2005;112(Suppl.):IV121-125.
42 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10.2: toxicology in ECC. Circulation. 2005;112(Suppl.):IV126-132.
43 Ong ME, Ornato JP, Edwards DP, et al. Use of an automated, load-distributing band chest compression device for out-of-hospital cardiac arrest resuscitation. JAMA. 2006;295:2629-2637.
44 Hallstrom A, Rea TD, Sayre MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA. 2006;295:2620-2628.
45 Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563.
46 Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556.
47 Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31:2041-2051.
48 Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118-121.
49 Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29-39.
50 Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
51 Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461.
52 Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629-1633.
53 Jacques T, Harrison GA, McLaws ML, et al. Signs of critical conditions and emergency responses (SOCCER): a model for predicting adverse events in the inpatient setting. Resuscitation. 2006;69:175-183.
54 Buist M, Bernard S, Nguyen TV, et al. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62:137-141.
55 Harrison GA, Jacques T, McLaws ML, et al. Combinations of early signs of critical illness predict in-hospital death-the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71:327-334.
56 Zandbergen EG, de Haan RJ, Stoutenbeek CP, et al. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998;352:1808-1812.