CHAPTER 386 Adjuvant Endovascular Management of Brain Arteriovenous Malformations
Clinical Significance and Treatment Plan
Treatment of AVMs often requires a multidisciplinary approach with a team consisting of an interventional neuroradiologist, a vascular neurosurgeon, a vascular neurologist, and a stereotactic radiosurgeon.1–5 Such a team provides a balanced approach to management of the AVM.
Patient Selection
The discovery of an AVM in a patient does not represent an immediate indication for treatment. Each AVM does not carry the same risk for future hemorrhage.6 An incidentally discovered cortical micro-AVM in a young patient does not have the same prognosis and natural history as a large thalamic AVM in a young patient with a progressive neurological deficit or hemorrhage.
Clinical Analysis
Age
In general, the younger the patient at the time of diagnosis, the more likely treatment should be considered. Diagnosis at a young age represents an early imbalance between the patient (i.e., host) and the AVM, and the patient has a greater number of years at risk for hemorrhage. Increasing age and age older than 60 years have also been found to be risk factors for hemorrhage.6,7 Older patients likewise have a greater risk for hemorrhage-associated morbidity and mortality than younger individuals do; therefore, treatment may even be warranted in the elderly.
Gender
The gender of the patient should not influence the decision to treat a brain AVM. Although there has been controversy about the risk for hemorrhage or other symptoms in women, especially during pregnancy, this association has not been well established. The majority of studies demonstrate that pregnancy or delivery does not seem to increase the risk for hemorrhage or the risk for progression of neurological symptoms.8–11
Initial Symptoms
Hemorrhage
Although some controversy exists regarding the rebleeding rate during the first year after hemorrhage, it is generally believed to be similar to or just slightly higher than the natural history risk of 2% to 4% per year.12–20 Each episode of hemorrhage is associated with a 10% risk for mortality and a 30% to 50% morbidity rate.35 A study by Ondra and coworkers analyzed the outcome of symptomatic AVMs. They reported a severe morbidity rate of 1.7%, annual mortality rate of 1%, and a mean rebleeding interval of greater than 6 years.18
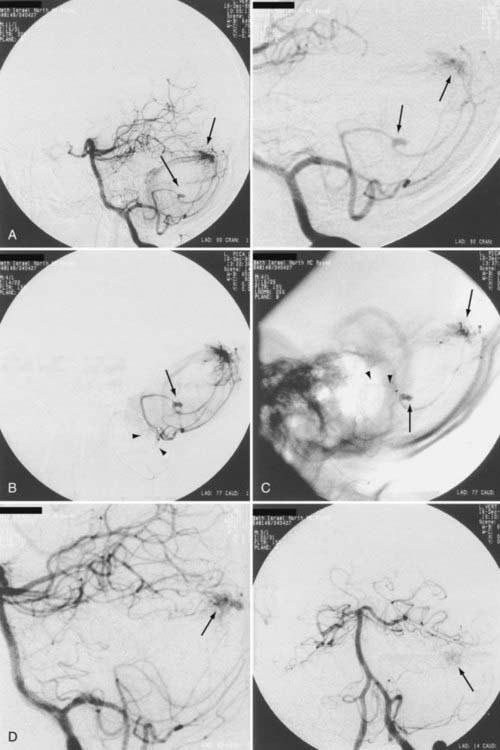
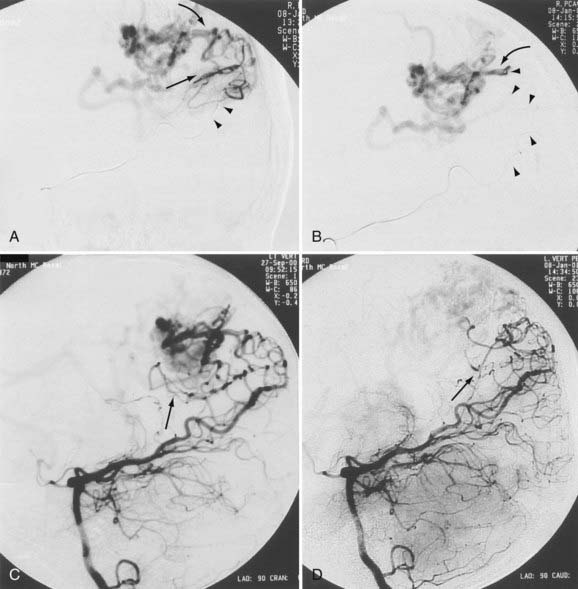
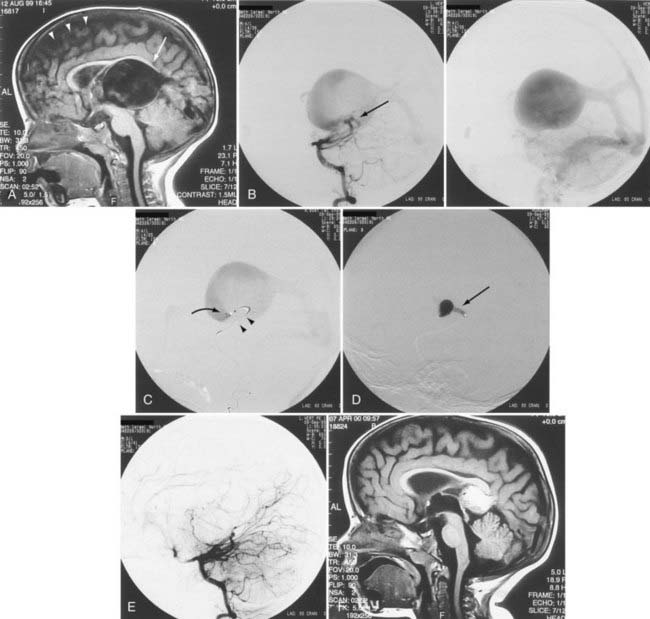
When the benefits of treatment outweigh the risks associated with treatment in patients with a history of AVM hemorrhage, the angioarchitecture of the AVM is meticulously analyzed in search of areas of anatomic weakness. The pattern of hemorrhage may correspond with a particular feature, such as a feeding artery or perinidal aneurysm. If such an abnormality is discovered, targeted embolization can be performed (Figs. 386-1 to 386-3).8,21 If the source of hemorrhage can be occluded by embolization, surgical resection may be deferred to allow more complete recovery. If embolization of high-risk or causative malformations is technically impossible or unsuccessful, early surgical resection may be necessary. Although treatment philosophies vary depending on local expertise, the universal goal should be to reduce or eliminate the risk for future hemorrhage with acceptable treatment-associated risk.
Seizures
Many patients initially seen with a seizure disorder can be treated successfully with antiepileptic medications and rarely require immediate intervention. Patients with seizures carry the same future risk for hemorrhage as those with other symptomatic AVMs. Seizures do not alter the risk for future hemorrhage.18 When indicated, both microsurgery and radiosurgery have demonstrated similar results in reducing the incidence of seizures attributable to AVMs and the need for anticonvulsant therapy.22–24 Embolization has little role in the specific treatment algorithm of patients with seizures, except as an adjuvant to definitive treatment planning.
Headaches
Headache is a nonspecific symptom. Some patients with AVMs that have a dural or posterior cerebral artery supply complain of headaches that resolve or improve after embolization.25 Other patients with parietal lobe AVMs complain of worsening headache after embolization of anterior and middle cerebral artery feeders if there is a compensatory increased supply through the posterior cerebral arteries. Headaches by themselves do not represent an indication for treatment of patients with AVMs because the risk for hemorrhage is not changed from that of the general population.
Neurological Deficit
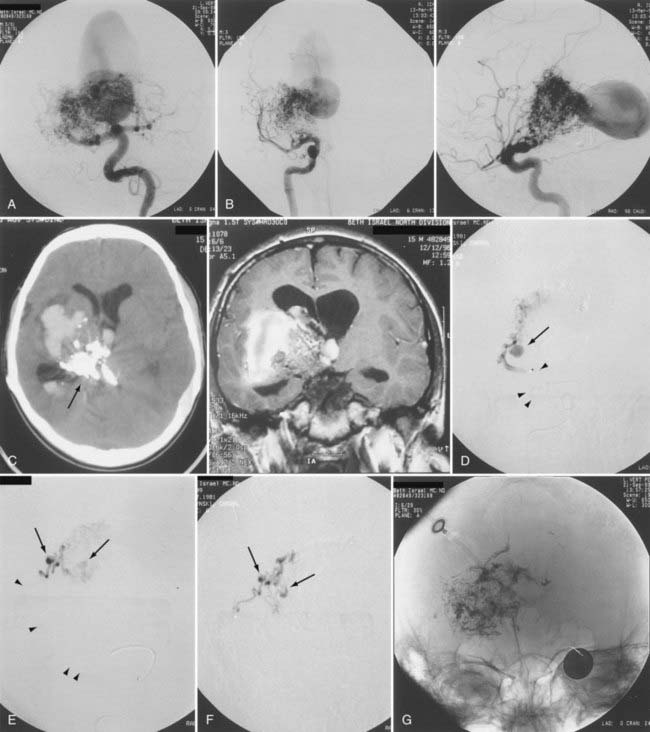
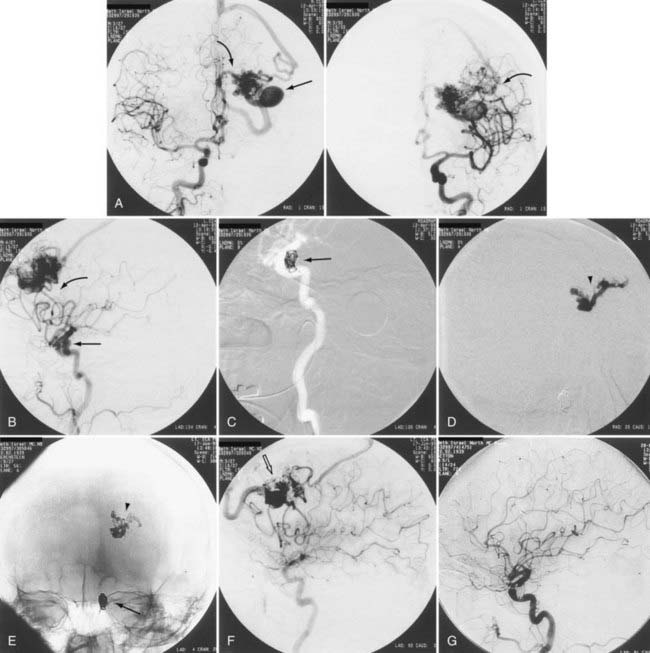
Up to 40% of patients with AVMs have neurological deficits,26 but neurological deficits are uncommon in patients without history of hemorrhage. In our experience and in the reports of others, neurological deficits are present in more than half of patients.25 Although arterial steal and mass effect have been implicated as possible causes,27 venous congestion and ischemia are also probable mechanisms (Fig. 386-4).25 Magnetic resonance imaging (MRI) has demonstrated changes in the venous drainage patterns of adjacent brain tissue with associated edema and partial thrombosis of draining veins of the AVM. If treated by partial embolization, these changes may be reversible in lobar AVMs causing progressive neurological deficits. Partial embolization of deep thalamic and basal ganglia AVMs in patients with progressive neurological deficits has been reported to result in stabilization in 27% and reversal of symptoms in 64% (see Fig. 386-3).28 Further definitive therapy should be carried out to completely obliterate the AVM and remove the risk for future hemorrhage, when possible.
Architecture of the Arteriovenous Malformation
A decision to treat depends, in part, on the demonstration of areas of weakness in the angioarchitecture indicating potential instability. If there is an associated arterial aneurysm on the feeding pedicle or in the nidus, evidence of venous thrombosis, restriction of outflow, venous hypertension, venous pouches or dilations, venous pseudoaneurysm, or compromised venous outflow, treatment may be indicated and can achieve cure in certain circumstances, or targeted treatment as an adjuvant to definitive treatment may be beneficial (Fig. 386-5; see also Figs. 386-1 to 386-4).
A number of radiologic variables have been associated with an increased risk for hemorrhage, including small AVM size, elevated feeding artery pressure, periventricular or intraventricular location, basal ganglia location, deep venous drainage, impaired venous drainage, single draining vein, intranidal aneurysm, multiple aneurysms, and vertebrobasilar blood supply.15,29–36
Data from the New York Islands AVM Hemorrhage Study, an ongoing, prospective, population-based survey to determine the incidence of AVM-related hemorrhage and the associated rates of morbidity and mortality in a zip code–defined population of 10 million people, showed that increasing age (hazard ratio [HR], 1.05; 95% confidence interval [CI], 1.03 to 1.08), initial hemorrhagic AVM manifestation (HR, 5.38; 95% CI, 2.64 to 10.96), deep brain location (HR, 3.25. 95% CI, 1.30 to 8.16), and exclusive deep venous drainage (HR, 3.25; 95% CI, 1.01 to 5.67) were independent predictors of subsequent hemorrhage. Annual hemorrhage rates on follow-up ranged from 0.9% in patients without a hemorrhagic AVM manifestation, deep AVM location, or deep venous drainage to as high as 34.4% in those harboring all three risk factors.6,12,20 The authors also studied predictors of residual dysplastic vessels on cerebral angiography after AVM treatment. The number of patients showing angiographic evidence of dysplastic vessels was significantly associated with increasing size of the AVM, and symptomatic postoperative intracerebral hemorrhage was associated with dysplastic vessels on the postoperative angiogram.37
Location, Size, and Deep Venous Drainage
Although the location, size, and deep venous drainage of a malformation are important factors in determining the risks associated with microsurgical or radiosurgical therapy,38 these factors are of lesser concern in the endovascular treatment of AVMs. In performing embolization of AVMs, meticulous technique is important to prevent embolization of nontarget tissue and stroke (see Fig. 386-5).
Associated Lesions
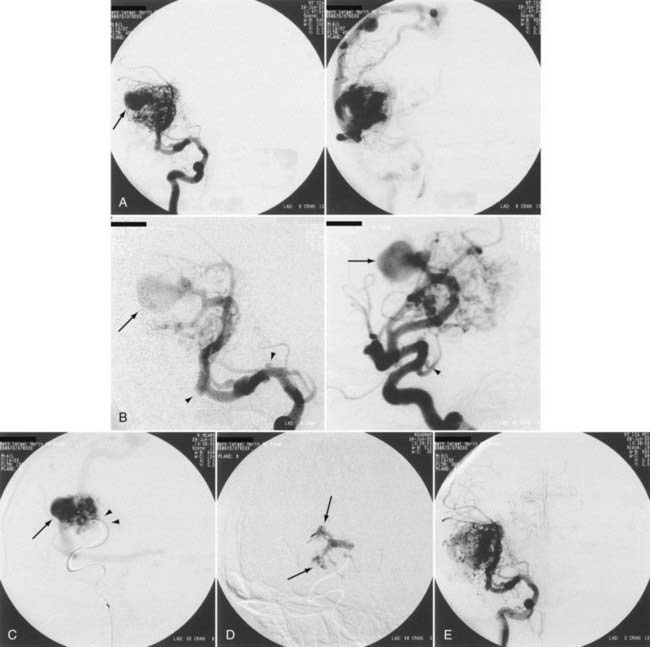
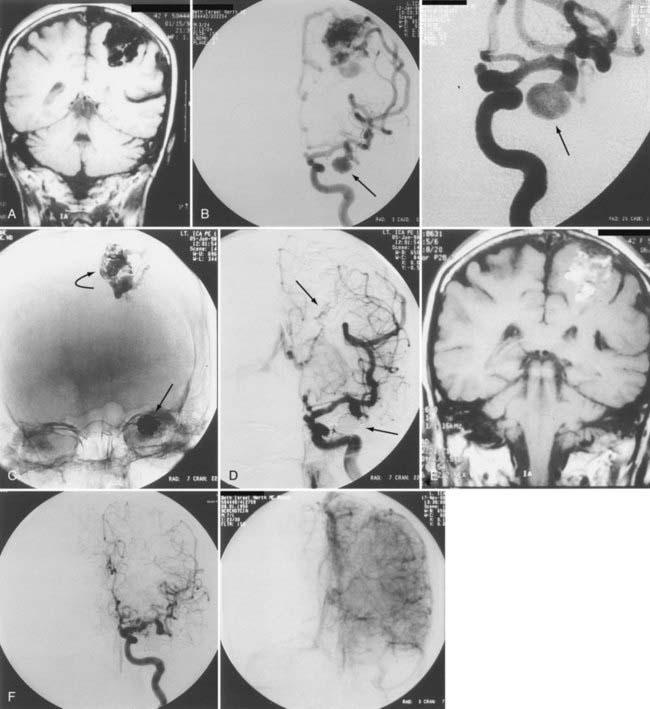
Aneurysms are associated with AVMs in 10% to 40% of patients.39–42 Such aneurysms may be flow related (i.e., proximal or distal on a feeder vessel), intranidal, or remote and apparently unrelated (Figs. 386-6 and 386-7; see also Fig. 386-2).39–42 This weakness represents a marker for future hemorrhage and increases the risk for hemorrhage from 3% to 7%.13 Patients with subarachnoid hemorrhage, intracerebral hemorrhage, or both and an angiographically demonstrated AVM with an associated aneurysm should have therapy directed first toward the lesion that is suspected to be the source of hemorrhage. In the presence of acute subarachnoid hemorrhage, the source of hemorrhage is often a feeding artery aneurysm. When an intracerebral hematoma exists, the more likely source of hemorrhage is the AVM, including nidal and perinidal locations (see Fig. 386-3). Enlarging pseudoaneurysms represent an unstable situation and deserve special attention to prevent early hemorrhage. Ventricular hemorrhage or parenchymal hemorrhage may be venous in origin.
High blood flow to an AVM may induce aneurysms to form on feeding arteries. Such “flow-related” aneurysms may regress after complete or partial obliteration of the AVM by endovascular embolization, and regression is more likely to occur the closer the aneurysm is to the nidus of the AVM.41 If the aneurysm is located close to the AVM, it should be obliterated at the time of embolization. If the aneurysm is within the planned resection field, it may be clipped at the time of microsurgery. If on follow-up angiography 6 to 12 months after AVM embolization there is no regression of the associated aneurysm, active treatment of the aneurysm should be considered. We recommend treating large, irregular, proximal aneurysms early as part of a global treatment plan (see Figs. 386-4 and 386-5). However, the treatment-associated morbidity must be low to justify the procedure in light of the unknown natural history of the aneurysm in the absence of the AVM.
Treatment Goals
Endovascular embolization is most commonly used as an adjunct to surgical resection or stereotactic radiosurgery. Embolization can be performed as the sole treatment (curative or palliative) in carefully selected patients, or it may result in excessive complications.43
Curative Embolization
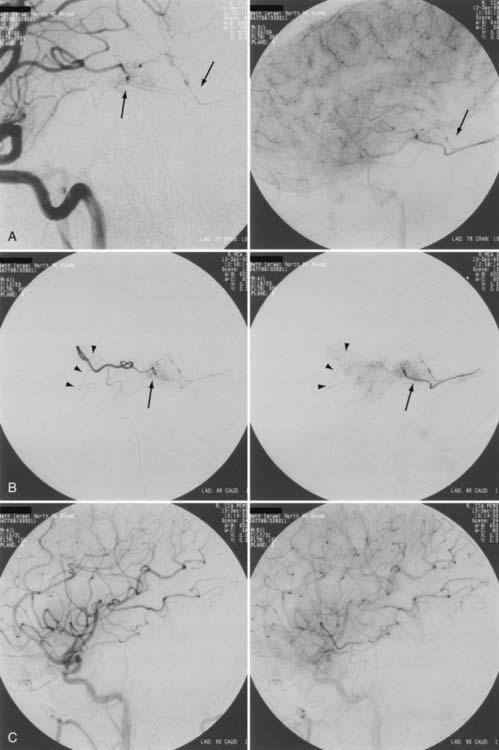
Curative embolization is discussed in greater detail elsewhere in this textbook. The prerequisite for curing an AVM with endovascular embolization techniques alone is angioarchitecture that permits solid casting of the AVM nidus with permanent embolization material. Curative embolization is complete anatomic obliteration of the malformation by endovascular embolization, occlusion of the nidus, and early venous shunting, and it therefore requires the use of a permanent, nonbiodegradable embolic agent to form a cast within the pathologic angioarchitecture (Fig. 386-8). Particles or resorbable agents should not be used for curative embolization.
In addition to the immediate postembolization angiogram, 6-month and, preferably, 12- to 24-month postprocedural angiograms are useful to confirm the durability of treatment. Long-term angiographic follow-up is needed to detect small remnants that may not have been appreciated on the immediate postembolization angiogram and to exclude recanalization caused by the radiopaque embolic material mixing with nonopaque autologous blood at the time of embolization.44–46 Another important reason for long-term angiographic follow-up is to rule out the potential recruitment of arterial collaterals. These collaterals probably develop by a mechanism of nonsprouting angiogenesis because of insufficient penetration of the embolic agent and lack of obliteration of the nidal-venous junction, with occlusion of only the proximal feeding vessel. Although delayed thrombosis may occur, it must be confirmed on long-term angiographic follow-up.44 After thrombosis of the venous outlets has occurred, no new veins can be recruited and a cure has been achieved. In general, when long-term follow-up angiography demonstrates complete obliteration of the malformation without residual opacification of the lesion, no arteriovenous shunting, and no stasis in the nidus, future recanalization is not expected.
Complete obliteration of an AVM is possible if it is a small lesion with a single pedicle and a terminal feeder (see Fig. 386-8). Our experience in total obliteration of small malformations is limited. We emphasize the need for late angiographic follow-up to confirm the lack of residual small shunts. With the future development of permanent embolic agents that are nonpolymerizing in nature, a higher cure rate may be achieved. Currently, studies using Onyx (ethylene vinyl copolymer, eV3, Plymouth, MN) have not demonstrated increased cure rates with primary embolization. In general, complication rates appear to be higher and patients are exposed to greater radiation,47–51 but further studies may demonstrate improved outcomes.
Palliative Targeted Embolization
If embolization was performed to reverse a progressive neurological deficit, clinical and MRI follow-up is recommended to determine whether the goal has been achieved and whether additional treatment stages are necessary (see Fig. 386-4). We believe that reduction of venous pressure and venous hypertension is the goal of this mode of treatment and can be documented by angiography and MRI. This management strategy should be discussed with the patient before treatment because it can facilitate patient compliance during the treatment and follow-up stages.
Preoperative Embolization
During the past 2 decades, the role of presurgical embolization of AVMs has become well accepted and firmly established.26,52–56 The goal of preoperative embolization is to facilitate surgical removal of the AVM (see Fig. 386-6). Studies have demonstrated that preoperative embolization leads to decreased operative time, blood loss, and morbidity and mortality in cerebral AVM surgery.46,55,57 To provide the best outcome with the lowest overall morbidity, communication between the neurointerventionalist and the vascular neurosurgeon regarding the specific goals of embolization is imperative.
The goals of adjuvant embolization are staged reduction of blood flow in an AVM by stepwise occlusion of the AVM nidus over a period of several weeks; elimination of deep-feeding arteries such as the lenticulostriate arteries, which could require extension of the resection plane beyond the margin of the nidus; and occlusion of any other feeding arteries not readily accessible during surgery, such as skull base dural collaterals (see Fig. 386-6).
Embolization And Radiosurgery
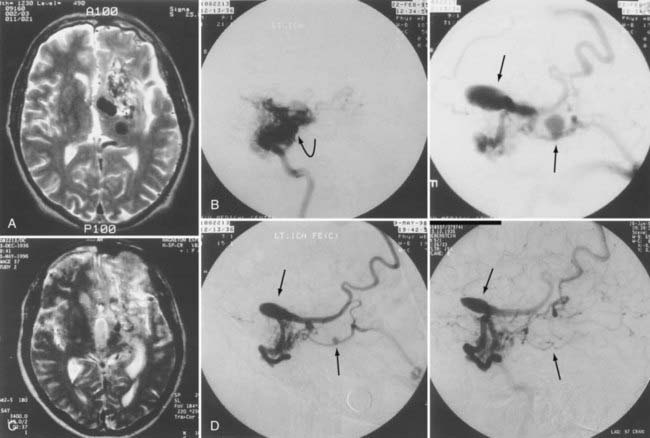
The two major goals of embolization before stereotactic radiosurgery are a reduction in overall AVM size and targeted embolization of specific angioarchitectural abnormalities (see Fig. 386-7). The use of embolization before radiosurgery is controversial.23 Mixed results have been reported regarding the effect of endovascular procedures on radiosurgical morbidity. One study found a lack of prior embolization to be predictive of neurological deficit and edema,58 whereas others have found that a history of prior embolization was associated with decreased obliteration rates, increased morbidity, or both.59–61 The latency period of obliteration after radiosurgery may be 1 to 5 years,62,63 and embolization before radiosurgery may increase the risk for hemorrhage during this period.
Embolization of an AVM before radiosurgery may be performed to decrease the size of the lesion to a volume suitable for radiosurgery. The volume of the AVM is one of the most important indicators predicting the success of stereotactic radiosurgery in achieving complete obliteration.23,60,64 However, global size reduction by endovascular embolization of large-volume AVMs is not always possible. This is most difficult to achieve in lesions with feeders from multiple territories and in lesions located in areas with marked potential for collateral supply (i.e., the occipital and temporal lobes). In such cases, to avoid induction of collateral supply, intranidal deposition of embolic material is mandatory (see Fig. 386-7). Although limited data are available concerning the success rate of embolization for size reduction as the primary goal of treatment,65 it remains the only method available to reduce AVM size to within the range amenable to radiosurgery.
Targeted AVM embolization before radiosurgery may be performed in patients with angioarchitectural abnormalities believed to increase the risk for hemorrhage. Distal flow-related aneurysms, intranidal pseudoaneurysms, venous aneurysms, and restriction of venous outflow should be targeted with a liquid adhesive. Large, irregular, multilobulated, proximal aneurysms should be occluded with microcoils such as the Guglielmi detachable coils to eliminate the risk for future rupture and subarachnoid hemorrhage (see Figs. 386-6 and 386-7). In deep AVM structures in which there is both a surgically accessible arterial supply (choroidal or circumferential) and a surgically inaccessible perforator supply, one can wait for the results of embolization to conclude the final treatment plan.
We believe that a permanent embolic agent such as N-butyl-2-cyanoacrylate (NBCA) should be used for preradiosurgical embolization, regardless of the indication (i.e., size reduction or targeted embolization). There is no role for embolization with temporary embolic material such as polyvinyl alcohol (PVA) particles or proximal occlusion agents such as platinum coils because no permanent obliteration of the nidus can be achieved. Conversely, permanent occlusive agents such as NBCA should not be used for occlusion of only proximal feeders because it leads to induction of a collateral supply and destabilization of the lesion.46,66
Techniques Of Embolization
Catheter Selection
Early attempts at catheterization and occlusion of blood vessels involved aneurysm patients and were done via an open surgical approach, which has many of the same risks and morbidity as open neurosurgical procedures.21 These techniques were transitioned for use in AVM patients. The first microcatheters that allowed access to AVM feeders were flow directed. Mounted calibrated leak balloons helped control flow and allowed the release of contrast material and cyanoacrylate embolic material, but these balloons were easy to overinflate and had a high rate of vessel perforation.67
Subsequently, a torqueable guidewire system that allowed passage of a catheter was developed (Advanced Cardiovascular System, Santa Clara, CA). Modifications were also made in the microcatheters, including softer distal segments with improved versatility.68,69 A thick-walled proximal catheter segment controls torque and transmits longitudinal movements, the intermediate catheter segment is flexible but still transmits torque, and the distal segment is soft and thin walled. There are now many vendors of microcatheters designed for intracranial navigation. However, because the cerebral arteries are more tortuous and fragile than the extracranial systemic arteries, specialized training is required for safe navigation.
Control of Flow
When a microcatheter has been positioned in the feeding artery or nidus of an AVM, an individual compartment can be cast if the surrounding blood flow can be controlled. As discussed, high-velocity arteriovenous shunting, unsuitable nidal architecture (i.e., fistulous single-hole compartments), or both can increase the risk of off-target embolization. If the angiogram shows congestion of the nidus (i.e., slow or stationary dye) after the injection of embolic material, downstream (i.e., venous outflow) occlusion may have occurred. High-grade stenosis of draining veins is a risk factor for AVM rupture.70 Small amounts of glue passing through the shunt can occlude the stenotic portion of the draining vein. In multicompartmental AVMs with multiple feeders and one draining vein, casting of one compartment, including the venous outlet, can obstruct the drainage system for the other compartments. Because there is no significant stromal support for the pial arteries, particularly in or around the AVM nidus, hemorrhage is likely to occur. For this reason, venous occlusion must be avoided.
To overcome these difficulties, several techniques were historically proposed. First, some microcatheter designs permitted control of flow in the feeder during embolization (calibrated leak balloon catheter). Second, soft platinum wires (available as straight and coiled wires) were designed to be injected through the microcatheter into high-flow fistulas before glue embolization to slow the torrential blood flow.71,72 These liquid coils (Boston Scientific/Target Therapeutics, Fremont, CA) remain in the fistula site and prevent a large amount of glue from migrating through the fistula. Finally, embolization can be performed with the patient under induced systemic arterial hypotension. Although the liquid coils are no longer manufactured and systemic hypotension is rarely if ever used, these techniques have historical interest.
Embolic Agents
Cyanoacrylates
Cyanoacrylate polymers are the most widely used of the liquid agents. Zanetti and Sherman first introduced polymerizing cyanoacrylates as liquid embolic agents for endovascular embolization.73 Because of their low viscosity, cyanoacrylates can be delivered through small, flexible, flow-directed catheters that can easily be manipulated to allow penetration deep into the AVM nidus. They are delivered and polymerize quickly, generally in seconds. Cyanoacrylates have high resistance to in vivo biodegradation.74,75 An intense inflammatory response helps maintain vessel occlusion and stimulate fibrosis. Although recanalization can occur, it is rare after complete embolization. The risk for recanalization is increased if there is proximal occlusion with minimal or no penetration of the nidus.76 In such cases, when the nidus remains active, collateral changes commonly develop to revascularize the nidus and may be unfavorable for future endovascular occlusion and present additional challenges during surgical resection of the AVM.
The first cyanoacrylate used was isobutyl-2-cyanoacrylate, which was discontinued after studies demonstrated that it exhibited some carcinogenic potential in animals.77 Histoacryl blue (NBCA) is now the embolization material of choice. NBCA is mixed with low-viscosity oily contrast medium (e.g., Ethiodol Ultra-Fluid), with or without additional tantalum or tungsten powder for radiologic visualization. NBCA polymerizes immediately on contact with free hydrogen ion in blood. The delivery catheter is rinsed with dextrose to prevent premature initiation of the polymerization. The main disadvantage of NBCA is related to its adhesive properties and the risk of adherence of the catheter to the adjacent blood vessel wall, which can cause injury to the vessel or inhibit removal. Modifications in cyanoacrylate’s chemical structure endeavor to reduce its adhesiveness and increase its cohesiveness for AVM embolization.
Ethyl Alcohol Copolymer in Dimethyl Sulfoxide Solvent
In the late 1980s, ethyl alcohol–dimethyl sulfoxide (DMSO) was first used for the embolization of AVMs.78 This liquid precipitate is also approved for the embolization of AVMs and is currently available in the United States as Onyx. This agent is viscous and precipitates in the biologic environment as the DMSO organic solvent diffuses into the lipid-rich environment. Onyx can be used to fill AVM vessels, and it does not specifically adhere to the delivery catheter. This permits more time for operator control over volume and rate of delivery, but prolonged fluoroscopic observation and periodic control angiography to assess the status of obliteration of the AVM are necessary.
Preliminary studies found that DMSO induced vasospasm and angionecrosis.38,79 Further studies found that decreasing the volume of DMSO and the rate of introduction could reduce these effects.80 In a study of 23 AVM patients, Jahan and coauthors reported one complication related to distal vasospasm that developed during injection, as well as histopathologic evidence of angionecrosis in two AVM specimens resected 24 hours after embolization.66 A recent study reported angionecrosis in 42.9%, but subsequent studies have not commented on this effect.47,49–51,81 Although long-term data are lacking, Murayama and associates demonstrated no recanalization in swine after 6 months of follow-up,80 and Jahan and colleagues reported no recanalization in a limited number of patients imaged up to 20 months after embolization.66 In a series of 47 patients, Weber and coworkers reported 84% initial nidal reduction with recanalization in 11% noted on angiography at 3 months.51 A study of patients undergoing microsurgical resection after Onyx embolization reported recanalization of embolized vessels in 14.3% on inspection by pathology,82 but recanalization rates as determined by angiography or pathology have not been reported in other recent studies.47,49,50,81
Use of Onyx is not without complications. Authors have reported cerebral artery perforations because relatively stiff-walled microcatheters that can resist the high pressures needed to inject Onyx must be used.51 Catheters tend to become lodged in the Onyx cast as the polymer precipitates around the shaft, even though the precipitate is nonadhesive.51 Onyx AVM embolization requires longer fluoroscopic and procedure times than needed for NBCA, and further investigation is necessary to justify the increased radiation exposure and procedure time associated with Onyx.83 Because Onyx remains relatively new at the time of this writing, its role in the treatment of cerebral AVMs remains to be defined.
Polyvinyl Alcohol
PVA is the most commonly used particulate agent historically for the embolization of AVMs. PVA particles (50 to 1000 µm) can be used in various size ranges at the discretion of the operator. A microcatheter with a diameter large enough to accommodate the particle size selected is necessary to prevent occlusion of the microcatheter. Larger catheters have decreased flexibility in the tortuous cerebral vasculature and thus make superselective angiography of small or distal vessels more difficult.2
Recanalization is also more likely with particulate embolization84–86 than with cyanoacrylate embolization, and particulates are therefore indicated only for preoperative embolization when surgical resection will follow closely. In a randomized clinical trial comparing embolization with NBCA versus PVA, the trial investigators found no difference in the degree of nidal reduction, number of vessels embolized, surgical resection times, need for transfusions and fluid replacement, and outpatient outcomes. There was a significant increase in the incidence of postsurgical hematomas in the PCA group.2 This study lead to Food and Drug Administration approval of NBCA with an indication for preoperative occlusion of brain AVMs.
Alternative Embolysates
A number of other agents have been used for the embolization of AVMs, including Silastic pellets, blood clots, dura, silk sutures, and Gelfoam.87–90 Undiluted absolute ethyl alcohol (98% dehydrated alcohol injection, U.S. Pharmacopeia) has also been used for the embolization of AVMs. Of the 17 patients treated with embolization, 7 were cured by embolization alone, 3 patients after microsurgery, and 1 additional patient after radiosurgery. Despite these promising results, 2 patients with partially treated lesions died, and 8 patients experienced complications related to therapy. Absolute ethanol also results in significant brain edema and can precipitate vasospasm. These significant side effects have not led to widespread use of ethanol for the embolization of brain AVMs.
Postoperative Monitoring And Follow-Up
Careful postoperative monitoring in the intensive care unit is a cornerstone of postoperative management. Monitoring of vital signs and neurological status is essential in preventing and detecting hemodynamic or neurological changes. We advocate axial imaging, even without any detectable neurological deficit, to visualize the residual malformation and surrounding change in parenchymal signal, to observe the venous drainage pathways, and to detect silent unexpected hemorrhage (see Figs. 386-4 and 386-7).
When curative embolization is the goal of therapy, long-term follow-up may be necessary. Complete angiographic embolization of an AVM does not guarantee that future hemorrhage will not occur because the nidus of the AVM remains active and there is a possibility of delayed recanalization through collateral channels.91 Permanent, complete endovascular cure is considered possible with the use of cyanoacrylate; however, the degree of permanent obliteration is probably high but remains unknown.91
Outcomes And Complications
Treatment of AVMs is a multidisciplinary team effort that combines several stages and modalities. In accordance with the experience reported in the literature, only complete obliteration of a malformation can be considered a cure that abolishes future risk. In most published series, the number of completely and permanently embolized AVMs is small. In overall cohorts of patients treated by embolization, the success rate for curative embolization is chiefly in the range of 5% to 20% but varies greatly from 0% to 70%.28,54,56,65,70,76,92–100 Curative embolization rates have been similar in patients treated with Onyx and NBCA and range between approximately 15% and 50%.47–51 Curative rates are higher in patients chiefly selected for curative embolization, but rates are still less than 70% and risks may be higher. For these reasons, curative embolization is reserved for only a select number of patients. In contrast, embolization as an adjunctive treatment is a cornerstone of AVM treatment.
In the literature there has been a wide range of reported rates of morbidity (0% to 50%) and mortality (1% to 4%) after embolization of AVMs.* Rates are dependent on many factors: patient selection, embolic agents, means of delivering the occlusive agent, preexisting neurological morbidity, the goals of embolization (i.e., primary curative or adjuvant therapy), and the time at which outcome is assessed.† Typically, neurological deficits occur in 10% to 14% of patients, disabling deficits in 2% to 5%, and death in approximately 1%.2,43,52–54,56,57,85,100,116–121 Recent studies using Onyx have reported similar results.47,49–51,66,82 When embolization is used as a primary treatment, adjuvant therapy, or palliative therapy, the following factors are predictive of postembolization deficits: number of embolization procedures, number of arterial feeders embolized, eloquent location, location in the basal ganglia, deep venous drainage, size smaller than 3 cm, size larger than 6 cm, Spetzler-Martin grade III to V, venous penetration of the glue cast, and AVMs with a pure fistula or a nidus with a fistulous component.43,54,116,118,121 Additionally, a patient’s age and general medical condition can alter the expected procedural risks. Although previous studies have not found these factors to be significantly predictive of postembolization deficits, this is most likely due to patient selection. Atherosclerotic vessels increase the risk for embolic complications from dislodged atherosclerotic plaque. Additionally, risks from inappropriate endovascular technique and insufficient neurointerventional equipment are evident and not discussed.
We have reviewed our treatment of 275 AVM patients between 1997 and 2006 to assess the frequency, severity, and predictors of neurological deficits after adjuvant embolization for cerebral AVMs.26 Two hundred two patients were treated in 377 embolization procedures. There were a total of 29 new clinical deficits after embolization (8% of procedures; 14% of patients), 19 of which were moderate or significant and 10 were mild or transient.
The most common and serious complication of embolization is intraprocedural or delayed hemorrhage (Fig. 386-9). Hemorrhagic complications associated with embolization occur in 1% to 2% of procedures (3% to 15% of patients).55,120,122,123 Hemorrhage during or after embolization may be due to vessel perforation during catheterization, AVM rupture, intranidal aneurysm rupture, or hemodynamic changes as a result of alterations in feeder pressure, normal perfusion breakthrough, or obstruction to venous outflow.120,124–127 We have found that hemorrhage during or after embolization is more likely with AVMs smaller than 3 cm in diameter or larger than 6 cm in diameter.26 Small AVMs may be technically more difficult to embolize than medium-sized AVMs. The increased propensity of small, untreated AVMs to bleed has been demonstrated in many studies and may be due to increased pressure within small AVMs.30,31,36 Deficits and hemorrhage are more likely to occur after the embolization of larger AVMs because it is more difficult to achieve complete embolization. Although accessibility and a reduced number of feeding vessels are associated with decreased complications, we have found small (<3 cm) versus medium (3 to 6 cm) size to be predictive of neurological deficits, intraprocedural hemorrhage, and infarction after embolization used as adjuvant therapy to microsurgery or radiosurgery.
In our series, postembolization deficits resolved in a significant number of patients (P < .0001) over a mean follow-up of 43.4 ± 34.6 months.26 Five patients suffered persistent neurological deficits on long-term follow-up (1% of procedures; 2% of patients). In multivariate analysis, the following variables significantly predicted the development of new neurological deficit after embolization: complex AVM with a treatment plan specifying more than one embolization procedure (odds ratio [OR], 2.7; 95% CI, 1.4 to 8.6), diameter smaller than 3 cm (OR, 3.2; 95% CI, 1.2 to 9.1), diameter larger than 6 cm (OR, 6.2; 95% CI, 1.0 to 57.0), deep venous drainage (OR, 2.7; 95% CI, 1.1 to 6.9), and eloquent location (OR, 2.4; 95% CI, 1.0 to 5.7).
Andrade-Souza YM, Ramani M, Scora D, et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007;60:443-451.
, 1999 Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
Debrun G, Vinuela F, Fox A, et al. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56:615-627.
Duong DH, Young WL, Vang MC, et al. Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke. 1998;29:1167-1176.
Frizzel RT, Fisher WS3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031-1039.
Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
Haw CS, terBrugge K, Willinsky R, et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226-232.
Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60-69.
Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58:602-611.
Liscak R, Vladyka V, Simonova G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1014.
Lundqvist C, Wikholm G, Svendsen P. Embolization of cerebral arteriovenous malformations: part II—aspects of complications and late outcome. Neurosurgery. 1996;39:460-467.
Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068.
, 2002 N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23:748-755.
Ogilvy CS, Stieg PE, Awad I, et al. AHA Scientific Statement: recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458-1471.
Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
Purdy PD, Batjer HH, Samson D. Management of hemorrhagic complications from preoperative embolization of arteriovenous malformations. J Neurosurg. 1991;74:205-211.
Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg. 1987;67:17-28.
Starke RM, Komotar RJ, Otten ML, et al. Adjuvant embolization with n-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke. 2009:2783-2790.
Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med. 1990;323:96-101.
Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163-168.
Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131-214.
Vinuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: part I—technique, morphology, and complications. Neurosurgery. 1996;39:448-457.
1 Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
2 N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23:748-755.
3 Gentili F, Schwartz M, TerBrugge K, et al. A multidisciplinary approach to the treatment of brain vascular malformations. Adv Tech Stand Neurosurg. 1992;19:179-207.
4 Ogilvy CS, Stieg PE, Awad I, et al. AHA Scientific Statement: Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458-1471.
5 Richling B, Killer M, Al-Schameri AR, et al. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59:S148-S157.
6 Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
7 Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
8 Amias AG. Cerebral vascular disease in pregnancy. I. Haemorrhage. J Obstet Gynaecol Br Commonw. 1970;77:100-120.
9 Horton JC, Chambers WA, Lyons SL, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867-871.
10 Robinson JL, Hall CJ, Sedzimir CB. Subarachnoid hemorrhage in pregnancy. J Neurosurg. 1972;36:27-33.
11 Robinson JL, Hall CS, Sedzimir CB. Arteriovenous malformations, aneurysms, and pregnancy. J Neurosurg. 1974;41:63-70.
12 Berman MF, Sciacca RR, Pile-Spellman J, et al. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47:389-396.
13 Brown RDJr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
14 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
15 Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:931-934.
16 Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995;37:851-855.
17 Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068.
18 Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
19 Stapf C, Labovitz DL, Sciacca RR, et al. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population-based stroke survey. Cerebrovasc Dis. 2002;13:43-46.
20 Stapf C, Mohr JP, Pile-Spellman J, et al. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus. 2001;11(5):e1.
21 Bristol RE, Albuquerque FC, McDougall CG. The evolution of endovascular treatment for intracranial arteriovenous malformations. Neurosurg Focus. 2006;20(6):E6.
22 Piepgras DG, Sundt TMJr, Ragoowansi AT, et al. Seizure outcome in patients with surgically treated cerebral arteriovenous malformations. J Neurosurg. 1993;78:5-11.
23 Starke RM, Komotar RJ, Hwang BY, et al. A comprehensive review of radiosurgery for cerebral arteriovenous malformations: outcomes, predictive factors, and grading scales. Stereotact Funct Neurosurg. 2008;86:191-199.
24 Yeh HS, Tew JMJr, Gartner M. Seizure control after surgery on cerebral arteriovenous malformations. J Neurosurg. 1993;78:12-18.
25 Berenstein A, Lasjaunias P. Surgical Neuroangiography: Endovascular Treatment of Cerebral Lesions, Vol 4. Berlin: Springer-Verlag. 1992.
26 Starke RM, Komotar RJ, Otten ML, et al. Adjuvant embolization with n-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke. 2009:2783-2790.
27 Carter LP, Gumerlock MK. Steal and cerebral arteriovenous malformations. Stroke. 1995;26:2371-2372.
28 Hurst RW, Berenstein A, Kupersmith MJ, et al. Deep central arteriovenous malformations of the brain: the role of endovascular treatment. J Neurosurg. 1995;82:190-195.
29 Batjer H, Suss RA, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986;18:29-35.
30 Duong DH, Young WL, Vang MC, et al. Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke. 1998;29:1167-1176.
31 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
32 Kader A, Young WL, Pile-Spellman J, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801-807.
33 Marks MP, Lane B, Steinberg GK, et al. Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology. 1990;176:807-813.
34 Miyasaka Y, Yada K, Ohwada T, et al. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:239-243.
35 Turjman F, Massoud TF, Vinuela F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37:856-860.
36 Waltimo O. The change in size of intracranial arteriovenous malformations. J Neurol Sci. 1973;19:21-27.
37 Stapf C, Connolly ES, Schumacher HC, et al. Dysplastic vessels after surgery for brain arteriovenous malformations. Stroke. 2002;33:1053-1056.
38 Chaloupka JC, Vinuela F, Vinters HV, et al. Technical feasibility and histopathologic studies of ethylene vinyl copolymer (EVAL) using a swine endovascular embolization model. AJNR Am J Neuroradiol. 1994;15:1107-1115.
39 Cunha e Sa MJ, Stein BM, Solomon RA, et al. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77:853-859.
40 Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793-800.
41 Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539-546.
42 Starke RM, Komotar RJ, Haque R, et al. Arteriovenous malformations and associated intracranial aneurysms: relationship to history of hemorrhage, disability on presentation, and long-term outcome after treatment. Paper presented at the Congress of Neurological Surgeons, New Orleans, LA: 2009.
43 Jayaraman MV, Marcellus ML, Hamilton S, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29:242-246.
44 Debrun G, Vinuela F, Fox A, et al. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56:615-627.
45 Debrun GM, Aletich V, Ausman JI, et al. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40:112-120.
46 DeMeritt JS, Pile-Spellman J, Mast H, et al. Outcome analysis of preoperative embolization with N-butyl cyanoacrylate in cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1995;16:1801-1807.
47 Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589-597.
48 Florio F, Lauriola W, Nardella M, et al. Endovascular treatment of intracranial arterio-venous malformations with Onyx embolization: preliminary experience. Radiol Med (Torino). 2003;106:512-520.
49 Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518-523.
50 van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol. 2007;28:172-177.
51 Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61:244-252.
52 Hartmann A, Mast H, Mohr JP, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431-2435.
53 Hartmann A, Pile-Spellman J, Stapf C, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816-1820.
54 Haw CS, terBrugge K, Willinsky R, et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226-232.
55 Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60-69.
56 Vinuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
57 Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg. 1987;67:17-28.
58 Liscak R, Vladyka V, Simonova G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1014.
59 Andrade-Souza YM, Ramani M, Scora D, et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007;60:443-451.
60 Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
61 Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239-1247.
62 Shin M, Kawahara N, Maruyama K, et al. Risk of hemorrhage from an arteriovenous malformation confirmed to have been obliterated on angiography after stereotactic radiosurgery. J Neurosurg. 2005;102:842-846.
63 Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.
64 Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med. 1990;323:96-101.
65 Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
66 Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984-995.
67 Kerber C. Balloon catheter with a calibrated leak. A new system for superselective angiography and occlusive catheter therapy. Radiology. 1976;120:547-550.
68 Berenstein A. Technique of catheterization and embolization of the lenticulostriate arteries. J Neurosurg. 1981;54:783-789.
69 Latchaw RE, Gold LH. Polyvinyl foam embolization of vascular and neoplastic lesions of the head, neck, and spine. Radiology. 1979;131:669-679.
70 Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131-214.
71 Berenstein A, Lasjaunias P. Surgical Neuroangiography, Vol 4. New York: Springer. 1991.
72 Richling B, Knosp E. Ein fuhrungsdrahtgesteuertes Kathersystem zur Begehung und Embolisation intrazerebraler Arterien. In: Schneider GH, Vogler E, editors. Digitale dildgenbende Verfahren, Interventionelle Verfahren, Integrierte digitale Radiologie. Berlin: Springer, 1988.
73 Zanetti PH, Sherman FE. Experimental evaluation of a tissue adhesive as an agent for the treatment of aneurysms and arteriovenous anomalies. J Neurosurg. 1972;36:72-79.
74 Richling B. Homologous controlled-viscosity fibrin for endovascular embolization. Part I. Experimental development of the medium. Acta Neurochir (Wien). 1982;62:159-170.
75 Richling B. Homologous controlled-viscosity fibrin for endovascular embolization. Part II: catheterization technique, animal experiments. Acta Neurochir (Wien). 1982;64:109-124.
76 Fournier D, TerBrugge KG, Willinsky R, et al. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg. 1991;75:228-233.
77 Fiorella D, Albuquerque FC, Woo HH, et al. The role of neuroendovascular therapy for the treatment of brain arteriovenous malformations. Neurosurgery. 2006;59:S163-S177.
78 Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163-168.
79 Sampei K, Hashimoto N, Kazekawa K, et al. Histological changes in brain tissue and vasculature after intracarotid infusion of organic solvents in rats. Neuroradiology. 1996;38:291-294.
80 Murayama Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43:1164-1175.
81 Jankowitz BT, Vora N, Jovin T, et al. Treatment of pediatric intracranial vascular malformations using Onyx-18. J Neurosurg Pediatr. 2008;2:171-176.
82 Natarajan SK, Ghodke B, Britz GW, et al. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with Onyx: single-center experience and technical nuances. Neurosurgery. 2008;62:1213-1225.
83 Velat GJ, Reavey-Cantwell JF, Sistrom C, et al. Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63:ONS73-78.
84 Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16:299-306.
85 Sorimachi T, Koike T, Takeuchi S, et al. Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: angiographic reappearance and complications. AJNR Am J Neuroradiol. 1999;20:1323-1328.
86 Standard SC, Guterman LR, Chavis TD, et al. Delayed recanalization of a cerebral arteriovenous malformation following angiographic obliteration with polyvinyl alcohol embolization. Surg Neurol. 1995;44:109-112.
87 Deveikis JP, Manz HJ, Luessenhop AJ, et al. A clinical and neuropathologic study of silk suture as an embolic agent for brain arteriovenous malformations. AJNR Am J Neuroradiol. 1994;15:263-271.
88 Luessenhop AJ, Rosa L. Cerebral arteriovenous malformations. Indications for and results of surgery, and the role of intravascular techniques. J Neurosurg. 1984;60:14-22.
89 Porstmann W, Wierny L, Warnke H, et al. Catheter closure of patent ductus arteriosus. 62 cases treated without thoracotomy. Radiol Clin North Am. 1971;9:203-218.
90 Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303-306.
91 Connors JJ, Wojak JC. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia: Saunders; 1999.
92 Cockroft KM, Hwang SK, Rosenwasser RH. Endovascular treatment of cerebral arteriovenous malformations: indications, techniques, outcome, and complications. Neurosurg Clin N Am. 2005;16:367-380. x
93 Deruty R, Pelissou-Guyotat I, Mottolese C, et al. The combined management of cerebral arteriovenous malformations. Experience with 100 cases and review of the literature. Acta Neurochir (Wien). 1993;123:101-112.
94 Frizzel RT, Fisher WS3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031-1039.
95 Paulsen RD, Steinberg GK, Norbash AM, et al. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery. 1999;44:991-996.
96 Valavanis A, Christoforidis G. Endovascular management of cerebral arteriovenous malformations. Neurointerventionist. 1999;1:34-40.
97 Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: part I—technique, morphology, and complications. Neurosurgery. 1996;39:448-457.
98 Willinsky R, Goyal M, TerBrugge K, et al. Embolization of small (<3 cm) brain arteriovenous malformations. Correlation of angiographic results to a proposed angioarchitecture grading system. Intervent Neuroradiol. 2001;7:19-27.
99 Wilms G, Goffin J, Plets C, et al. Embolization of arteriovenous malformations of the brain: preliminary experience. J Belg Radiol. 1993;76:299-303.
100 Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol. 2004;25:1139-1143.
101 Deruty R, Pelissou-Guyotat I, Amat D, et al. Complications after multidisciplinary treatment of cerebral arteriovenous malformations. Acta Neurochir (Wien). 1996;138:119-131.
102 Hartmann A, Stapf C, Hofmeister C, et al. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000;31:2361-2364.
103 Lundqvist C, Wikholm G, Svendsen P. Embolization of cerebral arteriovenous malformations: part II—aspects of complications and late outcome. Neurosurgery. 1996;39:460-467.
104 Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16:299-306.
105 Meisel HJ, Mansmann U, Alvarez H, et al. Effect of partial targeted N-butyl-cyano-acrylate embolization in brain AVM. Acta Neurochir (Wien). 2002;144:879-887.
106 Merland JJ, Rufenacht D, Laurent A, et al. Endovascular treatment with isobutyl cyano acrylate in patients with arteriovenous malformation of the brain. Indications, results and complications. Acta Radiol Suppl. 1986;369:621-622.
107 Morgan MK, Zurin AA, Harrington T, et al. Changing role for preoperative embolisation in the management of arteriovenous malformations of the brain. J Clin Neurosci. 2000;7:527-530.
108 Pasqualin A, Scienza R, Cioffi F, et al. Treatment of cerebral arteriovenous malformations with a combination of preoperative embolization and surgery. Neurosurgery. 1991;29:358-368.
109 Paulsen RD, Steinberg GK, Norbash AM, et al. Embolization of rolandic cortex arteriovenous malformations. Neurosurgery. 1999;44:479-484.
110 Purdy PD, Samson D, Batjer HH, et al. Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol. 1990;11:501-510.
111 Schmutz F, McAuliffe W, Anderson DM, et al. Embolization of cerebral arteriovenous malformations with silk: histopathologic changes and hemorrhagic complications. AJNR Am J Neuroradiol. 1997;18:1233-1237.
112 Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.
113 Vinuela F, Fox AJ, Debrun G, et al. Progressive thrombosis of brain arteriovenous malformations after embolization with isobutyl 2-cyanoacrylate. AJNR Am J Neuroradiol. 1983;4:1233-1238.
114 Wikholm G, Lundqvist C, Svendsen P. The Goteborg cohort of embolized cerebral arteriovenous malformations: a 6-year follow-up. Neurosurgery. 2001;49:799-805.
115 Wikholm G, Lundqvist C, Svendsen P. Transarterial embolization of cerebral arteriovenous malformations: improvement of results with experience. AJNR Am J Neuroradiol. 1995;16:1811-1817.
116 Cronqvist M, Wirestam R, Ramgren B, et al. Endovascular treatment of intracerebral arteriovenous malformations: procedural safety, complications, and results evaluated by MR imaging, including diffusion and perfusion imaging. AJNR Am J Neuroradiol. 2006;27:162-176.
117 Kim LJ, Albuquerque FC, Spetzler RF, et al. Postembolization neurological deficits in cerebral arteriovenous malformations: stratification by arteriovenous malformation grade. Neurosurgery. 2006;59:53-59.
118 Oran I, Parildar M, Derbent A. Ventricular/paraventricular small arteriovenous malformations: role of embolisation with cyanoacrylate. Neuroradiology. 2005;47:287-294.
119 Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58:602-611.
120 Purdy PD, Batjer HH, Samson D. Management of hemorrhagic complications from preoperative embolization of arteriovenous malformations. J Neurosurg. 1991;74:205-211.
121 Purdy PD, Batjer HH, Samson D, et al. Intraarterial sodium Amytal administration to guide preoperative embolization of cerebral arteriovenous malformations. J Neurosurg Anesthesiol. 1991;3:103-106.
122 Starke RM, Komotar RJ, Otten ML, et al. Adjuvant embolization in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Paper presented at the Congress of Neurological Surgeons. Orlando, FL: 2008.
123 Picard L, Da Costa E, Anxionnat R, et al. Acute spontaneous hemorrhage after embolization of brain arteriovenous malformation with N-butyl cyanoacrylate. J Neuroradiol. 2001;28:147-165.
124 Jafar JJ, Rezai AR. Acute surgical management of intracranial arteriovenous malformations. Neurosurgery. 1994;34:8-12.
125 Massoud TF, Hademenos GJ, Young WL, et al. Can induction of systemic hypotension help prevent nidus rupture complicating arteriovenous malformation embolization? Analysis of underlying mechanism achieved using a theoretical model. AJNR Am J Neuroradiol. 2000;21:1255-1267.
126 Sorimachi T, Takeuchi S, Koike T, et al. Blood pressure monitoring in feeding arteries of cerebral arteriovenous malformations during embolization: a preventive role in hemodynamic complications. Neurosurgery. 1995;37:1041-1047.
127 Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.