54 Adjunctive Respiratory Therapy
Many critically ill patients are unable to effectively clear secretions that accumulate in the central and peripheral airways. This can be due to factors such as increased secretion production, impaired cough reflex, weakness, and pain. The presence of an endotracheal tube prevents closure of the glottis to generate the high expiratory pressures necessary for an effective cough, thereby promoting the retention of secretions. In addition, in critically ill patients, cilia in the pulmonary tree are impaired in function and reduced in number.1,2 This leads to an increased risk of aspiration, atelectasis, and pneumonia, which are all detrimental in the critically ill patient.
Adjunctive respiratory therapy is able to prevent and treat respiratory complications that are encountered in the critically ill patient. As highlighted in Table 54-1, measures available range from those that are simple to institute, such as proper body positioning and suctioning, to more complex interventions such as chest physiotherapy, bronchoscopy, and use of aerosolized/inhaled medications that act directly on the pulmonary system.
TABLE 54-1 Adjunctive Respiratory Therapies
| Methods to Improve Pulmonary Mucociliary Clearance |
| Methods to Improve Lung Expansion |
| Methods to Improve Oxygenation and Ventilation |
 Methods to Improve Pulmonary Mucociliary Clearance
Methods to Improve Pulmonary Mucociliary Clearance
Percussion
Percussion of the chest can aid in secretion clearance. It is performed by clapping cupped hands over the thorax in a rhythmic fashion or using mechanical devices that mimic the same action. The energy of the force generated by the cupped hands is transmitted through the thorax to dislodge secretions. When used in conjunction with postural drainage, this is an effective method to mobilize secretions from the pulmonary tract. It is a technique often used in the daily management of cystic fibrosis patients3 and those with severe bronchiectasis.
High-Frequency Chest Compression
High-frequency chest compression (HFCC) relies on rapid pressure changes to the respiratory system during expiration to enhance movement of mucus from the peripheral airways to the central airways for clearance. This method employs an automated vest device worn by the patient. The vest is attached to an air-pulse generator, and small volumes of gas are introduced into it at a rapid rate ranging from 5 to 25 Hz, producing pressures up to 50 cm H2O. This technique, mainly used in cystic fibrosis patients, is equivalent to conventional chest physiotherapy techniques of percussion and postural drainage.4–6 One study examined the use of HFCC in nine long-term mechanically ventilated patients.7 In this small observational study, HFCC was compared to percussion and postural drainage. No difference was seen in the amount of sputum production, oxygen saturation, or patient comfort between the two methods, but HFCC was determined to be safe and felt to save staff time. It is difficult to apply this technique to most critically ill patients because of the size of the vest; covering the thorax may prevent adequate monitoring.
Manual Hyperinflation
Manual hyperinflation with an inflation bag and using high tidal volumes involves disconnecting the patient from the ventilator. Typically the lungs are inflated slowly to 1.5 to 2 times the tidal volume or to peak airway pressures of 40 cm H2O (as measured by a manometer) and then at end inspiration with an inspiratory pause to allow for filling of alveoli with slow time constants. This is followed by a quick release to allow for rapid expiration. The goal of manual hyperinflation is to recruit atelectatic lung regions to improve oxygenation and improve clearance of secretions. Similar to recruitment maneuvers described with mechanical ventilators, manual hyperinflation leads to only transient improvements in oxygenation, without any long-term clinically significant improvement in outcomes.8–12 It also has the disadvantage of requiring a ventilator disconnect, and this method can be mimicked by a mechanical ventilator.13
Positioning and Mobilization
Mobilization of patients in the intensive care unit (ICU) either through active or passive limb exercises may improve overall patient well being and, in the long term, may lead to better patient outcomes. In a recent randomized controlled trial of ventilated patients, the addition of early physiotherapy and occupational therapy to daily interruption of sedation resulted in slightly more ventilator-free days and improved functional capacity.14
Positioning also plays an important role in improving physiology and outcome in critically ill patients. Position of the patient with the head of the bed elevated at least 30 degrees significantly reduces the risk of aspiration and ventilator-associated pneumonia.15 Upright positioning of patients in whom there is no contraindication improves lung volumes and therefore gas exchange and work of breathing, especially in those where the supine or semirecumbent position leads to increased work of breathing. In some individuals with unilateral lung disease, positioning with the affected side up can lead to improved ventilation/perfusion (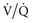 ) matching by increasing perfusion to the dependent “good” side.16,17 If atelectasis secondary to retained secretions is the cause, having the affected side up leads to improved postural drainage.
) matching by increasing perfusion to the dependent “good” side.16,17 If atelectasis secondary to retained secretions is the cause, having the affected side up leads to improved postural drainage.
Postural drainage involves positioning the body to allow gravity to assist in the movement of secretions and is indicated in patients with sputum production of more than 25 to 30 mL/day who have difficulty clearing their secretions.18 In cystic fibrosis, postural drainage with percussion is an effective method to clear pulmonary secretions and is associated with improved lung function.19,20
Tracheal Suction
Used in conjunction with other techniques to mobilize secretions from the peripheral to the central airways, suctioning is an effective way of removing secretions to improve bronchial hygiene. It can be performed using open methods where the patient is disconnected from the ventilator and a disposable suction catheter is placed. The closed system involves a suction catheter placed in a protective sheath and directly connected to the ventilator circuit. No disconnect is required, and the risk of environmental cross-contamination is reduced. Routine changes of in-line suction catheters are not required and are cost-effective.21,22 Overall, the risk of nosocomial pneumonia between the two systems is not different.23–25
Continuous Rotation Therapy
Continuous rotational or kinetic therapy extends the practice of regular 2-hourly repositioning of patients from one side to the other by placing the patient on a bed that moves to preprogrammed angles on a more frequent basis or through the use of air mattresses that deflate alternatively from side to side to provide postural position changes. Most studies demonstrate a lower incidence of nosocomial pneumonia or atelectasis.26–32 Only one small randomized trial found a reduction in duration of mechanical ventilation and length of stay, which was not confirmed in other prior studies.33
Bronchoscopy
Fiberoptic bronchoscopy has the advantage of providing direct visualization of the airways and permits suctioning of specific segments where secretions may be retained, causing problems such as atelectasis. The role of bronchoscopy in the ICU is reviewed elsewhere, but it can be considered an adjunctive therapy for the treatment of atelectasis or removal of secretions. As a recent review highlighted,29,34 bronchoscopy is a moderately effective technique for the treatment of atelectasis in the critically ill patient, with success rates ranging from 19% to 89% depending on the extent of atelectasis (lobar atelectasis responds better than subsegmental atelectasis). When compared with aggressive multimodal chest physiotherapy in the only randomized trial, no difference in the rate of resolution was seen between the two methods.35 Because bronchoscopy is an invasive procedure, it is not without associated risks and complications: sedation required for the procedure, transient increases in intracranial pressure, hypoxemia, and hemodynamic consequences/arrhythmias. Therefore bronchoscopy cannot be recommended as first-line therapy except in situations such as extensive unilateral atelectasis leading to significant difficulties in oxygenating or ventilating that have not resolved with other methods such as suctioning.
Chest Physiotherapy
Chest physiotherapy is a multimodal therapy with the goals of improving pulmonary function (gas exchange, improved lung compliance, and improved pulmonary mucus clearance). Techniques include percussive therapies (manual or mechanical chest percussion), postural drainage, chest vibration, manual hyperinflation, mobilization, suctioning, and rotational therapy. Overall, chest physiotherapy provides transient improvements in oxygenation and lung compliance, likely secondary to airway clearance and recruitment of atelectatic regions. In specific situations, it may improve outcome and clinical course, such as preventing ventilator-associated pneumonia36 or acute lobar atelectasis.37
 Aerosol Therapies
Aerosol Therapies
Aerosolization
Factors that influence the efficacy of aerosol delivery in the mechanically ventilated patient include38:
Bronchodilators
Bronchodilators are the most frequently administered aerosolized therapy in critically ill patients. Inhaled β2-agonists, such as albuterol or fenoterol, are generally well tolerated in the critically ill patient and are known to improve lung mechanics in patients with and without airflow obstruction. In acute lung injury, β2-agonists may improve lung edema clearance and have additional antiinflammatory properties, although the clinical significance of such therapy has yet to be established.39–42 Adverse effects (e.g., arrhythmias, hypokalemia) can occur in patients receiving excessive doses where significant systemic absorption is likely. Other bronchodilators including ipratropium bromide can also be effective in patients with increased airway reactivity, especially when used in conjunction with a β2-agonist. Bronchodilators administered via MDI are equally as effective as a nebulizer in spontaneously breathing patients.38 In mechanically ventilated patients, the use of nebulization is either equally as good as43 or less effective44,45 than an MDI with a spacer. MDI administration has the advantage of easier use without the risk of bacterial contamination and need for adjustment of flow rates.38
Antibiotics
Aerosolization of antibiotics as a form of topical treatment for pulmonary infections has been studied for over 20 years. Theoretical advantages of aerosolized antibiotics include direct therapy to the site of infection at higher concentrations, with a lower risk of systemic absorption and side effects. In chronic pulmonary infective states such as cystic fibrosis and severe bronchiectasis,46–48 aerosolized antibiotics have a role in reducing bacterial concentrations in the sputum, but they have only be shown to provide clinical long-term benefit in cystic fibrosis.48 In the acute infective state, aerosolized antibiotics have no additional benefit compared to parenteral antibiotics.49–51
In the intubated or tracheostomized patient, the risk of colonization of the airway is high, with a significant increase in the risk for nosocomial pneumonia. In an observational study of six chronically ventilated patients, aerosolized aminoglycosides (tobramycin or amikacin) eradicated the colonizing bacteria 67% of the time and significantly reduced the levels of inflammatory markers in the sputum.52 As a preventive measure, a recent meta-analysis of prospective clinical trials of aerosolized aminoglycosides suggested a significant reduction in the development of ventilator-associated pneumonia but no difference in overall mortality.53 As an adjuvant for treatment of ventilator-associated pneumonia, a meta-analysis of five randomized controlled trials suggested a significant improvement in the clinical resolution of pneumonia.54 Despite the findings, limitations of these analyses must be considered, given the heterogeneity of the trials. In addition, concerns of bacterial resistance must also be considered. Side effects reported in spontaneously breathing patients treated with inhaled tobramycin include increased cough, dyspnea, and chest pain.46
Mucoactive Agents
In chronic inflammatory lung conditions such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, bronchiectasis, and intubation/tracheostomy, overproduction of mucus and impaired clearance results in complications such as airflow obstruction, atelectasis, and infection. Mucus is primarily composed of water, mucin glycoprotein, cellular debris, neutrophil-derived filamentous actin and DNA, and bacteria.55 Mucoactive agents can help improve the clearance of mucus secretions.
Expectorant methods such as simple hydration together with oral expectorant medications (e.g., guaifenesin, bromhexine) that act via the vagal-mediated increase in airway secretion to decrease mucus viscosity have not been shown to be effective methods of clearing secretions.56,57 Oral iodine preparations (e.g., saturated solution of potassium iodide), although described as mucoactive agents, are similarly ineffective and may be associated with significant side effects such as hypothyroidism or hyperkalemia.55
Mucolytic agents reduce the viscosity of mucus by breaking down the mucin glycoprotein network or free DNA strands, thereby improving mucus rheology to improve clearance. Aerosolized N-acetylcysteine (NAC) breaks down the disulfide bonds of the mucin glycoprotein network and is associated with improved mucus clearance. However, because of increased incidence of bronchospasm with its use, therapy with NAC is not frequently initiated but may be used in conjunction with an inhaled bronchodilator.55 Free DNA can significantly increase the viscosity of mucus and therefore impede clearance from the airways. Recombinant human DNase (rhDNase, dornase alpha) improves pulmonary function in the chronic management of cystic fibrosis patients but has no significant effect in acute exacerbations of cystic fibrosis.58,59 In bronchiectasis not due to cystic fibrosis, rhDNase is not effective and may potentially be harmful.60
 Methods to Improve Lung Expansion
Methods to Improve Lung Expansion
Deep breathing and incentive spirometry involve coached inspiratory maneuvers to voluntarily increase lung volumes to greater than the vital capacity of the patient. These techniques require an awake, cooperative patient who is able to tolerate the maneuver. The only advantage of using an incentive spirometer is that it provides visual feedback and a reminder to the patient to continue these maneuvers. Incentive spirometry and deep breathing are equally effective in reducing postoperative pulmonary complications compared to chest physiotherapy.61,62
Intermittent positive-pressure breathing to improve lung expansion has fallen out of favor as a preventive measure in postoperative patients because of its expense, lack of difference in outcomes compared to deep breathing or incentive spirometry, and complications such as abdominal distension.62,63
 Methods to Improve Oxygenation and Ventilation
Methods to Improve Oxygenation and Ventilation
Nitric Oxide
Nitric oxide (NO) was first described as a vascular-derived relaxing factor that caused vasodilation via vascular smooth muscle relaxation. It is a highly lipid-soluble gas that allows for rapid diffusion through the alveolar-blood barrier into the pulmonary circulation and smooth muscle cells of the vasculature. The main action of NO is mediated by activating guanylate cyclase, increasing intracellular cyclic guanylate monophosphate (cGMP), thereby causing smooth muscle and subsequent vasomotor relaxation.64 The beneficial effects observed with inhaled NO are mediated primarily through its actions on pulmonary vascular smooth muscle. A reduction in pulmonary vascular resistance from arteriolar and venous vasodilation leads to reduced intravascular pressure at the level of the capillaries, with the potential benefit of reduced fluid leak into the alveoli. Additional benefits observed include a reduction in platelet aggregation and neutrophil adhesion/sequestration in the lungs.65–67 NO is rapidly inactivated by binding to the heme moiety of hemoglobin. Because of its short half-life, NO does not enter the systemic circulation, making it an ideal selective pulmonary vasodilator.
The most common use of NO in the ICU is in the setting of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Numerous clinical observational studies in ALI/ARDS have demonstrated improvements in oxygenation by improving 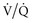 mismatch as demonstrated by a 10% to 20% increase in PaO2:FIO2 ratio and a reduction in pulmonary vascular resistance and mean pulmonary arterial pressures by at least 5 to 8 mm Hg.68 These physiologic benefits in both animal and clinical observational studies suggested that the use of NO in critically ill patients could be beneficial. Randomized control trials of varying sample size and design had similar findings69–72 and showed that NO improved the PaO2 and PaO2:FIO2 ratios acutely, but by 24 to 72 hours, those in the control group achieved the same level of improvement. Similarly, a reduction in mean pulmonary artery pressure was also observed in these trials with the use of NO. Only 60% of ALI/ARDS patients had a response with improved oxygenation after institution of inhaled NO.69 However, the improvement in oxygenation did not translate into clinically meaningful outcomes such as decrease in mortality, reduction in organ failure, or increased numbers of days free of mechanical ventilation. A trend towards a benefit was seen in a post hoc analysis in one trial in the more severe forms of ARDS, but further studies are needed.69 In fact, a meta-analysis of twelve randomized controlled trials did not support the routine use of inhaled NO in ALI and even suggested a possible increase in renal dysfunction.73
mismatch as demonstrated by a 10% to 20% increase in PaO2:FIO2 ratio and a reduction in pulmonary vascular resistance and mean pulmonary arterial pressures by at least 5 to 8 mm Hg.68 These physiologic benefits in both animal and clinical observational studies suggested that the use of NO in critically ill patients could be beneficial. Randomized control trials of varying sample size and design had similar findings69–72 and showed that NO improved the PaO2 and PaO2:FIO2 ratios acutely, but by 24 to 72 hours, those in the control group achieved the same level of improvement. Similarly, a reduction in mean pulmonary artery pressure was also observed in these trials with the use of NO. Only 60% of ALI/ARDS patients had a response with improved oxygenation after institution of inhaled NO.69 However, the improvement in oxygenation did not translate into clinically meaningful outcomes such as decrease in mortality, reduction in organ failure, or increased numbers of days free of mechanical ventilation. A trend towards a benefit was seen in a post hoc analysis in one trial in the more severe forms of ARDS, but further studies are needed.69 In fact, a meta-analysis of twelve randomized controlled trials did not support the routine use of inhaled NO in ALI and even suggested a possible increase in renal dysfunction.73
Almitrine bismesylate enhances pulmonary vasoconstriction in areas of hypoxic vasoconstriction, thereby enhancing redistribution of blood flow from shunt areas to lung units with normal 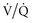 ratios.74,75 This effect of almitrine therefore potentates the effects of inhaled NO on gas exchange. Almitrine is not readily available in North America and requires further study to define its role in combination with NO.
ratios.74,75 This effect of almitrine therefore potentates the effects of inhaled NO on gas exchange. Almitrine is not readily available in North America and requires further study to define its role in combination with NO.
In addition to ALI/ARDS, other clinical conditions where NO use may be beneficial are listed in Table 54-2. Inhaled NO has been used post heart and lung transplants as a method to reduce right ventricular afterload in the setting of elevated pulmonary artery pressures.76 In lung transplants, NO has been described to reduce the risk of ischemia-reperfusion injury. But this effect was not supported by a recent randomized clinical trial in which NO was instituted early after lung transplantation.77
TABLE 54-2 Clinical Conditions Where Inhaled Nitric Oxide May Be Used
Inhaled NO is typically started at low doses ranging from 1 to 2 ppm and gradually increased until the desired effect is achieved. One method recommended in the United Kingdom, based on American-European Consensus Conference on ALI/ARDS guidelines, is to perform a dose/response test starting at 20 ppm and reducing the concentrations to 10, 5, and 0 ppm to find the lowest effective dose.78 A significant response should be considered a 20% increase in the PaO2:FIO2 ratio or at least a 5 mm Hg decrease in mean pulmonary artery pressure (PAP). Improvements in gas exchange are usually seen at lower doses than are reductions in PAP. The usual dose of inhaled NO ranges from 10 to 40 ppm. Doses greater than 80 ppm are associated with a higher risk for adverse effects. From the clinical trials, longer administration is generally safe with no evidence of the effect diminishing. However, inhaled NO should be weaned as soon as possible as a patient’s condition improves.
Adverse effects of NO include the formation of methemoglobin and spontaneous oxidation to nitrogen dioxide (NO2). NO2 is known to be toxic to the respiratory system with maximum exposure limited to 5 ppm. Complications from NO2 exposure include airway irritation and hyperreactivity with levels as low as 1.5 ppm and pulmonary edema and pulmonary fibrosis developing after exposure to higher levels. Despite these adverse effects, the development of methemoglobinemia or other toxicities related to NO2 during acute or prolonged NO inhalation has been unusual, especially when NO has been administered at concentrations less than 80 ppm.79
To reduce the risk of exposure to NO2, NO should be stored at concentrations no higher than 1000 ppm in a pure nitrogen environment and only exposed to oxygen at the time of administration. NO should be delivered into the ventilator circuit as close to the patient as possible. NO and NO2 levels should be monitored closely on the inspiratory side of the Y-piece when using doses above 2 ppm. Care should be taken to prevent abrupt discontinuation of NO. Rebound pulmonary vasoconstriction can occur with sudden discontinuation, leading to rapid worsening of 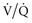 mismatch and pulmonary hypertension with significant hemodynamic collapse.80 Backup supplies of NO and delivery systems should be readily available.
mismatch and pulmonary hypertension with significant hemodynamic collapse.80 Backup supplies of NO and delivery systems should be readily available.
An absolute contraindication to NO therapy is methemoglobinemia reductase deficiency (congenital or acquired). Relative contraindications include bleeding diathesis (secondary to reports of altered platelet function and bleeding time with iNO), intracranial hemorrhage, and severe left ventricular failure (NHA grade III or IV).78
Inhaled Prostaglandins
Inhaled prostaglandins I2 (PGI2) and E1 (PGE1) have similar effects to inhaled nitric oxide, with minimal systemic effects. For PGI2, doses ranging from 1 to 50 ng/kg/min are favorably tolerated and reduce pulmonary artery pressures and improve oxygenation similar to iNO.81–83 PGE1 has the advantage of a more rapid degradation by pulmonary endothelial cells, providing a selective advantage over PGI2 at higher doses.84 Additional studies are required to define a role for these agents, but they can be considered as alternatives for rescue therapy when used for conditions similar to those treated with iNO. As with iNO, care must be taken to avoid abrupt discontinuation of PGI2 or PGE1, because rebound pulmonary hypertension and cardiovascular collapse can result.
Heliox
Helium is an inert gas with significantly lower density than room air (1.42 g/L for oxygen versus 0.17 g/L for helium). By substituting helium for nitrogen in the helium-oxygen mix (heliox), the degree of reduction in density of the gas is directly proportional to the fraction of the inspired oxygen concentration in the mix. Heliox reduces the Reynolds number, permitting more laminar flow and reducing airflow resistance and the work of breathing and dynamic hyperinflation associated with high airway resistance. Clinical situations where heliox may be used include conditions with high airflow resistance such as severe acute asthma or COPD exacerbations, bronchiolitis, bronchopulmonary dysplasia, and extrathoracic or tracheal obstruction. Heliox has been used to improve lung compliance during noninvasive ventilation in COPD patients, to reduce the work of breathing, to avoid intubation,85,86 and to improve aerosolized drug delivery.87 In the management of moderate to severe asthma exacerbations, routine use of heliox is not supported by systematic reviews of the literature but can be considered as an adjuvant in severe cases.88–90 In COPD exacerbation, two multicentered trials found no difference in intubation rate or length of stay in the ICU when heliox was added to noninvasive ventilation.91,92 However, there appeared to be a cost benefit resulting from a shorter overall hospital length of stay associated with the use of heliox.91 Heliox is generally well tolerated and produces no significant adverse effects. Disadvantages of its use in critically ill patients include cost of therapy and the high concentrations of helium required. Most studies utilize helium/oxygen mixes of 80:20 or 70:30 to achieve therapeutic benefit. At higher concentrations of oxygen, the effect of helium declines, and therefore heliox is limited in use to patients who are not severely hypoxemic. When used in conjunction with nebulized medications, higher flows of heliox may be required to ensure adequate delivery of the medication, though this may be offset by the smaller particle size generated in a heliox mixture.87,93 Ventilators also require recalibration for measured FIO2, flows, and tidal volumes when using heliox.94
Key Points
Kollef MH, Prentice D, Shapiro SD, et al. Mechanical ventilation with or without daily changes of in-line suction catheters. Am J Respir Crit Care Med. 1997;156(2 Pt 1):466-472.
Ntoumenopoulos G, Presneill JJ, McElholum M, Cade JF. Chest physiotherapy for the prevention of ventilator-associated pneumonia. Intensive Care Med. 2002;28(7):850-856.
Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26(1):15-23.
Meade MO, Granton JT, Matte-Martyn A, et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167(11):1483-1489.
Maggiore SM, Richard JC, Abroug F, et al. A multicenter, randomized trial of noninvasive ventilation with helium-oxygen mixture in exacerbations of chronic obstructive lung disease. Crit Care Med. 2010;38(1):145-151.
Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874-1882.
1 Konrad F, Schreiber T, Brecht-Kraus D, Georgieff M. Mucociliary transport in ICU patients. Chest. 1994;105(1):237-241.
2 Konrad F, Schiener R, Marx T, Georgieff M. Ultrastructure and mucociliary transport of bronchial respiratory epithelium in intubated patients. Intensive Care Med. 1995;21:482-489.
3 Thomas J, Cook DJ, Brooks D. Chest physical therapy management of patients with cystic fibrosis. A meta-analysis. Am J Respir Crit Care Med. 1995;151:846-850.
4 Arens R, Gozal D, Omlin KJ, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:1154-1157.
5 Scherer TA, Barandun J, Martinez E, Wanner A, Rubin EM. Effect of high-frequency oral airway and chest wall oscillation and conventional chest physical therapy on expectoration in patients with stable cystic fibrosis. Chest. 1998;113:1019-1027.
6 Varekojis SM, Douce FH, Flucke RL, et al. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir Care. 2003;48:24-28.
7 Whitman J, Van Beusekom R, Olson S, Worm M, Indihar F. Preliminary evaluation of high-frequency chest compression for secretion clearance in mechanically ventilated patients. Respir Care. 1993;38:1081-1087.
8 Hodgson C, Keating JL, Holland AE, et al. Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database Syst Rev 2009;CD006667.
9 Patman S, Jenkins S, Stiller K. Manual hyperinflation–effects on respiratory parameters. Physiother Res Int. 2000;5:157-171.
10 Ntoumenopoulos G, Gild A, Cooper DJ. The effect of manual lung hyperinflation and postural drainage on pulmonary complications in mechanically ventilated trauma patients. Anaesth Intensive Care. 1998;26:492-496.
11 Hodgson C, Denehy L, Ntoumenopoulos G, Santamaria J, Carroll S. An investigation of the early effects of manual lung hyperinflation in critically ill patients. Anaesth Intensive Care. 2000;28:255-261.
12 Denehy L. The use of manual hyperinflation in airway clearance. Eur Respir J. 1999;14:958-965.
13 Berney S, Denehy L. A comparison of the effects of manual and ventilator hyperinflation on static lung compliance and sputum production in intubated and ventilated intensive care patients. Physiother Res Int. 2002;7:100-108.
14 Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874-1882.
15 Keenan SP, Heyland DK, Jacka MJ, Cook D, Dodek P. Ventilator-associated pneumonia. Prevention, diagnosis, and therapy. Crit Care Clin. 2002;18:107-125.
16 Ibanez J, Raurich JM, Abizanda R, Claramonte R, Ibanez P, Bergada J. The effect of lateral positions on gas exchange in patients with unilateral lung disease during mechanical ventilation. Intensive Care Med. 1981;7:231-234.
17 Rivara D, Artucio H, Arcos J, Hiriart C. Positional hypoxemia during artificial ventilation. Crit Care Med. 1984;12:436-438.
18 AARC (American Association for Respiratory Care) clinical practice guideline. Postural drainage therapy. Respir Care. 1991;36:1418-1426.
19 Desmond KJ, Schwenk WF, Thomas E, Beaudry PH, Coates AL. Immediate and long-term effects of chest physiotherapy in patients with cystic fibrosis. J Pediatr. 1983;103:538-542.
20 Reisman JJ, Rivington-Law B, Corey M, et al. Role of conventional physiotherapy in cystic fibrosis. J Pediatr. 1988;113:632-636.
21 Kollef MH, Prentice D, Shapiro SD, et al. Mechanical ventilation with or without daily changes of in-line suction catheters. Am J Respir Crit Care Med. 1997;156:466-472.
22 Stoller JK, Orens DK, Fatica C, et al. Weekly versus daily changes of in-line suction catheters: impact on rates of ventilator-associated pneumonia and associated costs. Respir Care. 2003;48:494-499.
23 Subirana M, Sola I, Benito S. Closed tracheal suction systems versus open tracheal suction systems for mechanically ventilated adult patients. Cochrane Database Syst Rev 2007;CD004581.
24 Deppe SA, Kelly JW, Thoi LL, et al. Incidence of colonization, nosocomial pneumonia, and mortality in critically ill patients using a Trach Care closed-suction system versus an open-suction system: prospective, randomized study. Crit Care Med. 1990;18:1389-1393.
25 Zeitoun SS, de Barros AL, Diccini S. A prospective, randomized study of ventilator-associated pneumonia in patients using a closed vs. open suction system. J Clin Nurs. 2003;12:484-489.
26 Clemmer TP, Green S, Ziegler B, et al. Effectiveness of the kinetic treatment table for preventing and treating pulmonary complications in severely head-injured patients. Crit Care Med. 1990;18:614-617.
27 deBoisblanc BP, Castro M, Everret B, Grender J, Walker CD, Summer WR. Effect of air-supported, continuous, postural oscillation on the risk of early ICU pneumonia in nontraumatic critical illness. Chest. 1993;103:1543-1547.
28 Fink MP, Helsmoortel CM, Stein KL, Lee PC, Cohn SM. The efficacy of an oscillating bed in the prevention of lower respiratory tract infection in critically ill victims of blunt trauma. A prospective study. Chest. 1990;97:132-137.
29 Gentilello L, Thompson DA, Tonnesen AS, et al. Effect of a rotating bed on the incidence of pulmonary complications in critically ill patients. Crit Care Med. 1988;16:783-786.
30 Kirschenbaum L, Azzi E, Sfeir T, Tietjen P, Astiz M. Effect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilatory care. Crit Care Med. 2002;30:1983-1986.
31 Raoof S, Chowdhrey N, Feuerman M, King A, Sriraman R, Khan FA. Effect of combined kinetic therapy and percussion therapy on the resolution of atelectasis in critically ill patients. Chest. 1999;115:1658-1666.
32 Wang JY, Chuang PY, Lin CJ, Yu CJ, Yang PC. Continuous lateral rotational therapy in the medical intensive care unit. J Formos Med Assoc. 2003;102:788-792.
33 Staudinger T, Bojic A, Holzinger U, et al. Continuous lateral rotation therapy to prevent ventilator-associated pneumonia. Crit Care Med. 2010;38:486-490.
34 Kreider ME, Lipson DA. Bronchoscopy for atelectasis in the ICU: a case report and review of the literature. Chest. 2003;124:344-350.
35 Marini JJ, Pierson DJ, Hudson LD. Acute lobar atelectasis: a prospective comparison of fiberoptic bronchoscopy and respiratory therapy. Am Rev Respir Dis. 1979;119:971-978.
36 Ntoumenopoulos G, Presneill JJ, McElholum M, Cade JF. Chest physiotherapy for the prevention of ventilator-associated pneumonia. Intensive Care Med. 2002;28:850-856.
37 Stiller K, Geake T, Taylor J, Grant R, Hall B. Acute lobar atelectasis. A comparison of two chest physiotherapy regimens. Chest. 1990;98:1336-1340.
38 Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156:3-10.
39 Groshaus HE, Manocha S, Walley KR, Russell JA. Mechanisms of beta-receptor stimulation-induced improvement of acute lung injury and pulmonary edema. Crit Care. 2004;8:234-242.
40 Manocha S, Gordon AC, Salehifar E, Groshaus H, Walley KR, Russell JA. Inhaled beta-2 agonist salbutamol and acute lung injury: an association with improvement in acute lung injury. Crit Care. 2006;10:R12.
41 Perkins GD, McAuley DF, Thickett DR, Gao F. The Beta-Agonist Lung Injury Trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281-287.
42 Roca O, Gomez-Olles S, Cruz MJ, Munoz X, Griffiths MJ, Masclans JR. Effects of salbutamol on exhaled breath condensate biomarkers in acute lung injury: prospective analysis. Crit Care. 2008;12:R72.
43 Duarte AG, Momii K, Bidani A. Bronchodilator therapy with metered-dose inhaler and spacer versus nebulizer in mechanically ventilated patients: comparison of magnitude and duration of response. Respir Care. 2000;45:817-823.
44 Ikeda A, Nishimura K, Koyama H, et al. Comparison of the bronchodilator effects of salbutamol delivered via a metered-dose inhaler with spacer, a dry-powder inhaler, and a jet nebulizer in patients with chronic obstructive pulmonary disease. Respiration. 1999;66:119-123.
45 Marik P, Hogan J, Krikorian J. A comparison of bronchodilator therapy delivered by nebulization and metered-dose inhaler in mechanically ventilated patients. Chest. 1999;115:1653-1657.
46 Barker AF, Couch L, Fiel SB, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162:481-485.
47 Lin HC, Cheng HF, Wang CH, Liu CY, Yu CT, Kuo HP. Inhaled gentamicin reduces airway neutrophil activity and mucus secretion in bronchiectasis. Am J Respir Crit Care Med. 1997;155:2024-2029.
48 Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23-30.
49 Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest. 2006;130:1503-1510.
50 Flume PA, Mogayzel PJJr, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
51 Stephens D, Garey N, Isles A, Levison H, Gold R. Efficacy of inhaled tobramycin in the treatment of pulmonary exacerbations in children with cystic fibrosis. Pediatr Infect Dis. 1983;2:209-211.
52 Palmer LB, Smaldone GC, Simon SR, O’Riordan TG, Cuccia A. Aerosolized antibiotics in mechanically ventilated patients: delivery and response. Crit Care Med. 1998;26:31-39.
53 Falagas ME, Siempos II, Bliziotis IA, Michalopoulos A. Administration of antibiotics via the respiratory tract for the prevention of ICU-acquired pneumonia: a meta-analysis of comparative trials. Crit Care. 2006;10:R123.
54 Ioannidou E, Siempos II, Falagas ME. Administration of antimicrobials via the respiratory tract for the treatment of patients with nosocomial pneumonia: a meta-analysis. J Antimicrob Chemother. 2007;60:1216-1226.
55 Rubin BK. The pharmacologic approach to airway clearance: mucoactive agents. Respir Care. 2002;47:818-822.
56 Clarke SW, Thomson ML, Pavia D. Effect of mucolytic and expectorant drugs on tracheobronchial clearance in chronic bronchitis. Eur J Respir Dis Suppl. 1980;110:179-191.
57 Shim C, King M, Williams MHJr. Lack of effect of hydration on sputum production in chronic bronchitis. Chest. 1987;92:679-682.
58 Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637-642.
59 Wilmott RW, Amin RS, Colin AA, et al. Aerosolized recombinant human DNase in hospitalized cystic fibrosis patients with acute pulmonary exacerbations. Am J Respir Crit Care Med. 1996;153:1914-1917.
60 O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest. 1998;113:1329-1334.
61 Guimaraes MM, El DR, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database Syst Rev 2009;CD006058.
62 Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Phys Ther. 1994;74:3-10.
63 Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. Am Rev Respir Dis. 1984;130:12-15.
64 Gianetti J, Bevilacqua S, De Caterina R. Inhaled nitric oxide: more than a selective pulmonary vasodilator. Eur J Clin Invest. 2002;32:628-635.
65 Gries A, Bode C, Peter K, et al. Inhaled nitric oxide inhibits human platelet aggregation, P-selectin expression, and fibrinogen binding in vitro and in vivo. Circulation. 1998;97:1481-1487.
66 Gries A, Herr A, Motsch J, et al. Randomized, placebo-controlled, blinded and cross-matched study on the antiplatelet effect of inhaled nitric oxide in healthy volunteers. Thromb Haemost. 2000;83:309-315.
67 Sato Y, Walley KR, Klut ME, et al. Nitric oxide reduces the sequestration of polymorphonuclear leukocytes in lung by changing deformability and CD18 expression. Am J Respir Crit Care Med. 1999;159:1469-1476.
68 Hart CM. Nitric oxide in adult lung disease. Chest. 1999;115:1407-1417.
69 Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15-23.
70 Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group on Inhaled Nitric Oxide. Intensive Care Med. 1999;25:911-919.
71 Michael JR, Barton RG, Saffle JR, et al. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med. 1998;157:1372-1380.
72 Troncy E, Collet JP, Shapiro S, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483-1488.
73 Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334:779.
74 Payen DM, Gatecel C, Plaisance P. Almitrine effect on nitric oxide inhalation in adult respiratory distress syndrome. Lancet. 1993;341:1664.
75 Reyes A, Roca J, Rodriguez-Roisin R, Torres A, Ussetti P, Wagner PD. Effect of almitrine on ventilation-perfusion distribution in adult respiratory distress syndrome. Am Rev Respir Dis. 1988;137:1062-1067.
76 Date H, Triantafillou AN, Trulock EP, Pohl MS, Cooper JD, Patterson GA. Inhaled nitric oxide reduces human lung allograft dysfunction. J Thorac Cardiovasc Surg. 1996;111:913-919.
77 Meade MO, Granton JT, Matte-Martyn A, et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483-1489.
78 Cuthbertson BH, Dellinger P, Dyar OJ, et al. UK guidelines for the use of inhaled nitric oxide therapy in adult ICUs. American-European Consensus Conference on ALI/ARDS. Intensive Care Med. 1997;23:1212-1218.
79 Weinberger B, Laskin DL, Heck DE, Laskin JD. The toxicology of inhaled nitric oxide. Toxicol Sci. 2001;59:5-16.
80 Christenson J, Lavoie A, O’Connor M, Bhorade S, Pohlman A, Hall JB. The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med. 2000;161:1443-1449.
81 van Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL. Dose-response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest. 2000;117:819-827.
82 Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:991-996.
83 Zwissler B, Kemming G, Habler O, et al. Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:1671-1677.
84 Meyer J, Theilmeier G, Van Aken H, et al. Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg. 1998;86:753-758.
85 Jaber S, Fodil R, Carlucci A, et al. Noninvasive ventilation with helium-oxygen in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1191-1200.
86 Jolliet P, Tassaux D, Thouret JM, Chevrolet JC. Beneficial effects of helium:oxygen versus air:oxygen noninvasive pressure support in patients with decompensated chronic obstructive pulmonary disease. Crit Care Med. 1999;27:2422-2429.
87 Goode ML, Fink JB, Dhand R, Tobin MJ. Improvement in aerosol delivery with helium-oxygen mixtures during mechanical ventilation. Am J Respir Crit Care Med. 2001;163:109-114.
88 Rodrigo G, Pollack C, Rodrigo C, Rowe BH. Heliox for nonintubated acute asthma patients. Cochrane Database Syst Rev 2006;CD002884.
89 Ho AM, Lee A, Karmakar MK, Dion PW, Chung DC, Contardi LH. Heliox vs air-oxygen mixtures for the treatment of patients with acute asthma: a systematic overview. Chest. 2003;123:882-890.
90 Rodrigo GJ, Rodrigo C, Pollack CV, Rowe B. Use of helium-oxygen mixtures in the treatment of acute asthma: a systematic review. Chest. 2003;123:891-896.
91 Jolliet P, Tassaux D, Roeseler J, et al. Helium-oxygen versus air-oxygen noninvasive pressure support in decompensated chronic obstructive disease: A prospective, multicenter study. Crit Care Med. 2003;31:878-884.
92 Maggiore SM, Richard JC, Abroug F, et al. A multicenter, randomized trial of noninvasive ventilation with helium-oxygen mixture in exacerbations of chronic obstructive lung disease. Crit Care Med. 2010;38:145-151.
93 Hess DR, Acosta FL, Ritz RH, Kacmarek RM, Camargo CAJr. The effect of heliox on nebulizer function using a beta-agonist bronchodilator. Chest. 1999;115:184-189.
94 Tassaux D, Jolliet P, Thouret JM, Roeseler J, Dorne R, Chevrolet JC. Calibration of seven ICU ventilators for mechanical ventilation with helium-oxygen mixtures. Am J Respir Crit Care Med. 1999;160:22-32.
95 Krasuski RA, Warner JJ, Wang A, Harrison JK, Tapson VF, Bashore TM. Inhaled nitric oxide selectively dilates pulmonary vasculature in adult patients with pulmonary hypertension, irrespective of etiology. J Am Coll Cardiol. 2000;36:2204-2211.
96 Beghetti M, Adatia I. Inhaled nitric oxide and congenital cardiac disease. Cardiol Young. 2001;11:142-152.
97 Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double-blind study. Lancet. 2000;356:1464-1469.
98 Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F. Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med. 1997;23:1089-1092.
99 Schmid ER, Burki C, Engel MH, Schmidlin D, Tornic M, Seifert B. Inhaled nitric oxide versus intravenous vasodilators in severe pulmonary hypertension after cardiac surgery. Anesth Analg. 1999;89:1108-1115.
100 Solina A, Papp D, Ginsberg S, et al. A comparison of inhaled nitric oxide and milrinone for the treatment of pulmonary hypertension in adult cardiac surgery patients. J Cardiothorac Vasc Anesth. 2000;14:12-17.
101 Kieler-Jensen N, Lundin S, Ricksten SE. Vasodilator therapy after heart transplantation: effects of inhaled nitric oxide and intravenous prostacyclin, prostaglandin E1, and sodium nitroprusside. J Heart Lung Transplant. 1995;14:436-443.
102 Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Curr Opin Hematol. 2003;10:99-107.
103 Weiner DL, Hibberd PL, Betit P, Cooper AB, Botelho CA, Brugnara C. Preliminary assessment of inhaled nitric oxide for acute vaso-occlusive crisis in pediatric patients with sickle cell disease. JAMA. 2003;289:1136-1142.

