Chapter 88 Acute Retinal Necrosis Syndrome
Definition
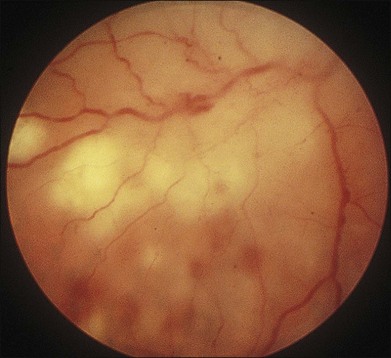
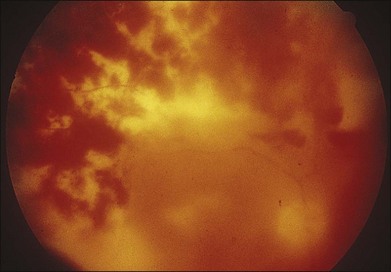
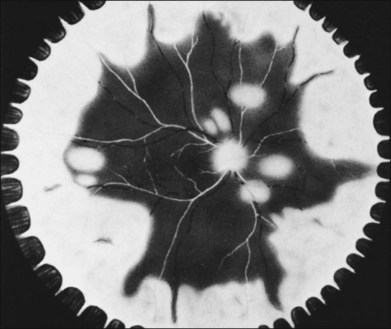
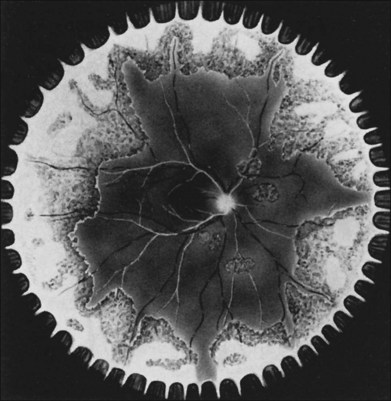
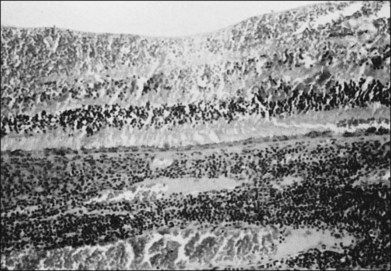
In 1971 Urayama and coworkers1 first reported six cases of an apparently new syndrome characterized by acute necrotizing retinitis, vitritis, retinal arteritis, choroiditis, and late-onset rhegmatogenous retinal detachment. Young and Bird2 described two similar cases in 1978 and gave the syndrome the acronym BARN (bilateral acute necrosis syndrome). With the subsequent recognition of unilateral and asynchronous bilateral cases, the disease has been termed simply acute retinal necrosis syndrome, or ARN syndrome. Acute retinal necrosis syndrome is characterized by the initial onset of episcleritis or scleritis, periorbital pain, and anterior uveitis, which may be granulomatous or stellate in appearance. This is followed by decreased vision resulting from vitreous opacification, necrotizing retinitis, and, in some cases, optic neuritis or neuropathy. The retinitis appears as deep, multifocal, yellow-white patches, typically beginning in the peripheral fundus (Figs 88.1, 88.2) and then becoming concentrically confluent and spreading toward the posterior pole (Figs 88.3–88.5); the macula frequently is spared. An active vasculitis is present, with perivascular hemorrhages, sheathing, and terminal obliteration of arterioles by thrombi. The phase of active retinitis usually lasts 4–6 weeks.3–8
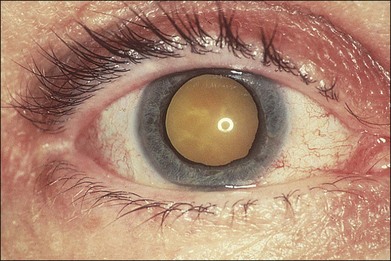
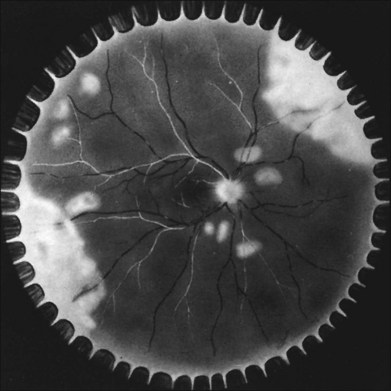
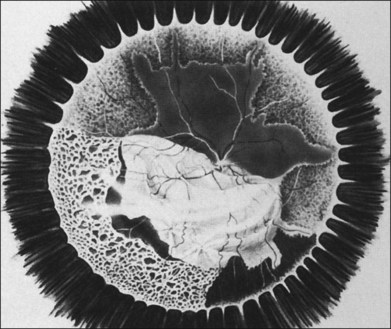
With resolution, pigmentation of the peripheral lesions begins at their posterior margins, leaving a scalloped appearance (Fig. 88.6), frequently accompanied by retinal breaks at the junction of normal and necrotic retina. Giant retinal pigment epithelial tears may develop.9 A rhegmatogenous retinal detachment has been observed in approximately 75% of untreated eyes, generally within 1–2 months after the onset of the disease. Earlier exudative and rhegmatogenous detachments have been noted, particularly in cases associated with herpes simplex virus (HSV).3,6,8,10 Vitreous inflammation may lead to organization and proliferative vitreoretinopathy,11 adding a traction component to the retinal detachment (Fig. 88.7). Vision may suddenly decrease as a result of anterior ischemic optic neuropathy. Macular pucker also may occur.
Computed tomography12–14 and ultrasonography14 have revealed enlargement of the optic nerve sheath in ARN cases associated with prominent optic disc edema. Since ARN patients can develop meningoencephalitis, it is helpful to keep this possibility in mind, look for symptoms and signs of neurologic disease, and refer the patient for further investigation as appropriate.15 Even in ARN patients who are not immunocompromised and who have no clinical evidence of encephalitis, magnetic resonance imaging of selected cases has shown lesions of the lateral geniculate, optic tracts, and chiasm,16 suggesting viral spread through the central nervous system by axoplasmic transport from retinal ganglion cells. An explanation for this has been shown in mouse models, where there is evidence to show that HSV1 infection may progress through the optic chiasm from one eye to the other and along the visual pathways leading to meningoencephalitis.17
The contralateral eye is involved in approximately one-third of untreated ARN cases, usually within 6 weeks of the onset of disease in the first eye.9,18 However, the second eye can be affected as late as 34 years after involvement of the first eye,19 and the fellow eye has become involved even after acyclovir treatment for ARN in the first eye.20 Mild forms of ARN syndrome, characterized by patchy, peripheral retinal opacification, recently have been reported. These mild cases did not become rapidly confluent or lead to detachment.21 It is unclear whether this atypical presentation is a result of intense acyclovir and corticosteroid therapy or a reflection of a wide range of disease severity. In addition, less involved forms of ARN have been reported after primary varicella, without concomitant therapy.22
Patient population
ARN syndrome occurs most commonly in otherwise healthy patients of either sex and of any age; in general, patients are not immunocompromised or systemically ill. However, ARN patients may demonstrate subclinical immune dysfunction. In one review of 216 patients with ARN, impaired cellular immunity was noted in 16%.23 Skin testing of patients with ARN revealed anergy in five of seven tested cases and abnormal lymphocyte proliferative indices in one-third.24 The significance of cutaneous anergy is unclear, however, since patients with zoster infections frequently demonstrate anergy.25 Specific HLA haplotypes may increase the relative risk of developing ARN syndrome, such as the HLA-DQw7 antigen and phenotype Bw62, DR4 in white patients in the United States26 and HLA-Aw33, B44, and DRw6 in Japanese patients.27 Pleocytosis of the cerebrospinal fluid frequently accompanies the syndrome,9,13,28 and intrathecal production of antibodies against herpesviruses has been demonstrated in selected cases.4 Some patients present with ARN before, after, or at the same time as they show skin manifestations of varicella zoster infection29,30 (e.g., primary varicella, herpes zoster ophthalmicus, or Ramsay Hunt syndrome). Unilateral ARN also has been noted after herpes simplex keratitis.31 Diffuse cerebral atrophy and labyrinthine deafness have been reported following ARN,32 and some investigators have suggested that ARN be considered as one of the uveomeningeal syndromes.14
With the increase in intravitreal steroid injections for a variety of retinal diseases, there have been reports of ARN occurring in immunocompetent patients and other patients who have medical comorbidities. As a result, this increased risk has led some to increase their vigilance for the development of viral retinitis following intravitreal injection 33–35
Acute necrotizing retinitis that is clinically identical to ARN or shares many features with ARN has been reported in immunocompromised patients. ARN was first described in immunocompromised patients in 1985;36 a recent case series described 26 cases of ARN in AIDS patients, noting a generally fulminant course.37 The cause of the retinitis in these immunocompromised patients may be particularly diverse or multifactorial. For example, an AIDS patient died after an ARN-like syndrome with concurrent encephalitis, and herpes simplex viral antigens were localized in the central nervous system at postmortem examination.38 In contrast, several immunocompromised patients have presented with ARN in association with skin manifestations of zoster.39 Although ARN syndrome initially was defined as manifesting in otherwise healthy patients, many authorities have broadened the diagnostic criteria to include immunocompromised hosts.39–42 It is the evolution of clinical signs and symptoms, and not the specific pathogen or immune status of the patient, that serves as the sole basis by which ARN syndrome has been defined (see Differential Diagnosis, below).
Etiology
Considerable evidence points to multiple members of the herpesvirus family in the etiology of ARN syndrome. In numerous studies, varicella zoster virus (VZV) has been the most frequent virus isolated,43 sometimes in up to two-thirds of patients.44 VZV was isolated in tissue culture from the vitreous of a blind eye enucleated early in the course of the disease, and specific varicella zoster antigens were identified in retinal tissue by immunocytologic staining45 (Fig. 88.8). VZV was identified by electron microscopy in necrotic retinal tissue (Fig. 88.9) in two other cases of ARN.46 Varicella zoster DNA in intraocular fluids of ARN cases has been confirmed by polymerase chain reaction (PCR),47 and Witmer quotients of paired serum to intraocular fluid antibody levels have been diagnostic of varicella zoster in numerous cases.48,49 Varicella zoster antigens have been demonstrated in vitreous aspirates of patients with ARN syndrome.50
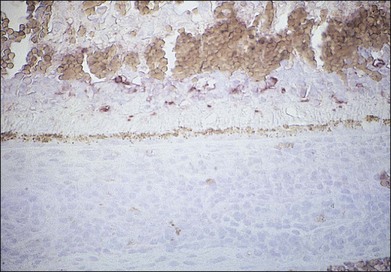
Fig. 88.8 Varicella zoster antigens (brown) are seen in cells scattered in all layers of a necrotic retina.
Restriction endonuclease patterns of the ARN virus isolate were similar to those of typical varicella zoster strains and showed similar sensitivities to a panel of antiviral drugs.51 Although strain heterogeneity has been observed in the varicella zoster viruses associated with ARN syndrome,52 these data thus do not support the notion that the ARN virus represents a mutant strain of VZV with significant alterations in either the viral thymidine kinase or DNA polymerase genes. It therefore remains enigmatic why an “old virus” should give rise to a “new” syndrome.
Some reports have suggested that other members of the herpesvirus family may cause ARN, in about 20% of cases according to one study.44 A number of studies have implicated HSV because of concurrent herpes simplex skin lesions,53 the detection of herpes simplex antigens on vitreous cells,7 the presence of immune complexes containing herpes simplex antigens in aqueous or serum,54 the documentation of intraocular antibody synthesis directed against HSV,49 diagnostic changes in serum antibody levels to herpes simplex, polymerase chain reaction detection of herpes simplex,55 or the culture of herpes simplex from vitreous humor.3,8,45 Several cases have been described of ARN caused by reactivation of HSV type 2.47,55,56 Interestingly, patients with herpes simplex type 2 appear to be much younger (mean 21 years) than those with either herpes simplex type 1 or varicella zoster (mean age 40 years).49,57,58 The proportion of ARN syndrome patients with disease caused by HSV-2 may be higher in Japan than in the United States, perhaps coincident with changing epidemiologic distributions of HSV-1 versus HSV-2 in this population.59, 60 Patients with ARN syndrome caused by herpes simplex type 1 appear to have a higher risk of encephalitis or meningitis than those with disease caused by VZV.57,61–65
In one study of a case of ARN,66 cytomegalovirus (CMV) was cultured and CMV antigens were demonstrated in retinal tissue, but megalic cells or electron-dense cytoplasmic inclusions, which have uniformly typified CMV infection of the retina, were not identified. In another case the polymerase chain reaction yielded positive results for CMV in one immunocompetent individual, whose vitreous was negative for VZV and HSV types 1 and 2.67 Epstein–Barr virus has also been postulated in some cases of ARN syndrome,68,69 but definitive linkage of this nearly ubiquitous virus to disease remains problematic. Indeed, EBV has been found in 20% of normal cadaveric eyes,70 and while one study found EBV by PCR in about one-sixth of their ARN cases, each case was also positive for VZV by PCR.44
Pathologic features
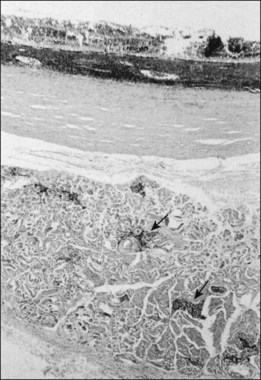
Studies of blind eyes enucleated early in the course of ARN have demonstrated retinal necrosis, hemorrhage, and considerable vitreous debris. The retinal necrosis is full-thickness, and an underlying choroiditis is present that may be granulomatous (Fig. 88.10). VZV DNA and antigens have been identified in lymphoid cells within choroidal infiltrates in an eye enucleated in the late stage of disease.71 Eosinophilic intranuclear inclusions within cells of all layers of retina and retinal pigment epithelium were the first clues suggesting a possible viral etiology by a member of the herpes group.46 There is histologic evidence of retinal arteritis (Fig. 88.11), although no virus particles have been detected in vascular endothelium. Deposits of immune complexes containing varicella zoster viral antigens have been demonstrated in retinal vessel walls by immunocytologic methods and may play a role in the vasculitis seen during active stages. The perivasculitis is not restricted to retinal vessels alone and can involve the extraocular muscles (Fig. 88.12). Immune complexes with herpes simplex also have been identified in intraocular fluids in ARN cases.
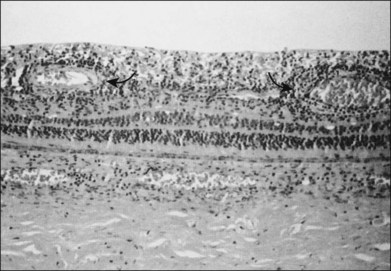
Fig. 88.11 Histologic evidence of retinal vasculitis (arrows) in acute retinal necrosis.
(Courtesy of Robert Y. Foos.)
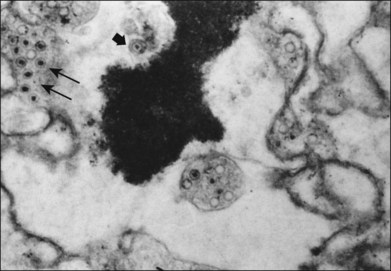
Ultrastructural studies of retinal tissue have revealed 180 nanometer virions containing icosahedral capsids, consistent with particles of the herpes group46 (see Fig. 88.8). The optic nerve may be largely necrotic and heavily infiltrated with plasma cells, but no virus particles or antigens have been seen in the optic nerve. It is possible that the absence of viral antigens or particles reflects more the time point in the course of the disease when enucleation was performed in these rare cases than conclusive evidence regarding acute optic nerve infection. The multifocal, deep retinitis observed early in the course of ARN is compatible with either blood-borne spread of virus or transmission to the eye through a bilateral nerve pathway. An animal model of ARN using herpes simplex virus suggests that spread to the contralateral eye occurs by retrograde axonal transport between the suprachiasmatic nucleus of the hypothalamus and the contralateral retina.72
Differential diagnosis
The American Uveitis Society released its criteria for diagnosis of acute retinal necrosis syndrome in 1994.42 Under this definition, diagnosis of ARN syndrome is dependent solely on the observed clinical findings and their progression. Neither the identification of an etiologic agent nor knowledge of the patient’s immune status is required to render a diagnosis of ARN syndrome. The diagnostic criteria are divided into mandatory and supporting categories and are summarized in Table 88.1. By this definition, retinal lesions presumptively caused by herpesviruses (but not part of other well-recognized syndromes such as CMV retinitis or progressive outer retinal necrosis [see below]) are covered under the umbrella term necrotizing herpetic retinopathy.
Table 88.1 American Uveitis Society criteria for diagnosis of acute retinal necrosis syndrome
| Required clinical criteria | Supporting clinical criteria |
|---|---|
| One or more foci of retinal necrosis with discrete borders, located in peripheral retina | Optic neuropathy/atrophy |
| Rapid progression of disease in the absence of therapy | Scleritis |
| Circumferential spread of disease | Pain |
| Evidence of occlusive vasculopathy and arteriolar involvement | |
| A prominent inflammatory reaction in the vitreous and anterior chamber |
The differential diagnosis of ARN includes CMV retinopathy, syphilitic retinitis, toxoplasmosis (particularly in immunocompromised hosts), large cell lymphoma, and acute multifocal hemorrhagic retinal vasculitis. A more extensive differential diagnosis includes toxocariasis, fungal or bacterial retinitis, pars planitis, Behçet disease, sarcoidosis, commotio retinae, central retinal artery or ophthalmic artery occlusion, ocular ischemic syndrome, collagen-vascular disease, intraocular leukemia/lymphoma (including T-cell-mediated73) and retinoblastoma.
The syndrome of ARN is readily distinguished from a separate form of varicella zoster retinitis reported in human immunodeficiency virus (HIV)-infected patients, sometimes referred to as “progressive outer retinal necrosis syndrome” or PORN.54,74 This latter entity shares an etiologic agent with some cases of ARN but is otherwise completely distinct. It is characterized by multifocal, patchy choroidal, and deep posterior retinal opacification that initially may be parafoveal. Additional features differentiating this syndrome from ARN include the absence of vitreous or anterior chamber inflammation or signs of active vasculitis. PORN progresses rapidly from the posterior pole to involve the entire retina, resulting in widespread retinal necrosis and atrophy. Despite a common etiologic agent, varicella zoster retinitis should not be referred to as the syndrome of ARN. Indeed, the two diseases may even affect different eyes of the same patient.75
Although the syndrome of ARN is a clinical diagnosis established on the basis of a constellation of evolving signs and symptoms, which may be pathognomonic in many cases, in atypical or difficult diagnostic cases ancillary clinical history or laboratory tests can support the diagnosis. It is important to assess the patient’s level of immunocompetence, since knowledge of seropositivity to HIV and syphilis may help to establish the appropriate specific diagnosis. Diagnostic vitrectomy is appropriate in cases of uncertain diagnosis. The most sensitive and specific method for the detection of herpes viruses in vitreous specimens is the polymerase chain reaction (PCR). PCR assays are capable of detecting a single varicella zoster virion from vitreous biopsy.76 Simultaneous PCR for multiple herpesviruses has been used to screen for an etiologic agent in atypical cases of ARN and has implicated both CMV76 and HSV47 as potentially causative. PCR can be performed in multiplex for VZV, HSV, CMV, and toxoplasmosis.77 Sensitivity for each, even in multiplex testing, is at least 10 genomes per microliter. With application of quantitative PCR (qPCR), accurate estimates of pathogen load can be achieved.78,79 These data suggest significant heterogeneity in the pathogen load among individual cases of disease. The great sensitivity of PCR can be problematic, however; assays that yield false-positive results, likely through amplification of latent virus in host tissue, have been reported.76 False-negative results can also occur. Consensus has not yet been reached regarding the negative predictive value of a negative PCR test for viral DNA. PCR requires meticulous technical performance and confirmation by probe hybridization to avoid specificity problems and is best performed by a laboratory with considerable experience with this method. In many cases, aqueous and/or vitreous sampling for PCR is becoming the diagnostic test of choice when faced with viral retinitis.44,80
In cases in which PCR is negative but clinical suspicion is high, endoretinal biopsy may be appropriate. Taking the biopsy from the transition zone between normal and necrotic retina during the acute phase of the disease greatly increases its diagnostic yield. In the past, obtaining paired serum and intraocular fluid specimens and applying a modified Witmer quotient was performed for diagnostic value even in the early stages of ARN syndrome. It also seemed to develop increasing sensitivity with time.48,81 With the increased use of PCR, Witmer quotient appears to be used less frequently.44,79,80 Quantitative antiviral antibody levels in acute and chronic sera from ARN patients are consistent with a specific etiologic diagnosis in a minority of cases and have been falsely negative in cases in which vitreous cultures were positive for herpesviruses.
Treatment and prognosis
Treatment of ARN syndrome is complex and must be individualized in response to the many pathogenic and temporal facets of the disorder, as well as to the specific vitreoretinal pathologic findings at hand. Cases of ARN associated with mild or minimal inflammation have been reported in which ARN did not progress to extensive retinal necrosis, traction, or detachment.21,22 Controlled, randomized, prospective treatment studies on ARN have not been conducted given the relative rarity of this disease, so current recommendations are based solely on anecdotal data and case series. Early studies indicated that the natural history of classic ARN syndrome carries a generally poor prognosis in untreated eyes,9 with only 28% of affected eyes obtaining a final vision better than 20/200 because of rhegmatogenous retinal detachment (75% of affected eyes), optic nerve dysfunction, or macular abnormality. The advent of antiviral therapy and vitrectomy techniques82 has decreased this level of vision loss to less than one-third of cases in recent years.12 In one series of cases that were detected early and treated aggressively with acyclovir and laser photocoagulation, outcome for 13 eyes of 12 patients showed 20/40 or better vision in 46% of eyes and 20/400 or better in 92%.83 However, the use of prophylactic laser photocoagulation has become more controversial than it was in the past.84
In light of the characterization and the determination of antiviral sensitivities of the varicella zoster isolate, acyclovir has been used in an effort to limit the direct cytopathic effect of virus on retinal tissue. Based on the dose required for a 50% reduction of virus plaques in tissue culture (ED50), oral administration of acyclovir results in subtherapeutic serum levels; in contrast, intravenous acyclovir (13 mg/kg every 8 hours) results in an intravitreal acyclovir level4 over three times the ARN ED50. After intravenous administration of acyclovir to ARN patients (1500 mg/m2/day in three divided doses), retinal lesions were first noted to regress 3.9 days after the beginning of therapy; new lesions did not develop, and existing lesions did not progress.20 Historically, intravenous therapy for 5–10 days was followed by oral acyclovir at the zoster dosage (800 mg orally five times daily, assuming normal renal function) for up to 6 weeks after the onset of infection, and longer in immunocompromised patients. Valacyclovir (1 g orally three times daily) or famciclovir85,86 (500 mg orally three times daily) may also be used following intravenous acyclovir administration. Side-effects of acyclovir include decreased renal function, gastrointestinal irritation, phlebitis, central nervous system dysfunction, and hypersensitivity reactions. Acyclovir has potent antiviral action against VZV, HSV types 1 and 2 (both of which have been implicated in ARN), and Epstein–Barr virus, but it has low activity against CMV. Valganciclovir is active against VZV, HSV, and CMV, but is FDA approved at present only for CMV retinitis.
In recent years many clinicians have moved to outpatient use of oral antivirals for treatment induction of ARN.87–89 Prodrugs such as valacyclovir and famciclovir have high systemic absorption, are active against VZV and HSV, and may be used for prophylaxis against involvement of the second eye.3,90 Valganciclovir, another prodrug, has been used in other cases.91 Treatment algorithms have used oral valacyclovir 1 g 3 times daily, oral famciclovir 500 mg 3 times daily, or valganciclovir 450–900 mg 2 times daily until complete resolution of retinitis was observed. Some clinicians have used higher doses of valacyclovir 2 g 3–4 times daily to achieve resolution.92 These medications are then generally slowly tapered over 1.5–75.7 months.84,93 Recent studies have not found a significant superiority of intravenous to oral treatment induction, but these studies have been retrospective and limited by the relative rarity of this disease. Given the risk of encephalitis, particularly in patients with HSV-1-associated disease, close monitoring of patient mental status is warranted.
Recent studies have suggested an adjunctive role for intravitreal antiviral medication in the treatment of ARN syndrome. In one small series, three patients treated with intravitreal ganciclovir as well as intravenous foscarnet, ganciclovir, or acyclovir led to excellent outcomes,94 while another three patients responded to intravitreal antiviral treatment despite progression on intravenous acyclovir.95 A similar approach has been employed for progressive outer retinal necrosis.96,97 Early administration of intravitreal antivirals also provides an opportunity for vitreous sampling (akin to the “tap and inject” for endophthalmitis), allowing for PCR-based identification of causative virus.
Acute decreases in vision in ARN patients can result from ischemic optic neuropathy and have led to trials of anticoagulants such as aspirin, along with high-dose oral steroids early after initiation of antiviral therapy. Systemic corticosteroids also may limit intraocular inflammation and the vitreous reaction, but are generally begun only after 24–48 hours of intravenous acyclovir. Virus particles or antigens have not been observed in the optic nerve in ARN, and it appears that the optic nerve dysfunction is caused by ischemia from swollen vascular endothelial cells, thrombotic arteriolar occlusion, and infiltration of the optic nerve by inflammatory cells. Hyperaggregation of platelets has been reported in six of seven patients with bilateral ARN studied, as determined by adenosine 5-diphosphate aggregation testing and partial prothrombin times.98 Optic nerve dysfunction has been reported in ARN patients despite anticoagulation therapy or antiplatelet therapy. Studies have employed optic nerve sheath fenestration in conjunction with acyclovir therapy in a small group of ARN patients with optic neuropathy and disc edema, with reported improvement in final vision in six of eight eyes.14 Better results were obtained in the subgroup that underwent decompression within 12 days of the onset of the optic neuropathy.
In early studies prior to current antiviral management techniques, the second eye became involved in 36% of ARN patients, usually within 6 weeks of the first eye’s involvement.20 More recent studies have suggested a fellow-eye involvement rate of ~3% with appropriate antiviral treatment.84 The duration of systemic treatment to prevent fellow-eye involvement is not well established but generally risk is felt to be decreased in 6–12 weeks. However, bilateral ARN has been reported up to 34 years after the first eye was affected,19 raising a question as to utility of very long-term or life-long prophylaxis in patients who have lost vision in one eye from ARN syndrome. Risk of second-eye involvement may also be dependent on the etiologic agent causing disease. Additionally, rare cases of reactivation in the originally affected eye have been reported.99
Retinal tears at the junction of normal and necrotic retina, as well as subsequent proliferative retinopathy creating a complicated combined traction-rhegmatogenous retinal detachment, pose a difficult problem in the management of ARN. Whereas Peyman et al.7,18 obtained good results in selected patients with intravenous and intravitreal acyclovir in conjunction with prophylactic vitrectomy and scleral buckles in an uncontrolled study of active ARN cases that had not yet developed detachment, others have not been able to prevent retinal detachment by similar management,100 while still others have found decreased incidence of secondary detachment, but no improvement in final mean visual acuity.101 In a study of 13 eyes of 12 ARN patients, the incidence of retinal detachment despite intravenous acyclovir therapy was 84%, suggesting that antiviral therapy alone does not effectively preclude retinal detachment.
The use of prophylactic laser has been an issue of great debate in recent years. Several studies have demonstrated the benefit of prophylactic laser photocoagulation posterior to areas of active retinitis,28,46,83,102 while others have found no decrease in the risk of retinal detachment.103,104 One reason for this variability may be the small sample sizes used in these studies. Investigators have also postulated that generally the eyes receiving prophylactic photocoagulation have less vitritis, which allows for treatment. It is thought that vitritis over necrotic retina increases the risk of retinal detachment. They further reason that the eyes unable to receive prophylactic photocoagulation because of media opacity are at higher risk for retinal detachment than those with less vitritis and subsequently clearer media.84 Without prospective randomized controlled studies, this question may not be definitively answered. Unfortunately, given the infrequency of this disease, it may be difficult to perform such a study.
One study found that retinal detachments appear to happen anywhere from 9 to 148 days from the onset of symptoms, so these eyes should be carefully examined at each office visit.44 With the use of modern microsurgical techniques, including intravitreal silicone oil, air–fluid exchange, demarcating laser photocoagulation, and a long-acting gas tamponade, a high percentage of retinas can be anatomically reattached. However, it must be kept in mind that patients with minimal vision secondary to optic atrophy, macular pucker, or retinal dysfunction before detachment are unlikely to obtain improved visual function despite anatomically successful retinal reattachment.
As ARN syndrome appears to be due to reactivation of pre-existing herpes viral infection with VZV or HSV, it is logical to ask whether vaccination might reduce the incidence of ARN syndrome. Zostavax® is an attenuated live herpes zoster virus vaccine that carries indication for reduction of incidence of shingles in individuals over age 60. In a large study of over 38 000 individuals, the vaccine reduced the incidence of shingles episodes by approximately 50%, with greater effect seen in the cohort of 60–70-year-olds than in those over age 80.105 However, as this population is older than the typical ARN syndrome population, it is unclear whether use of this vaccine will impact rates of disease. Of greater interest is the question of whether childhood chickenpox vaccination with attenuated live virus will impact development of ARN syndrome. Reactivation of attenuated vaccine resulting in herpetic meningitis has been described106 but to date no cases of ARN syndrome associated with vaccine reactivation have been reported. Similar questions will continue to arise with development of vaccines against HSV-1 and HSV-2.107
1 Urayama A, Yamada N, Sasaki T, et al. Unilateral acute uveitis with retinal periarteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607–619.
2 Young NJ, Bird AC. Bilateral acute retinal necrosis. Br J Ophthalmol. 1978;62(9):581–590.
3 Duker JS, Nielsen JC, Eagle RC, Jr., et al. Rapidly progressive acute retinal necrosis secondary to herpes simplex virus, type 1. Ophthalmology. 1990;97(12):1638–1643.
4 el Azazi M, Samuelsson A, Linde A, et al. Intrathecal antibody production against viruses of the herpesvirus family in acute retinal necrosis syndrome. Am J Ophthalmol. 1991;112(1):76–82.
5 Lewis ML, Culbertson WW, Post JD, et al. Herpes simplex virus type 1. A cause of the acute retinal necrosis syndrome. Ophthalmology. 1989;96(6):875–878.
6 Margolis T, Irvine AR, Hoyt WF, et al. Acute retinal necrosis syndrome presenting with papillitis and arcuate neuroretinitis. Ophthalmology. 1988;95(7):937–940.
7 Peyman GA, Goldberg MF, Uninsky E, et al. Vitrectomy and intravitreal antiviral drug therapy in acute retinal necrosis syndrome. Report of two cases. Arch Ophthalmol. 1984;102(11):1618–1621.
8 Watanabe J, Ashida M, Funaki A, et al. [A case of acute retinal necrosis syndrome caused by herpes simplex virus type 1.]. Nippon Ganka Gakkai Zasshi. 1989;93(1):65–71.
9 Fox GM, Blumenkranz M. Giant retinal pigment epithelial tears in acute retinal necrosis. Am J Ophthalmol. 1993;116(3):302–306.
10 Matsuo T, Date S, Tsuji T, et al. Immune complex containing herpesvirus antigen in a patient with acute retinal necrosis. Am J Ophthalmol. 1986;101(3):368–371.
11 Ahmadieh H, Soheilian M, Azarmina M, et al. Surgical management of retinal detachment secondary to acute retinal necrosis: clinical features, surgical techniques, and long-term results. Jpn J Ophthalmol. 2003;47(5):484–491.
12 Litoff D, Catalano RA. Herpes zoster optic neuritis in human immunodeficiency virus infection. Arch Ophthalmol. 1990;108(6):782–783.
13 Sergott RC, Anand R, Belmont JB, et al. Acute retinal necrosis neuropathy. Clinical profile and surgical therapy. Arch Ophthalmol. 1989;107(5):692–696.
14 Sergott RC, Belmont JB, Savino PJ, et al. Optic nerve involvement in the acute retinal necrosis syndrome. Arch Ophthalmol. 1985;103(8):1160–1162.
15 Cardine S, Chaze PA, Bourcier F, et al. [Bilateral acute retinal necrosis syndrome associated with meningoencephalitis caused by herpes simplex virus 2. A case report.]. J Fr Ophtalmol. 2004;27(7):795–800.
16 Farrell TA, Wolf MD, Folk JC, et al. Magnetic resonance imaging in a patient with herpes zoster keratouveitis and contralateral acute retinal necrosis. Am J Ophthalmol. 1991;112(6):735–736.
17 Labetoulle M, Kucera P, Ugolini G, et al. Neuronal pathways for the propagation of herpes simplex virus type 1 from one retina to the other in a murine model. J Gen Virol. 2000;81(Pt 5):1201–1210.
18 Carney MD, Peyman GA, Goldberg MF, et al. Acute retinal necrosis. Retina. 1986;6(2):85–94.
19 Falcone PM, Brockhurst RJ. Delayed onset of bilateral acute retinal necrosis syndrome: a 34-year interval. Ann Ophthalmol. 1993;25(10):373–374.
20 Blumenkranz MS, Culbertson WW, Clarkson JG, et al. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296–300.
21 Matsuo T, Nakayama T, Koyama T, et al. A proposed mild type of acute retinal necrosis syndrome. Am J Ophthalmol. 1988;105(6):579–583.
22 Kelly SP, Rosenthal AR. Chickenpox chorioretinitis. Br J Ophthalmol. 1990;74(11):698–699.
23 Rochat C, Herbort CP. [Acute retinal necrosis syndrome. Lausanne cases, review of the literature and new physiopathogenetic hypothesis.]. Klin Monatsbl Augenheilkd. 1994;204(5):440–449.
24 Rochat C, Polla BS, Herbort CP. Immunological profiles in patients with acute retinal necrosis. Graefes Arch Clin Exp Ophthalmol. 1996;234(9):547–552.
25 Pepose JS. Skin test with varicella-zoster virus antigen for ophthalmic herpes zoster. Am J Ophthalmol. 1984;98(6):825–827.
26 Holland GN, Cornell PJ, Park MS, et al. An association between acute retinal necrosis syndrome and HLA-DQw7 and phenotype Bw62, DR4. Am J Ophthalmol. 1989;108(4):370–374.
27 Ichikaw T, Sakai J, Usui M. HLA antigens of patients with Kirisawa’s uveitis and herpetic keratitis. Atarashii Ganka. 1989;6:107–114.
28 Sternberg P, Jr., Han DP, Yeo JH, et al. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology. 1988;95(10):1389–1393.
29 Browning DJ, Blumenkranz MS, Culbertson WW, et al. Association of varicella zoster dermatitis with acute retinal necrosis syndrome. Ophthalmology. 1987;94(6):602–606.
30 Yeo JH, Pepose JS, Stewart JA, et al. Acute retinal necrosis syndrome following herpes zoster dermatitis. Ophthalmology. 1986;93(11):1418–1422.
31 Sado K, Kimura T, Hotta Y, et al. Acute retinal necrosis syndrome associated with herpes simplex keratitis. Retina. 1994;14(3):260–263.
32 Severin M, Neubauer H. Bilateral acute vascular retinal necrosis. Ophthalmologica. 1981;182(4):199–203.
33 Shah AM, Oster SF, Freeman WR. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149(3):433–440.
34 Aggermann T, Stolba U, Brunner S, et al. Endophthalmitis with retinal necrosis following intravitreal triamcinolone acetonide injection. Ophthalmologica. 2006;220(2):131–133.
35 Toh T, Borthwick JH. Acute retinal necrosis post intravitreal injection of triamcinolone acetonide. Clin Experiment Ophthalmol. 2006;34(4):380–382.
36 Neetens A, Stevens W, Taelman R, et al. Immune deficiency and necrotising retinopathy. Bull Soc Belge Ophtalmol. 1985;215:73–86.
37 Batisse D, Eliaszewicz M, Zazoun L, et al. Acute retinal necrosis in the course of AIDS: study of 26 cases. AIDS. 1996;10(1):55–60.
38 Freeman WR, Thomas EL, Rao NA, et al. Demonstration of herpes group virus in acute retinal necrosis syndrome. Am J Ophthalmol. 1986;102(6):701–709.
39 Jabs DA, Schachat AP, Liss R, et al. Presumed varicella zoster retinitis in immunocompromised patients. Retina. 1987;7(1):9–13.
40 Chambers RB, Derick RJ, Davidorf FH, et al. Varicella-zoster retinitis in human immunodeficiency virus infection. Case report. Arch Ophthalmol. 1989;107(7):960–961.
41 Freeman WR, Wiley CA, Gross JG, et al. Endoretinal biopsy in immunosuppressed and healthy patients with retinitis. Indications, utility, and techniques. Ophthalmology. 1989;96(10):1559–1565.
42 Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117(5):663–667.
43 Muthiah MN, Michaelides M, Child CS, et al. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452–1455.
44 Lau CH, Missotten T, Salzmann J, et al. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114(4):756–762.
45 Culbertson WW, Blumenkranz MS, Pepose JS, et al. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology. 1986;93(5):559–569.
46 Culbertson WW, Blumenkranz MS, Haines H, et al. The acute retinal necrosis syndrome. Part 2: Histopathology and etiology. Ophthalmology. 1982;89(12):1317–1325.
47 Cunningham ET, Jr., Short GA, Irvine AR, et al. Acquired immunodeficiency syndrome: associated herpes simplex virus retinitis. Clinical description and use of a polymerase chain reaction-based assay as a diagnostic tool. Arch Ophthalmol. 1996;114(7):834–840.
48 Pepose JS, Flowers B, Stewart JA, et al. Herpesvirus antibody levels in the etiologic diagnosis of the acute retinal necrosis syndrome. Am J Ophthalmol. 1992;113(3):248–256.
49 Van Gelder RN, Willig JL, Holland GN, et al. Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology. 2001;108(5):869–876.
50 Soushi S, Ozawa H, Matsuhashi M, et al. Demonstration of varicella-zoster virus antigens in the vitreous aspirates of patients with acute retinal necrosis syndrome. Ophthalmology. 1988;95(10):1394–1398.
51 Pepose JS, Biron K. Antiviral sensitivities of the acute retinal necrosis syndrome virus. Curr Eye Res. 1987;6(1):201–205.
52 Abe T, Sato M, Tamai M. Variable R1 region in varicella zoster virus in fulminant type of acute retinal necrosis syndrome. Br J Ophthalmol. 2000;84(2):193–198.
53 Ludwig IH, Zegarra H, Zakov ZN. The acute retinal necrosis syndrome. Possible herpes simplex retinitis. Ophthalmology. 1984;91(12):1659–1664.
54 Margolis TP, Lowder CY, Holland GN, et al. Varicella-zoster virus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1991;112(2):119–131.
55 Rahhal FM, Siegel LM, Russak V, et al. Clinicopathologic correlations in acute retinal necrosis caused by herpes simplex virus type 2. Arch Ophthalmol. 1996;114(11):1416–1419.
56 Thompson WS, Culbertson WW, Smiddy WE, et al. Acute retinal necrosis caused by reactivation of herpes simplex virus type 2. Am J Ophthalmol. 1994;118(2):205–211.
57 Ganatra JB, Chandler D, Santos C, et al. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–172.
58 Tan JCH, Byles D, Stanford MR, et al. Acute retinal necrosis in children caused by herpes simplex virus. Retina. 2001;21(4):344–347.
59 Hashido M, Lee FK, Nahmias AJ, et al. An epidemiologic study of herpes simplex virus type 1 and 2 infection in Japan based on type-specific serological assays. Epidemiol Infect. 1998;120(2):179–186.
60 Itoh N, Matsumura N, Ogi A, et al. High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol. 2000;129(3):404–405.
61 Gain P, Chiquet C, Thuret G, et al. Herpes simplex virus type 1 encephalitis associated with acute retinal necrosis syndrome in an immunocompetent patient. Acta Ophthalmol Scand. 2002;80(5):546–549.
62 Gaynor BD, Wade NK, Cunningham ET, Jr. Herpes simplex virus type 1 associated acute retinal necrosis following encephalitis. Retina. 2001;21(6):688–690.
63 Kim C, Yoon YH. Unilateral acute retinal necrosis occurring 2 years after herpes simplex type 1 encephalitis. Ophthalmic Surg Lasers. 2002;33(3):250–252.
64 Tada Y, Negoro K, Morimatsu M, et al. Findings in a patient with herpes simplex viral meningitis associated with acute retinal necrosis syndrome. AJNR Am J Neuroradiol. 2001;22(7):1300–1302.
65 Maertzdorf J, Van der Lelij A, Baarsma GS, et al. Herpes simplex virus type 1 (HSV-1)-induced retinitis following herpes simplex encephalitis: indications for brain-to-eye transmission of HSV-1. Ann Neurol. 2000;48(6):936–939.
66 Rungger-Brandle E, Roux L, Leuenberger PM. Bilateral acute retinal necrosis (BARN). Identification of the presumed infectious agent. Ophthalmology. 1984;91(12):1648–1658.
67 Silverstein BE, Conrad D, Margolis TP, et al. Cytomegalovirus-associated acute retinal necrosis syndrome. Am J Ophthalmol. 1997;123(2):257–258.
68 Kramer S, Brummer C, Zierhut M. Epstein–Barr virus associated acute retinal necrosis. Br J Ophthalmol. 2001;85(1):114.
69 Gallego-Pinazo R, Harto M, Garcia-Medina JJ, et al. Epstein–Barr virus and acute retinal necrosis in a 5-year-old immunocompetent child. Clin Ophthalmol. 2008;2(2):451–455.
70 Chodosh J, Gan YJ, Sixbey JW. Detection of Epstein–Barr virus genome in ocular tissues. Ophthalmology. 1996;103(4):687–690.
71 Rummelt V, Wenkel H, Rummelt C, et al. Detection of varicella zoster virus DNA and viral antigen in the late stage of bilateral acute retinal necrosis syndrome. Arch Ophthalmol. 1992;110(8):1132–1136.
72 Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32(9):2462–2472.
73 Levy-Clarke GA, Buggage RR, Shen D, et al. Human T-cell lymphotropic virus type-1 associated t-cell leukemia/lymphoma masquerading as necrotizing retinal vasculitis. Ophthalmology. 2002;109(9):1717–1722.
74 Forster DJ, Dugel PU, Frangieh GT, et al. Rapidly progressive outer retinal necrosis in the acquired immunodeficiency syndrome. Am J Ophthalmol. 1990;110(4):341–348.
75 Gariano RF, Berreen JP, Cooney EL. Progressive outer retinal necrosis and acute retinal necrosis in fellow eyes of a patient with acquired immunodeficiency syndrome. Am J Ophthalmol. 2001;132(3):421–423.
76 Short GA, Margolis TP, Kuppermann BD, et al. A polymerase chain reaction-based assay for diagnosing varicella-zoster virus retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1997;123(2):157–164.
77 Dabil H, Boley ML, Schmitz TM, et al. Validation of a diagnostic multiplex polymerase chain reaction assay for infectious posterior uveitis. Arch Ophthalmol. 2001;119(9):1315–1322.
78 Dworkin LL, Gibler TM, Van Gelder RN. Real-time quantitative polymerase chain reaction diagnosis of infectious posterior uveitis. Arch Ophthalmol. 2002;120(11):1534–1539.
79 Asano S, Yoshikawa T, Kimura H, et al. Monitoring herpesvirus DNA in three cases of acute retinal necrosis by real-time PCR. J Clin Virol. 2004;29(3):206–209.
80 Cottet L, Kaiser L, Hirsch HH, et al. HSV2 acute retinal necrosis: diagnosis and monitoring with quantitative polymerase chain reaction. Int Ophthalmol. 2009;29(3):199–201.
81 Luyendijk L, vd Horn GJ, Visser OH, et al. Detection of locally produced antibodies to herpes viruses in the aqueous of patients with acquired immune deficiency syndrome (AIDS) or acute retinal necrosis syndrome (ARN). Curr Eye Res. 1990;9(Suppl:7–11.
82 Blumenkranz M, Clarkson J, Culbertson WW, et al. Vitrectomy for retinal detachment associated with acute retinal necrosis. Am J Ophthalmol. 1988;106(4):426–429.
83 Crapotta JA, Freeman WR, Feldman RM, et al. Visual outcome in acute retinal necrosis. Retina. 1993;13(3):208–213.
84 Tibbetts MD, Shah CP, Young LH, et al. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824.
85 Figueroa MS, Garabito I, Gutierrez C, et al. Famciclovir for the treatment of acute retinal necrosis (ARN) syndrome. Am J Ophthalmol. 1997;123(2):255–257.
86 Klein JL, Sandy C, Migdal CS, et al. Famciclovir in AIDS-related acute retinal necrosis. AIDS. 1996;10(11):1300–1301.
87 Figueroa MS, Garabito I, Gutierrez C, et al. Famciclovir for the treatment of acute retinal necrosis (ARN) syndrome. Am J Ophthalmol. 1997;123(2):255–257.
88 Aslanides IM, De Souza S, Wong DT, et al. Oral valacyclovir in the treatment of acute retinal necrosis syndrome. Retina. 2002;22(3):352–354.
89 Emerson GG, Smith JR, Wilson DJ, et al. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology. 2006;113(12):2259–2261.
90 Pepose JS. The potential impact of the varicella vaccine and new antivirals on ocular disease related to varicella-zoster virus. Am J Ophthalmol. 1997;123(2):243–251.
91 Savant V, Saeed T, Denniston A, et al. Oral valganciclovir treatment of varicella zoster virus acute retinal necrosis. Eye (Lond). 2004;18(5):544–545.
92 Guex-Crosier Y, Meylan PR. High dosage of oral valaciclovir as an alternative treatment of varicella zoster acute retinal necrosis syndrome. Eye (Lond). 2006;20(2):247.
93 Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114(2):307–312.
94 Chau Tran TH, Cassoux N, Bodaghi B, et al. Successful treatment with combination of systemic antiviral drugs and intravitreal ganciclovir injections in the management of severe necrotizing herpetic retinitis. Ocul Immunol Inflamm. 2003;11(2):141–144.
95 Luu KK, Scott IU, Chaudhry NA, et al. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am J Ophthalmol. 2000;129(6):811–813.
96 Scott IU, Luu KM, Davis JL. Intravitreal antivirals in the management of patients with acquired immunodeficiency syndrome with progressive outer retinal necrosis. Arch Ophthalmol. 2002;120(9):1219–1222.
97 Roig-Melo EA, Macky TA, Heredia-Elizondo ML, et al. Progressive outer retinal necrosis syndrome: successful treatment with a new combination of antiviral drugs. Eur J Ophthalmol. 2001;11(2):200–202.
98 Ando F, Kato M, Goto S, et al. Platelet function in bilateral acute retinal necrosis. Am J Ophthalmol. 1983;96(1):27–32.
99 Matsuo T, Nakayama T, Baba T. Same eye recurrence of acute retinal necrosis syndrome. Am J Ophthalmol. 2001;131(5):659–661.
100 Blumenkranz M, Clarkson J, Culbertson WW, et al. Visual results and complications after retinal reattachment in the acute retinal necrosis syndrome. The influence of operative technique. Retina. 1989;9(3):170–174.
101 Hillenkamp J, N’lle B, Bruns C, et al. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009;116(10):1971–1975.
102 Han DP, Lewis H, Williams GA, et al. Laser photocoagulation in the acute retinal necrosis syndrome. Arch Ophthalmol. 1987;105(8):1051–1054.
103 McDonald HR, Lewis H, Kreiger AE, et al. Surgical management of retinal detachment associated with the acute retinal necrosis syndrome. Br J Ophthalmol. 1991;75(8):455–458.
104 Tibbetts MD, Shah CP, Young LH, et al. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824.
105 Oxman M. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284.
106 Han J, Hanson D, Way S. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30(3):266–268.
107 Pepose J, Keadle T, Morrison L. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol. 2006;141(3):547–557.