Chapter 98 Acute respiratory failure in children
Established or imminent respiratory failure is the commonest reason for admission to neonatal and paediatric intensive care units (ICUs). A number of structural and functional factors contribute to the high incidence of respiratory failure, particularly in the first year of life. In addition, respiratory failure is frequently a consequence of pathology primarily affecting other organ systems, e.g. congenital heart disease or central nervous system (CNS) disease.
PREDISPOSING FACTORS
STRUCTURAL IMMATURITY OF THE THORACIC CAGE1
In the neonate, the diaphragm and intercostal muscles have a lower percentage of type 1 (slow twitch and high oxidative) muscle fibres and therefore fatigue more readily. Diaphragmatic muscle mass is relatively reduced. Intercostal muscle activity is inhibited during rapid eye movement sleep, further reducing ventilatory efficiency. Increased respiratory work is poorly sustained in the face of increased load and may culminate in exhaustion and apnoea.2
INCREASED SUSCEPTIBILITY TO INFECTION
The immaturity and inexperience of the immune system (cellular and humoral) result in a markedly increased susceptibility to infection in the first 6 months of life. The neonate’s T-cells are unable to produce certain cytokines which compromises the interaction between T-cells and B-cells. There is greater reactivity of T-suppressor cells to T-helper cells. Complement activation is impaired in premature and full-term neonates. The neonate’s phagocytes have diminished motility, adherence and chemotaxis but bacterial killing by polymorphonuclear leukocytes is intact. In addition, there are many immune deficiency syndromes that may first reveal themselves early in postnatal life.
AETIOLOGY
Acute respiratory failure may result from upper or lower airway obstruction, alveolar disease, pulmonary compression, neuromuscular disease or injury (Table 98.1). Upper respiratory tract obstruction is discussed in Chapter 97.
Table 98.1 Causes of respiratory insufficiency in infancy and childhood
| Site | Neonate | Older infant and child |
|---|---|---|
| Upper airway obstruction | ||
| See Chapter 103 | ||
| Lower airway obstruction | ||
| Tracheal | Tracheomalacia | Foreign body |
| Vascular anomalies | ||
| Tracheal stenosis | Mediastinal tumour | |
| Bronchial | Bronchomalacia | Foreign body |
| Bronchiolar | Meconium aspiration | |
| Congenital cystic adenomatoid malformation | Acute viral bronchiolitis | |
| Lobar emphysema | ||
| Disorders of lung function | ||
| Aspiration syndromes | Pneumonia | |
| Cystic fibrosis | ||
| Hyaline membrane disease | ||
| Bronchopulmonary dysplasia | Aspiration syndromes | |
| Perinatal pneumonia | ||
| Massive pulmonary haemorrhage | Congenital heart disease | |
| Pulmonary oedema | Near-drowning | |
| Pulmonary hypoplasia | Trauma | |
| Diaphragmatic hernia | Burns | |
| Acute respiratory distress syndrome | ||
| Pulmonary compression | ||
| Diaphragmatic hernia | Pneumothorax | |
| Pneumothorax | Pleural effusion | |
| Repaired exomphalos or gastroschisis | Empyema | |
| Neurological and muscular disorders | ||
| Diaphragmatic palsy | Poisoning | |
| Birth asphyxia | Meningitis | |
| Convulsions | Encephalitis | |
| Apnoea of prematurity | Status epilepticus | |
| Trauma | ||
| Guillain–Barré syndrome | ||
| Envenomation | ||
TRACHEOMALACIA, TRACHEAL STENOSIS AND VASCULAR COMPRESSION
Division of the vascular ring and ligation or repositioning of the aberrant vessel, while removing the cause of the obstruction, do not immediately re-establish normal airway dimensions or stability. Although severity of symptoms may be alleviated by surgery, problems may persist for some years. Tracheomalacia may sometimes be stabilised by a prolonged period of nasotracheal intubation or tracheostomy with continuous positive airway pressure (CPAP). Tracheopexy, which suspends the anterior tracheal wall from the posterior sternal surface and great vessels, is occasionally useful. A slide tracheoplasty may be required to correct tracheal stenosis associated with complete tracheal rings.3 A range of stenting devices have also been developed for complex airways and employed with mixed success.
MECONIUM ASPIRATION SYNDROME
Most of these infants require oxygen therapy. Severely affected infants require controlled mechanical ventilation (CMV), which may be difficult because of the high pressures required, the non-uniformity of ventilation, and danger of pneumothorax. Improved outcomes are now achieved using surfactant (may cause transient deterioration), inhaled nitric oxide and high-frequency oscillation.4 Extracorporeal membrane oxygenation (ECMO) is also effective in those centres that have the facility, although its use has declined since the introduction of the above therapies. Cerebral effects of severe intrapartum asphyxia contribute to overall morbidity and mortality.
HYALINE MEMBRANE DISEASE
Clinical signs appear shortly after birth and consist of tachypnoea, chest-wall retraction, expiratory grunting and a progressive increase in oxygen requirements. The chest X-ray reveals a reticulogranular pattern (ground-glass appearance) with air bronchograms. In uncomplicated cases, the disease is self-limiting and resolves in 4–5 days. Respiratory failure may require increasing inspired oxygen concentrations (FiO2), CPAP, intermittent mandatory ventilation (IMV) or CMV. CPAP is known to improve oxygenation, the pattern and regularity of respiration, retard the progression of the disease and reduce morbidity, particularly with early application in the extremely preterm infant. In infants with persistent pulmonary hypertension, transitional circulation and requiring high airway pressures, the use of inhaled nitric oxide and high-frequency oscillatory ventilation are beneficial.
Instillation of surfactant into the trachea has been shown to improve oxygenation and compliance (despite some initial deterioration) and reduce the risk of pneumothorax, early mortality and morbidity.5–8 Two types of surfactant are used: synthetic (Exosurf), and bovine (Survanta) or porcine (Curosurf).
PNEUMONIA
Bacterial pneumonia also occurs. Pneumococcal pneumonia is common and usually responds dramatically to appropriate antibiotic therapy. Staphylococcal pneumonia is relatively uncommon, but may result in life-threatening respiratory failure, and is often associated with complications (e.g. empyema, pneumatocele, tension pneumothorax and suppuration in other organs). Aspiration of an effusion may be useful for diagnostic purposes. Parapneumonic effusions (empyema) may require tube thoracostomy or video-assisted thoracoscopic drainage. Resolution of the effusion can be enhanced by the instillation of thrombolytic agents such as urokinase or tissue plasminogen activator.9 In severe cases with bronchopleural fistula, surgical resection of the necrotic area offers the best chance of survival.
PULMONARY OEDEMA
Pulmonary oedema in the newborn period is due mostly to congenital heart disease, especially coarctation of the aorta, patent ductus arteriosus, critical aortic stenosis and, rarely, obstructed total anomalous pulmonary venous drainage. Pulmonary oedema due to circulatory overload may also occur in erythroblastosis fetalis, the placental transfusion syndrome, or as a result of inappropriate fluid therapy. Myocarditis is a further cause seen throughout infancy and childhood. Prolonged supraventricular tachycardia may result in ventricular dysfunction and biventricular failure, including pulmonary oedema.
DIAPHRAGMATIC HERNIA
Neonates presenting in the first 4 hours of life have major degrees of lung hypoplasia and have a mortality of 40% despite maximal supportive therapy. Those presenting after 4 hours of age should all survive. CMV may be complicated by tension pneumothorax and bronchopleural fistula on either side. Pulmonary hypertension with persistent fetal circulation and difficulties of CMV present major challenges. Many centres use ECMO or high-frequency ventilation in these infants. Nitric oxide is a useful pulmonary vasodilator in this condition.4 Prolonged ventilatory support is often required but the outlook for survivors is excellent.
DIAPHRAGMATIC PALSY
Paralysis of the hemidiaphragm may cause respiratory failure in infants, particularly if associated with another disorder affecting lung function. Problems are greatest in children under 3 years, due to the poor stability of the chest wall. CPAP may be an effective method of increasing lung volume, stabilising the rib cage and reducing paradoxical movement. Surgical plication of the diaphragm is usually effective in cases that fail to resolve with conservative management.
BIRTH ASPHYXIA
Birth asphyxia may result from placental failure, obstetric difficulties or maternal sedation. The resulting central respiratory depression, convulsions, intracerebral haemorrhage, meconium aspiration and transitional circulation contribute to respiratory failure. The Apgar score at 1 minute is an objective means of evaluating the degree of asphyxia; the score at 5 minutes is a guide to prognosis. Delayed spontaneous respiration (> 5 minutes) is also a poor prognostic sign. Further postnatal hypoxaemia, hypercapnia and brain ischaemia must be avoided. Severely asphyxiated infants need CMV after delivery, with correction of acidosis and hypovolaemia; inotropic drugs may be needed to maintain cerebral blood flow. It is important to prevent hypoglycaemia and control convulsions. A recent randomised control trial has shown improved cerebral outcomes with the use of early topical hypothermia, although the benefit is probably restricted to infants with less severe degrees of hypoxic–ischaemic encephalopathy.10
APNOEA OF PREMATURITY
Many premature infants also suffer obstructive or mixed (central and obstructive) apnoea, both disorders of respiratory and airway musculature control. The infant airway collapses easily, and this is aggravated by neck flexion. Ex-premature infants may develop recurrent apnoea with intercurrent infection or following general anaesthesia and surgical procedures. This risk may persist until a postconceptual age of 46 weeks, and demands close monitoring.11 The risk may be greater in infants with a history of apnoeic episodes, bronchopulmonary dysplasia, anaemia or neurological disease. This is transient and remarkably responsive to theophylline or caffeine loading. Ongoing maintenance therapy is often unnecessary.
STATUS ASTHMATICUS
Clinical and radiological features and management of asthma in small children are similar to that in adults (see Chapter 35). Blood gas estimation is indicated for any child with acute severe asthma, if pulsus paradoxus greater than 20 mmHg (2.6 kPa) is present, or if the child fails to respond to optimal drug therapy. Hypoxaemia due to intrapulmonary shunt and 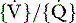 mismatching is the usual finding, and is the main cause of morbidity and mortality. High inspired oxygen therapy is therefore important. Hypocapnia in response to hypoxic drive is the rule; normocapnia or a rising PaCO2 are signs of worsening asthma or fatigue and require increased medical therapy or mechanical ventilation.
mismatching is the usual finding, and is the main cause of morbidity and mortality. High inspired oxygen therapy is therefore important. Hypocapnia in response to hypoxic drive is the rule; normocapnia or a rising PaCO2 are signs of worsening asthma or fatigue and require increased medical therapy or mechanical ventilation.
Nebulised and/or i.v. β2-sympathomimetic amines, i.v. aminophylline and corticosteroids form the mainstay of drug therapy; maximal therapy should be introduced early. Salbutamol is nebulised with oxygen as 0.05 mg/kg of 0.5% solution diluted to 4 ml with sterile water, given 2–4-hourly initially, or more frequently in severe cases. A greater and more sustained response may be achieved by more frequent or continuous salbutamol nebulisation.12 Inhaled ipratropium may have additional benefit, even in patients receiving maximal therapy with β2-sympathomimetic amines.
Some centres abandoned the use of aminophylline because of its narrow therapeutic range and because of the belief that it did not add benefit to maximal therapy with salbutamol. There is evidence, however, that aminophylline does have a place in the management of severe acute asthma in children that is unresponsive to initial treatment.13 Aminophylline is also known to possess anti-inflammatory properties. A loading dose of 5–10 mg/kg aminophylline over 1 hour produces serum levels in the therapeutic range; lower loading doses may be employed to reduce the occurrence of nausea and vomiting. Children under 9 years have increased metabolism, and require higher doses of theophylline (0.85 mg/kg per hour, equivalent to an aminophylline infusion of 1.1 mg/kg per hour). Serum concentrations should be measured (therapeutic range is 60–110 μmol/l).
Particular attention should be paid to fluid balance. Dehydration may lead to inspissation of secretions, but the risks of inappropriate antidiuretic hormone (ADH) secretion and pulmonary oedema must be noted.14,15
With aggressive medical therapy, the need for CMV should be rare. Its use should be based predominantly on clinical features rather than solely on blood gas analysis. It should not, however, be withheld out of fear of difficulties. CMV may worsen air trapping and lead to hypotension or pneumothorax. Controlled hypoventilation (permissive hypercapnia) with a long expiratory time is advocated to minimise airway pressures and air trapping. A trial of positive end-expiratory pressure (PEEP) may be justified to reduce intrinsic (auto) PEEP and air trapping, although evidence in adults suggests that PEEP may be associated with increased air trapping.16
ACUTE VIRAL BRONCHIOLITIS
Management consists of minimal handling and oxygen therapy. If respiratory distress is marked, feeds should be withheld and fluids administered i.v. Progression of the disease leads to exhaustion and respiratory failure in 1–2% of cases. CPAP and/or aminophylline therapies are reported to reduce the work of breathing, lower PaCO2 and eliminate recurrent apnoea. Nebulised adrenaline may also be beneficial.17 CMV is required in some cases, particularly when the disease occurs in association with congenital heart or chronic lung disease. High airway pressures may be required, increasing the risk of pneumothorax. The antiviral agent ribavirin is difficult to administer and has not been shown to improve outcome. Bacterial coinfection is common, and justifies the use of broad-spectrum antibiotics in severe cases.18
RESPIRATORY FAILURE SECONDARY TO CONGENITAL HEART DISEASE
Infants with congenital heart disease may develop respiratory failure for a number of reasons.
TYPE OF CARDIAC LESION
Congenital heart lesions producing acute respiratory failure fall into four main groups.
MENINGITIS AND ENCEPHALITIS
Meningitis is common in the early years of life. After the neonatal period, the usual causative organisms are Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumoniae. Immunisation has markedly reduced the incidence in developed countries. Rapid diagnosis and appropriate antibiotic therapy form the cornerstone of treatment. Respiratory failure is mainly associated with uncontrolled convulsions, altered conscious state or raised intracranial pressure (ICP).
GENERAL MEASURES
PHYSIOTHERAPY
The role of conventional chest physiotherapy with posturing, percussion and vibration in the paediatric ICU setting is unproven. Physiotherapy must be applied cautiously in children with cardiovascular instability or raised ICP. Chest compression and vibration in the newborn may result in rib fractures and possibly intracranial haemorrhage. Physiotherapy may cause a significant fall in PaO2. FiO2 should be increased in anticipation (caution is required in premature infants who are at risk of retinopathy of prematurity). In infants, periodic gentle pharyngeal suction removes pharyngeal secretions and may stimulate coughing. Effective bagging, tracheal suction and positioning are the most useful techniques in the intubated patient.
MONITORING AND ASSESSMENT
TRANSILLUMINATION
Transillumination of the thorax with a cold fibreoptic light source is a useful technique to detect pneumothorax in newborn infants. Transillumination occurs on the side of pneumothorax, but does not quantify the amount of air present. Bilateral pneumothoraces may cause confusion. In severe pulmonary interstitial emphysema, increased transillumination may be seen. A chest X-ray should be performed to confirm the pneumothorax when time permits.
SPECIFIC MEASURES
OXYGEN THERAPY
Older children tolerate appropriately sized facemasks but FiO2 is rarely known. Masks incorporating a reservoir bag will deliver high concentrations in young children. Restless children do not tolerate facemasks and oxygen delivery may become intermittent. Once positioned, nasal cannulae are usually well tolerated. A single catheter in the postnasal space (1–2 l/min) is also an effective method of delivery. With nasal cannulae, FiO2 depends on flow rate, size of the nasopharynx, the presence or absence of mouth-breathing and peak inspiratory flow rate. Using a single nasal cannula, a flow rate of 150 ml/kg per min provides FiO2 about 0.5 in children under 2 years.20 Effective humidification is difficult with nasal cannulae, and drying of mucosa and secretions may be a problem.
INTUBATION
MECHANICAL VENTILATORY SUPPORT
MECHANICAL VENTILATION
Paediatric ventilators are discussed elsewhere in this volume. The risks of barotrauma, volutrauma and oxygen toxicity demand that specific ventilator settings be prescribed for rate, peak inspiratory pressure, PEEP, CPAP, flow rate, inspiratory time, minute volume and inspired oxygen. If pulmonary function progressively deteriorates, a stepwise increase of each setting should be considered. The least harmful alternative should be undertaken first (e.g. increasing FiO2 is probably a safer alternative than increasing PEEP). Unless contraindicated, a PEEP of 3–5 cmH2O (0.3–0.5 kPa) is recommended in all ventilated infants to prevent airway closure. In the presence of reduced cerebral compliance, PEEP may be associated with increased intracranial pressure. Oxygenation is a priority, however, and other means of maintaining cerebral perfusion pressure should be employed if PEEP is required. Removing or reducing PEEP must be considered if there is evidence of barotrauma. Increased PEEP demands a similar increase in peak inspiratory pressure if the same tidal volume is to be maintained. Mean airway pressure is the main determinant of oxygenation. It is dependent on rate, peak inspiratory pressure, flow rate, inspiratory time and PEEP.
CONTINUOUS POSITIVE AIRWAY PRESSURE
Benefits of CPAP include the following:
SEDATION AND ANALGESIA
Sedation and analgesia should be used to reduce restlessness and discomfort, and to minimise the work of breathing (see Chapter 100). In the brain injured, sedation prevents coughing, straining, unwanted autonomic responses and increases in intracranial pressure. Heavy sedation (with or without muscle relaxants) is recommended, provided supervision and monitoring facilities are adequate.
COMPLICATIONS
Complications of mechanical ventilatory support include:
Mechanical ventilation must be approached cautiously if air leak is a particular risk (e.g. in immature lungs and diseases characterised by air trapping). Permissive hypercapnia can often be tolerated, provided that oxygenation, perfusion and acid–base balance are acceptable.
HIGH-FREQUENCY OSCILLATORY VENTILATION
Animal studies using HFOV and an ‘open lung strategy’ have convincingly demonstrated less of the histological changes of ARDS and less barotrauma.22,23 Although a multicentre trial of HFOV in preterm infants failed to show benefit, a number of single centre randomised or rescue studies have demonstrated less barotrauma in very low birth weight infants, a decreased incidence of chronic lung disease (BPD) and reduced requirement for ECMO.24,25 The combined use of HFOV and nitric oxide therapy has led to reduced use of ECMO in many centres. HFOV has an important role in the management of severe pulmonary air leak syndromes.
1 Muller NL, Bryan AC. Chest wall mechanics and respiratory muscles in infants. Pediatr Clin North Am. 1979;26:503-526.
2 Keens TG, Bryan AC, Levison H, et al. Developmental pattern of muscle fibre types in human ventilatory muscles. J Appl Physiol Respir Environ Exercise Physiol. 1978;44:909-913.
3 Kyocyildirim E, Kanani M, Roebuck D, et al. Long-segment tracheal stenosis: slide tracheoplasty and a multidisciplinary approach improve outcomes and reduce costs. J Thorac Cardiovasc Surg. 2004;128:876-882.
4 Kinsella JP, Neish SR, Dunbar ID, et al. Clinical responses to prolonged treatment of persistent pulmonary hypertension of the newborn with low doses of inhaled nitric oxide. J Pediatr. 1993;123:103-108.
5 Bose C, Corbet A, Bose G, et al. Improved outcome at 28 days of age for very low birth weight infants treated with a single dose of a synthetic surfactant. J Pediatr. 1990;117:947-953.
6 Long W, Corbet A, Cotton R, et al. A controlled trial of synthetic surfactant in infants weighing 1250 g or more with respiratory distress syndrome. N Engl J Med. 1991;325:1696-1703.
7 Stevenson D, Walther F, Long W, et al. Controlled trial of a single dose of synthetic surfactant at birth in premature infants weighing 500–699 grams. J Pediatr. 1992;120:S3-12.
8 Jobe A. Pulmonary surfactant therapy. N Engl J Med. 1993;328:861-868.
9 Park CS, Chung WM, Lim MK, et al. Transcatheter instillation into loculated pleural effusion: analysis of treatment effect. Am J Roentgenol. 1996;167:649-652.
10 Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663-670.
11 Sims C, Johnson CM. Postoperative apnoea in infants. Anaesth Intensive Care. 1994;22:40-45.
12 Robertson CF, Smith F, Beck R, et al. Response to frequent low doses of nebulized salbutamol in acute asthma. J Pediatr. 1985;106:672-674.
13 Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79:405-410.
14 Baker JW, Yerger SY, Segar WE. Elevated plasma antidiuretic hormone levels in status asthmaticus. Mayo Clin Proc. 1976;51:31-34.
15 Stalcup SA, Mellins RB. Mechanical forces producing pulmonary edema in acute asthma. N Engl J Med. 1977;297:592-596.
16 Tuxen DV. Detrimental effects of positive end-expiratory pressure during controlled mechanical ventilation of patients with severe airflow limitation. Am Rev Resp Dis. 1989;140:5-9.
17 Sanchez I, De Koster J, Powell RE, et al. Effect of racemic epinephrine and salbutamol on clinical score and pulmonary mechanics in infants with bronchiolitis. J Pediatr. 1993;122:145-151.
18 Korppi M, Leinonen M, Koskela M, et al. Bacterial coinfection in children hospitalized with respiratory syncytial virus infections. Pediatr Infect Dis J. 1989;8:687-692.
19 Lanteri CJ, Kano S, Duncan AW, et al. Changes in respiratory mechanics in children undergoing cardiopulmonary bypass. Am J Respir Crit Care Med. 1996;152:1893-1900.
20 Shann F, Gatchalian S, Hutchinson R. Nasopharyngeal oxygen in children. Lancet. 1988;1:1238-1240.
21 Clough JB, Duncan AW, Sly PD. The effect of sustained positive airway pressure on derived cardiac output in children. Anaesth Intensive Care. 1994;22:30-34.
22 Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol. 1983;55:131-138.
23 DeLemos RA, Coalson JJ, Gerstmann DR, et al. Ventilation management of infant baboons with hyaline membrane disease: the use of high-frequency ventilation. Pediatr Res. 1987;21:594-602.
24 Clark RH, Yoder BA, Sell MS. Prospective, randomised comparison of high-frequency oscillation and conventional ventilation in candidates for extracorporeal membrane oxygenation. J Pediatr. 1994;124:447-454.
25 Clark RH, Gerstmann DR, Null DM, et al. Prospective randomised comparison of high-frequency oscillatory and conventional ventilation in respiratory distress syndrome. Pediatrics. 1992;89:5-12.