Chapter 42 Acute Renal Failure
3 How is ARF classified?
The main categories are prerenal, intrarenal or parenchymal, and postrenal or obstructive (Table 42-1).
| Prerenal | Postrenal | Parenchymal |
|---|---|---|
| Dehydration | Ureter | Glomerular |
| Impaired cardiac function | Bladder | Interstitial |
| Vasodilation | Urethra | Allergic interstitial nephritis |
| Renal vascular obstruction | Vascular | |
| Hepatorenal syndrome | ATN |
ATN, Acute tubular necrosis.
5 What are the implications of urinary electrolytes in the differential diagnosis of ARF?
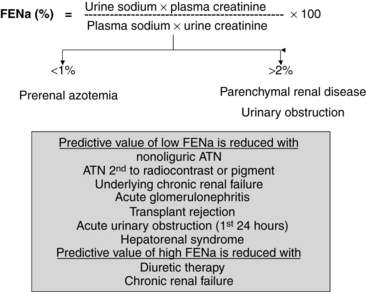
The determination of urine electrolyte and creatinine concentrations may be helpful in the differential diagnosis of ARF. When used with serum values, urinary diagnostic indexes can be generated. Understanding the concepts behind the interpretation of these indexes is easier and better than trying to remember specific numbers. Quite simply, if the tubule is working well in the setting of decreased GFR, tubular reabsorption of sodium and water is avid, and the relative clearance of sodium to creatinine is low. Conversely, if the tubule is injured and cannot reabsorb sodium well, the relative clearance of sodium to creatinine is not low. Therefore, with prerenal azotemia, the ratio of the clearance of sodium to the clearance of creatinine, which is also called the fractional excretion of sodium (FENa) (FENa = [Urinary sodium]/[Urinary creatinine] × [Plasma creatinine]/[Plasma sodium] × 100), is typically less than 1.0, whereas with parenchymal or obstructive causes of ARF, the FENa is generally greater than 2.0 (Fig. 42-1).
10 Which critical electrolyte disorders accompany ARF?
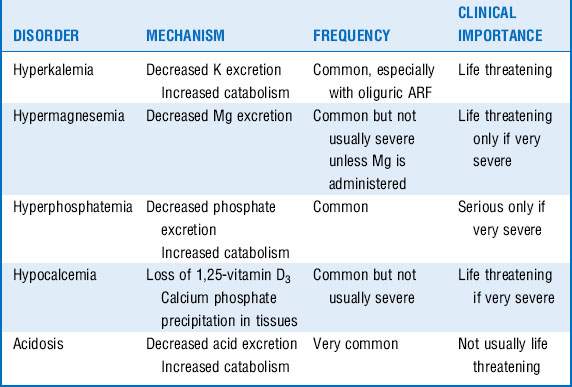
The most common electrolyte disorders that accompany ARF include hyperkalemia, hypermagnesemia, hyperphosphatemia, hypocalcemia, and acidosis (Table 42-2). Of these disorders, hyperkalemia is the most common and probably the one that is usually most serious.
14 What are the options for nonconservative therapy of ARF?
The three main options for nonconservative therapy of ARF are hemodialysis, peritoneal dialysis, and continuous renal replacement therapy (CRRT). Each option has advantages and disadvantages (Box 42-1), and, of course, variations exist of each of these modalities.
15 What is CRRT?
Key Points Acute Renal Failure
1. Serum creatinine concentration may be insensitive to the loss of renal function.
2. ARF due to acute tubular necrosis may be initiated by one mechanism and maintained by another different mechanism.
3. Results elicited by measures to prevent ATN are far superior to those yielded by efforts to provide treatment.
4. Urinalysis and urine electrolyte and creatinine estimation can provide important information about the cause of ARF.
5. Hyperkalemia is an important life-threatening complication of ARF requiring urgent management.
6. Renal replacement therapy, in the form of dialysis, may be required for correction of volume status, electrolyte imbalance, and acidosis, if conservative therapy fails.
1 Asif A., Epstein M. Prevention of radiocontrast induced nephropathy. Am J Kidney Dis. 2004;44:12–24.
2 Bellomo R., Cass A., Cole L., et al. Intensity of continuous renal replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638.
3 Briglia A.E. Choosing the best dialysis option in patients with acute renal failure and in intensive care unit. In: Henrich W.L., ed. Principles and Practice of Dialysis. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:219–240.
4 Conger J. Hemodynamic factors in acute renal failure. Adv Ren Replace Ther. 1997;4:25–37.
5 Esson M.L., Schrier R.W. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–752.
6 Khosla N., Mehta R.L. Continuous dialysis therapeutic techniques. In: Henrich W.L., ed. Principles and Practice of Dialysis. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:196–218.
7 Lameire N. The pathophysiology of acute renal failure. Crit Care Clin. 2005;21:197–210.
8 Miller T.R., Anderson R.J., Linas S.L., et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89:47–50.
9 Nowicki M., Zwiech R., Szklarek M. Acute renal failure: the new perspective. Annales Academiae Medicae Bialostocensis. 2004;49:145–150.
10 Palevsky P.M. Renal replacement therapy: indications and timing. Crit Care Clin. 2005;21:347–356.
11 Saxena R., Toto R.D. Approach to the patient with kidney disease. In: Brenner B.M., ed. Brenner & Rector’s The Kidney. 8th ed. Philadelphia: Saunders; 2008:705–723.
12 Venkataraman R., Kellum J.A. Defining Acute Renal Failure: the RIFLE Criteria. J Intensive Care Med. 2007;22:187–193.
13 Wiseman A.C., Linas S. Disorders of potassium and acid-base balance. Am J Kidney Dis. 2005;45:941–949.