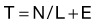135 Acute Radiation Emergencies
• The nature and extent of an acute radiologic emergency should be identified to determine whether hospital disaster plans should be activated.
• Large-scale radiologic emergencies require triage of multiple patients based on the estimated radiation dose, presence of traumatic injuries, and availability of local resources.
• An emergency physician can use the symptoms and findings on physical examination to estimate the whole-body radiation dose (rad or gray) for predicting a patient’s course and subsequent need for treatment.
• A complete blood count with differential at triage and 24 to 48 hours later can be used to guide the diagnosis, treatment, and prognosis of acute radiation syndrome.
• Decontamination is an important aspect of acute radiation emergencies but should not supersede stabilization of unstable patients.
• Recognition of patients who have been exposed to moderate to high doses of radiation and triage to early treatment of acute radiation syndrome can significantly decrease mortality.
Epidemiology
Acute radiologic emergencies are a rare occurrence, yet with increasing use of radioisotopes in medicine, research, and industry, as well as ongoing use in national defense and electrical power generation, it is imperative that emergency physicians (EPs) be versed in acute medical management after radiologic emergencies. The relative magnitude of the situation and the resources needed to address an emergency vary depending on the scale of the exposure event. Small-scale events include contained exposure in hospitals or research facilities or contained breaches at nuclear power plants in which small amounts of material may lead to exposure or contamination of a limited number of individuals. Large-scale events involve relatively large quantities of radioactive material or potential widespread exposure or contamination of a significant population. Examples of large-scale events include nuclear reactor meltdowns (e.g., Fukushima Daiichi, Japan, in March 2011; the Chernobyl meltdown in April 1986), detonation of nuclear weapons, or terrorist attacks with a radiologic dispersal device—otherwise known as a “dirty bomb.”1,2 Hospitals are required by the Joint Commission to have disaster plans in place for mass casualty events, including radiologic emergencies, and EPs and emergency medical service personnel will be at the forefront of decision making and patient care when a radiologic event occurs.
The first priority during an acute radiation emergency is to establish the reality and type of radiologic event. Information received from the field is essential in mounting an effective response in the emergency department (ED) and the hospital. Important information to obtain from the field, if possible, is listed in the Documentation box. With large events, EDs should anticipate patients arriving from multiple field triage and stabilization sites, as well as individuals who arrive on their own accord without first being triaged or evaluated for injury or radiation exposure or contamination.
![]() Documentation
Documentation
Important Information to Obtain from the Field after Acute Radiologic Emergencies
Type of radioactive material involved. Different types of radioactive material can expose patients in different ways. Important specifics include the type of radioisotope or source (α-, β-, γ-emitters, or neutrino), the form that it was in when the exposure occurred, and whether those exposed were also contaminated via inhaled dust or ingestion
Types of traumatic injuries occurring with the radiation exposure (blast or burn)
Type of decontamination procedures taking place at the scene or in field triage
Number of patients expected to require triage, evaluation, and treatment
What local, regional, or federal resources have been activated and notified of the acute radiologic emergency
Communication between the field triage team and the ED must be clear because no federal or internationally agreed-upon medical triage systems have been established specifically for radiation mass casualty incidents, and thus existing mass casualty emergency triage algorithms should be used with modifications for the impact of the radiation exposure or contamination.1,3–5 The Scarce Resources for a Nuclear Detonation Project has published new triage recommendations for the first 4 days following a possible nuclear detonation, and formal guidelines will probably be recommended in the near future.5–7
The disaster response coordinator should prepare the ED to receive patients triaged at all levels with blast, thermal, and radiation injuries. Importantly, standard personal protective equipment sufficient to protect the emergency personnel treating these patients in the decontamination area and in the ED must be used.1,8–11 Those triaging and caring for patients with possible contamination should be using strict isolation precautions.
In any acute radiation emergency, initial mass casualty field triage should not be confused with the subsequent clinical triage used by EPs and other providers for more definitive medical management. It is essential that those involved in triage and provision of emergency care for radiologic emergencies understand that lifesaving medical stabilization takes precedence over other concerns, including external radiation decontamination.1,4,10–12
Pathophysiology
Radioactivity refers to the loss of particles or energy from an unstable atom that is decaying. There are two distinct types of radiation: ionizing radiation (e.g., α and β particles from a decaying radioisotope and nonparticulate electromagnetic γ-rays and x-rays) and nonionizing radiation (e.g., all forms of the electromagnetic spectrum, except for γ-rays and x-rays).The main means of exposure to a dose of radiation are (1) from an external radiation source, (2) by loose radioactive material deposited on the skin or into wounds, and (3) by ingestion or inhalation of radioactive material.1 Significant radiation injuries can occur from irradiation with or without contamination (contact of the substance directly with human tissue). A contaminated patient will have been exposed to radioactive material that could still be on or inside the patient’s body. Irradiated patients without contamination have been exposed but do not have radioactive material on or in them. Irradiated patients are not “radioactive.” Such differentiation in a radiologic emergency situation is important because it dictates whether decontamination is needed. A radioactivity meter (most commonly a Geiger counter is the initial screening tool) and the patient’s history should be used to determine whether a patient has been contaminated.
To comprehend the physiologic effects of radiation, it is important to understand the units of measurement for types of doses (see the Facts and Formulas box). Radiation damage occurs within microseconds of exposure and most significantly affects rapidly reproducing types of cells (e.g., intestinal crypt cell, stem cells) or cells with a large nucleus, such as lymphocytes. The threshold of 2 Gy of whole-body irradiation is often used as the reference point for when cells have been irreparably damaged and symptoms of acute radiation syndrome (ARS) develop.1,12
![]() Facts and Formulas
Facts and Formulas
Dose Equivalent or Equivalent Dose (SI Terms)
Triage Formula for Irradiated Patients When CBC with Differential Is Available (Adapted from AFRRI, 2010; REAC/TS, 2011)
In otherwise healthy adult patients who have been exposed to external irradiation without trauma or burn, a clinically detrimental radiation dose can be distinguished from a dose lower than 1 Gy 4 hours after exposure by using a triage score involving two criteria: the N/L ratio and whether emesis has occurred in the 4 hours since exposure
The triage score T is assigned as follows:
Given: N = WBC × (% neutrophils); L = WBC × (% lymphocytes); E = 0 if no emesis is present by 4 hours; E = 2 if emesis is present by 4 hours
If T is higher than 3.7, the patient should be referred for further evaluation; otherwise can be discharged with follow-up if clinically stable
Presenting Signs and Symptoms
Depending on the cause of the radiologic emergency (spill or leak versus explosion), the emergency provider may be in the position of having to stabilize and treat traumatic injuries and burns before assessing patients for signs of ARS. In cases of blast and thermal injury, patients’ traumatic injuries are of primary concern because poor outcomes after radiation injury are compounded by traumatic injury and thermal burns (e.g., combined injury).12,13
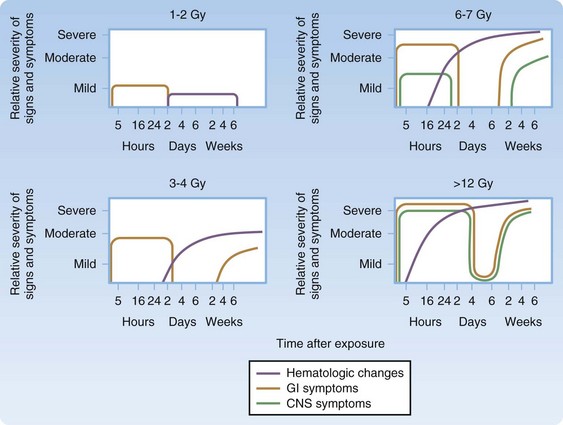
ARS has four subsyndromes—hematopoietic, gastrointestinal, cutaneous, and neurovascular—and the time until onset and severity are related to the dose for all syndromes. The hematopoietic system is most vulnerable, with at least mild changes occurring at whole-body exposure to doses of less than 1 Gy, and acute treatment is typically considered at 2 Gy and higher.4,11,14 The cutaneous, gastrointestinal, and cardiovascular subsyndromes generally occur with whole-body exposure to doses of greater than 4 Gy, greater than 6 Gy, and greater than 10 Gy, respectively.11
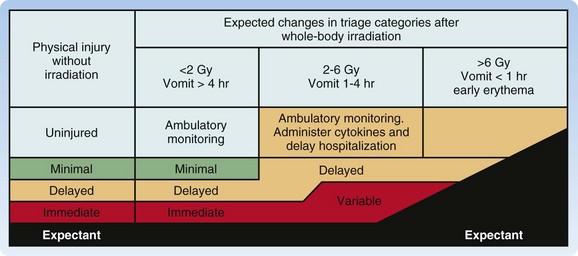
ARS and the subsyndromes typically follow four predictable phases. The first, or prodromal, phase can include the symptoms of nausea and vomiting, diarrhea, fatigue, fever, erythema, conjunctivitis, and respiratory difficulty. Altered mental status occurs in the cardiovascular subsyndrome in association with very high radiation doses (>10 Gy). This prodromal phase indicates that more serious manifestations may follow and provides important clues for triage.5,9–12,14,15 Next is the latent phase, in which the symptoms subside; it lasts days to weeks, with patients exposed to higher doses typically having shorter latency. The latent phase is followed by the manifest illness phase, in which the four ARS subsyndromes are evident and patients require intensive medical management. The final phase is eventual recovery or death (Fig. 135.1; Table 135.1).
Differential Diagnosis and Medical Decision Making
Given that modern supportive care is effective in increasing LD50/60 doses (lethal dose for 50% of the population surviving at 60 days) in nontrauma patients from 3.5 to 4 Gy (without medical care) to up to 6 to 8 Gy (treated with transfusions, antibiotics, colony-stimulating factors, and intensive care), it is of the utmost importance to triage and treat patients appropriately early in the postexposure period.1,5,10,11 The goal is to provide the highest level of care possible to patients who may have had radiation exposure, are possibly contaminated, and may or may not have sustained concomitant trauma or burns.
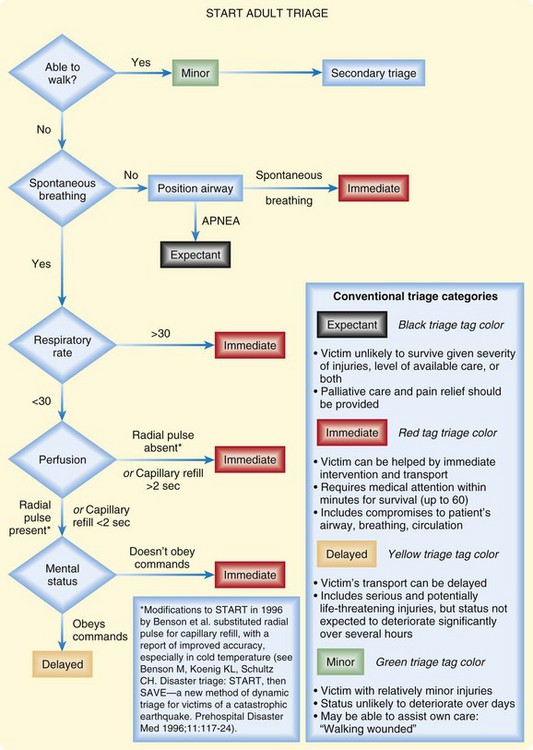
Although several well-known mass casualty triage systems such START (Figs. 135.2 and 135.3) and JumpSTART have been devised, no federal or internationally agreed-up on medical triage systems have currently been established specifically for radiation mass casualty incidents.4,6,14, Existing mass casualty emergency triage algorithms are currently being used with modifications for the impact of radiation and need for decontamination.5,8,14 However, large interorganizational efforts are currently being made at the federal level to further delineate triage protocols for different types of radiologic emergencies and for different levels of resource scarcity. See the Radiation Emergency Medical Management (REMM) website9 for up-to-date triage recommendations.
A significant aspect of triage is determining the degree to which the patient may have been irradiated and whether external or internal contamination is present.2,8–10,16 A Geiger counter and nasal swabs should be used at initial triage to determine whether a patient is externally (or potentially internally) contaminated. This should help dictate the amount and type of decontamination required to minimize the radiation dose. Removal of clothing alone is typically responsible for 70% to 90% of the external decontamination. In the setting of trauma and burn injury, additional wound decontamination and early wound care are necessary1,2,8–11,16 (see the Tips and Tricks box).
Tips and Tricks
Recommendations for Radiologic Decontamination
Principles of Radiologic Decontamination
Ideally, decontamination takes place at field triage (area away from the emergency department [ED]), and patients arrive at the ED for evaluation and treatment already largely decontaminated. The exception should be when patients require medical stabilization and resources are available for the expected volume of severely injured patients.
The hazard of radiation exposure by health care providers from a radiologically contaminated patient is probably negligible when recommended personal protective equipment is used.
Decontamination begins after the patient is medically stabilized.
Patients with a risk for external or internal contamination should be evaluated with a Geiger counter and the nares swabbed and tested to evaluate the risk for inhalation of radioactive material.
Decontamination priorities are (1) wounds, (2) body orifices around the face, and (3) intact skin.
Patients should be reassessed with a Geiger counter after every attempt at decontamination (e.g., removal of clothing, washing, and wound cleansing). Decontamination procedures should be continued until less than two times the normal amount of background radiation is present.
All wound care material, irrigation fluid, and water used for washing or showering should be contained and disposed of properly as radioactive waste.
Decontamination Techniques
Clothing is removed by rolling the clothes off in an outward direction and away from the patient’s skin and airway so that any radioactive contamination is trapped in the rolled clothing, and the clothing is placed in a sealed bag. Clothing should be cut, not torn.
Wound dressings are to be removed and the skin adjacent to the wound cleaned before cleaning the wound to prevent contamination of the wound. Areas around wounds should be draped to prevent contamination of the skin during copious wound irrigation.
Partial-thickness burns should be cleaned well with nonirritating solutions. Blisters should be left closed; open blisters should be irrigated well and treated as all other open partial-thickness burns.
Full-thickness burns with radioactive contaminants warrant typical care because of contamination sloughing with the eschar.
Radiation injury and traumatic injury (blast and burn) interact synergistically. Patients with traumatic or burn injury who have been subjected to significant whole-body irradiation (>2 Gy) have a substantially worse prognosis because of effects on the hematopoietic syndrome and high risk for infection and poor healing. Consequently, they require a higher triage priority than do patients who are only irradiated (with or without contamination) and should be triaged and treated according to the Armed Forces Radiobiology Research Institute (AFRRI) treatment protocol16 (Fig. 135.4).
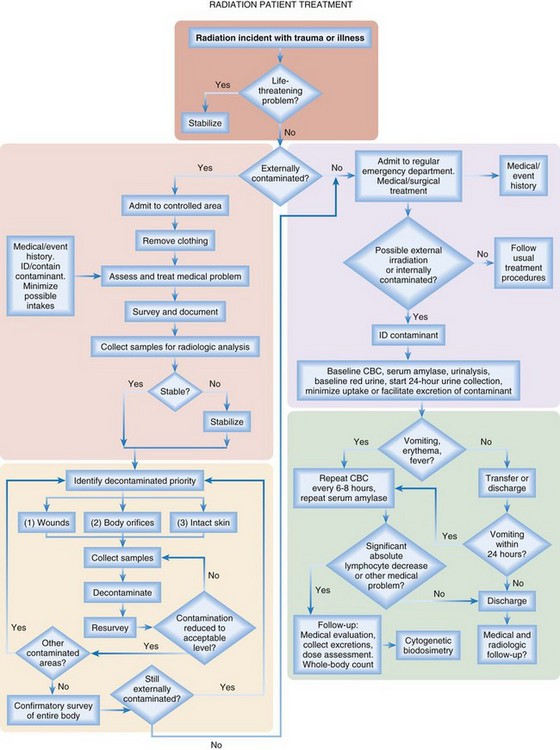
Fig. 135.4 Treatment algorithm for patients with combined radiation and trauma or burn injury.
CBC, Complete blood count; ID, identify.
(Adapted from Armed Forces Radiobiology Research Institute. AFRRI pocket guide: emergency radiation medicine response. Bethesda, Md: Uniformed Services University of the Health Sciences; 2011.)
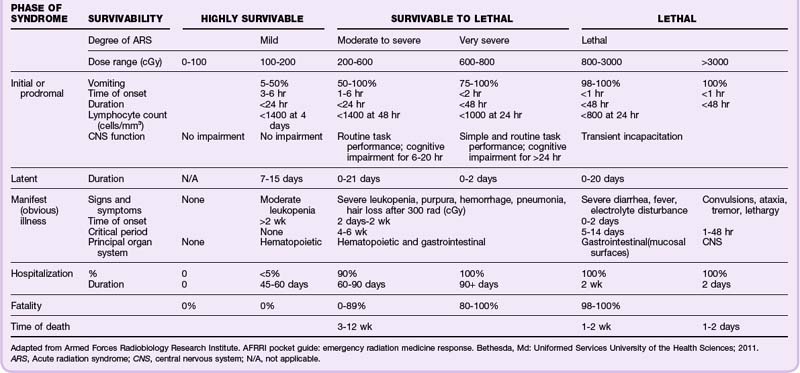
Although the REMM website9,17 has very useful biodosimetry tools to assist in estimation of the radiation dose, it requires that blood samples be collected for a complete blood count (CBC) with differential and other laboratory tests (Box 135.1). However, because early in a large-scale acute radiologic emergency it may not be possible to process large numbers of blood samples, it may be necessary to use the history and symptoms (mainly time from exposure to the onset of persistent vomiting) (Table 135.2) with other findings on physical examination (Table 135.3) to estimate the whole-body radiation dose for triaging patients to the four levels of care—expectant, immediate, delayed, and minimal. Ideally, all patients triaged higher than “minimal” should undergo additional work-up, including blood work and urine testing for more sensitive and specific biodosimetric radiation dose estimation. Current recommendations include obtaining a CBC with differential at baseline and every 6 hours for at least the first 48 hours because lymphocyte depletion follows dose-dependent first-order kinetics and the neutrophil-lymphocyte (N/L) ratio increases over the first 48 hours after exposure—a sensitive indicator of the radiation dose (Table 135.4).
Box 135.1
Recommended Testing in Acute Radiation Emergencies
Recommended initial laboratory tests (all patients with suspected irradiation):
If the patient is vomiting less than 3 hours after exposure:
If the history indicates internal contamination (inhalation or gastrointestinal):
Table 135.2 Radiation Dose Estimate by Time to Emesis After Irradiation
| TIME TO EMESIS | UPPER BOUND DOSE ESTIMATE |
|---|---|
| >2 hr | <9 Gy |
| >4 hr | <4 Gy |
| >10 hr | <2 Gy |
Adapted from Parker DD, Parker JC. Estimating radiation dose from time to emesis and lymphocyte depletion. Health Phys 2007;93:701-4.
Table 135.3 Findings in Patients in the Prodromal Phase of Acute Radiation Syndrome for Dose Estimation Without Biodosimetry
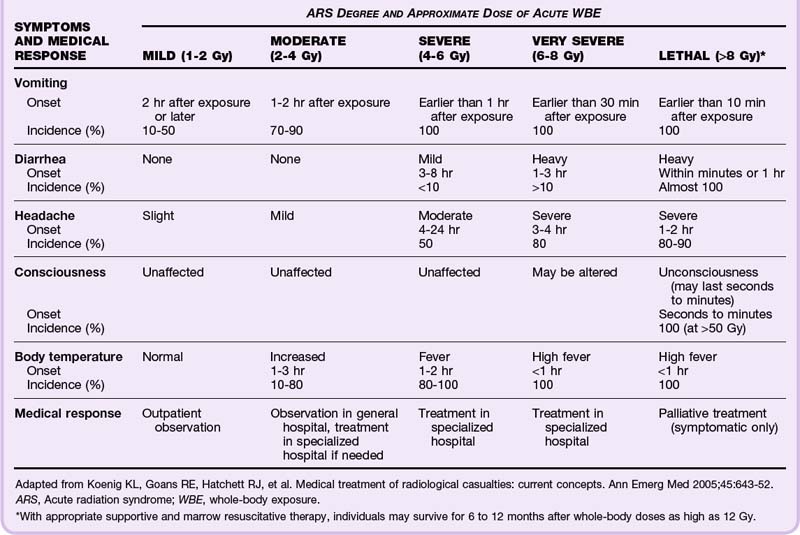
Table 135.4 Expected Patient Outcome Based on Absolute Lymphocyte Count at 48 Hours
| MINIMAL LYMPHOCYTE COUNT 48 HOURS AFTER EXPOSURE | APPROXIMATE ABSORBED RADIATION DOSE (Gy) | PROGNOSIS |
|---|---|---|
| 1000-3000 | 0-0.5 | No significant injury |
| 1000-1500 | 1-2 | Significant but probably nonlethal, good prognosis |
| 500-1000 | 2-4 | Severe injury, fair prognosis |
| 100-500 | 4-8 | Very severe injury, poor prognosis |
| <100 | >8 | High lethality even with full treatment with CSFs |
CSFs, Colony-stimulating factors.
Adapted from Goans RE, Halloway EC, Berger ME, et al. Early dose assessment in criticality accidents. Health Phys 2001;81:446-9.
The U.S. Department of Defense has used the Radiation Emergency Assistance Center/Training Site (REAC/TS) database of patients with radiation exposure and symptoms combined with the N/L ratio to create a triage score that differentiates patients exposed to a high radiation dose from those exposed to less than 1 Gy. It can be used in patients seen 4 or more hours after an exposure and has a sensitivity of 89% and specificity of 93%1,2 (see the Facts and Formulas box).
Treatment
In ARS diagnosed by the onset of symptoms or biodosimetry assessment, treatment is based on the whole-body radiation dose. In general, treatment of ARS is not indicated for (1) a low exposure dose (<1 Gy) because the risk for complications is low and (2) a very high exposure dose (>10 Gy) because the prognosis is very poor in this population and palliative care is most appropriate.1,11 With exposure to greater than 2 to 3 Gy, medical treatments are both supportive (appropriate use of intravenous fluids, antiemetics, and nutritional support) and preventive (prophylactic antibiotics, colony-stimulating factors, and transfusion of blood products)2,10,11 (Box 135.2).
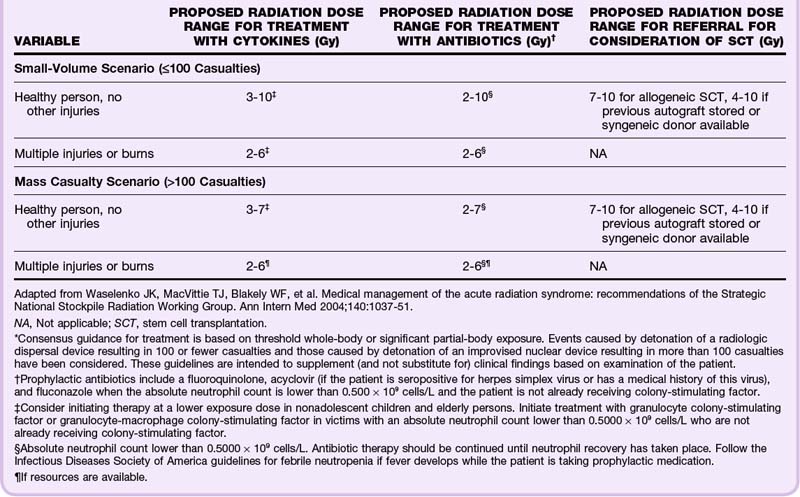
Hospital treatment of ARS is focused on supportive measures and management of the hematopoietic subsyndrome to minimize the complications of sepsis and bleeding. The goal is to reduce both the depth and duration of neutropenia and thrombocytopenia, as well as to prevent or manage neutropenic fever and sepsis.2,10,11 Treatment recommendations for antibiotics (fluoroquinolone, acyclovir, and fluconazole) and cytokine therapy (hematopoietic colony-stimulating factors) vary depending on the estimated dose of radiation exposure, the presence of traumatic injury or burn, and the size of the radiologic emergency, which is addressed in Table 135.5. Note that the current recommendations are based on healthy adults and that a lower threshold for treatment may be advisable for children younger than 12 years and the elderly, in whom few data are available for evidence-based recommendations.5,11 When treatment is indicated, it should continue until the absolute neutrophil count is higher than 1.0 × 109 cells/L after recovery from the nadir.
For those with trauma and burns, there are caveats for decontamination of wounds (see the Tips and Tricks box). Because of the increased likelihood of infection and bleeding as a result of the hematopoietic effects of radiation exposure, any orthopedic reductions, splinting, casting, or surgery (orthopedic and otherwise) should be completed within the initial 48 hours of significant radiation exposure (>2 Gy).1,2,9
In addition to the aforementioned general recommendations for treatment of radiologic emergencies, specific approaches for treating internal contamination (ingestion or inhalation) have been established. Reduction of the internal radionuclide content is called radionuclide decorporation and can be helpful in reducing the absorbed radiation dose.18 Approaches to decorporation include (1) reducing or inhibiting absorption in the gastrointestinal tract, (2) blocking uptake to the organ predisposed to injury (e.g., potassium iodide for blocking uptake by the thyroid), (3) diluting the isotope and increasing elimination, (4) altering the chemistry of the substance, (5) displacing the isotope from receptors, (6) using chelation techniques to enhance elimination, (7) excising radionuclide from contaminated traumatic wounds early to minimize absorption, and (8) performing bronchoalveolar lavage for severe cases of insoluble inhaled particles.1,2
Treatment options are available if the radioactive contaminant is known and antidotes exist.2,9,10,19,20 Poison control centers can provide help in identifying potential antidotes for the ingestion of known radionuclides. A limited selection of radionuclide-specific decorporation treatments are included in Table 135.6. In general, the risk versus benefit balance of using these decorporation agents depends on the estimated dose ingested and the time since the ingestion. Estimation of ingested radionuclide doses is typically beyond the scope of EPs and ED staff, and in many situations these treatments are not helpful early in the medical response following a large-scale radiologic disaster.5 Therefore, guidance from poison control, the Centers for Disease Control and Prevention, or REMM (or any combination of these resources) is recommended before beginning treatment.5,18,21
Table 135.6 Specific Decorporation Treatments in Patients with Internal Radiation Contamination*
| RADIONUCLIDE | THERAPY† |
|---|---|
| Tritium | Dilution: force fluids |
| Iodine 125 or 131 | Blocking: saturated solution of potassium iodide |
| Mobilization: antithyroid drugs | |
| Cesium 134 or 137 | Blocking: Prussian blue |
| Strontium 89 or 90 | Decrease absorption: aluminum phosphate gel antacids |
| Blocking: strontium lactate | |
| Displacement: oral phosphate | |
| Mobilization: ammonium chloride or parathyroid extract | |
| Plutonium and other transuranic elements | Chelating: Zn or Ca-DTPA (investigational) |
| Unknown ingestion | Reduce absorption; consider emetics, gastric lavage, charcoal, whole-bowel irrigation |
* Seek guidance from the Centers for Disease Control and Prevention or Radiation Emergency Medical Management (REMM) when administering any of these interventions because many have an unfavorable risk-to-benefit ratio in those with ingestion of a low to moderate dose. Treatment is strongly recommended for ingestion doses 10 times the annual limit of intake (REMM, 2011).
† Only potassium iodide, diethylenetriaminepentaacetic acid (DTPA), and Prussian blue are approved by the Food and Drug Administration for decorporation (REMM, 2011).
Adapted from Koenig KL, Goans RE, Hatchett RJ, et al. Medical treatment of radiological casualties: current concepts. Ann Emerg Med 2005;45:643-52.
Importantly, after a nuclear detonation or nuclear reactor incident, radioactive iodine is a significant concern, and specific treatment might include potassium iodide (KI).2,8,19,22 The decision to treat with KI will probably involve multiple agencies, including the U.S. Centers for Disease Control and Prevention, the U.S. Food and Drug Administration, and the U.S. Department of Health and Human Services, and guidance should be sought before treating.9,21 If indicated after a nuclear disaster, KI should be administered before or immediately coincident with passage of the radioactive cloud, although KI may still have a substantial protective effect even if taken 3 or 4 hours after exposure. Consult the REMM website or desktop application for prescribing details and note that pregnant women and children deserve special consideration for KI given their increased risk for malignancy and thyroid disease over time.10,20
The psychologic impact of a nuclear event can be overwhelming22,23,24 and should not be underestimated. It has been suggested that if possible, educational material and counselors should be available at triage and receiving centers, as well as in the ED, and patients should be given instructions to see a health care provider if they are having signs of psychologic stress.1,8,25
Follow-Up, Next Steps in Care, and Patient Education
Disposition after a radiologic emergency depends on several factors, including the estimated radiation dose and associated trauma or burn. Patients who remain asymptomatic or only minimally symptomatic without persistent vomiting were probably triaged as “minimal” (radiation exposure < 1 Gy) and can be discharged home safely from the triage area after decontamination, with follow-up by a primary care provider. Importantly, they should be registered with the designated governmental agency that is tracking patients and outcomes of acute radiation emergencies.1,9 All patients not admitted to the hospital or transferred to a higher level of care should also receive (1) reassurance, (2) informative patient handouts on radiologic emergencies and symptoms that require additional care, (3) recommendations for follow-up, and (4) instruction on how to safely deal with possible contamination at home. Basic patient handouts are available from the REMM website.25
In mass casualty situations, mildly symptomatic patients typically receive acute first aid and symptomatic treatment of nausea and vomiting and are discharged with strict instructions to return in 24 to 48 hours for revaluation of their hematopoietic system and possible need for treatment of ARS. Patients are to return earlier if the symptoms worsen.1,10,22 Moderately symptomatic patients or those who have absorbed more than 2 Gy and are experiencing symptoms are hospitalized for observation and treatment of ARS and any injuries. Patients with moderate injury or burn (>10% body surface area) and moderate to severe ARS should taken to surgery (if needed) within 36 to 48 hours, as well as be treated for ARS in an inpatient setting.2,10,12 Within these recommendations for disposition, it is specifically noted that any transfers to larger facilities for surgery or management of severe ARS should take place in the first 48 hours when possible to enable the greatest likelihood of a good outcome.
Severely symptomatic patients with gastrointestinal and cardiovascular syndromes (>10 Gy) of absorbed radiation are treated with as many resources as reasonable for the situation in recognition of the fact that the prognosis is very poor regardless of interventions. In the setting of a mass casualty, these patients are managed expectantly (i.e., with comfort measures for pain, nausea or vomiting, and anxiety). Similarly, patients with moderate to severe trauma or burns and more than 6 to 8 Gy of absorbed radiation dose will often be managed expectantly. In isolated cases, empiric antibiotic therapy, colony-stimulating factor therapy, transfusions, and a search for bone marrow donors might ensue in a heroic attempt to save the patient’s life.2,10,12 Unfortunately, data and experience in identifying the optimal disposition of children and the elderly who have experienced irradiation and trauma are limited, so clinicians will have to decide each case individually.12
Armed Forces Radiobiology Research Institute. Medical management of radiological casualties. Bethesda, Md: Uniformed Services University of the Health Sciences; 2010. p. 51. Available at http://www.usuhs.mil/afrri/outreach/pdf/3edmmrchandbook.pdf
Koenig KL, Goans RE, Hatchett RJ, et al. Medical treatment of radiological casualties: current concepts. Ann Emerg Med. 2005;45:643–652.
. Radiation Emergency Assistance Center/Training Site (REAC/TS) at Oak Ridge Institute for Science and Education (ORISE). Available at http://orise.orau.gov/reacts/default.aspx
Radiation Emergency Assistance Center/Training Site (REAC/TS). The medical aspects of radiation incidents. Oak Ridge, Tenn: Department of Energy; 2011. p. 56. Available at http://orise.orau.gov/reacts/resources/radiation-accident-management.aspx
U.S. Department of Health and Human Services Radiation Emergency Management. Available at http://www.remm.nlm.gov/
Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051.
1 Radiation Emergency Assistance Center/Training Site (REAC/TS). The medical aspects of radiation incidents. Oak Ridge, Tenn: Department of Energy; 2011. p. 56
2 Armed Forces Radiobiology Research Institute. Medical management of radiological casualties. Bethesda, Md: Uniformed Services University of the Health Sciences; 2010. p. 51
3 U.S. Department of Health and Human Services. Radiation emergency medical management. Prototype for adult medical facility orders during a radiation event. http://www.remm.nlm.gov/adultorderform.htm#countermeasures, July 6, 2010. Available at
4 Hrdina CM, Coleman CN, Bogucki S, et al. The “RTR” medical response system for nuclear and radiological mass-casualty incidents: a functional TRiage-TReatment-TRansport medical response model. Prehosp Disaster Med. 2009;24:167–178.
5 National Security Staff, Interagency Policy Coordination Subcommittee for Preparedness & Response to Radiological and Nuclear Threats. Planning guidance for response to a nuclear detonation. Washington, DC: Federal Emergency Management Agency; 2010.
6 Coleman CN, Weinstock DM, Casagrande R, et al. Triage and treatment tools for use in a scarce resources–crisis standards of care setting after a nuclear detonation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S111–S121.
7 DiCarlo AL, Maher C, Hick JL, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–S44.
8 International Atomic Energy Agency. Generic procedures for medical response during a nuclear or radiologic emergency. International Atomic Energy Agency; 2005. p. 287
9 U.S. Department of Health and Human Services. Radiation emergency medical management. Radiologic emergencies triage guidelines. http://www.remm.nlm.gov/radtriage.htm, July 23, 2011. Available at
10 Koenig KL, Goans RE, Hatchett RJ, et al. Medical treatment of radiological casualties: current concepts. Ann Emerg Med. 2005;45:643–652.
11 Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051.
12 Berger M, Leonard RB, Ricks RC, et al. Hospital triage in the first 24 hours after a nuclear or radiological disaster. Oak Ridge, Tenn: Radiation Emergency Assistance Center/Training Site (REAC/TS); 2004. p. 28
13 Fliedner TM, Chao NJ, Bader JL, et al. Stem cells, multiorgan failure in radiation emergency medical preparedness: a U.S./European Consultation Workshop. Stem Cells. 2009;27:1205–1211.
14 Coleman CN, Hrdina C, Bader JL, et al. Medical response to a radiologic/nuclear event: integrated plan from the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Ann Emerg Med. 2009;53:213–222.
15 Parker DD, Parker JC. Estimating radiation dose from time to emesis and lymphocyte depletion. Health Phys. 2007;93:701–704.
16 Armed Forces Radiobiology Research Institute. AFRRI pocket guide: emergency radiation medicine response. Bethesda, Md: Uniformed Services University of the Health Sciences; 2011.
17 U.S. Department of Health and Human Services. Radiation emergency medical management. Dose estimator for exposure: 3 biodosimetry tools. http://www.remm.nlm.gov/ars_wbd.htm, March 14, 2011. Available at
18 Marcus C. Administration of decorporation drugs to treat internal radionuclide contamination: medical emergency response to radiologic incidents. RSO Magazine 2004. p. 9–15.
19 Gourmelon P, et al. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98:825–832.
20 U.S. Department of Health and Human Services. Radiation emergency medical management. Potassium iodide (KI). http://www.remm.nlm.gov/potassiumiodide.htm, May 20, 2011. Available at
21 U.S. Department of Health and Human Services. Radiation emergency medical management. Caution and comments about radiation countermeasures for treatment. http://www.remm.nlm.gov/ali-warning.htm, April 7, 2011. Available at
22 Smith JM, Spano MA. Interim guidelines for hospital response to mass casualties from a radiologic incident. Atlanta: Centers for Disease Control and Prevention; 2003. p. 47
23 Weisdorf D, Chao N, Waselenko JK, et al. Acute radiation injury: contingency planning for triage, supportive care, and transplantation. Biol Blood Marrow Transplant. 2006;12:672–682.
24 Vazquez M, Jordan O, Kuper E, et al. Management of acute traumatic stress in nuclear and radiological emergencies. Health Phys. 2010;98:795–798.
25 U.S. Department of Health and Human Services. Radiation emergency medical management. Follow up instructions for individuals involved in a radiological/nuclear incident. http://www.remm.nlm.gov/followup.htm, March 14, 2011. Available at