Pancreas typically enlarged and edematous with loss of normal fatty lobulation
• Necrotizing pancreatitis (20-30% of cases): Areas of parenchymal necrosis which are either nonenhancing or severely hypoenhancing
• Complications
 Infected pancreatic necrosis: Ectopic gas, in absence of intervention, highly suggestive of infected necrosis
Infected pancreatic necrosis: Ectopic gas, in absence of intervention, highly suggestive of infected necrosis
 Central necrosis: Necrosis of central portion of gland/duct with intact pancreas/duct in head and tail
Central necrosis: Necrosis of central portion of gland/duct with intact pancreas/duct in head and tail
 Infected pancreatic necrosis: Ectopic gas, in absence of intervention, highly suggestive of infected necrosis
Infected pancreatic necrosis: Ectopic gas, in absence of intervention, highly suggestive of infected necrosis Central necrosis: Necrosis of central portion of gland/duct with intact pancreas/duct in head and tail
Central necrosis: Necrosis of central portion of gland/duct with intact pancreas/duct in head and tail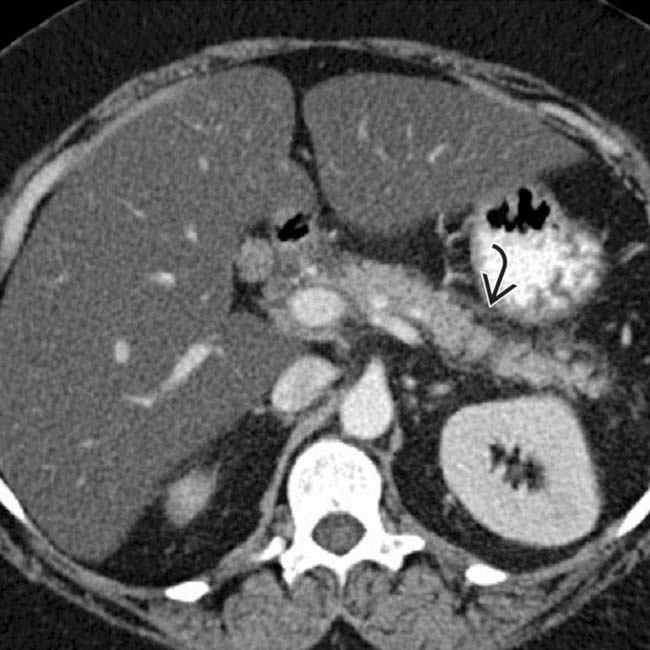
 and edema, compatible with mild edematous pancreatitis.
and edema, compatible with mild edematous pancreatitis.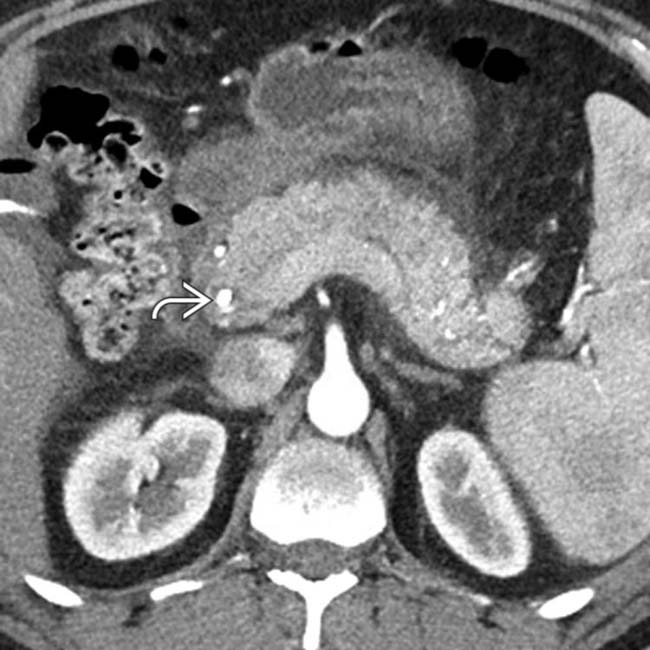
 demonstrates enlargement of the pancreas, edema with loss of normal fatty lobulation, and peripancreatic fat stranding and fluid, compatible with acute edematous pancreatitis.
demonstrates enlargement of the pancreas, edema with loss of normal fatty lobulation, and peripancreatic fat stranding and fluid, compatible with acute edematous pancreatitis.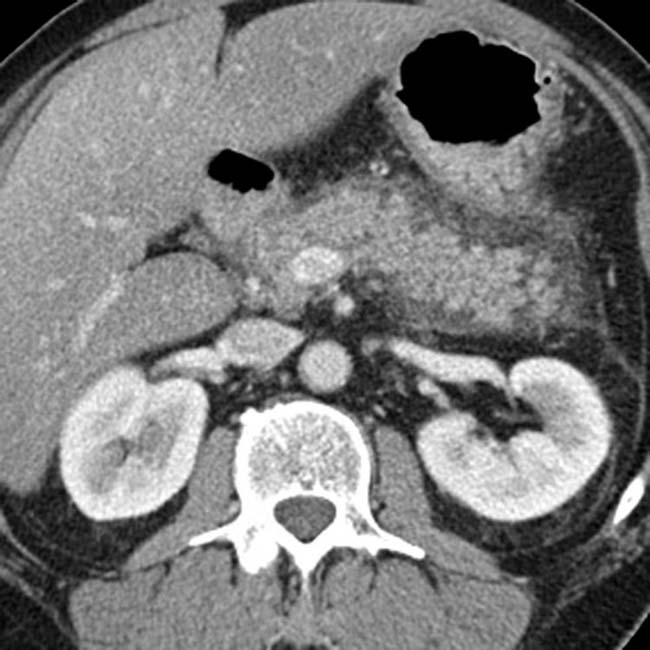
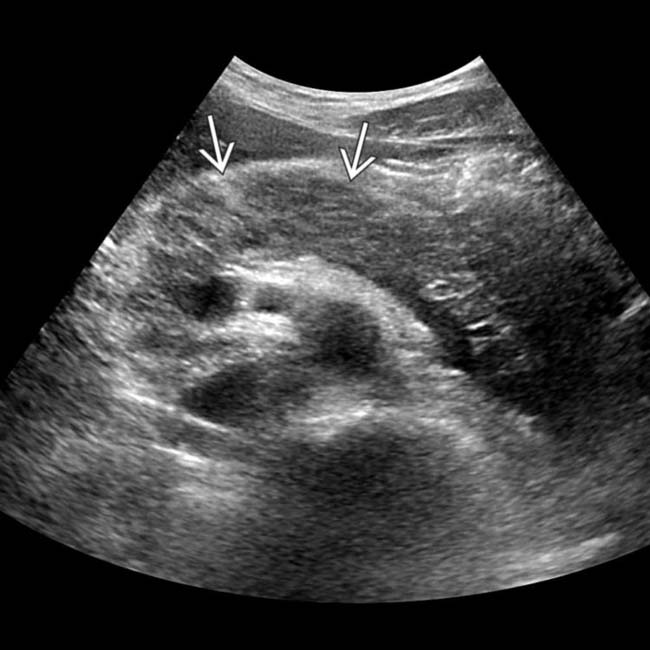
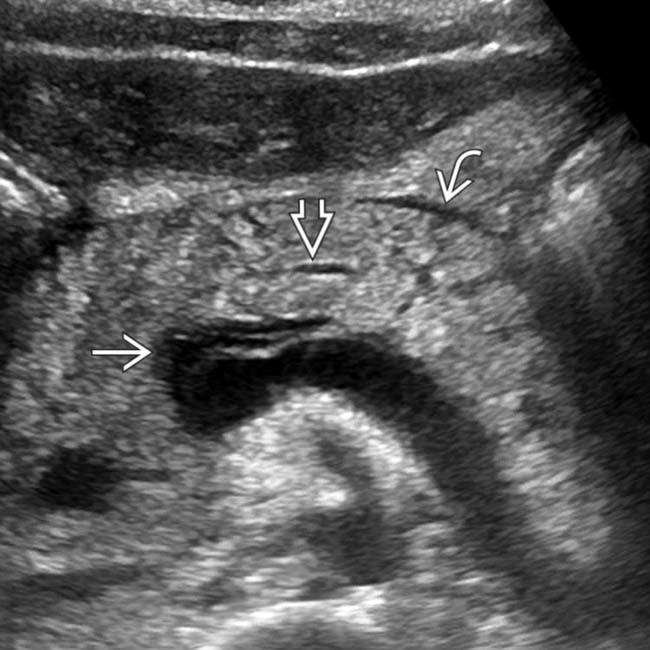
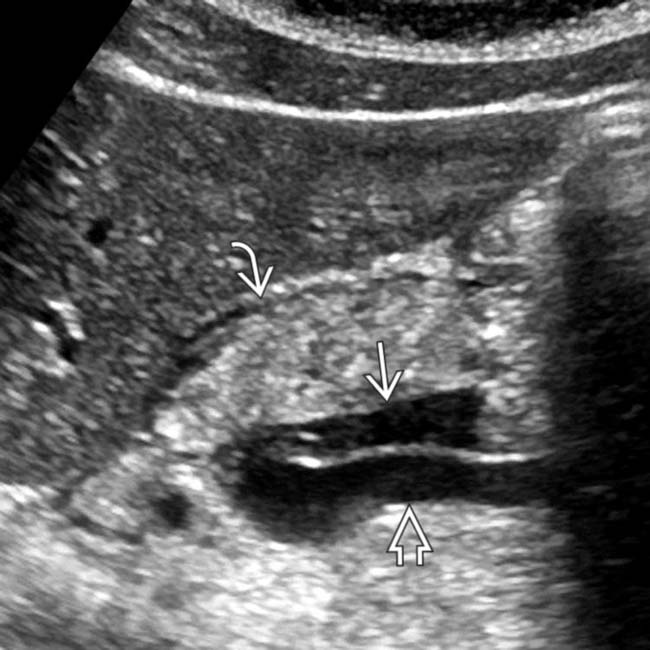
 , which appears abnormally hypoechoic, compatible with acute pancreatitis in this patient with a markedly elevated lipase level.
, which appears abnormally hypoechoic, compatible with acute pancreatitis in this patient with a markedly elevated lipase level.IMAGING
General Features
CT Findings
• Revised Atlanta classification in 2012 standardized nomenclature used to describe acute pancreatitis
• 2 primary subtypes of acute pancreatitis
 Interstitial edematous pancreatitis (70-80% of cases)
Interstitial edematous pancreatitis (70-80% of cases)
 Interstitial edematous pancreatitis (70-80% of cases)
Interstitial edematous pancreatitis (70-80% of cases)
– Peripancreatic fat stranding, edema, and free fluid (with fluid most often localized to lesser sac, anterior pararenal spaces, and paracolic gutters)
• Complications
 Infected pancreatic necrosis
Infected pancreatic necrosis
 Central necrosis (disconnected duct syndrome)
Central necrosis (disconnected duct syndrome)
 Extrapancreatic fat necrosis
Extrapancreatic fat necrosis
 Pseudoaneurysm
Pseudoaneurysm
 Venous thrombosis
Venous thrombosis
 Fluid collections
Fluid collections
 Infected pancreatic necrosis
Infected pancreatic necrosis
 Central necrosis (disconnected duct syndrome)
Central necrosis (disconnected duct syndrome)
– Necrosis of central portion of gland and pancreatic duct with intact upstream and downstream pancreas/duct in head and tail
 Extrapancreatic fat necrosis
Extrapancreatic fat necrosis
 Pseudoaneurysm
Pseudoaneurysm
– Small contrast-filled outpouching arising next to artery ± adjacent hematoma (due to leak or rupture)
 Venous thrombosis
Venous thrombosis
 Fluid collections
Fluid collections
– Acute peripancreatic fluid collection: Fluid collection first 4 weeks after acute edematous pancreatitis
– Pseudocyst: Fluid collection persisting > 4 weeks after acute edematous pancreatitis
– Acute postnecrotic fluid collection: Fluid collection first 4 weeks after acute necrotizing pancreatitis
MR Findings
• Pancreas appears enlarged with increased signal on T2WI and abnormally low signal on T1WI due to edema
• T2WI offers advantage (over CT) of allowing differentiation of simple fluid collections from collections with internal solid debris (i.e., walled off necrosis)
DIFFERENTIAL DIAGNOSIS
Infiltrating Pancreatic Carcinoma
• Heterogeneous, hypoenhancing mass with abrupt obstruction of upstream pancreatic duct and upstream pancreatic atrophy
• Presence of dilated pancreatic duct or biliary obstruction should prompt further investigation for underlying mass
• Pancreatic cancer infiltrates dorsally into retroperitoneum, unlike pancreatitis, which infiltrates anteriorly and laterally
• Usually other signs of malignancy, including vascular encasement, metastatic disease (most often liver), etc.
PATHOLOGY
General Features
• Etiology
 Many other causes, including metabolic disorders (e.g., hypertriglyceridemia, hypercalcemia), infection, trauma, drugs, anatomic variants (e.g., pancreatic divisum, annular pancreas), neoplasm (e.g., pancreatic adenocarcinoma or IPMN), iatrogenic (e.g., ERCP), etc.
Many other causes, including metabolic disorders (e.g., hypertriglyceridemia, hypercalcemia), infection, trauma, drugs, anatomic variants (e.g., pancreatic divisum, annular pancreas), neoplasm (e.g., pancreatic adenocarcinoma or IPMN), iatrogenic (e.g., ERCP), etc.
 Exact pathogenesis of acute pancreatitis unclear and may vary depending on etiology
Exact pathogenesis of acute pancreatitis unclear and may vary depending on etiology
 Many other causes, including metabolic disorders (e.g., hypertriglyceridemia, hypercalcemia), infection, trauma, drugs, anatomic variants (e.g., pancreatic divisum, annular pancreas), neoplasm (e.g., pancreatic adenocarcinoma or IPMN), iatrogenic (e.g., ERCP), etc.
Many other causes, including metabolic disorders (e.g., hypertriglyceridemia, hypercalcemia), infection, trauma, drugs, anatomic variants (e.g., pancreatic divisum, annular pancreas), neoplasm (e.g., pancreatic adenocarcinoma or IPMN), iatrogenic (e.g., ERCP), etc. Exact pathogenesis of acute pancreatitis unclear and may vary depending on etiology
Exact pathogenesis of acute pancreatitis unclear and may vary depending on etiology
– Possibilities include reflux of pancreatic enzymes, bile, and duodenal contents into pancreatic duct, increased ductal pressure due to ampullary obstruction, or activation of intracellular/extracellular homeostatic factors
CLINICAL ISSUES
Presentation
• Most common signs/symptoms
Natural History & Prognosis
• Prognosis
• Natural evolution of fluid collections
Treatment
• Initial treatment is conservative, including fluid resuscitation, pain control, n.p.o. (nothing by mouth) with nutritional support until patient can resume oral diet, and antibiotics (only if infection is suspected)
 Early ERCP and sphincterotomy in patients with suspected gallstone pancreatitis only in setting of cholangitis or cholestasis
Early ERCP and sphincterotomy in patients with suspected gallstone pancreatitis only in setting of cholangitis or cholestasis
 Early ERCP and sphincterotomy in patients with suspected gallstone pancreatitis only in setting of cholangitis or cholestasis
Early ERCP and sphincterotomy in patients with suspected gallstone pancreatitis only in setting of cholangitis or cholestasis• Infected pancreatic necrosis may require surgical debridement (necrosectomy), although surgery typically deferred until 4 weeks after presentation to allow collections to become walled off
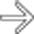
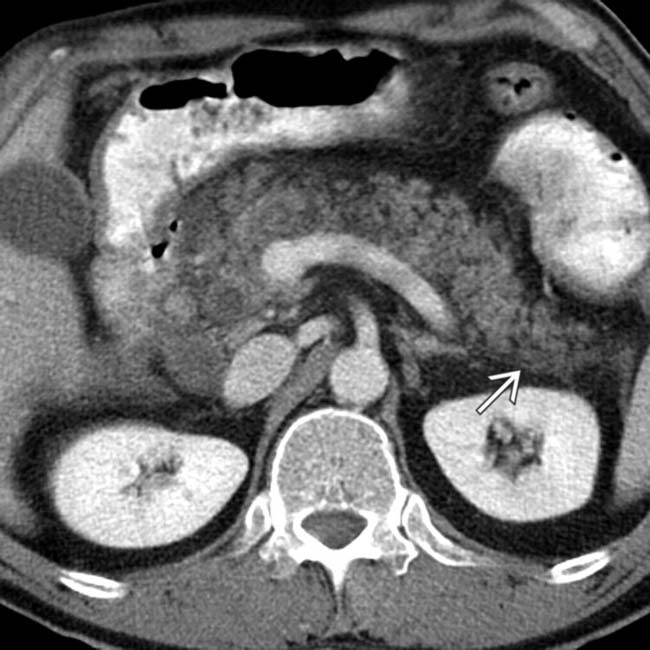
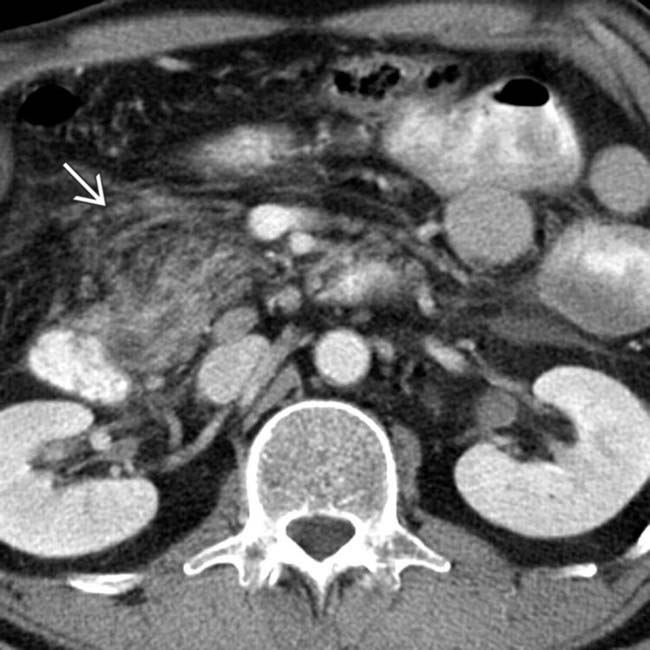
 . The main sign of acute pancreatitis is fluid anterior to the pancreas
. The main sign of acute pancreatitis is fluid anterior to the pancreas  as well as fluid anterior to the splenic vein
as well as fluid anterior to the splenic vein  .
.
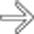
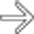
 tracking caudally down the superior mesenteric vein
tracking caudally down the superior mesenteric vein  . There is also fluid noted anterior to the body of the pancreas
. There is also fluid noted anterior to the body of the pancreas  , a strong clue to the diagnosis of acute pancreatitis.
, a strong clue to the diagnosis of acute pancreatitis.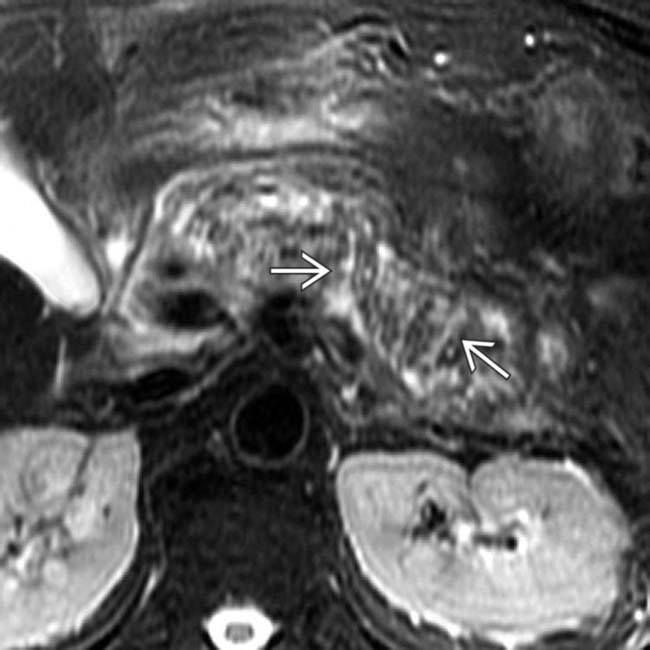
 .
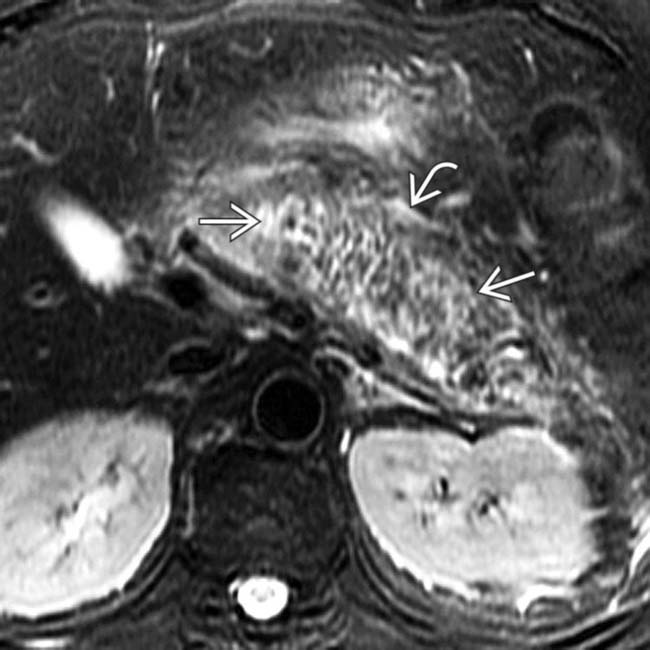
.
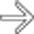
 and peripancreatic fluid
and peripancreatic fluid  consistent with edematous pancreatitis. Fat-suppressed T2WI is the most critical pulse sequence for highlighting changes of acute pancreatitis on MR.
consistent with edematous pancreatitis. Fat-suppressed T2WI is the most critical pulse sequence for highlighting changes of acute pancreatitis on MR.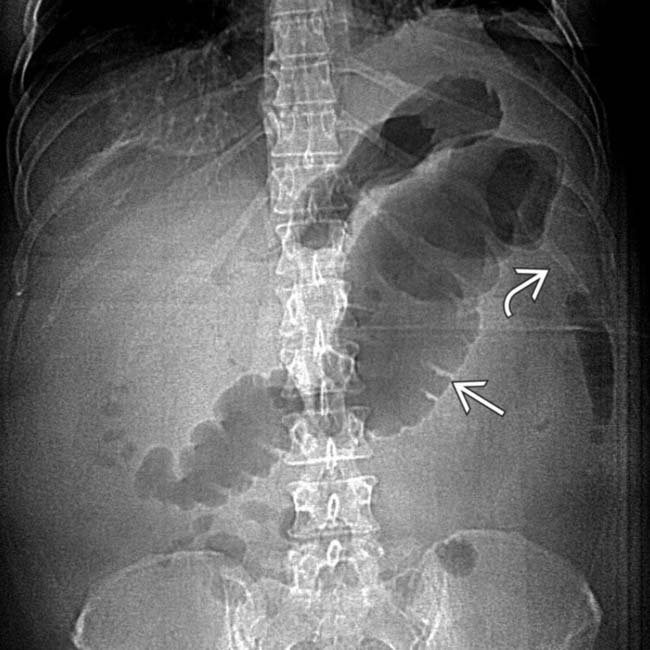
 with abrupt “cut-off” and narrowing
with abrupt “cut-off” and narrowing  of the colon at the splenic flexure.
of the colon at the splenic flexure.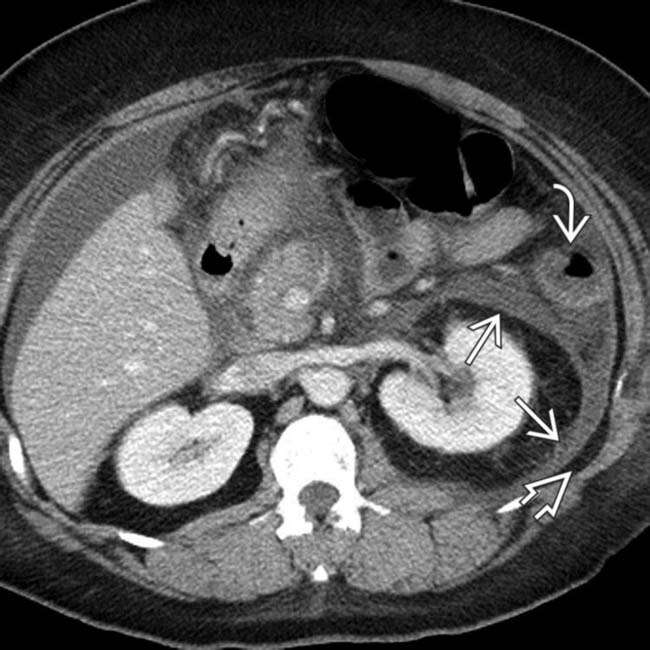
 as a result of the adjacent pancreatic inflammation, causing the colon cut-off sign. Note the posterior interfascial extension of fluid
as a result of the adjacent pancreatic inflammation, causing the colon cut-off sign. Note the posterior interfascial extension of fluid 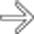 with preserved fat in the posterior pararenal space
with preserved fat in the posterior pararenal space  .
.
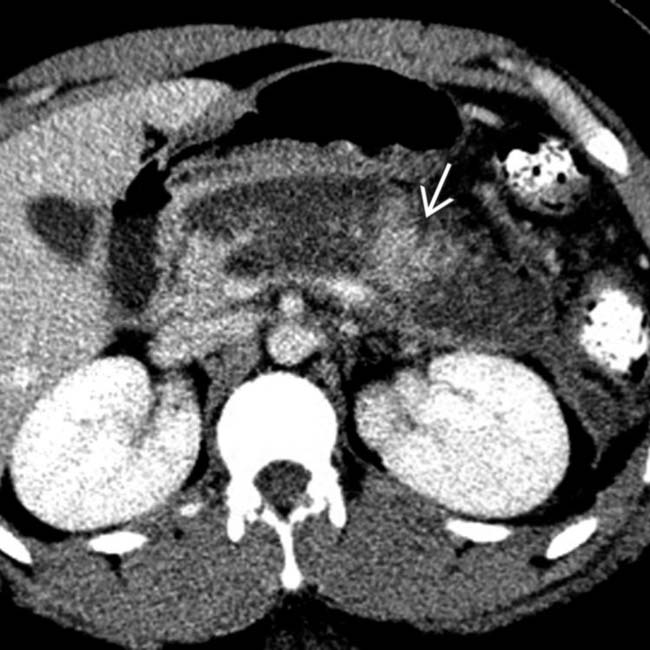
 still enhancing. Early CT (< 72 hours) can miss or underestimate pancreatic necrosis.
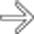
still enhancing. Early CT (< 72 hours) can miss or underestimate pancreatic necrosis.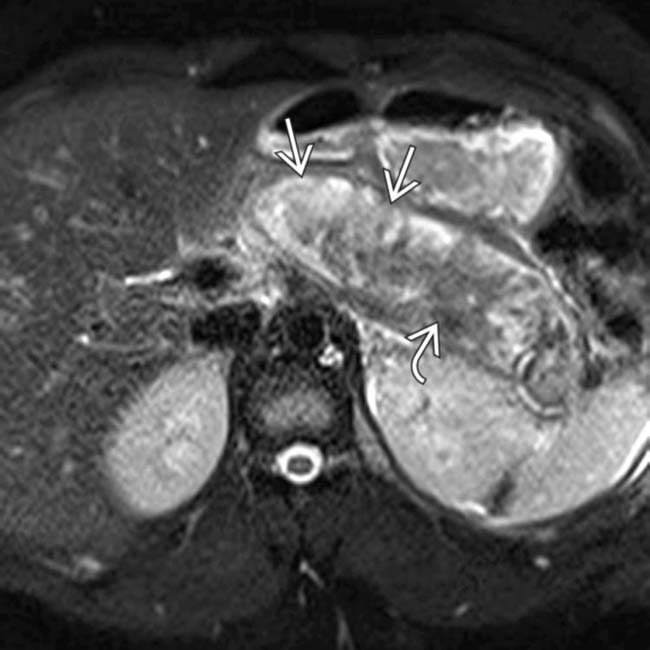
 due to necrotizing pancreatitis. The more hypointense T2 signal
due to necrotizing pancreatitis. The more hypointense T2 signal  in the pancreatic bed represents a combination of residual viable pancreatic tissue and necrotic debris.
in the pancreatic bed represents a combination of residual viable pancreatic tissue and necrotic debris.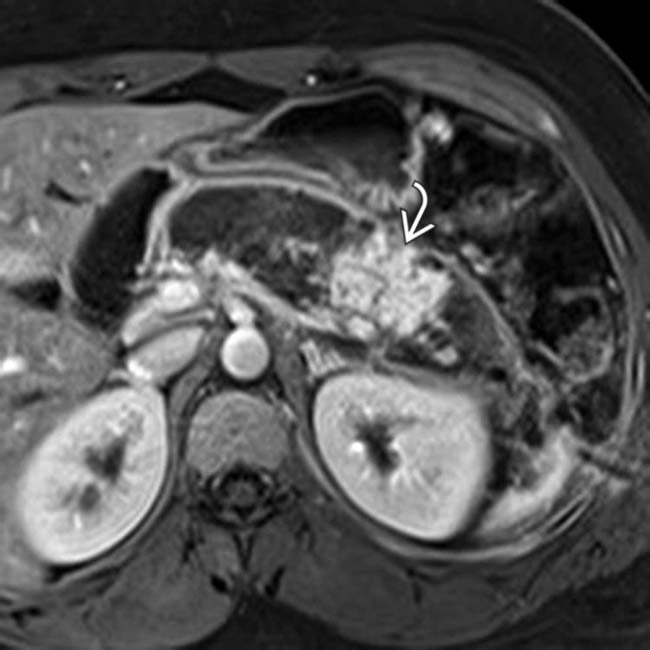
 still enhancing.
still enhancing.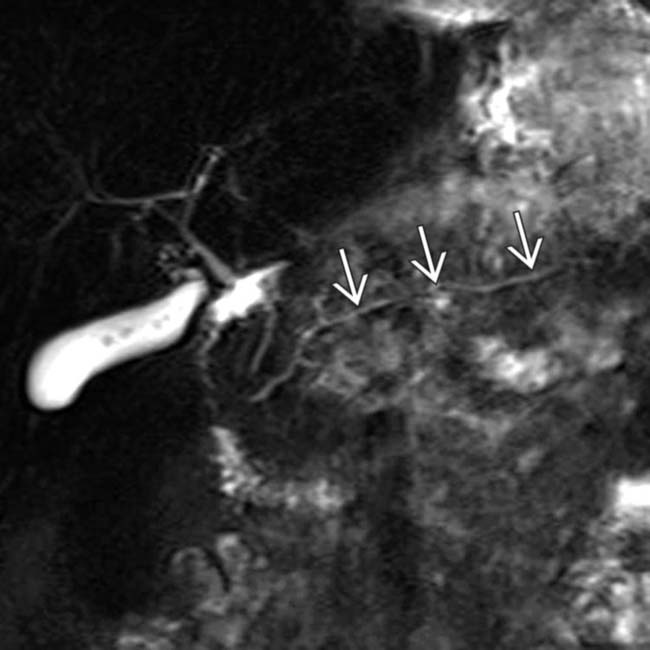
 still appears intact, a valuable piece of information for the gastroenterologist. Evaluation of the pancreatic duct is a significant advantage of MR compared to CT.
still appears intact, a valuable piece of information for the gastroenterologist. Evaluation of the pancreatic duct is a significant advantage of MR compared to CT.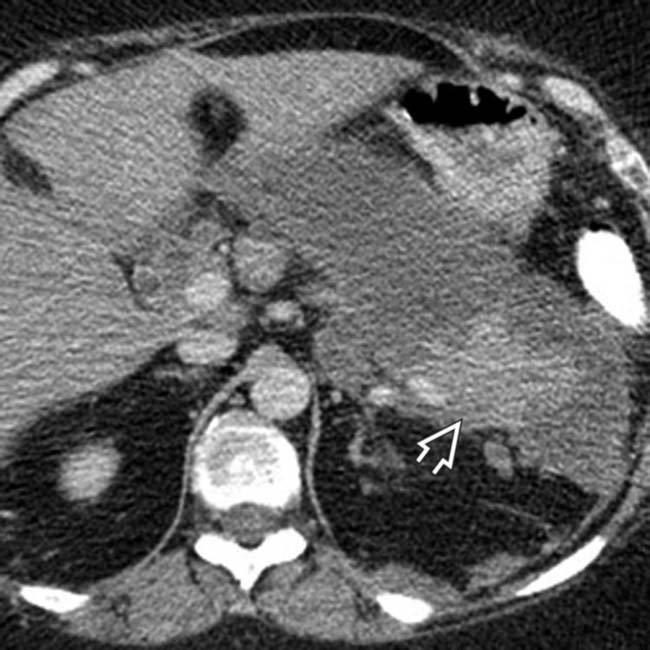
 is still normally enhancing, with nonenhancement of the remainder of the pancreas, compatible with necrotizing pancreatitis.
is still normally enhancing, with nonenhancement of the remainder of the pancreas, compatible with necrotizing pancreatitis.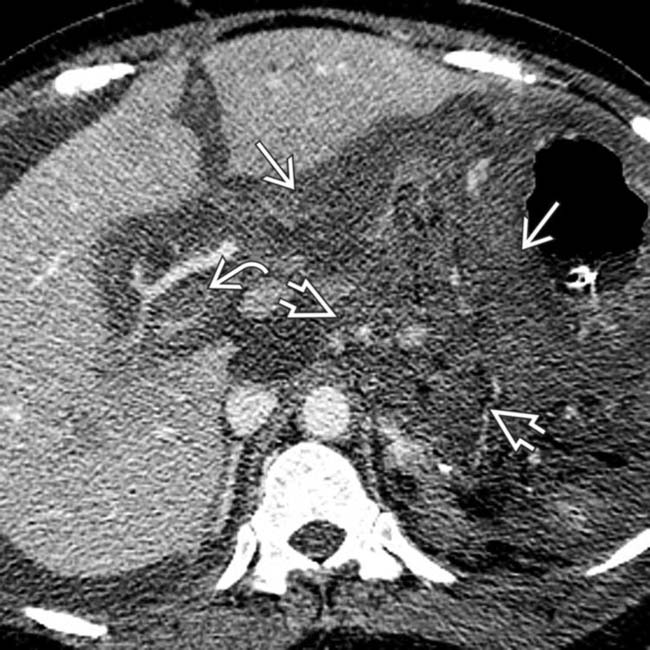
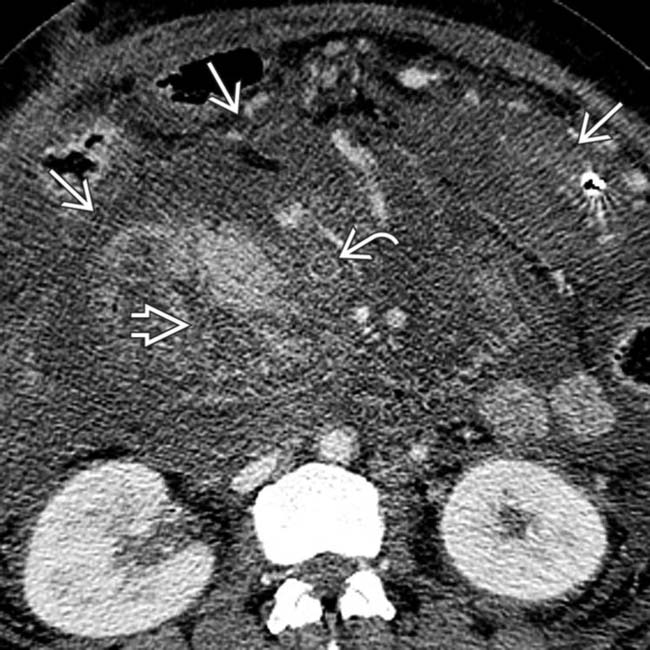
 . Note the marked peripancreatic inflammation
. Note the marked peripancreatic inflammation  and thrombus in the portal vein
and thrombus in the portal vein  .
.
 . Note the thrombus extending into the superior mesenteric vein
. Note the thrombus extending into the superior mesenteric vein  and marked inflammation of the mesentery
and marked inflammation of the mesentery  . The patient soon expired from multiorgan failure.
. The patient soon expired from multiorgan failure.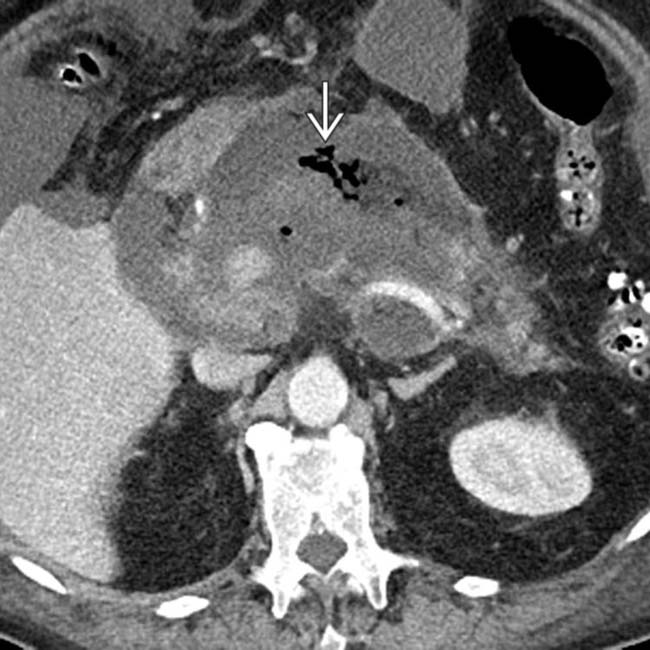
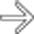 in the pancreatic bed is virtually diagnostic of infected necrosis, and the patient ultimately underwent necrosectomy.
in the pancreatic bed is virtually diagnostic of infected necrosis, and the patient ultimately underwent necrosectomy.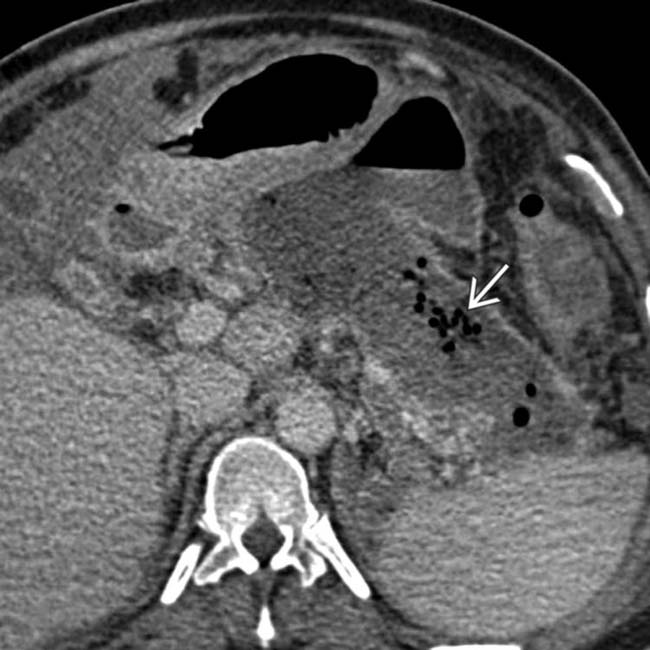
 in the pancreatic bed.
in the pancreatic bed.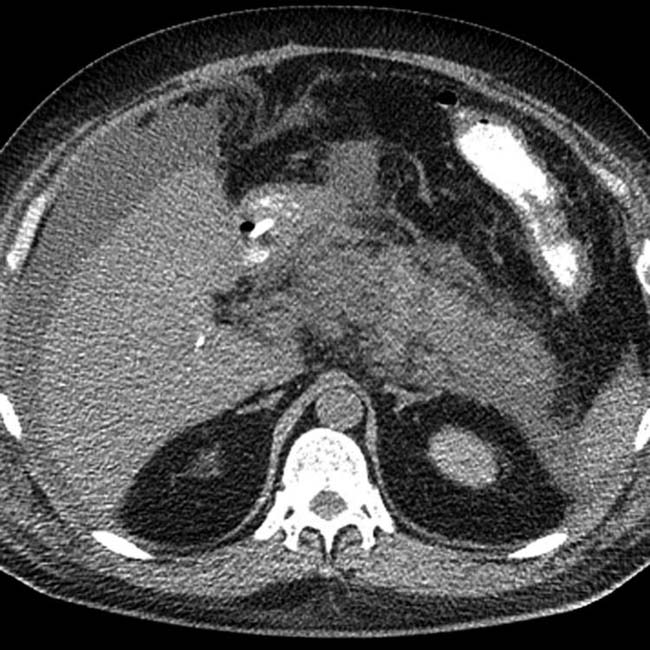
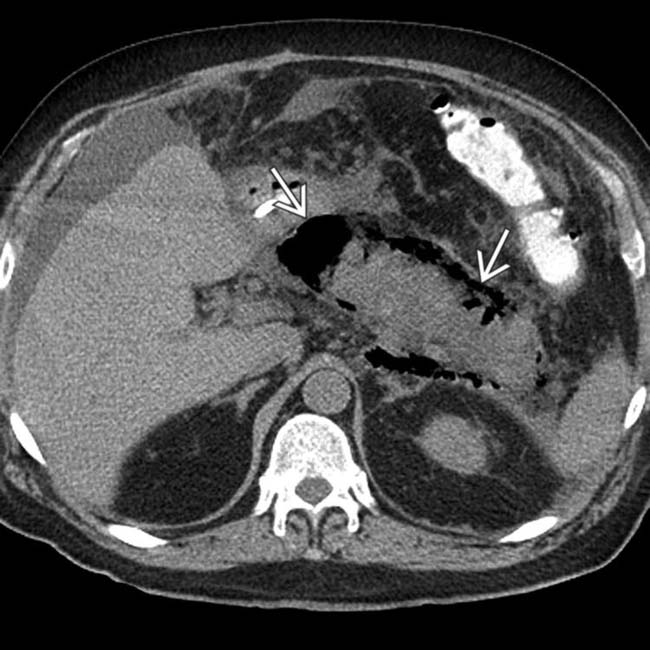
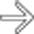 throughout the pancreatic parenchyma. At surgery, extensive infected necrosis of the pancreas was found and a necrosectomy was performed.
throughout the pancreatic parenchyma. At surgery, extensive infected necrosis of the pancreas was found and a necrosectomy was performed.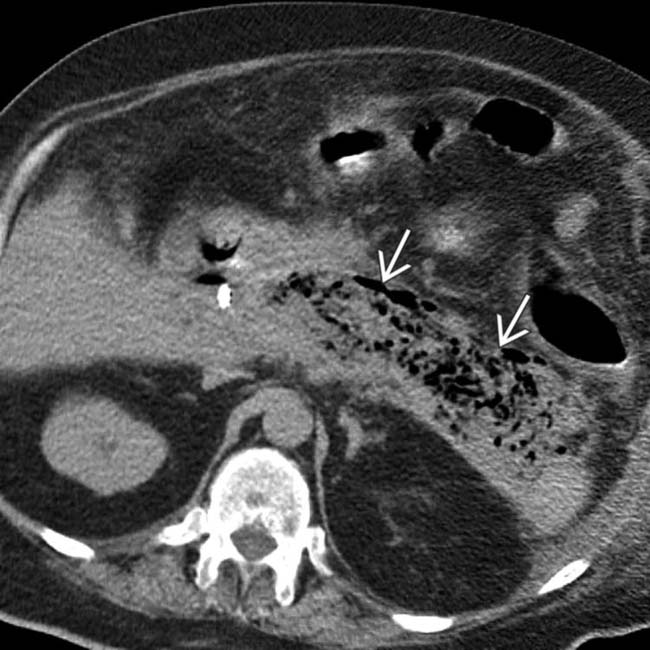
 compatible with infected necrosis. The patient in this case died as a result of his illness.
compatible with infected necrosis. The patient in this case died as a result of his illness.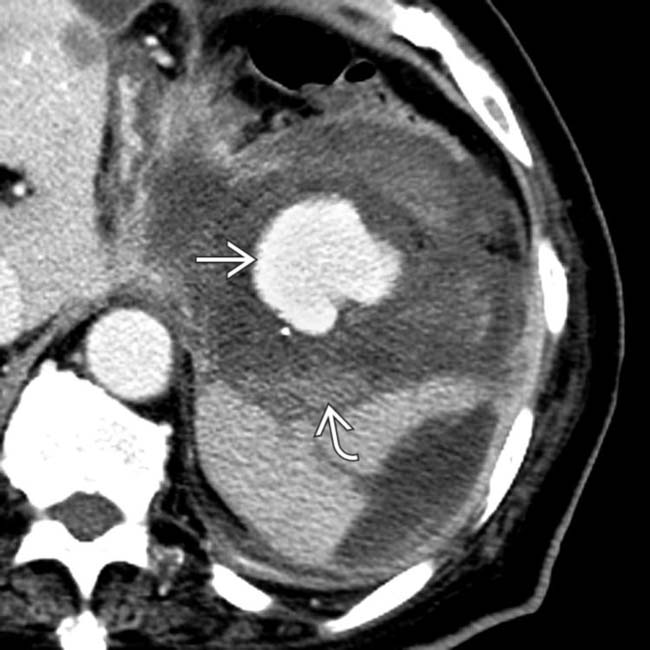
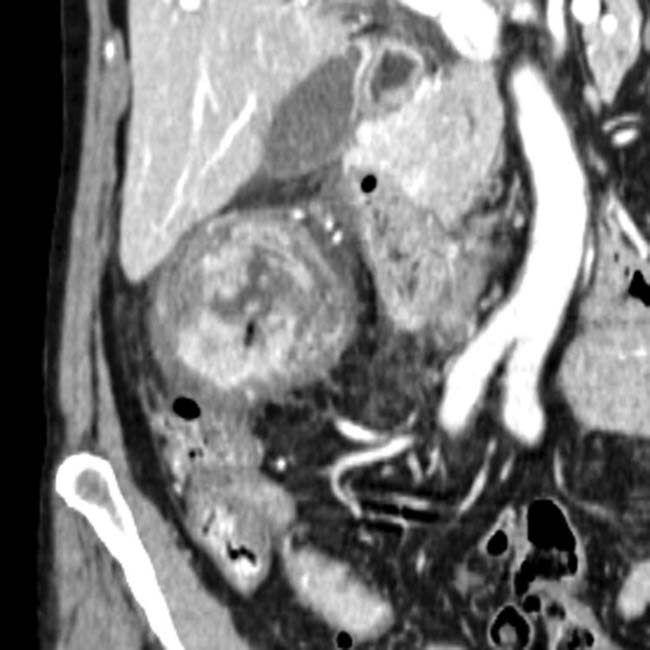
 with surrounding hemorrhage
with surrounding hemorrhage  in a patient who suffered a life-threatening bleed from a ruptured pseudoaneurysm of the splenic artery resulting from pancreatitis.
in a patient who suffered a life-threatening bleed from a ruptured pseudoaneurysm of the splenic artery resulting from pancreatitis.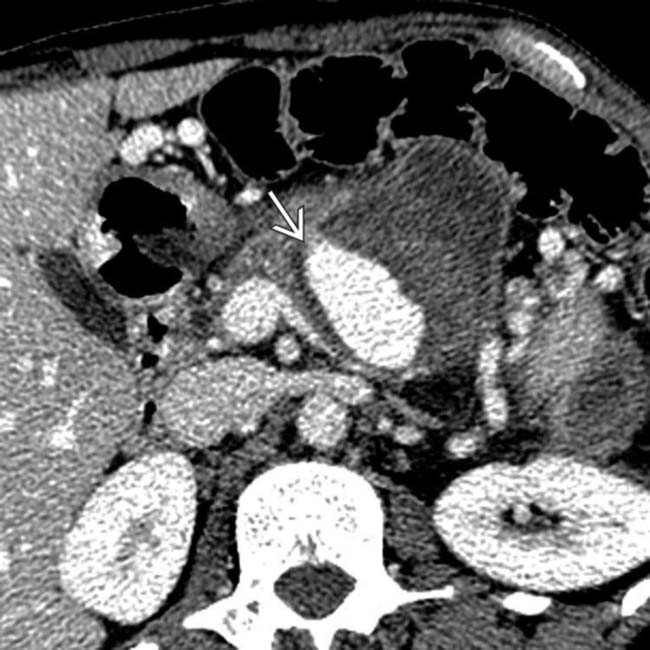
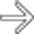 arising within a hemorrhagic pseudocyst in a patient with acute pancreatitis.
arising within a hemorrhagic pseudocyst in a patient with acute pancreatitis.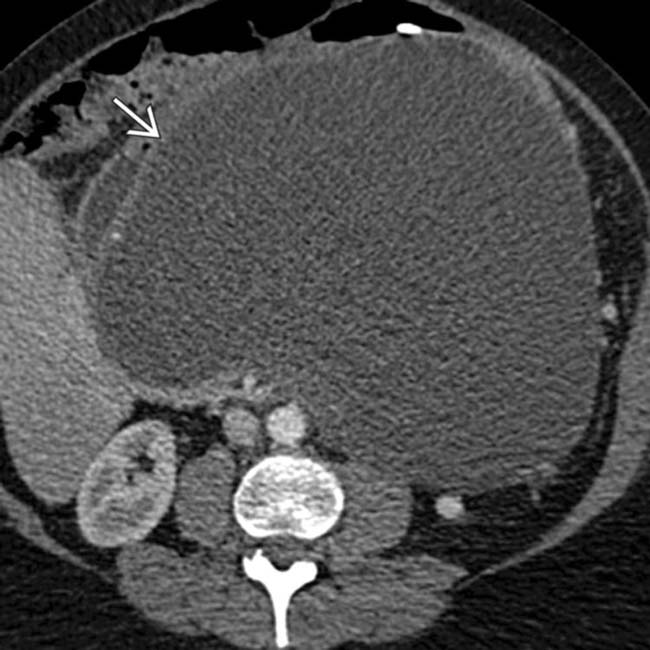
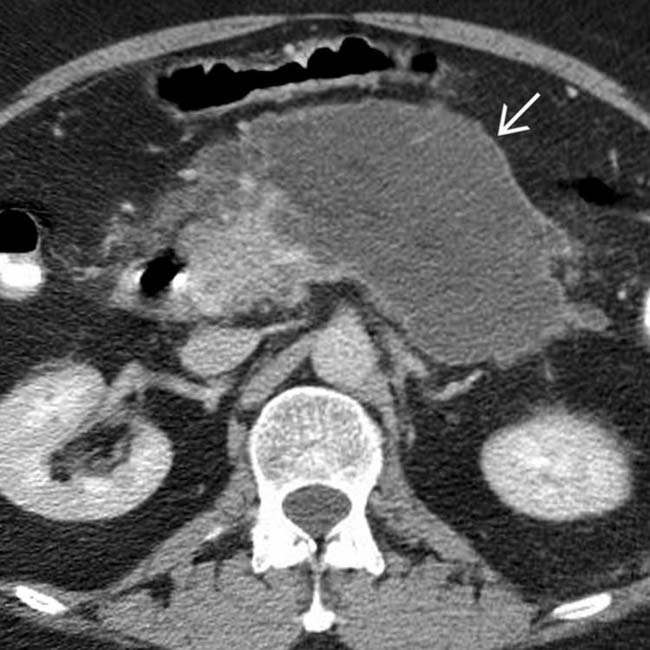
 several months after a bout of acute edematous pancreatitis. Notice that the collection is simple in appearance without debris or hemorrhage, compatible with a pseudocyst. The large size and mass effect of this pseudocyst necessitated drainage.
several months after a bout of acute edematous pancreatitis. Notice that the collection is simple in appearance without debris or hemorrhage, compatible with a pseudocyst. The large size and mass effect of this pseudocyst necessitated drainage.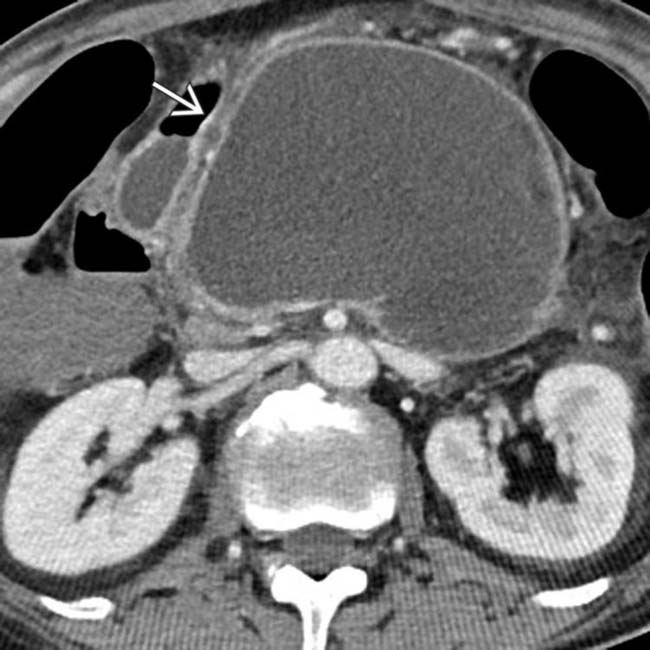
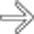 , with a well-defined wall, occupying the pancreatic bed.
, with a well-defined wall, occupying the pancreatic bed.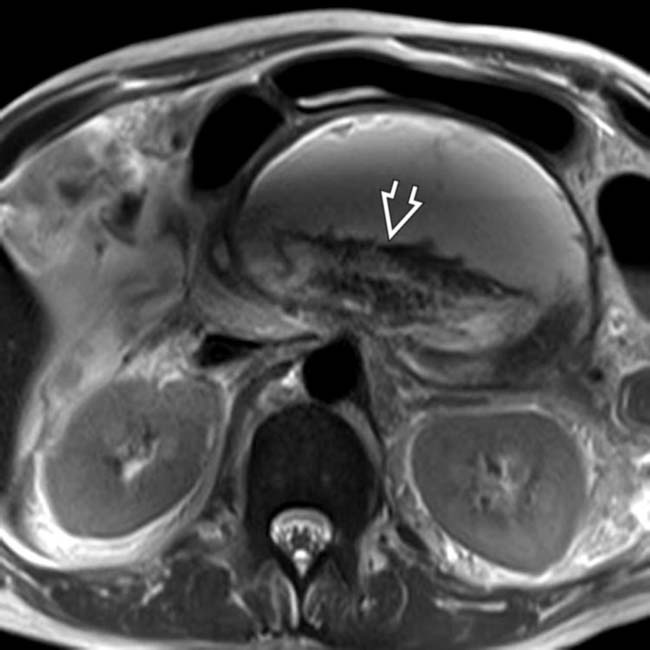
 , suggesting this represents walled-off necrosis rather than a pseudocyst. Walled-off necrosis, unlike a pseudocyst, often requires either a large bore catheter for drainage, or necrosectomy.
, suggesting this represents walled-off necrosis rather than a pseudocyst. Walled-off necrosis, unlike a pseudocyst, often requires either a large bore catheter for drainage, or necrosectomy.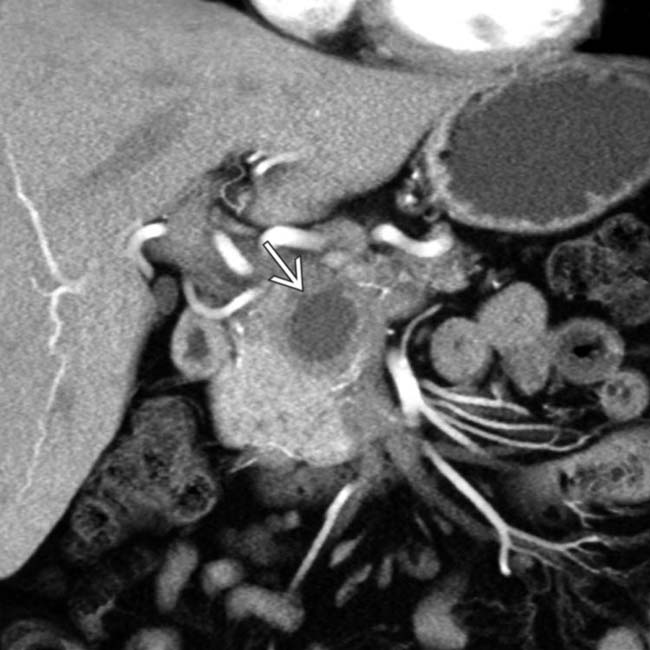
 replacing a portion of the pancreatic head, compatible with walled-off necrosis.
replacing a portion of the pancreatic head, compatible with walled-off necrosis.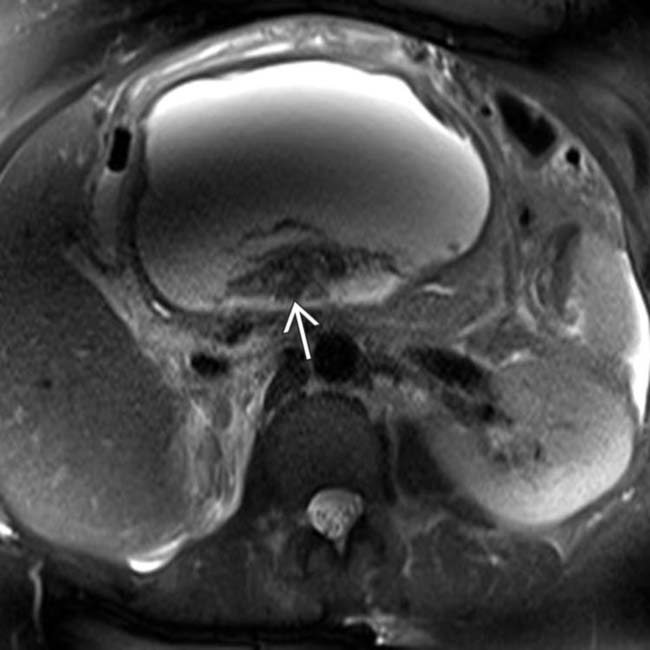
 within the collection suggests that this is walled-off necrosis, not a pseudocyst. Distinguishing these 2 entities is a major advantage of MR compared to CT.
within the collection suggests that this is walled-off necrosis, not a pseudocyst. Distinguishing these 2 entities is a major advantage of MR compared to CT.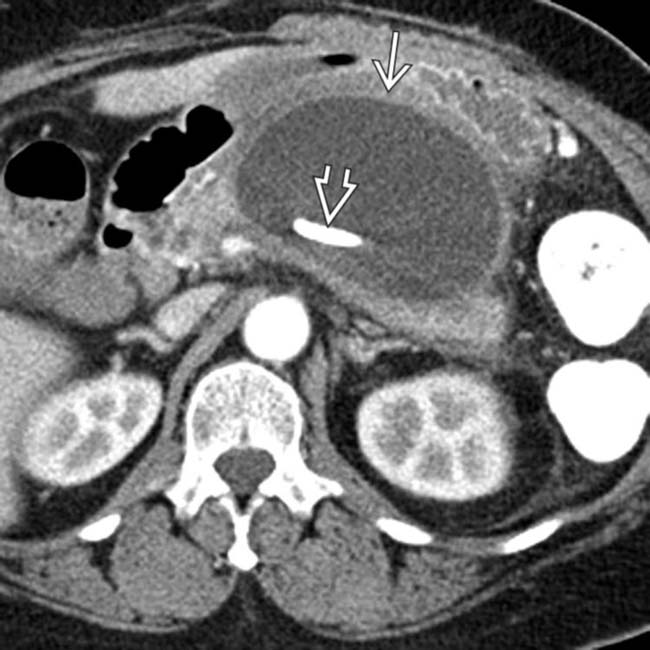
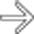 in the lesser sac with a well-defined wall and simple internal contents after a bout of pancreatitis, in keeping with a pseudocyst. An internal drain
in the lesser sac with a well-defined wall and simple internal contents after a bout of pancreatitis, in keeping with a pseudocyst. An internal drain  has been placed due to this pseudocyst’s mass effect on the stomach.
has been placed due to this pseudocyst’s mass effect on the stomach.
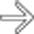 in the body of the pancreas with absence of normally enhancing pancreatic parenchyma in this location. The location of necrosis in this case raises concern for “disconnected duct” syndrome.
in the body of the pancreas with absence of normally enhancing pancreatic parenchyma in this location. The location of necrosis in this case raises concern for “disconnected duct” syndrome.
 that contains internal debris
that contains internal debris  representing fat necrosis and hemorrhage. Note the extension of the fluid into the interfascial retroperitoneal space between the perirenal and anterior pararenal spaces and into the muscles of the flank.
representing fat necrosis and hemorrhage. Note the extension of the fluid into the interfascial retroperitoneal space between the perirenal and anterior pararenal spaces and into the muscles of the flank.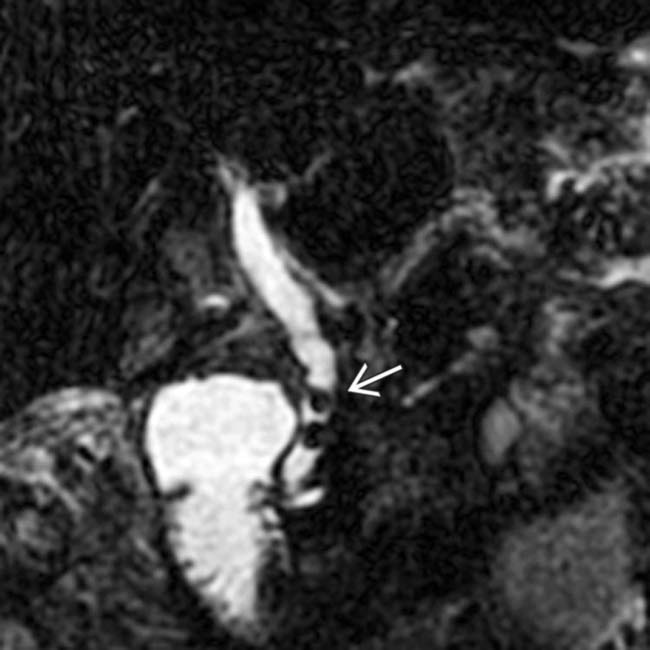
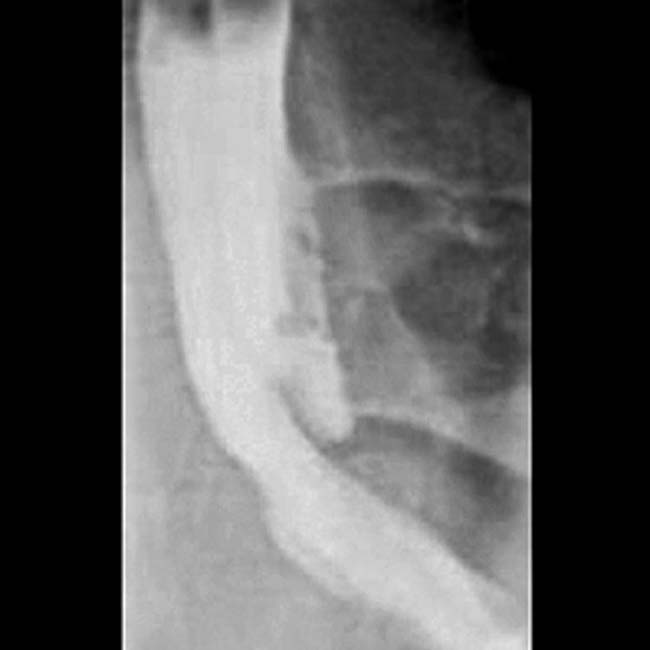
 as the cause of the patient’s acute pancreatitis. Emergent ERCP was performed to remove the common duct stones.
as the cause of the patient’s acute pancreatitis. Emergent ERCP was performed to remove the common duct stones.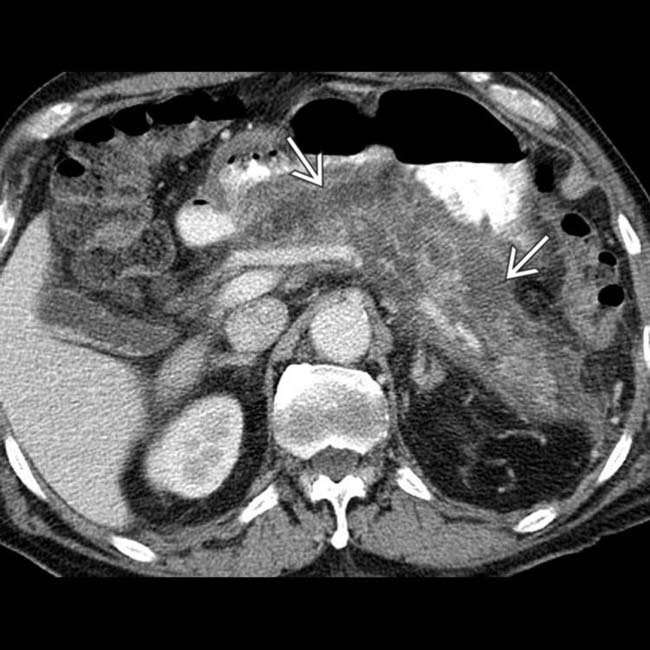
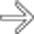 , consistent with severe acute necrotizing pancreatitis.
, consistent with severe acute necrotizing pancreatitis.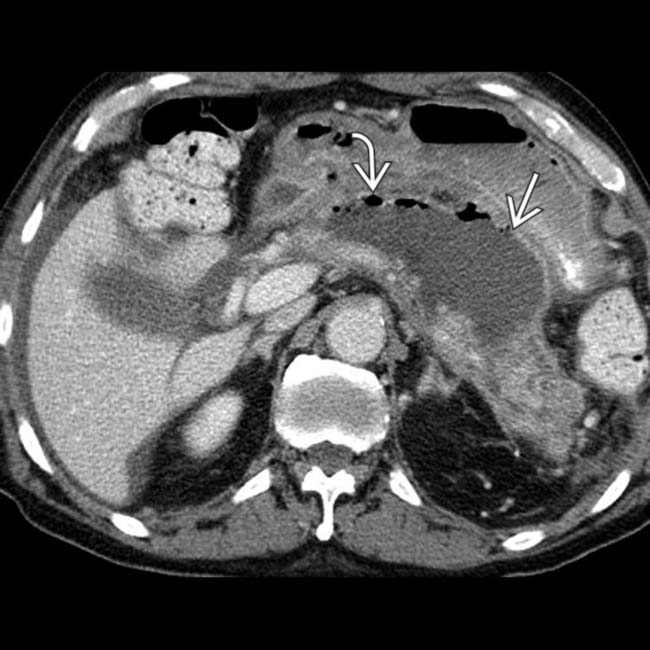
 with a well defined, peripherally enhancing wall. Note the multiple nondependent gas bubbles
with a well defined, peripherally enhancing wall. Note the multiple nondependent gas bubbles  , indicating infection.
, indicating infection.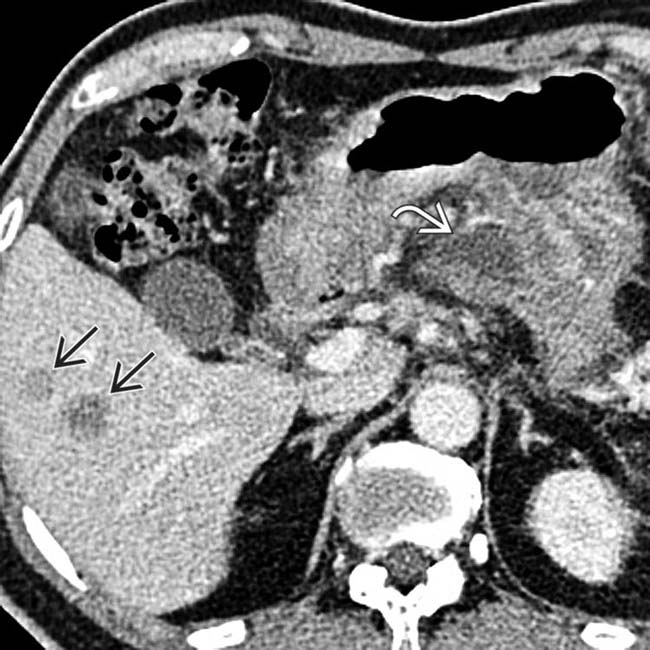
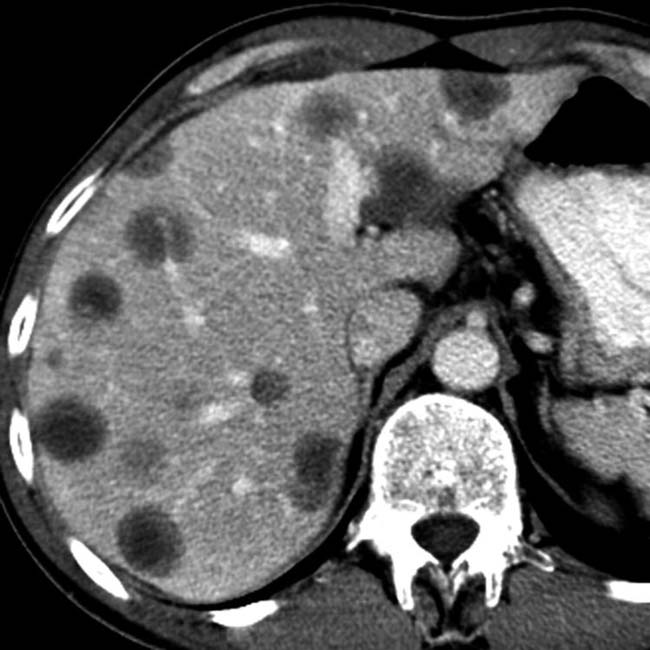
 , consistent with metastases. Note also the lesser sac fluid collection
, consistent with metastases. Note also the lesser sac fluid collection  from acute pancreatitis.
from acute pancreatitis.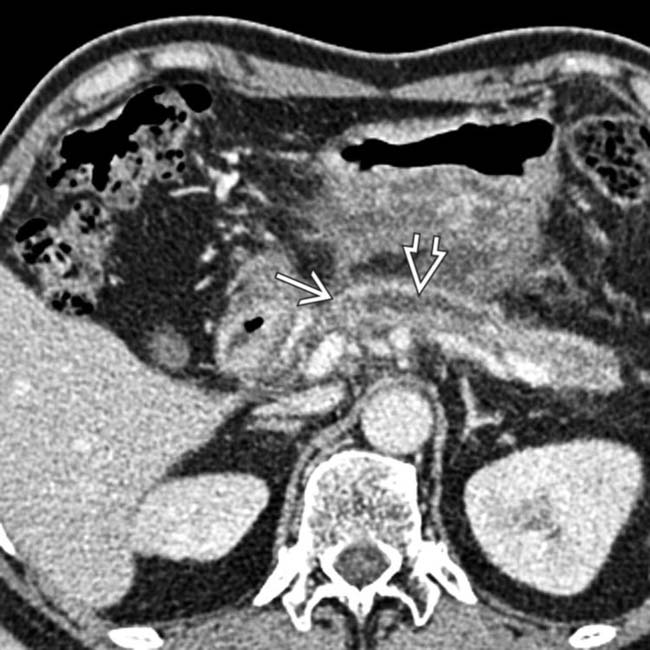
 . Note that the pancreatic duct is obstructed by an isodense mass
. Note that the pancreatic duct is obstructed by an isodense mass 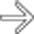 . Endoscopic ultrasound biopsy of the mass revealed adenocarcinoma, which presented as pancreatitis.
. Endoscopic ultrasound biopsy of the mass revealed adenocarcinoma, which presented as pancreatitis.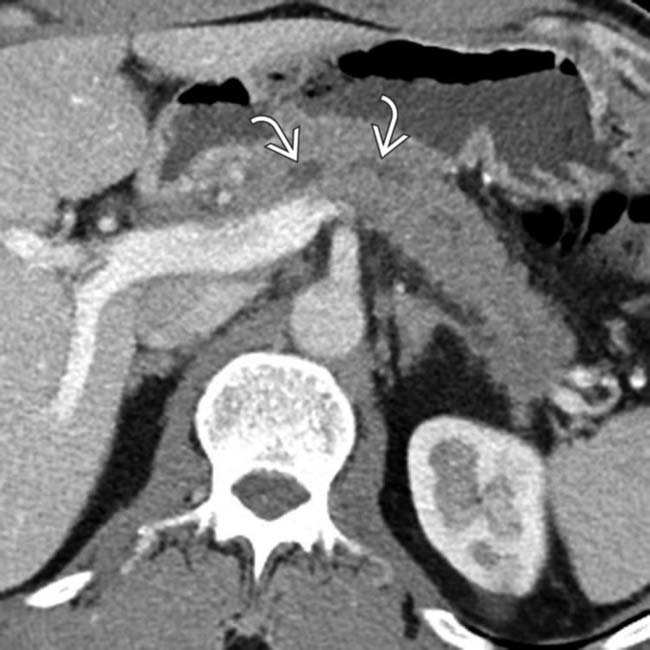
 , an unusual feature for acute pancreatitis.
, an unusual feature for acute pancreatitis.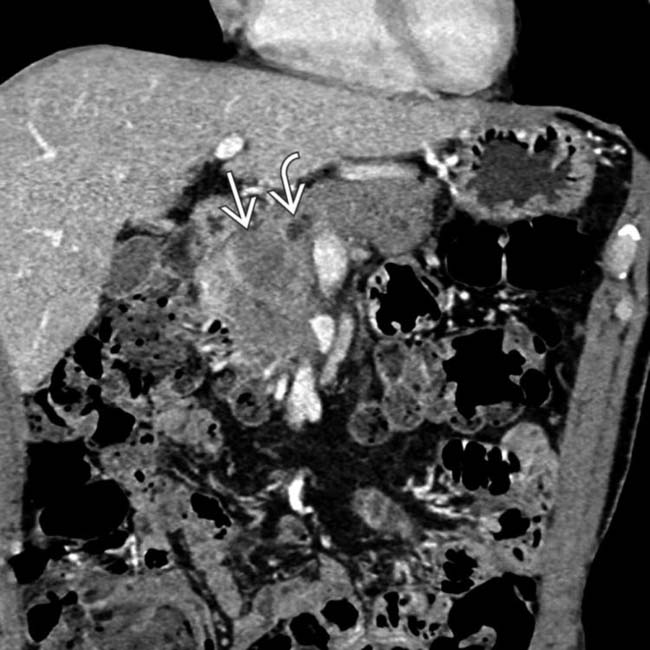
 obstructing the pancreatic duct
obstructing the pancreatic duct  . The presence of a dilated pancreatic duct in acute pancreatitis should always prompt search for an underlying mass.
. The presence of a dilated pancreatic duct in acute pancreatitis should always prompt search for an underlying mass.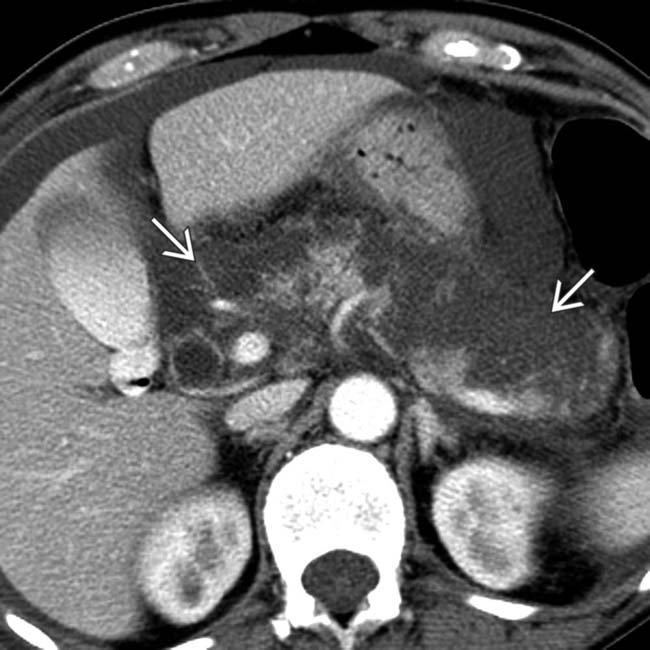
 of the body and tail of the pancreas.
of the body and tail of the pancreas.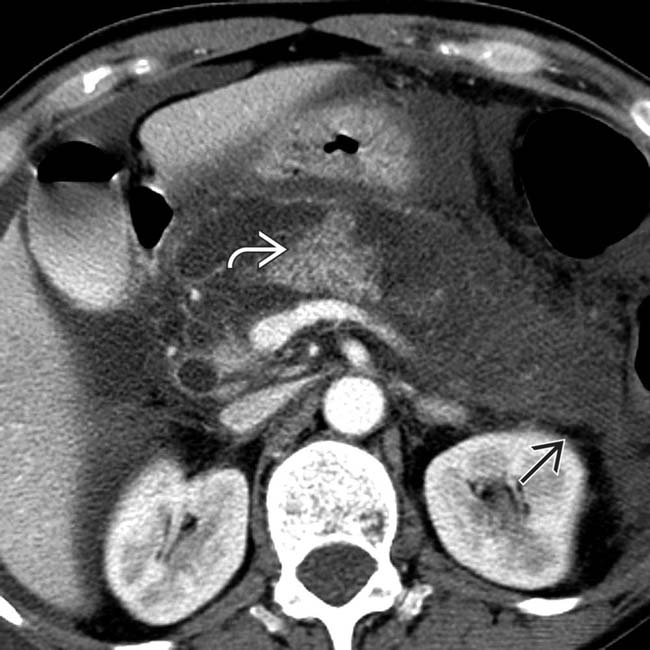
 with only a small area of a residual, normally enhancing pancreas
with only a small area of a residual, normally enhancing pancreas  .
.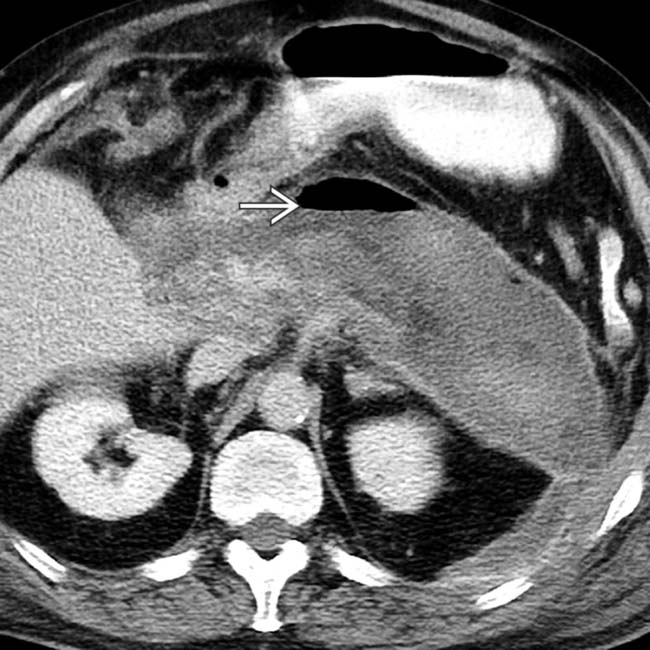
 , indicating a gas-forming infection.
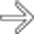
, indicating a gas-forming infection.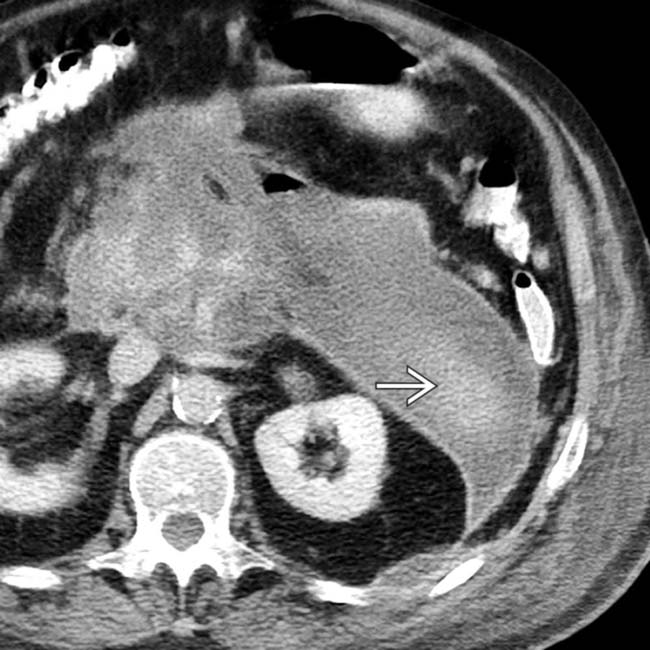
 extending into the left anterior pararenal space.
extending into the left anterior pararenal space.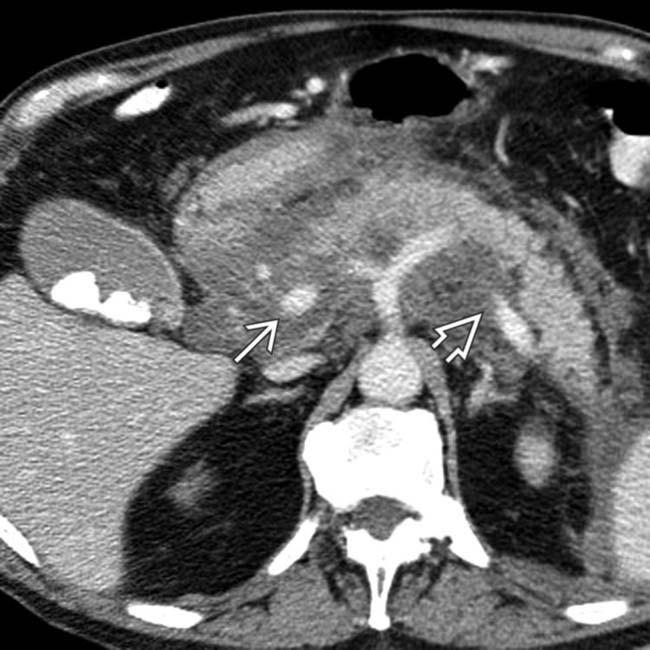
 are surrounded, the splenic vein
are surrounded, the splenic vein  is occluded, and the gastric wall is thickened.
is occluded, and the gastric wall is thickened.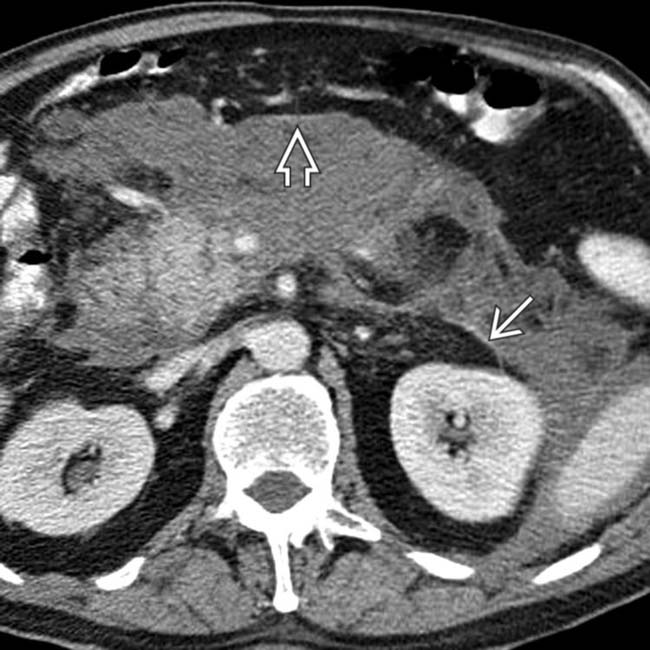
 and ventrally into the mesentery
and ventrally into the mesentery  .
.
 .
.
 .
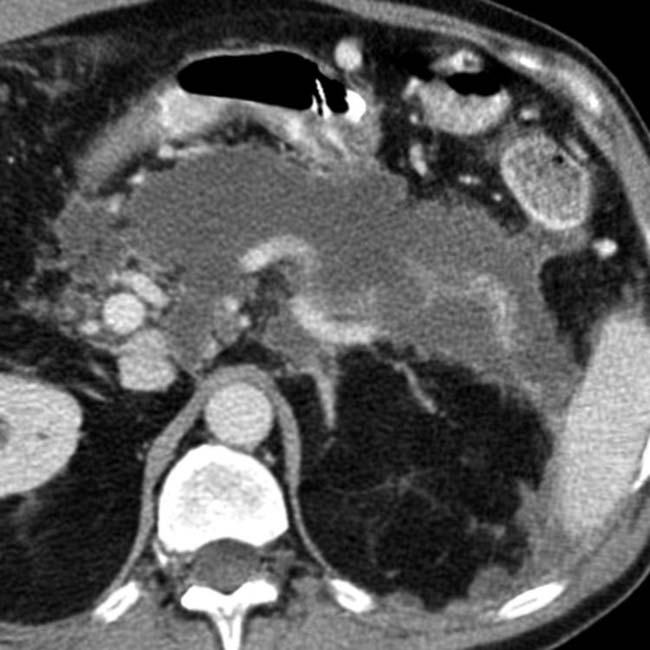
.
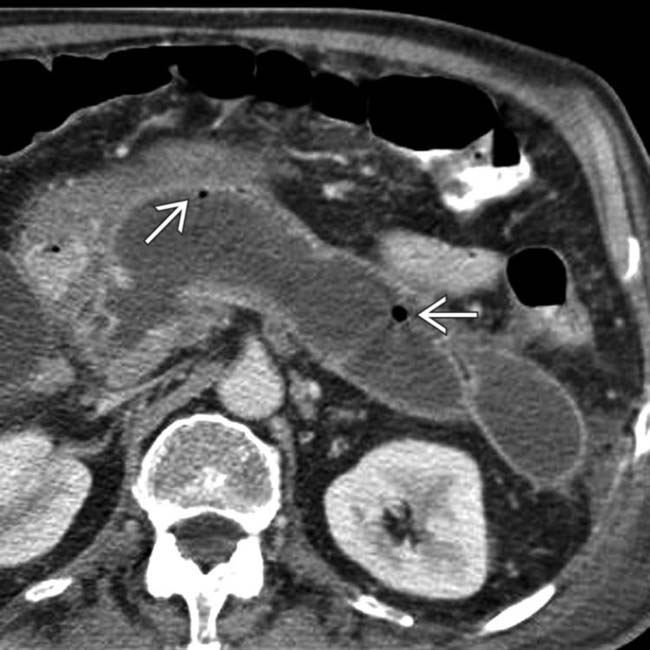
 , which indicate infection.
, which indicate infection.


































































































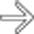
 .
.
 after pancreatitis.
after pancreatitis.