104 Acute Pancreatitis
The term acute pancreatitis describes a wide spectrum of disease ranging from a mild edematous form of acute pancreatitis to severe acute necrotizing pancreatitis. Acute pancreatitis is the third most common gastrointestinal disease requiring hospitalization in the United States and accounts for annual costs of more than $2 billion.1,2 The mild form of acute pancreatitis is a self-limited disease associated with little or no distant organ dysfunction; it has a mortality rate of less than 1% and usually resolves in 3 to 4 days. Patients with this form of acute pancreatitis rarely need intensive care unit (ICU) therapy or pancreatic surgery. Although most (80%) patients with acute pancreatitis have mild disease, 10% to 15% develop the systemic inflammatory response syndrome (SIRS) and run a fulminant clinical course leading to pancreatic necrosis and multisystem organ injury.3–5 The mortality rate for severe acute pancreatitis is 15% to 30%, whereas the overall mortality rate for all patients presenting with acute pancreatitis is less than 5%.4,5 The natural course of severe acute pancreatitis occurs in two phases. The first 7 to 14 days of this disease process are characterized by SIRS and resulting end-organ dysfunction. Inflammatory mediators are released into the systemic circulation, and patients manifest signs and symptoms of cardiorespiratory and renal failure.6 Pancreatic infection is uncommon during this early phase of acute pancreatitis and SIRS, but bacteremia and pneumonia have been identified at a median of 7 days.7 Attempts to modify the course of the disease by instituting therapy with protease inhibitors, octreotide, or platelet-activating factor receptor antagonists have been unsuccessful.8–10
Since the 1980s, the morbidity and mortality associated with acute pancreatitis have decreased substantially.11–14 The reasons for the decrease in mortality in severe acute pancreatitis are uncertain but may reflect improved critical care services and better strategies for surgical management. In general, mortality from severe acute pancreatitis is related to infection.13,14 Infection of the necrotic pancreas (and associated tissues) typically develops in the second and third weeks of the disease and is reported to occur in 40% to 70% of patients with pancreatic necrosis.7,13,14 Multiple organ system dysfunction syndrome is the main life-threatening complication, and mortality rates of 50% have been reported.15 Infected necrosis is the most important risk factor for death secondary to necrotizing pancreatitis.13–16 Prevention, diagnosis, and optimal treatment of infection in severe acute pancreatitis are crucial for improving outcome for patients with this disease.
This chapter discusses the etiology, pathophysiology, severity and staging, and management of patients with severe acute pancreatitis. Chronic pancreatitis is not discussed in this chapter. Several authors and/or societies have proposed guidelines and protocols for management of severe acute pancreatitis.4,14,17–19
 Etiology and Epidemiology
Etiology and Epidemiology
In 2001 in California, the rate of hospital admission with an initial attack of acute pancreatitis was 44 per 100,000 per year, an increase of more than 32% over the decade of the study. Overall rates of hospitalization in the Unites States over the last 20 years has increased from 40 per 100,000 to 80 per 100,000 and included both sexes and all age groups.20 The increasing incidence of acute pancreatitis is believed to be related to increases in alcohol consumption and gallstone disease in some societies. Acute pancreatitis is slightly more common in men than in women, with a male-to-female ratio of 1 : 1.2 to 1 : 1.5. Predisposing factors related to race have not been identified, but both hospitalization rates and emergency department visits for patients diagnosed with acute pancreatitis are higher for blacks than for whites. Pancreatitis can occur in any age group, but cases in the very young (<3 years) are likely to be related to a systemic disease such as hemolytic uremic syndrome or cystic fibrosis. On the other hand, alcohol-related acute pancreatitis has a peak incidence between 45 and 55 years of age, with a gradual decline thereafter. Gallstone pancreatitis can occur in any age group, but its frequency increases with age. Biliary pancreatitis is more common in women, and alcohol-related acute pancreatitis is more common in men.
Understanding the etiology of a particular case of pancreatitis is important; evaluation and treatment depend to some extent on the predisposing disease process.6,17 Gallstones are the leading cause of acute pancreatitis in developed countries and account for 45% of all cases. A biliary etiology should be suspected in female patients older than age 40 with a serum alanine aminotransferase level greater than three times the upper reference limit. Gallstone pancreatitis is the commonest form of pancreatitis in older patients. Since the frequency of gallstones increases with age, gallstones should be suspected in elderly patients.
Alcohol abuse typically accounts for about 35% of cases of acute pancreatitis; however, it is unclear whether acute alcoholic pancreatitis ever arises in the absence of chronic injury to the gland.21 Infrequent, but not rare, causes of pancreatitis include drug reactions (usually idiosyncratic), pancreatic and ampullary tumors, hypertriglyceridemia, hypercalcemia (almost always secondary to hyperparathyroidism), hypothermia, congenital abnormalities of the biliary or pancreatic duct (e.g., choledochal cyst), trauma (including acute pancreatitis after endoscopic retrograde cholangiopancreatography), and infectious or parasitic organisms. Rare causes include bites of certain spiders, scorpions, and the Gila monster lizard. Unidentified causes are termed idiopathic. The roles of sphincter of Oddi dysfunction, pancreas divisum, and bile crystals or sludge in the development of acute pancreatitis are less clear.20
 Pathogenesis and Genetic Susceptibility
Pathogenesis and Genetic Susceptibility
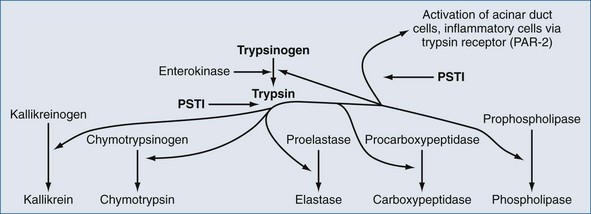
Regardless of the actual underlying cause, pancreatitis is an inflammatory process that can initiate SIRS.6 In spite of much investigation into the molecular pathogenesis of acute pancreatitis, the exact intracellular mechanisms initiating and accelerating pancreatitis are not completely understood. Three phenotypic responses occur in the acinar cell in the early phases of acute pancreatitis22,23: changes in secretions, intracellular activation of proteases, and generation of inflammatory mediators. Shortly after an appropriate stimulus, secretions are released from the apical cells into the pancreatic duct. This process entails exocytotic fusion of zymogen granules with the apical plasma membrane; the granules do not fuse with the basolateral membrane. However during acute pancreatitis, there is (1) markedly decreased apical secretion from the acinar cell, (2) disruption of the paracellular barrier in the pancreatic duct with leakage of contents into the paracellular space, and (3) redirection of secretion from zymogen granules from the apical pole to the basolateral regions of the acinar cell. Inappropriate activation of the proteolytic enzyme, trypsin, is thought to be the initial step in the development of acute pancreatitis. Trypsinogen activation is promotion by cationic trypsinogen mutations (PRSS1+), active trypsin, high calcium ion concentration, and low pH. Calcium levels are regulated in part by calcium-sensing receptors (CASR) and dysregulated by ethanol.22,23 Degradation of active trypsin is blocked by high calcium ion concentration. If trypsin in active within the pancreas, inflammation results and this up-regulates serine protease inhibitor Kazak 1 (SPINK1), which further blocks activation of trypsinogen.22 Trypsin also activates cells via the trypsin receptor, also known as protease-activated receptor 2 (PAR-2) (Figure 104-1).22 Pancreatic acinar and duct cells abundantly express PAR-2. Trypsin activity in the pancreas is controlled mainly by the pancreatic secretory trypsin inhibitor (PSTI), also called serine protease inhibitor Kazal type 1 (SPINK1).22 PSTI is synthesized in pancreas acinar cells and acts as a potent natural inhibitor of trypsin. Normally when trypsinogen is cleaved to release trypsin in the pancreas, PSTI immediately binds to the enzyme to prevent further activation of additional pancreatic enzymes. PSTI also blocks further activation of pancreatic cells via the trypsin receptor, PAR-2.
Several additional protective systems prevent pancreatic autodigestion by trypsin, and the genetic expression of these systems may contribute to the risk of developing acute pancreatitis or modulate the severity of the disease when it occurs. Trypsin-activated trypsinlike enzymes such as mesotrypsin degrade trypsinogen. Bicarbonate-rich pancreatic secretions are affected by abnormal expression of the cystic fibrosis transmembrane conductance receptor. A mutation in SPINK1, N34S, has been reported in people with familial pancreatitis,24 in children with idiopathic chronic pancreatitis,25,26 and in 2% of the control population.26 Because these mutations in SPINK1 are much more common than pancreatitis, this mutation probably is a disease modifier rather than a causative factor underlying the development of acute pancreatitis.
Genetic linkage and candidate gene studies have identified six pancreas-targeting factors that are associated with changes in susceptibility to acute and/or chronic pancreatitis, including cationic trypsinogen (PRSS1), anionic trypsinogen (PRSS2), serine protease inhibitor Kazal 1 (SPINK1), cy regulator (CFTR), chymotrypsinogen C (CTRC) and calcium-sensing receptor (CASR).22
 Diagnosis
Diagnosis
The diagnosis of acute pancreatitis is relatively straightforward when acute upper abdominal pain and tenderness, nausea, vomiting, and hyperamylasemia or hyperlipasemia are present.27 These clinical and biochemical signs are nonspecific, however, and can be present in many other acute intraabdominal conditions such as acute perforation of a hollow organ or mesenteric infarction. Many cases of acute pancreatitis still are diagnosed at autopsy. The diagnosis of acute pancreatitis can be particularly difficult in postoperative patients. Acute pancreatitis also can be hard to diagnose in patients receiving drugs for sedation and patients who are hypothermic or unable to complain of abdominal pain. The Cullen sign and the Grey Turner sign (periumbilical and flank bruising, respectively) are rare and can be present with any disease associated with retroperitoneal hemorrhage. Although hyperamylasemia is common in patients with acute pancreatitis, normal circulating amylase levels are present in 10% to 20% of all cases of acute pancreatitis. Normal serum amylase concentrations are seen predominantly in acute pancreatitis secondary to hyperlipidemia, acute exacerbations of chronic pancreatitis, and late in the course of acute pancreatitis.28 Advantages of serum amylase determination include its technical simplicity, wide availability, and sensitivity.29 This diagnostic test is plagued by low specificity, however. Serum lipase concentration increases within 4 to 8 hours of the onset of acute pancreatitis, peaks at 24 hours, and returns to normal after 8 to 14 days.29 The major advantage of serum lipase determination as a diagnostic test is its excellent sensitivity in acute alcoholic pancreatitis. Measurement of serum lipase activity also is valuable when patients present to an emergency department days after the onset of the disease, because serum lipase levels remain elevated longer than amylase levels.29 Although serum lipase formerly was believed to be a specific marker for acute pancreatitis, increased circulating levels of serum lipase can occur in many other diseases. Simultaneous estimation of amylase and lipase levels does not improve accuracy.29 Other pancreatic enzymes such as P-isoamylase, macroamylases, immunoreactive trypsinogen, and elastase generally are not considered useful for making the diagnosis of acute pancreatitis.
 Severity and Scoring
Severity and Scoring
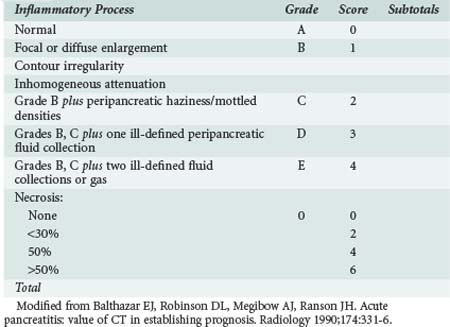
Prediction of the severity of the disease at the time of admission can be difficult, and patients can appear clinically well at admission but clinically deteriorate within 48 hours. Several different prognostic scoring systems with clinical, laboratory, and radiologic criteria have been proposed, yet none of the proposed scoring systems have a high sensitivity, specificity, positive predictive value, or negative likelihood ratio, and frequent clinical assessment is essential for identifying patients with severe disease.30 Ranson’s criteria (Table 104-1),31 the Imrie32 (Glasgow) score, the Acute Physiologic and Chronic Health Evaluation (APACHE) II and III scores,33 the simplified acute physiology score, and Balthazar’s computed tomography (CT) index (Table 104-2)34–36 are the most popular scoring systems and often are used to determine the need for admission to an ICU. Ranson’s criteria are based on 11 prognostic signs present at presentation and 48 hours later.31 A meta-analysis of studies using the Ranson criteria reported the following with regard to predicting severe acute pancreatitis (SAP): sensitivity, 74%: specificity, 77%; positive predictive value, 49%; and negative predictive value, 91%.30 The Glasgow (Imrie) severity score system collects data on 9 variables at admission but is not complete until 48 hours after admission. Many institutions routinely utilize the APACHE scoring system for all patients admitted to the ICU.33 Patients with SAP and an APACHE II score above 8 have severe disease and are likely to develop organ failure. Key statistical parameters related to APACHE II score of above 7 and the prediction of SAP are as follows: sensitivity, 65%; specificity, 76%; positive predictive value, 43%; and negative predictive value, 89%. Balthazar’s CT index34–36 uses both fluid collections and amount of pancreatic necrosis to predict outcome. A recent international group of experts concluded that an additional group of patients should be identified: those with moderately severe acute pancreatitis (MSAP).37 This is a large group of patients who meet the Atlanta classification of severe disease but do not develop organ failure. Patients in the MSAP group often develop local complications and often have long hospitalizations with significant morbidity but without mortality. In a strategy to identify those patients who will not need ICU care, Lankish et al. proposed and validated a “harmless acute pancreatitis score (HAPS).” Using this scoring system, 98% of 204 patients were correctly identified as having non-severe disease within 30 minutes of presentation.38 These simple measures included rebound or guarding on clinical examination, hematocrit greater than 43% in men and greater than 39.6 in women, and serum creatinine concentration above 2 mg/dL. Imamura and colleagues have recently proposed a simplified grading of early CT scans based on the presence or loss of enhancement of the renal rim fat. This simple assessment compared favorably with all the commonly used scoring systems.39
TABLE 104-1 Ranson’s Criteria for Patients with Non–Gallstone-Associated Pancreatitis
| At Presentation | During Initial 48 Hours |
|---|---|
| Age > 55 years | Hematocrit fall > 10% |
| White blood cell count > 16,000/µL | Blood urea nitrogen > 5 mg/dL |
| Blood glucose > 200 mg/dL | Serum calcium < 8 mg/dL |
| Serum alanine transferase > 250 U/dL | Arterial PO2 < 60 mm Hg |
| Serum lactate dehydrogenase > 350 IU | Base deficit > 4 mEq/L |
| Estimated fluid sequestration > 6 L |
Modified from Blamey SL, Imrie CW, O’Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut 1984;25:1340-6.
Many investigators have studied and proposed a variety of serum biomarkers as predictors of the severity and prognosis of acute pancreatitis.40–42 High circulating levels of C-reactive protein (CRP) (cutoff 150 mg/L) are associated with pancreatic necrosis, but there is a 48-hour latency before CRP increases, limiting its utility as an early predictor. This marker has a sensitivity and specificity of 80%. Although not ideal predictors of severity, serum concentrations of procalcitonin and interleukins (IL) 6 and 8 have some predictive value.40–42 Certain urinary markers also have some predictive value. While not used extensively clinically at the current time, procalcitonin appears to offer the greatest promise. Serum procalcitonin levels higher than 3.8 ng/mL accurately predict later organ dysfunction (sensitivity, 79%; specificity, 93%).42
The scoring systems mentioned help quantify the degree of illness, but it is essential that clinicians identify patients with impending or actual organ failure. Patients with signs of SIRS are especially at risk for further organ dysfunction.45 In a review of 259 patients with acute pancreatitis, mortality was significantly higher in patients who developed or had persistent SIRS at 48 hours (25.4%) than in patients who had transient SIRS (8%) or no SIRS in the first 48 hours (0.7%).37
An update of the Atlanta Classification system for severity of acute pancreatitis is expected soon; the system developed at the initial consensus meeting in 1992 has allowed comparisons among clinical trials and different treatment strategies.36 It defined SAP by its association with organ failure, local complications such as necrosis, abscess, or pseudocyst, or both. By consensus, the Atlanta Classification also defined SAP based upon the presence of ≥3 of Ranson’s criteria or an APACHE II score ≥ 8. Most often, SAP is a clinical expression of the development of pancreatic necrosis. Less commonly, patients with interstitial (edematous) pancreatitis can present with SAP. In addition to the previously proposed scoring systems, there is another very simple scoring system termed the Panc 3 Score.46 Three findings—hematocrit over 44 mg/dL, body mass index above 30 kg/m2, and a pleural effusion on chest x-ray—were the most sensitive predictors of overall severity. In the validation set of data, when all three of these findings were present and the pretest probability of pancreatitis was between 12% and 25%, the posttest likelihood of severe disease was 99%.43
Serum concentrations of CRP, neutrophil elastase, pancreatitis-associated peptide, IL-6, IL-8, IL-1, IL-10, and soluble tumor necrosis factor (TNF) receptors might be useful for the early prediction of severity of disease in acute pancreatitis.40 Circulating CRP concentration is an independent predictor of outcome in acute pancreatitis, but it is not predictive of severity at presentation.44 Laboratory tests also can be used for severity stratification; serum IL-6 concentration greater than 2.7 pg/mL within 48 hours from disease onset and a serum CRP level above 150 mg/L at 48 hours after pain onset can both be used. A recent meta-analysis of the role of procalcitonin in the identification of patients with SAP suggested that the test has a sensitivity of 0.72 for the diagnosis, a specificity of 0.86, and an area under the curve of 0.87, but the studies showed a fair amount of heterogeneity.42 Trypsinogen-2 can be measured via a simple serum immunofluorometric assay or urine dipstick assay, using a threshold of 50 µg/L.41
 Imaging
Imaging
Ultrasonography And Endoscopic Ultrasonography
Ultrasonography should be considered as an initial test in all patients with pancreatitis, especially if gallstones are suspected.5,17,19 By aiding in the diagnosis of gallstones, common bile duct stones, common bile duct dilation, and free peritoneal fluid, ultrasonography can be useful for determining the cause of pancreatitis.47 Ultrasonography currently has little role in the grading of severity of acute pancreatitis or determination of extent of pancreatic necrosis. However, this situation may change because of the evolution of contrast-enhanced ultrasonography. This technique employs microbubbles as a blood-pool contrast medium to allow visualization of tissue vascularization. Early in the course of pancreatitis, inflammation is associated with hyperemia. Later in the course of severe disease, contrast-enhanced ultrasonography can reveal confluent necrotic areas of devitalized pancreatic tissue.48 The value of ultrasonography is compromised by overlying bowel gas in at least 25% to 30% of cases.
Endoscopic ultrasonography (EUS) combines ultrasonography and endoscopic evaluation. It is less invasive than endoscopic retrograde cholangiopancreatography (ERCP) and has been shown to be clinically useful in diagnosing acute pancreatitis and choledocholithiasis.47 Endoscopic ultrasonography may be useful when CT and ultrasonography fail to show common bile duct stones. Endoscopic ultrasonography also may be useful for selecting patients who might benefit from endoscopic retrograde cholangiopancreatography and early stone extraction. Petrov et al. reviewed studies of patients randomized to EUS-guided ERCP (n = 213) versus ERCP alone (n = 210). These authors showed that ERCP could be avoided in 67.1% patients when EUS failed to identify gallstones.47 The use of EUS significantly reduced the risk of overall complications [relative risk (RR) 0.35, 95% confidence interval (CI) 0.20-0.62] and post-ERCP pancreatitis (RR 0.21, 95% CI 0.06-0.83). One additional advantage of endoscopic ultrasonography is that it can be performed in pregnant women, patients with metallic implants, and patients who are too unstable to be transported out of the ICU.47
Computed Tomography
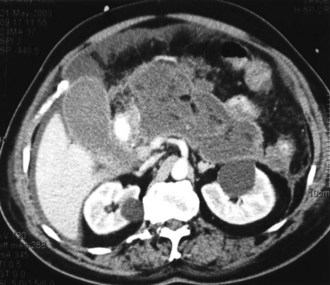
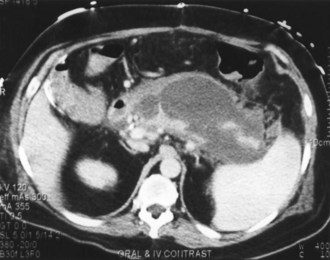
Contrast-enhanced CT is considered the gold standard for diagnosing pancreatic necrosis and peripancreatic collections and for grading acute pancreatitis (see Table 104-2).34–36 Necrosis is detected by CT as focal or diffuse areas of diminished pancreatic parenchymal contrast enhancement (<50 Hounsfield units). The accuracy of this test is greater than 90%. CT findings of acute pancreatitis include diffuse or segmental enlargement of the pancreas (interstitial edema), irregularity of the contour of the pancreas with obliteration of the peripancreatic fat planes, heterogeneous appearance with areas of decreased density within the pancreas, and variable ill-defined fluid collections (Figures 104-2 and 104-3).34–36 The Balthazar index ranges from 0 to 10 and is obtained by adding the points attributed to the extent of the inflammatory process to the volume of pancreatic necrosis. Although CT findings correlate with clinical course and severity of patients with acute pancreatitis,36 it is not necessary to obtain this study in patients with mild pancreatitis. In a recent Dutch observational study of 166 patients admitted with acute pancreatitis, early CT (within 4 days of admission) was performed in 47% of all patients. However, only 18 of the 166 patients had severe disease, and 11 eventually developed pancreatic necrosis. No changes in clinical management resulted from obtaining early CT scans. These data suggest that the use of early CT, especially in patients with mild disease, should be discouraged.49 CT can be helpful when the diagnosis is in doubt or when complications of pancreatitis may be developing. In general, contrast-enhanced CT scans should not be performed during the first 72 hours of the disease, because necrosis may not be fully established until after 96 hours, and there have been isolated reports of intravenous (IV) contrast material causing derangements of the pancreatic microcirculation.49 Contrast administration also can trigger or exacerbate renal insufficiency.
Endoscopic Retrograde Cholangiopancreatography
Endoscopic retrograde cholangiopancreatography is an effective means for treating common bile duct stones.47 Endoscopic retrograde cholangiopancreatography is not indicated for the management of mild pancreatitis or nonbiliary pancreatitis, and its overall use in patients with acute pancreatitis continues to be debated.50–58 Guidelines from England, Japan, and the United States indicate that ERCP is indicated in the management of patients with biliary pancreatitis and biliary obstruction or cholangitis.17–1952 There remains controversy regarding the role of ERCP for the management of patients with biliary pancreatitis but without bile duct obstruction. Five clinical trials have sought to determine whether ERCP plus sphincterotomy or conservative management is more appropriate for patients with acute pancreatitis.53–57 In a study of 121 patients randomized to ERCP or conservative treatment within 72 hours of onset, there was a significant reduction in morbidity (17% versus 34%; P=.03) but no significant difference in mortality (2% versus 8%; P=.23).53 The differences in morbidity seen in this trial cannot be explained by differences in the severity of pancreatitis between the two groups.53
In another study that enrolled 195 patients, ERCP performed within 24 hours was compared with conservative therapy. ERCP was associated with a significant reduction in morbidity (biliary sepsis; P = .001) without a significant reduction in mortality (five deaths with ERCP versus nine deaths with conservative treatment).54 Included in this study were patients with nonbiliary pancreatitis such as alcohol-related and parasite-related disease. In another trial with a similar design, 280 patients were randomized to receive ERCP within 24 hours or conservative treatment55; 75 of the 178 patients in the ERCP arm had impacted biliary stones. This study is the only one that showed a significant reduction in morbidity and mortality.
The study by Folsch and colleagues56 was a multicenter trial of ERCP versus conservative management. Patients with biliary sepsis and obstruction were excluded from study entry because efficacy in this group has been established. In contrast to the previous studies, this study showed a significant increase in complications in the ERCP group compared with the conservatively managed group (respiratory failure, 12% versus 4% [P = .03]; renal failure, 7% versus 4% [P = .10]). In addition, the mortality rate was higher in the ERCP group compared with the control group (11% versus 6%), requiring premature termination of the study.56 The results of this large clinical trial suggest that in the absence of biliary obstruction or sepsis, ERCP may be harmful, and a conservative approach is preferred.
Oria and colleagues studied 102 patients with acute pancreatitis and an APACHE II score higher than 6; the subjects were randomized to receive ERCP within 72 hours or conservative management. Three patients in each group suffered local complications.57 Petrov and colleagues performed a meta-analysis of these trials.58 These authors concluded that the early use of ERCP did not significantly reduce the risk of local pancreatic complications in patients with either mild or severe pancreatitis.
In contrast, Dutch investigators reported their observational results of the use of ERCP as part of another clinical trial on the use of probiotics in SAP. Of the 153 patients enrolled, 81 underwent ERCP and 72 received conservative management. Of the 153 patients, 78 patients with cholestasis had fewer complications when ERCP was utilized [ OR 0.35; 95% CI, 0.13-0.99], but there was no significant effect on mortality, and no reduction of complications or mortality if cholestasis was not present in patients with predicted SAP.51 The role of ERCP in idiopathic pancreatitis also is unclear. Advances in ultrasonography and magnetic resonance cholangiopancreatography (MRCP) suggest that these modalities may have a preferred role when diagnostic considerations are the issue in acute pancreatitis, especially in view of the potential for complications with ERCP.59 As noted previously, endoscopic ultrasound may have an increasing role in identifying patients with suspected choledocholithiasis who might benefit from ERCP.
Magnetic Resonance Cholangiopancreatography
Magnetic resonance imaging (MRI) and MRCP are noninvasive imaging modalities that are useful for depicting abnormalities of the pancreatic duct and parenchyma.60–62 These imaging techniques can identify acute fluid collections and necrosis in SAP. MRI has several advantages over CT: there is no risk from radiation with MRI, it can detect pancreatic duct disruption, and it can help identify the etiology of acute pancreatitis. Without injection of gadolinium, MRI can discriminate between normal pancreatic parenchyma, the presence of edema, and the presence of necrosis as well as differentiate between solid and liquid fluid collections. In a study of 90 patients, 28 had gallstones, 9 had common bile duct stones, and 10 had pancreatic divisum.60,62 MRCP can be performed when ERCP has failed or is not possible, although ERCP is not only a diagnostic modality but also a therapeutic one, because the endoscopic approach permits sphincterotomy and removal of common duct stones.61
Contrast-enhanced CT is the gold standard for documenting pancreatic necrosis and assessing the severity of acute pancreatitis. Nevertheless, results from a few studies suggest that MRCP compares favorably with contrast-enhanced CT for the diagnosis and grading of severe acute pancreatitis.60,62 The major advantage of MRCP for SAP is that MRCP obviates the necessity for the infusion of iodinated contrast media and thereby may lower the risk for acute renal dysfunction in these critically ill patients.60,62 Bowel peristalsis, vascular motion artifacts, gastrointestinal air, and the presence of metallic clips all can degrade the quality of the images obtained with MRCP. One disadvantage of MRI and MRCP is that acquisition of the image takes longer than with CT.
 Management
Management
General Support
Monitoring and Resuscitation
Several publications suggest that patients with SAP should be managed in an ICU, preferably by a specialist team.17–1952 Ongoing monitoring for signs of distant organ dysfunction is crucial. Resuscitation of intravascular volume is a key component of the initial management, regardless of the etiology and severity of acute pancreatitis. Sequestration of fluid into the so-called third space (i.e., the extravascular extracellular compartment) can lead to loss of as much as a third of plasma volume. Rapid restoration and maintenance of intravascular volume is essential because hypovolemia and shock probably are important factors contributing to the high incidence of acute renal failure among patients with severe acute pancreatitis.30,63 It is common for patients with SAP to require administration of crystalloid fluid at rates as great as 500 mL/h, at least for a while.
Recently, 76 patients with SAP were randomly assigned to receive rapid infusion of IV fluid at either 10 to 15 mL/kg/h or 5 to 10 mL/kg/h, both groups receiving more than 10 L of fluid during their first 3 days of ICU care.64 The investigators in this study suggested that several outcomes were better in the group that received more gradual fluid expansion. The results of this trial are interesting but require confirmation before there is widespread adoption of the authors’ recommendations.
Single-organ or multiorgan dysfunction is common, and monitoring of respiratory status is essential. Respiratory and cardiovascular dysfunction are common and require prompt identification and supportive care. Adequate oxygen delivery to tissues and prevention of splanchnic ischemia are essential to prevent further organ injury. Vasoactive agents may be required, but they should be considered only after ensuring that intravascular volume has been repleted. In addition, because rapid administration of large volumes of IV fluid may be indicated, abdominal compartment syndrome should be considered and assessed.65
Even when systemic signs of adequate resuscitation are present, local inflammation in the pancreas can continue, leading to ongoing production of cytotoxic mediators. Accordingly, investigators have been interested in targeting this aspect of the disease process. Treatment with protease inhibitors has been successful in experimental models of acute pancreatitis and is used via continuous arterial infusion in Japan.63,68 A trial was carried out that compared no infusion with continuous regional arterial infusion of the protease inhibitor, gabexate mesilate, plus antibiotics.66 Treatment with gabexate mesilate shortened the duration of abdominal pain, duration of SIRS, and decreased the length of hospital stay. Circulating levels of several markers of inflammation also were decreased with the protease inhibitor.66 A national survey of clinicians in Japan indicated the following: severe pain disappeared after a short period of time of infusion of a protease inhibitor; infected necrosis was less common when both a protease inhibitor and antibiotic infusion were infused; and mortality was lower when continuous arterial infusion was initiated within 2 days.67
Although there has been significant interest in decreasing cytokine production by administering an anti-TNF antibody, this approach has not been shown to be beneficial in clinical trials, perhaps owing to the early peak of TNF in the disease process. Similarly, although administration of an IL-1 receptor antagonist has been beneficial in animal models of SAP, this approach has not yet been applied successfully in clinical practice. One of the more interesting potential therapeutic approaches is directed at decreasing calcium ion–dependent cytokine release by using administering a calcium channel antagonist. In one animal study, treatment with a calcium channel blocker use was associated with a dramatic reduction in TNF release and an associated improvement in survival from 40% to 80%.69 However, these data are experimental, and although of interest, both further experimental data and results from clinical trials would be needed before this strategy could be advocated for the care of patients with SAP.
Pulmonary Dysfunction
Respiratory dysfunction is a major component of multiple organ system dysfunction syndrome secondary to acute pancreatitis, and most patients with this syndrome require ventilatory support.30,63 Acute respiratory distress syndrome (ARDS) is characterized by diffuse pulmonary infiltrates on the chest radiograph, arterial hypoxemia, pulmonary hypertension, and decreased pulmonary compliance.
Pulmonary Management
Patients with SAP must be monitored closely for hypoxic and/or hypercarbic respiratory failure. Supplemental oxygen is almost uniformly required, and mechanical ventilation is often required.17–19,54,63 Noninvasive positive-pressure ventilation (NIPPV) may be used to avoid endotracheal intubation in carefully selected patients; however, NIPPV usually is not well tolerated. SAP often is associated with marked abdominal distention and diminished functional residual capacity on this basis. Management of acute lung injury and ARDS secondary to SAP is similar to the management of these conditions associated with other primary problems (e.g., sepsis).
Pain Relief
Provision of pain relief to patients with severe acute pancreatitis is not only humane but also may improve pulmonary dysfunction.70–72 In studies outside the United States, buprenorphine was noted to have a superior effect to procaine and did not exacerbate acute pancreatitis by promoting contraction of the sphincter of Oddi.70 Pentazocine was found to have a superior analgesic effect to procaine. In a single trial comparing metamizole and morphine, no difference in analgesia was seen.72 Although IV narcotics are useful and effective, epidural analgesia with local anesthetics also should be considered.73
Specific Support
Nutrition
Traditionally, patients with acute pancreatitis have been managed by providing IV fluids and nutrition and avoiding enteral feeding to “rest” the inflamed pancreas and prevent stimulation of exocrine function and the release of proteolytic enzymes.17–1954 Nevertheless, most patients with mild acute pancreatitis can begin oral supplementation within a few days of their presentation with pain and do not require supplemental nutrition.54
In the past, the primary approach for providing nutritional support for patients with SAP was total parenteral nutrition (TPN). TPN is expensive, however, and may increase the risk of sepsis or metabolic derangements.74 TPN also has been associated with alterations in gut barrier function.74,75 Accumulating data support the view that enteral nutrition is safe and cost-effective in patients with SAP.76–83 A meta-analysis of several trials of enteral versus parenteral nutrition for patients with SAP revealed that enteral nutrition reduced the frequency of infections, decreased the need for surgery, and shortened length of hospital stay.76 One trial demonstrated that enteral nutrition instead of TPN markedly decreased medical costs per capita.81 Similarly, the Cochrane Group reviewed 8 trials of enteral versus parenteral nutrition (total of 348 patients) and concluded that the relative risk of death with enteral nutrition was 0.50 (95% CI 0.28-0.91), RR for multiple organ failure was 0.55 (95% CI 0.37-0.81), RR for systemic infection was 0.39 (95% CI 0.23-0.65), RR for operative interventions was 0.44 (95% CI 0.29-0.67), RR for local septic complications was 0.74 (95% CI 0.40-1.35), and RR for other local complications was 0.70 (95% CI 0.43-1.13). Mean length of stay was reduced by 2.37 days (95% CI 7.18-2.44 ) in the enteral group. The main findings were also sustained in a subgroup of patients with severe AP.80
If a nasoduodenal or nasojejunal tube is placed, care should be taken if blind manipulation through the duodenum is attempted. Although blind placement is possible, the duodenum is often distorted in patients with acute pancreatitis, and the risk of perforation is increased. Fluoroscopic or endoscopic guidance of the tube into a postpyloric, even jejunal, position may be preferable. Petrov et al. reviewed four studies totaling 92 patients with acute pancreatitis who received nasogastric rather than nasojejunal enteric feedings in randomized controlled trials.84 Patients were moderately to severely ill with evidence of end organ failure, typically respiratory failure, but were found to tolerate gastric feedings as well as nasojejunal feedings. By the 7th day, 78.8% of patients were able to achieve their goal feeding, and 79.3% of patients were able to sustain full tolerance of gastric enteral feeds. The total number of patients in the comparator groups, especially those with SAP, does not support a uniform suggestion to use nasogastric feeds rather than nasojejunal feeds at this time. Supplemental TPN may be valuable when nutritional requirements cannot be achieved using enteral nutrition alone or enteral access cannot be established. Ileus is not an absolute contraindication to enteral feeding, and most patients tolerate continuous feeding at a slow rate.85
Resting energy expenditure varies widely among patients with SAP, depending on the magnitude of the regional inflammatory process and the presence of additional complications, especially infection. Infection can increase energy expenditure by 5% to 20%, but overfeeding should be avoided, nutritional guidelines should be considered, and glucose control should be employed.85 Although triglyceride levels should be monitored and not be allowed to escalate to levels above normal, administration of lipids is safe and appropriate.79 Pancreatic secretion is not stimulated by IV lipids, whereas the anatomic site of nutrient administration determines the degree and extent of pancreatic stimulation after enteral nutrition. There is no proven causal relationship between infusion of exogenous fat and the development of pancreatitis.
 Pathogenesis of Pancreatic Infection and Antibiotic Prophylaxis
Pathogenesis of Pancreatic Infection and Antibiotic Prophylaxis
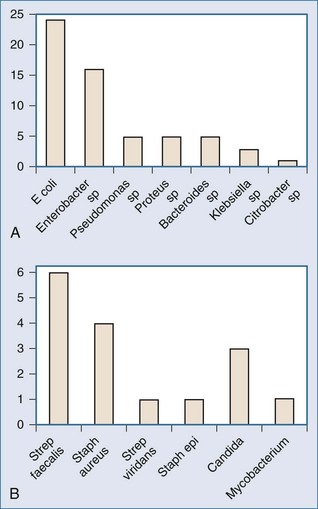
Microorganisms can gain access to necrotic pancreatic and peripancreatic tissue via several routes, bacterial translocation from the colon being the most likely. Failure of the intestinal barrier permits bacteria and yeast to translocate from the lumen of the gut into ascites, mesenteric lymph, the bloodstream, and the pancreatic phlegmon.74,75 The notion that pancreatic infection in acute pancreatitis is due to infection by gut-derived organisms is supported by the observation that most pancreatic infections are monomicrobial and caused by gram-negative bacteria, at least when prophylactic antibiotics have not been administered (Figure 104-4).74,75,86,87 Further support for the intestinal origin of pancreatic infection in acute pancreatitis derives from data obtained in a clinical trial of selective decontamination of the gut, wherein enteral administration of poorly absorbed antimicrobial agents was associated with a significant reduction in late mortality, principally owing to decreased incidence of pancreatic gram-negative infection.85 Microorganisms also can gain access to pancreatic necrosis through hematogenous dissemination from infected central venous catheters,88 via the biliary tree, or via the pancreatic duct from the lumen of the duodenum. Besselink and colleagues demonstrated clear links among intestinal barrier dysfunction, greater intestinal permeability, bacteremia, and infected necrosis.74 However, these authors were unable to demonstrate a connection between measured enterocyte damage and intestinal permeability.
The wisdom of using prophylactic antibiotics for managing acute pancreatitis has been debated for more than 50 years. This question has been addressed by many small (relatively underpowered) randomized controlled clinical trials,85–111 several meta-analyses of these same trials, and numerous observational or retrospective studies.85–111 When more than 30% of the gland is necrotic, pancreatic infection occurs in over 30% of patients with acute pancreatitis. Approximately 80% of deaths due to acute pancreatitis are related to infectious complications. Thus it is reasonable to consider whether administration of prophylactic antibiotics can decrease the incidence of either local or distant infections or the morbidity and mortality associated with pancreatic necrosis. Initial work in this area focused on the specific characteristics of antibiotics and whether or not the drugs penetrate into pancreatic tissue.109 Trials in the 1970s used antibiotics that either do not penetrate well into pancreatic tissue99 or did not have an adequate spectrum of antimicrobial activity. Aminoglycosides penetrate tissues poorly, whereas cephalosporins (e.g., cefotaxime), ureidopenicillins (e.g., piperacillin), fluoroquinolones (e.g., ciprofloxacin, ofloxacin, perfloxacin), metronidazole, and imipenem all penetrate well into pancreatic tissue.109
The most widely quoted trials in support of antibiotic prophylaxis for acute pancreatitis include the trial by Pederlozi et al.89 of 74 patients randomized to receive either imipenem (0.5 g every 8 hours for 14 days) or placebo, the trial by Sainio et al.90 of 60 patients randomized to receive either cefuroxime (1.5 g IV every 8 hours) or placebo, and the trial by Luiten et al.85 of 102 patients randomized to receive selective digestive decontamination versus standard therapy. In the Pederlozi trial,89 the secondary rate of pancreatic infection decreased from 30% in the control group to 12% in the imipenem group (P = .10). There were three deaths in each group, and there were no beneficial effects on organ failure, mortality, or avoidance of surgery. The trial by Saino and associates90 enrolled mostly young patients with alcoholic pancreatitis and found that infectious complications were more common in the group not treated with antibiotic prophylaxis compared with the group treated with cefuroxime (1.8 per patient versus 1 per patient; P=.10), as was mortality (7 versus 1; P=.03). Coagulase-negative Staphylococcus was cultured from unspecified sites in four of the eight patients who died. In the experimental arm of the selective digestive decontamination trial, colistin, amphotericin, and norfloxacin were administered via the oral and rectal routes. In addition, patients in this arm received a short course of therapy with cefotaxime. There were 18 deaths among the 52 patients in the control group (35%) and 11 deaths among the 50 patients in the selective digestive decontamination group (22%; P = .048).85
In a retrospective review of 180 patients with SAP, Ho and Frey94 found a mortality rate of 18% and a pancreatic infection rate of 76% among patients who did not receive prophylactic antibiotics, whereas the mortality rate was only 5%, and the infection rate was 27% among patients who were treated with prophylactic imipenem.
Two well-designed randomized trials in the last 5 years have failed to show benefit from antibiotic prophylaxis. Intravenous ciprofloxacin (Cip) and metronidazole (Met) were compared with placebo in 114 patients with SAP; 12% of patients in the Cip/Met group developed pancreatic infection, whereas 9% of placebo patients (P = 0.585) developed pancreatic infection. Mortality was not different (5% versus 7%, respectively).95 In a more recent trial of 100 randomized patients, meropenem (1 g/8 h) was compared with outcomes from a placebo group.97 There were no differences between the groups for these parameters: incidence of infected necrosis, need for surgical intervention, or mortality. Imipenem prophylaxis was studied in 72 patients with SAP, and use of imipenem was associated with fewer complications (12/35 versus 22/35) and infections (5/35 versus 16/35).97 However, the authors were unable to find a difference in the need for ICU care, overall hospital length of stay, need for surgical intervention, or 30-day mortality rate. Garcia-Barrasa et al. reported a randomized controlled trial of 21 patients randomized to either ciprofloxacin or placebo.99 They were unable to demonstrate a difference between the groups for any outcome measure.
The literature is replete with papers attempting to summarize benefits and justify antibiotic prophylaxis for SAP. A systematic review and several recent meta-analyses have been conducted to try to answer the question of whether or not antibiotic prophylaxis is beneficial.99–111 Using a fixed effects model, Hart et al. concluded that infected pancreatic necrosis (RR 0.72, 95% CI 0.46-1.16) and mortality (RR 0.71, 95% CI 0.41-1.23) were not dependent upon treatment group. However, these authors found that extrapancreatic infections were decreased when prophylaxis was used (RR 0.51, 95% CI 0.32-0.82).106 Xu et al. also concluded that mortality was not different (RR 0.76, 95% CI 0.5-1.18), nor was the need for surgical intervention (RR 0.90, 95% CI 0.66-1.23) reduced when patients were treated with prophylactic antibiotics. However these authors concluded that peripancreatic infection (RR 0.69, 95% CI 0.48-0.91) and extrapancreatic infection (RR 0.66, 95% CI 0.48-0.91) were reduced by administration of antibiotics.108
Instead of using IV antibiotics, a recent trial considered whether administration of probiotics could be used to decrease pancreatic infection.7 Infections occurred in 30% of patients in the probiotic group and 28% of patients in the placebo group. Death occurred in 16% of patients in the probiotic group and 6% of the patients in the placebo group. Importantly, nine patients in the probiotic group developed bowel ischemia, whereas none of the patients in the placebo group developed this complication. Based on this study, a probiotic strategy employing the multispecies product used in this study is not recommended. This same group of authors reported early nonpancreatic infection in 731 patients with pancreatitis over a 3-year period, with 173 patients developing a documented infection.112 Pneumonia was identified in 84 (11.5%) patients at a median of 9 days (interquartile range [IQR] 4-17) and bacteremia in 107 (14.6%) on day 10 (IQR 3-23). Infected necrosis was identified later at a median of 26 days (IQR 17-37). These data suggest that patients with SAP, like all ICU patients, are at risk for nosocomial infections. However, whether prophylactic antibiotics should be broadly applied for these indications is a controversial topic. One of the most concerning issues with respect to the routine use of prophylactic antibiotics is the change in microbial species over the past decade, with resistant bacterial species and fungal pathogens being commonly identified.5,87,112,113
In addition, several reports of SAP have documented changes in the microbial spectrum of pancreatic infections characterized by an increased incidence of fungal species and more antibiotic-resistant bacterial species.112,113 Fungal infection in SAP is a risk factor for morbidity and possibility mortality.113 These studies raise the possibility that prophylaxis with any broad-spectrum antibiotic may be associated with increased risk of infection with fungal species or resistant bacteria. If broad-spectrum bacterial agents are used, prophylactic use of an antifungal agent may be warranted.114 Although prophylactic antimicrobials administered IV or enterally are uniformly used in some institutions, I cannot recommend the widespread use of prophylactic antimicrobials without further data supporting the benefits of use over the apparent increase in antimicrobial resistance being reported in current series and seen in my own institution.
 Management of Pancreatic Necrosis and Abscess
Management of Pancreatic Necrosis and Abscess
Pancreatic necrosis is defined by the presence of diffuse or focal areas of nonviable pancreatic parenchyma, often associated with peripancreatic fat necrosis.36 Necrosis either can be sterile or infected; infection usually is confirmed by fine-needle aspiration.115,116 Pancreatic infection occurs in about 10% of all cases of acute pancreatitis, but in 30% to 70% of cases with necrosis. Contrast-enhanced CT is currently the gold standard for documenting the presence of nonperfused pancreatic parenchyma, although as noted earlier, MRI also can show both fluid collections and nonperfused pancreatic parenchyma. A pancreatic abscess is a circumscribed intraabdominal collection of pus, usually in close proximity to pancreatic necrosis, which arises as a consequence of acute pancreatitis.36
Infected pancreatic necrosis should be suspected in patients with acute pancreatitis with clinical signs of sepsis. This diagnosis also should be suspected when patients fail to improve with supportive therapy or regress after an initial period of improvement.47 Patients suspected of having infected pancreatic necrosis should undergo contrast-enhanced CT scan or ultrasound-guided fine-needle aspiration.112,115,116 This approach is a safe and reliable way to differentiate between sterile and infected necrosis. Complication rates of this procedure are low. Rare serious complications include bleeding and aggravation of acute pancreatitis. With Gram staining and culture of aspirated material, fine-needle aspiration by ultrasonography has a diagnostic sensitivity of 88% and specificity of 90%.115 Because there is a possibility of contamination of sterile necrosis, fine-needle aspiration is indicated only in these groups of patients: those with signs and symptoms of sepsis, those who fail to improve, and those who worsen after initial clinical improvement.117 Outside of a clinical trial, fine-needle aspiration should not be performed as a matter of routine for patients with SAP who are doing well.118,119 Studies have confirmed infection rates of 2.8% to 22% in the first week and 28.8% to 55% in the second to fourth weeks. The timing of fine-needle aspiration should be based on the probability of infection, based on time of onset from the disease and the current clinical condition of the patient. Some authors do not support the practice of needle aspiration of infection because they use prophylactic antibiotics and would not perform an “early” operation based on cultures obtained from a fine-needle aspirate. Rather, they wait 3 to 4 weeks and if the patient is unwell, operate at that time, whether or not presence of infection has been proven.118
Laboratory Markers Of Infected Necrosis
No reliable blood test has been developed to establish the diagnosis of infected necrosis.114–117 Measurement of serum CRP concentration was the best available blood test for identification of pancreatic necrosis; CRP concentrations greater than 120 mg/L are associated with necrosis.120 There is no correlation, however, between the serum CRP level and the presence of infected necrosis. Procalcitonin is a 116–amino acid propeptide of calcitonin that has been shown to be a marker for severe bacterial and fungal infection. In the meta-analysis by Mofidi et al., the sensitivity and specificity of procalcitonin for predicting infected pancreatic necrosis were 0.80 and 0.91, respectively.42
Clinicians must pursue the possibility of infected pancreatic necrosis in order to tailor the use of antibiotics and other forms of therapy. While fine-needle aspirates are an invasive modality and can be subject to sampling error, procalcitonin can be obtained noninvasively and is not altered by antibiotic therapy. Importantly, the clinician must recognize that procalcitonin elevation is a nonspecific marker of potential infection in critically ill patients, and if the procalcitonin level is elevated, a systematic search for all potential sites of infection should follow. However, Rau and colleagues reported that the magnitude of procalcitonin elevation was greatest in patients with intraabdominal as compared with respiratory or urinary tract infections.121
Indication And Timing Of Operative Intervention
Although there is no consensus about the timing of operative intervention for pancreatic necrosis, most experts now recommend delaying operation until infection has been identified.86,112,122,123 An intervention may be delayed until the third or fourth week of ICU care. In the past there was some belief that early surgery might improve outcome by removing necrotic tissue and decreasing the stimulus for systemic inflammation, but this notion has been disproved by clinical trials and experience and now is only of historic interest.124 Delaying as long as possible for any sort of invasive débridement has become the most common approach to managing patients with SAP and necrosis. Early in the course of the disease, the pancreatic tissue is friable, however, and nonviable tissues are not well demarcated. In addition, viable tissue usually is present, even when the gland grossly appears to be completely necrotic. Early operation should be reserved for patients with proven infected necrosis or patients with other surgical complications such as massive bleeding or bowel perforation.118
Sterile Necrosis
Before 1990, the standard surgical practice was to débride necrotic pancreatic tissue operatively, even in the absence of infection.123 Nonoperative management of sterile necrosis is now the standard of care according to several published guidelines. In selected cases, patients with extensive necrosis may not improve, and after a prolonged period of observation (6-8 weeks), operative débridement may be warranted.86,125,126 Sterile pancreatic necrosis has a mortality rate of 0% to 10% when managed using a conservative nonoperative approach.125–127
Operative Procedures
Although there is general agreement that infected necrosis requires operative débridement, there is no consensus about the best approach to achieve this goal, and there are increasing concerns about early open débridement.122,123 When a patient is very ill with sepsis, the primary treatment goal is to achieve drainage of infected material. Open necrosectomy has been associated with high rates of complications (34%-95%)122,123 and death (11%-39%).122,123 Recently the results of the Step-up Approach versus Open Necrosectomy trial were reported. In this trial, the “step-up approach” consisted of percutaneous drainage followed by minimally invasive retroperitoneal necrosectomy, if needed, and this strategy was compared with open necrosectomy.122 Thirty-five percent of patients were able to be treated with percutaneous drainage only. The trial’s primary endpoint was a composite of the complications related to the aggressiveness and type of therapy, such as multiple organ failure, bleeding, perforation, and enterocutaneous fistula. Patients who received the step-up approach were less likely to develop complications (40% versus 69%, respectively). Additionally, with the step-up approach, there was less organ failure (12% versus 40%), lower rate of incisional hernia (7% versus 24%), and lower incidence of new-onset diabetes mellitus (16% versus 38%). This important trial is unique because it was a randomized study of the surgical care of infected necrosis rather than a case series reported from one or more institutions. Interestingly, 35% of patients were able to progress to a clinical cure with percutaneous drainage alone. This finding suggests that drainage of purulent material allowed the necrosis to “regress” to sterile necrosis. The findings from this study also suggest that the step-up approach may be beneficial because of a lower level of surgical trauma and therefore activation of inflammatory mediators. The results of this trial are consistent with results of minimally invasive necrosectomy and other less invasive procedures.128–133 However, it is important to note that the trial did not compare open necrosectomy with minimally invasive retroperitoneal drainage.
As shown in older case series, percutaneous drainage of infected necrosis can be achieved in selected patients, especially when the infected material is not too viscous or too loaded with necrotic tissue. Drainage can be achieved via anterior or retroperitoneal approaches and is best achieved using a large-diameter catheter (12-14F).129–133
In recently reported studies, an endoscopic approach has been used to achieve drainage.131–133 Pancreatic drainage can be achieved using natural orifice transluminal endoscopic surgery (NOTES). Endoscopic ultrasound is used to identify collections through the wall of the stomach. Using a Seldinger-type (guidewire-based) technique, the collection is accessed and dilated serially with 10- to 15-mm balloons. The goal is to create a channel large enough to permit the endoscope to enter the cavity. Once the endoscope is in the cavity, débridement is accomplished using typical endoscopic instruments, paying careful attention to hemostasis. Copious irrigation is carried out before placing a drain. A nasocystic drain is then placed over the wire and is used to retain access and allow irrigation of the collection. Although a few patients have been successfully managed with this approach, it should be noted that they have been highly selected.
Open treatment of infected pancreatic necrosis is still the most commonly employed modality for débridement, but fewer surgeons are using an open approach as the initial treatment modality.122–124 However, even the open surgical treatment has been widely varied, and no study has systematically examined one open approach versus another. The surgeon may elect to perform open necrosectomy and either open or closed drainage, and may plan for selective or routine re-laparotomy. Irrigation and lavage may occur only in the operating room, or irrigation can be carried for intermittent periods postoperatively. Alternatively, irrigation can be performed continuously. Each of these techniques has been used successfully at different centers. Most experienced centers treating this disease now report mortality rates between 10% and 20% for infected pancreatic necrosis.
As previously noted in the step-up approach, the advent of minimally invasive techniques now allows several new approaches to drainage of infected pancreatic material.122 Video-assisted retroperitoneal drainage (VARD) has been popular since 2000.134 VARD drainage uses either a rigid nephroscope or a zero-degree laparoscope to access the retroperitoneum over a wire previously placed into the infected cavity, typically by CT scan. A 5-mm scope and instruments can be used in some patients. The lesser sac has also been approached via the base of the mesocolon with laparoscopic instruments, using hand access for débridement.134–138 A recent report of 18 patients with infected necrosis who were treated using this less invasive strategy demonstrated a length of stay of 16 days and a reduction in major wound complications.138 While VARD has the potential advantage of eliminating peritoneal contamination, commonly many procedures are needed, and the colon and other abdominal contents cannot be examined or treated if needed.
Conventional Resection
In the past, formal pancreatic resections were performed for acute pancreatitis. These procedures have been abandoned in the treatment of SAP, however, because of excessively high rates for complications and mortality.86,26 These procedures do not remove the surrounding necrotic tissue and needlessly remove healthy tissue.
Necrosectomy
Necrosectomy removes devitalized tissue from the pancreas and surrounding retroperitoneum and now can be performed by open or less invasive endoscopic or laparoscopic techniques.125–138 The tissue generally is removed by gentle finger fracture technique when an open approach is employed, and by gentle separation when a less invasive approach is used. Necrosectomy is designed to remove most of the devitalized tissue without injuring major blood vessels; hemostasis must be carefully obtained before the procedure is completed. Repeated drainage procedures may be necessary.
While the general approach for the management of infected pancreatic necrosis is to delay drainage and perform drainage in a less invasive manner, some patients may require open procedures. The open packing technique originally was popularized by Bradley87 et al. and was associated with a mortality of 15%, but morbidity was extensive and included external pancreatic fistulas in 46% of cases, hernias in 32% of cases, and massive venous hemorrhage in 7% of cases. Other centers have employed planned staged procedures or open drainage followed by the placement of drains. In case series using surgical management with drains, the overall mortality rate was only 6.2%. The authors noted a significantly better outcome when surgery was delayed beyond the fourth week. Alternatively, necrosectomy can be followed by closed-suction lavage of the retroperitoneum using 35 to 40 L/d of peritoneal lavage solution for each of the first 7 postoperative days. This approach was successfully used in 42 of 121 patients with pancreatic necrosis. Of the 121 patients, 12 (9.9%) died, including nine patients who were treated surgically and three patients who were treated conservatively.138 Morbidity included pancreatic fistulas in 8 of 42 (19%) surgically treated patients. Pancreatic fistulas after pancreatitis usually close spontaneously eventually if pancreatic ductal obstruction is not present. In a few cases, enteric internal drainage or pancreatic resection may be required to achieve closure of pancreatic fistulas.
Aside from pancreatic infections, patients with SAP are at risk for the usual gamut of nosocomial infections, including catheter-related bloodstream infections, urinary tract infections, and ventilator-associated pneumonia.7 Additional abdominal complications in patients with acute pancreatitis include concurrent biliary tract problems, stress gastritis and related bleeding, necrosis of the transverse colon, hemorrhage from gastric varices secondary to splenic vein thrombosis, and catastrophic bleeding from ruptured pseudoaneurysms involving the gastroduodenal artery or branches of the superior mesenteric artery. Should massive gastrointestinal bleeding occur, and a gastric or proximal duodenal source is excluded, arteriography should be considered. Necrosis of the transverse colon should be considered in a patient with abdominal tenderness and distention and sepsis. Patients with colonic necrosis are usually dramatically ill. Enterocutaneous fistulas are seen commonly when the open packing technique is used and less commonly when other methods of management are employed.
 Outcome
Outcome
With an increasing number of patients surviving SAP, attention has been focused on the quality of life and long-term outcome of surviving patients.140–143 This patient population is subject to a wide range of medical problems, including diabetes mellitus, symptoms of polyneuropathy, recurrent pancreatitis, and continual abdominal pain, with endocrine or exocrine dysfunction occurring in the majority of patients.141 Major social problems also can be an issue, especially among patients with alcohol-induced pancreatitis. Abdominal hernias may be present, especially in patients managed using open packing; future repairs may be needed. Chronic pancreatitis and related problems including pseudocysts, splenic vein thrombosis, and mesenteric pseudoaneurysms can occur but are not discussed here in further detail.
In one study, 35 patients after acute pancreatitis treated with open necrosectomy were evaluated for results on the Short-Form 36 assessment of health-related quality of life.139 Among this cohort of 35 patients, 32 were employed at the time of their SAP, and 12 patients returned to work within 6 months of discharge. SF-36 scores were above 60% in all patients, and 20 of 32 patients has a good quality of life (>70%).139 Patients with alcoholic pancreatitis had the worst outcomes. In 20 survivors of long-term (>30 days) hospital stay after an episode of SAP, 12 of 20 experienced morphologic or endocrine sequelae.141 Problems noted more than 6 months after discharge from the hospital included pancreatic fistulae, stenosis of both the pancreatic and biliary tree, and chronic abdominal pain.133
Key Points
Bradley EL3rd, Dexter ND. Management of severe acute pancreatitis: a surgical odyssey. Ann Surg. 2010;251:6-17.
Babu BI, Sheen AJ, Lee SH, et al. Open pancreatic necrosectomy in the multidisciplinary management of postinflammatory necrosis. Ann Surg. 2010;251:783-786.
Besselink MG, van Santvoort HC, Boermeester MA, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273.
Gaisano HB, Gorelick. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040-2044.
Pezzelli R. Pharmacotherapy for acute pancreatitis. Expert Opin Pharmacother. 2009;10:2999-3014.
van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et alDutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502.
1 Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver disease 2006. Am J Gastroenterol. 2006;101:2128-2138.
2 Fagenholz PJ, Fernandez Castillo C, Harris NS, Pellitier AJ, Camargo CAJr. Direct medical costs of acute pancreatitis hospitalization in the United States. Pancreas. 2007;35:302-307.
3 Charbonney E, Nathens AB. Severe acute pancreatitis: a review. Surg Infect (Larchmt). 2008;9(6):573-578.
4 Whitcomb DC. Acute Pancreatitis. N Engl J Med. 2006;354:2142-2150.
5 Tonsi AF, Bacchion M, Crippa S, Malleo G, Bassi C. Acute pancreatitis at the beginning of the 21st century: the state of the art. World J Gastroenterol. 2009;15(24):2945-2959.
6 Dambrauskas Z, Giese N, Gulbinas A, Giese T, Berberat PO, Pundzius J, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845-1853.
7 Laveda R, Martinez J, Munoz C, Penalva JC, Saez J, Belda G, et al. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World J Gastroenterol. 2005 Sep 14;11(34):5309-5313.
8 Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: A multicentre study. Lancet. 2000;355:1955-1960.
9 Besselink MG, van Santvoort HC, Boermeester MA, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273.
10 Pezelli R. Pharmacotherapy for acute pancreatitis. Expert Opin Pharmacother. 2009;10(18):2999-3014.
11 Uhl W, Buchler MW, Malfertheiner D, et al. A randomized double blind multi-centre trial of octreotide in moderate to severe acute pancreatitis. Gut. 1999;45:97-104.
12 Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2000;48:62-69.
13 Bank S, Singh P, Pooran N, et al. Evaluation of factors that have reduced mortality from acute pancreatitis over the past 20 years. J Clin Gastroenterol. 2002;35:50-60.
14 McKay CJ, Evans S, Sinclar TM, et al. High early mortality of acute pancreatitis in Scotland, 1984-95. Br J Surg. 1999;86:1302-1305.
15 Skipworth JRA, Pereira SP. Acute pancreatitis. Curr Opin Crit Care. 2008;14:172-178.
16 Haas B, Nathens AB. Surgical indications in acute pancreatitis. Curr Opin Crit Care. 2010;16:153-158.
17 Isenmann R, Buchler MW. Infection and acute pancreatitis. Br J Surg. 1994;81:1707-1708.
18 Gigout J, Desjeux A, Vitton V, Gasmi M, Subtil C, Grimaud JC, et al. What is the outcome for patients presenting with severe acute pancreatitis requiring a hospital stay of more than one month? Gastroenterol Clin Biol. 2009 Mar;33(3):210-216. Epub 2008 Oct 25
19 Banks PA. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-4000.
20 Takada T, Hirata K, Mayumi T, et al. Cutting-edge information for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:3-12.
21 Uhl W, Warshaw A, Imrie C, et al. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology. 2002;2:565-573.
22 Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology and prognosis. Curr Gastroenterol Rep. 2009;11:97-103.
23 Mitchell RMS, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447-1455.
24 Gaisano HB, Gorelick. New Insights Into the mechanisms of Pancreatitis. Gastroenterology. 2009;136:2040-2044.
25 Whitcomb DC. Genetic Aspects of Pancreatitis. Annu Rev Med. 2010;61:413-424.
26 Threadgold J, Greenhalf W, Ellis I, et al. The N34S mutation of SPINK1 (PSTI) is associated with a familial pattern of idiopathic chronic pancreatitis but does not cause the disease. Gut. 2002;50:675-681.
27 Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213-216.
28 Truninger K, Witt H, Kock J, et al. Mutations of the serine protease inhibitor, Kazal type 1 gene, in patients with idiopathic chronic pancreatitis. Am J Gastroenterol. 2002;97:1133-1137.
29 Keim V, Teich N, Fiedler F, et al. A comparison of lipase and amylase in the diagnosis of acute pancreatitis in patients with abdominal pain. Pancreas. 1998;16:45-49.
30 Clavien PA, Robert J, Meyer P, et al. Acute pancreatitis and normoamylasemia: Not an uncommon combination. Ann Surg. 1989;210:614-620.
31 Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309-1318.
32 Greer SE, Burchard KW. Acute pancreatitis and critical illness: a pancreatic tale of hypoperfusion and inflammation. Chest. 2009;136:1413-1419.
33 Ranson JH, Rifkind KM, Roses DF, et al. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81.
34 Blamey SL, Imrie CW, O’Neill J, et al. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340-1346.
35 Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818-829.
36 Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: Value of CT in establishing prognosis. Radiology. 1990;174:331-336.
37 Balthazar EJ. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am. 1989;27:19-37.
38 Bradley ELIII. A clinically based classification system for acute pancreatitis: Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13,1992. Arch Surg. 1993;128:586-590.
39 Vege SS, Gardner TB, Chari ST, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis. Am J Gastroenterol. 2009 Mar;104(3):710-715. Epub 2009 Feb 3
40 Lankisch PG, Weber-Dany B, Hebel K, Maisonneuve P, Lowenfels AB. The Harmless Acute Pancreatitis Score: A clinical algorithm for rapid initial stratification of non-severe disease. Clin Gastroenterol Hepatol. 2009 Feb 23.
41 Imamura Y, Hirota M, Ida S, Hayashi N, Watanabe M, Takamori H, et al. Significance of renal rim grade on computed tomography in severity evaluation of acute pancreatitis. Pancreas. 2010 Jan;39(1):41-46.
42 Kiriyama S, Ganata T, Takada T, et al. New diagnostic criteria of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:24-36.
43 Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery. 2009 Jul;146(1):72-81. Epub 2009 May 8
44 Jang T, Uzbielo A, Sineff S, Naunheim R, Scott MG, Lewis LM. Point-of-care urine trypsinogen testing for the diagnosis of pancreatitis. Acad Emerg Med. 2007;14:29-34.
45 Mofidi R, Patil PV, Suttie SA, Parks RW. Risk assessment in acute pancreatitis. Br J Surg. 2009;96:137-150.
46 Brown A, James-Stevenson T, Dyson T, Grunkenmeier D. The Panc 3 score: a rapid and accurate test for predicting severity on presentation in acute pancreatitis. J Clin Gastroenterol. 2007 Oct;41(9):855-858.
47 Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009 Jul-Aug;29(4):1003-1026.
48 Liu TH, Kwong KL, Tamm EP, et al. Acute pancreatitis in intensive care unit patients: Value of clinical and radiologic prognosticators at predicting clinical course and outcome. Crit Care Med. 2003;31:1026-1030.
49 Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967-974.
50 Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast Harmonic Echo-Endoscopic Ultrasound Improves Accuracy in Diagnosis of Solid Pancreatic Masses. Clin Gastroenterol Hepatol. 2010 Apr 24.
51 Spanier BWM, Nio Y, van der Hulst RWM, et al. Practice and Yield of Early CT Scan in Acute Pancreatitis: A Dutch Observational Multicenter Study. Pancreatology. 2010;10:222-228.
52 Uy MC, Daez ML, Sy PP, Banez VP, Espinosa WZ, Talingdan-Te MC. Early ERCP in acute gallstone pancreatitis without cholangitis: a meta-analysis. JOP. 2009 May 18;10(3):299-305.
53 van Santvoort HC, Besselink MG, de Vries AC, Boermeester MA, Fischer K, Bollen TL, et alDutch Acute Pancreatitis Study Group. Early endoscopic retrograde cholangiopancreatography in predicted severe acute biliary pancreatitis: a prospective multicenter study. Ann Surg. 2009 Jul;250(1):68-75.
54 Forsmark CE, Baillie J, AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022-2044.
55 Neoptolemos JP, Carr-Locke DL, London NJ, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983.
56 Fan ST, Lai EC, Mok FP, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232.
57 Nowak A, Nowakowska-Dutawa E, Marek T, et al. Patency of the Santori duct and acute biliary pancreatitis: A prospective ERCP study. Endoscopy. 1990;22:124-126.
58 Folsch UR, Nitsche R, Ludtke R, et al. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237-242.
59 Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. Ann Surg. 2007;245:10-17.
60 Petrov MS, van Santvoort HC, Besselink MG, et al. Early endoscopic retrograde cholangiopancreatography versus conservative management in acute biliary pancreatitis without cholangitis: a meta-analysis of randomized trials. Ann Surg. 2008;247:250-257.
61 Tanner AR, Dwarakanath AD, Tait NP. The potential impact of high-quality MRI of the biliary tree on ERCP workload. Eur J Gastroenterol Hepatol. 2000;12:773-776.
62 Viremouneix L, Monneuse O, Gautier G, et al. Prospective evaluation of non-enhanced mr imaging in acute pancreatitis. J Magn Reson Imaging. 2007;26:331-338.
63 Semelka RC, Kroeker MA, Shoenut JP, Kroeker R, Yaffe CS, Micflikier AB. Pancreatic disease: prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1991;181:785-791.
64 Amano Y, Oishi T, Takahashi M, Kumazaki T. Nonenhanced magnetic resonance imaging of mild acute pancreatitis. Abdom Imaging. 2001;26:59-63.
65 Hirota M, Takada T, Kitamura N. Fundamental and intensive care of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:45-52.
66 Mao EQ, Tang YQ, Fei J, Qin S, Wu J, Li L, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009 Jan 20;122(2):169-173.
67 Dambrauskas Z, Parseliunas A, Gulbinas A, Pundzius J, Barauskas G. Early recognition of abdominal compartment syndrome in patients with acute pancreatitis. World J Gastroenterol. 2009 Feb 14;15(6):717-721.
68 Ino Y, Arita Y, Akashi T, Kimura T, Igarashi H, Oono T, et al. Continuous regional arterial infusion therapy with gabexate mesilate for severe acute pancreatitis. World J Gastroenterol. 2008;14:6382-6387.
69 Takeda K, Matsuno S, Ogawa M, Watanabe S, Atomi Y. Continuous regional arterial infusion (CRAI) therapy reduces the mortality rate of acute necrotizing pancreatitis: result of a cooperative national survey in Japan. J Hepatobiliary Pancreat Surg. 2001;8:216-220.
70 Hughes CB, el-Din AB, Kotb M, Gaber LW, Gaber AO. Calcium channel blockade inhibits release of TNF alpha and improves survival in a rat model of acute pancreatitis. Pancreas. 1996 Jul;13(1):22-28. v
71 Jakobs R, Adamek MU, von Bubnoff AC, Riemann JF. Buprenorphine or procaine for pain relief in acute pancreatitis. A prospective randomized study. Scand J Gastroenterol. 2000;35:1319-1323.
72 Kahl S, Zimmermann S, Pross M, Schulz HU, Schmidt U, Malfertheiner P. Procaine hydrochloride fails to relieve pain in patients with acute pancreatitis. Digestion. 2004;69:5-9.
73 Peiro AM, Martinez J, Martinez E, de Madaria E, Llorens P, Horga JF, et al. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. 2008;8:25-29.
74 Bernhardt A, Kortgen A, Nisel HCH, Goertz A. Using epidural anesthesia in patients with acute pancreatitis—prospective study of 121 patients. Anaesthesiol Reanim. 2002;27:16-22.
75 Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J Parenter Enteral Nutr. 1995;19:453-460.
76 Besselink MG, van Santvoort HC, Renooji W, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg. 2009;250:712-719.
77 Cao Y, Xu Y, Lu T, Gao F, Mo Z. Meta-analysis of enteral nutrition versus total parenteral nutrition in patients with severe acute pancreatitis. Ann Nutr Metab. 2008;53(3-4):268-275. Epub 2009 Jan 9
78 Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, Windsor JA, Gooszen HG. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Arch Surg. 2008 Nov;143(11):1111-1117.
79 Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial. Br J Surg. 1997;84:1665-1669.
80 Meier R, Beglinger C, Layer P, et al. ESPEN guidelines on nutrition in acute pancreatitis. Clin Nutr. 2002;21:173-183.
81 Al-Omran M, Groof A, Wilke D: Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev 2003;1: CD002837.
82 McClave SA, Greene LM, Snider HL, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. J Parenter Enteral Nutr. 1997;21:14-20.
83 Olah A, Belagyi T, Issekutz A, et al. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103-1107.
84 Eatock FC, Brombacher GD, Steven A, et al. Nasogastric feeding in severe acute pancreatitis may be practical and safe. Int J Pancreatol. 2000;28:23-29.
85 Bradley EL3rd, Dexter ND. Management of severe acute pancreatitis: a surgical odyssey. Ann Surg. 2010 Jan;251(1):6-17.
86 Petrov MS, Correia ITD, Windsor JA. Nasogastric tube feeding in predicted severe pancreatitis: a systematic review of the literature to determine safety and tolerance. JOP (online). 2008;9(4):440-448.
87 De Waele JJ. Use of antibiotics in severe acute pancreatitis. Expert Rev Anti Infect Ther. 2010 Mar;8(3):317-324.
88 García-Barrasa A, Borobia FG, Pallares R, et al. A double-blind, placebo-controlled trial of ciprofloxacin prophylaxis in patients with acute necrotizing pancreatitis. J Gastrointest Surg. 2009;13:768-774.
89 Luiten EJ, Hop WC, Lange JF, et al. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995;222:57-65.
90 Pederzoli P, Bassi C, Vesentini S, et al. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480-483.
91 Sainio V, Kemppainen E, Puolakkainen P, et al. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995;346:663-667.
92 Schwarz M, Isenmann R, Meyer H, et al. Antibiotic use in necrotizing pancreatitis: Results of a controlled study. Dtsch Med Wochenschr. 1997;122:356-361.
93 Delcenserie R, Yzet T, Ducroix JP. Prophylactic antibiotics in treatment of severe acute alcoholic pancreatitis. Pancreas. 1996;13:198-201.
94 Takeda K, Matsuno S, Sunamura M, et al. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. Am J Surg. 1996;171:394-398.
95 Ho HS, Frey CF. The role of antibiotic prophylaxis in severe acute pancreatitis. Arch Surg. 1997;132:487-493.
96 Nordback I, Sand J, Saaristo R, Paajanen H. Early treatment with antibiotics reduces the need for surgery in acute necrotizing pancreatitis: A single-center randomized study. J Gastrointest Surg. 2001;5:113-118.
97 Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, et alGerman Antibiotics in Severe Acute Pancreatitis Study Group. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004 Apr;126(4):997-1004.
98 Dellinger EP, Tellado JM, Soto NE, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674-683.
99 Røkke O, Harbitz TB, Liljedal J, Pettersen T, Fetvedt T, Heen LØ, et al. Early treatment of severe pancreatitis with imipenem: a prospective randomize clinical trial. Scand J Gastroenterol. 2007;42:771-776.
100 Xue P, Deng LH, Zhang ZD, Yang XN, Wan MH, Song B, Xia Q. Effect of antibiotic prophylaxis on acute necrotizing pancreatitis: results of a randomized controlled trial. J Gastroenterol Hepatol. 2009 May;24(5):736-742. Epub 2009 Feb 12
101 Sharma VK, Howden CW. Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: A meta-analysis. Pancreas. 2001;22:28-31.
102 Golub R, Siddiqi F, Pohl D. Role of antibiotics in acute pancreatitis: A meta-analysis. J Gastrointest Surg. 1998;2:496-503.
103 Avard B, Fergusson J. Necrotizing pancreatitis and antibiotic prophylaxis. To use or not to use … that is the question. J Gastroenterol Hepatol. 2009 May;24(5):710-711.
104 Bai Y, Gao J, Zou DW, Li ZS. Antibiotics prophylaxis in acute necrotizing pancreatitis: an update. Am J Gastroenterol. 2010 Mar;105(3):705-707.
105 Wittau M, Hohl K, Mayer J, Henne-Bruns D, Isenmann R. The weak evidence base for antibiotic prophylaxis in severe acute pancreatitis. Hepatogastroenterology. 2008 Nov-Dec;55(88):2233-2237.
106 Jafri NS, Mahid SS, Idstein SR, Hornung CA, Galandiuk S. Antibiotic prophylaxis is not protective in severe acute pancreatitis: a systematic review and meta-analysis. Am J Surg. 2009 Jun;197(6):806-813. Epub 2009 Feb 13
107 Hart PA, Bechtold ML, Marshall JB, Choudhary A, Puli SR, Roy PK. Prophylactic antibiotics in necrotizing pancreatitis: a meta-analysis. South Med J. 2008 Nov;101(11):1126-1131.
108 Petrov MS. Meta-analyses on the prophylactic use of antibiotics in acute pancreatitis: many are called but few are chosen. Am J Gastroenterol. 2008 Jul;103(7):1837-1838. author reply 1838-9
109 Xu T, Cai Q. Prophylactic antibiotic treatment in acute necrotizing pancreatitis: results from a meta-analysis. Scand J Gastroenterol. 2008;43(10):1249-1258.
110 de Vries AC, Besselink MG, Buskens E, Ridwan BU, Schipper M, van Erpecum KJ, et al. Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology. 2007;7(5-6):531-538. Epub 2007 Sep 27
111 Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD002941. Update in: Cochrane Database Syst Rev. 2010;5:CD002941. PubMed PMID: 17054156.
112 Heinrich S, Schäfer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006 Feb;243(2):154-168.
113 Buchler M, Malfertheiner P, Friess H, et al. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology. 1992;103:1902-1910.
114 Besselink MG, Timmerman HM, Buskens E, Nieuwenhuijs VB, Akkermans LM, Gooszen HG, Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]. BMC Surg. 2004 Sep 29;4:12.
115 Bakker OJ, van Santvoort HC, Besselink MG, van der Harst E, Hofker HS, Gooszen HG. Prevention, detection, and management of infected necrosis in severe acute pancreatitis. Curr Gastroenterol Rep. 2009 Apr;11(2):104-110.
116 Kochhar R, Ahammed SK, Chakrabarti A, Ray P, Sinha SK, Dutta U, et al. Prevalence and outcome of fungal infection in patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2009 Feb 4.
117 De Waele JJ, Vogelaers D, Blot S, Colardyn F. Fungal infection in patients with severe acute pancreatitis and the use of prophylactic therapy. Clin Infect Dis. 2003;37:208-213.
118 Rau B, Pralle U, Mayer JM, Beger HG. Role of ultrasonographically guided fine-needle aspiration cytology in the diagnosis of infected pancreatic necrosis. Br J Surg. 1998;85:179-184.
119 Gerzof SG, Banks PA, Robbins AH, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology. 1987;93:1315-1320.
120 Banks P. Is computerized Tomographic Fine Needle Aspiration Helpful in the Management of Infected Pancreatic Necrosis. Pro Am J Gastroenterol. 2005;100:2371-2374.
121 Pappas T. Is computerized Tomographic Fine Needle Aspiration Helpful in the Management of Infected Pancreatic Necrosis. Con Am J Gastroenterol. 2005;100:2371-2374.
122 Hartwig W, Werner J, Uhl W, et al. Management of infection in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:423-428.
123 Wilson C, Heads A, Shenkin A, et al. C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg. 1989;76:177-181.
124 Rau BM, Kemppainen EA, Gumbs AA, Buchler MW, Wegscheider K, Bassi C, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007;245:745-754.
125 van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et alDutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010 Apr 22;362(16):1491-1502.
126 Babu BI, Sheen AJ, Lee SH, et al. Open Pancreatic Necrosectomy in the Multidisciplinary Management of Postinflammatory Necrosis. Ann Surg. 2010;251:783-786.
127 Mier J, Leon EL, Castillo A, et al. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71-75.
128 Beger HG, Buchler M, Bittner R, OettingerW, Block S, Nevalainen T. Necrosectomy and postoperative local lavage in patients with necrotizing pancreatitis: results of a prospective clinical trial. World J Surg. 1988;12:255-262.
129 Traverso LW, Kozarek RA. Pancreatic necrosectomy: definitions and technique. J Gastrointest Surg. 2005;9:436-439.
130 Rau B, Bothe A, Beger HG. Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19-year, single-center series. Surgery. 2005;138:28-39.
131 Ross A, Gluck M, Irani S, Hauptmann E, Fotoohi M, Siegal J, et al. Combined endoscopic and percutaneous drainage of organized pancreatic necrosis. Gastrointest Endosc. 2010 Jan;71(1):79-84. Epub 2009 Oct 27
132 Navaneethan U, Vege SS, Chari ST, Baron TH. Minimally invasive techniques in pancreatic necrosis. Pancreas. 2009 Nov;38(8):867-875.
133 Ang TL, Teo EK, Fock KM. Endoscopic drainage and endoscopic necrosectomy in the management of symptomatic pancreatic collections. J Dig Dis. 2009 Aug;10(3):213-224.
134 Gupta R, Wig JD, Bhasin DK, Singh P, Suri S, Kang M, et al. Severe acute pancreatitis: the life after. J Gastrointest Surg. 2009 Jul;13(7):1328-1336. Epub 2009 May 5
135 Becker V, Huber W, Meining A, Prinz C, Umgelter A, Ludwig L, et al. Infected necrosis in severe pancreatitis–combined nonsurgical multi-drainage with directed transabdominal high-volume lavage in critically ill patients. Pancreatology. 2009;9(3):280-286. Epub 2009 Apr 29
136 Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009 Sep;58(9):1260-1266. Epub 2009 Mar 11
137 Risse O, Auguste T, Delannoy P, Cardin N, Bricault I, Létoublon C. Percutaneous video-assisted necrosectomy for infected pancreatic necrosis. Gastroenterol Clin Biol. 2004;28:868-871.
138 Cheung MT, Ho CN, Siu KW, Kwok PC. Percutaneous drainage and necrosectomy in the management of pancreatic necrosis. ANZ J Surg. 2005;75:204-207.
139 Haan JM, Scalea TM. Laparoscopic debridement of recurrent pancreatic abscesses in the hostile abdomen. Am Surg. 2006;72:511-514.
140 Bucher P, Pugin F, Morel P. Minimally invasive necrosectomy for infected necrotizing pancreatitis. Pancreas. 2008;36:113-119.
141 Cinquepalmi L, Boni L, Dionigi G, Rovera F, Diurni M, Benevento A, et al. Long-term results and quality of life of patients undergoing sequential surgical treatment for severe acute pancreatitis complicated by infected pancreatic necrosis. Surg Infect (Larchmt). 2006;7(Suppl 2)):S113-S116.
142 Hochman D, Louie B, Bailey R. Determination of patient quality of life following severe acute pancreatitis. Can J Surg. 2006 Apr;49(2):101-106.
143 Soran A, Chelluri L, Lee KK, Tisherman SA. Outcome and quality of life of patients with acute pancreatitis requiring intensive care. J Surg Res. 2000 Jun 1;91(1):89-94.