Chapter 32 Acute Nerve Injuries
• Efficient identification of the injured nerve(s) and characterization of the nature and severity of injury facilitate the optimal evaluation and treatment of acute peripheral nerve injuries: obtaining a pertinent history and performing a directed physical examination are paramount. Each peripheral nerve is composed of fibers from more than one spinal nerve root; correspondingly, each spinal nerve contributes fibers to more than one peripheral nerve. Consequently, whereas spinal nerve lesions manifest in the clinical picture of radiculopathies with dermatomal sensory disturbances and mild to moderate weakness of muscles supplied by the spinal nerve, peripheral nerve lesions manifest in more precise borders of sensory disturbances acutely and more severe atrophy and paresis of muscles supplied solely by the peripheral nerve as time elapses.
• Use of electrodiagnostic and radiographic studies can augment the clinical evaluation when performed at the appropriate time and for specific indications. For example, the presence of intraoperative nerve action potentials (NAPs) across an involved segment implies that neurolysis alone is adequate for neural recovery.
• A preganglionic injury implies injury proximal to the dorsal root ganglion with permanent paralysis of the muscles innervated by the avulsed roots, complete sensory loss of the corresponding dermatomes, and most importantly, the preclusion of spontaneous recovery. A postganglionic injury potentially retains function of the cell body within the ventral horn of the spinal cord, and these neurons may regenerate axons in the appropriate conditions.
• Surgical options for nerve repair/reconstruction include neurolysis, nerve repair with and without graft, and nerve transfers. Direct (end-to-end) repair is possible only if a short nerve gap exists after resection of the nonfunctioning neural segment. Direct repair is preferred over indirect (graft) repair because of better functional results. Tension across the repair must be avoided to reduce the risk of failure of functional regeneration.
• Management of patient expectations is critical because recovery after nerve injury can take months to years. Nerve repair/reconstruction can be augmented with muscle/tendon transfers to maximize functional outcome.
Acute peripheral nerve injury may result from penetrating trauma, blunt trauma, compression, electrical and iatrogenic causes. The peripheral nerve is severed sharply in 30% of soft tissue lacerations,1 or it can remain grossly in continuity but with varying degrees of intraneural trauma from contusion and stretch. In 15% of nerve injuries associated with a potentially transecting mechanism, the peripheral nerve is only partially severed.2 Despite appearances, the injured peripheral nerve ultimately manifests in sensory and motor disturbances. The subsequent evaluation and treatment of acute peripheral nerve injuries rely upon the efficient identification of the nerve and the site affected and the characterization of the nature and severity of the injury. The challenge to the nerve surgeon remains in the optimal management of nerve injuries that will maximize functional outcomes.
Evaluating the Patient With an Acute Peripheral Nerve Injury
Anatomy and Physiology
A few basic principles underlie the anatomy of the peripheral nervous system. Each peripheral nerve is composed of fibers from more than one spinal nerve root; correspondingly, each spinal nerve contributes fibers to more than one peripheral nerve. Consequently, whereas spinal nerve lesions manifest in the clinical picture of radiculopathies with dermatomal sensory disturbances and mild to moderate weakness of muscles supplied by the spinal nerve, peripheral nerve lesions manifest in more precise borders of sensory disturbances acutely and more severe atrophy and paresis of muscles supplied solely by the peripheral nerve as time elapses.
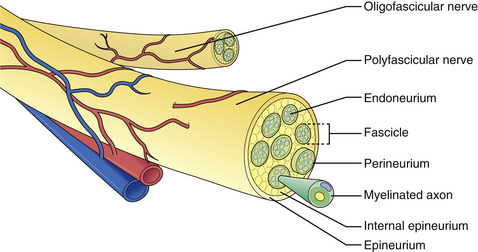
The microscopic anatomy of the peripheral nerve comprises nerve fibers, fascicles, connective tissue (epi-, peri-, and endoneurium), blood vessels, lymphatics, and nervi nervorum (Fig. 32.1). Although the fascicular pattern may change as the nerve courses more distally, the general structure of the nerve remains constant. After a peripheral nerve is injured, a coordinated sequence of events occurs to remove the damaged tissue and ultimately initiates the regenerative process. When the nerve is disrupted, the severed ends retract owing to the elasticity of the endoneurium. Trauma to the vasa nervorum leads to robust inflammation triggering fibroblasts to proliferate to form the basis for a dense scar at the injury site: in the most severe cases, the nerve ends become markedly disorganized, with fibroblasts, macrophages, capillaries, Schwann cells, and collagen fibers within which the regenerating axons form disorganized masses known as neuromas. The degree of damage sustained by the proximal segment and neuronal cell body depends on the distance of the zone of injury from the cell body. The nucleus migrates to the periphery of the cell and select cytoplasmic elements (e.g., Nissl granules, endoplasmic reticulum) undergo chromatolysis. Cell survival relies upon the Schwann cells and trophic molecules present in the immediate environment.
When a peripheral nerve is injured, the accompanying muscle is denervated. Denervation leads to a series of structural changes and atrophy of the muscle if neural regeneration does not occur. Atrophy is seen as a mean 70% reduction in the cross-sectional area after 2 months.3 Sodium channels regress toward embryonic forms with altered biochemical properties, and acetylcholine receptors redistribute to cover the entire muscle surface. This supersensitivity to acetylcholine manifests clinically as spontaneous uncoordinated muscle activity, otherwise known as fibrillation. Death of muscle fibers generally does not occur, but when it does, dropout occurs between 6 and 12 months after denervation.
Pertinent History of Injury
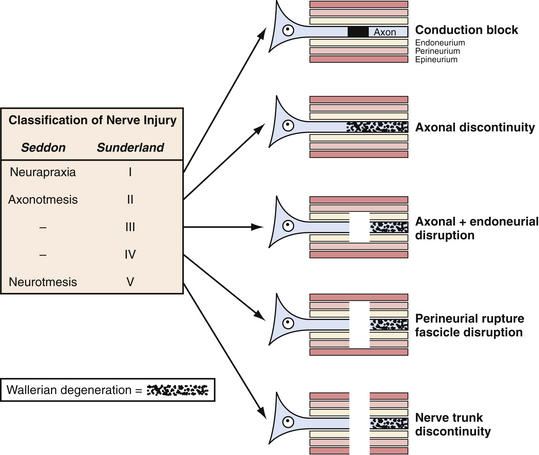
The description of the mechanism of injury can also yield information about the severity of injury to the nerve and guide treatment. For instance, if the injury involved low/no impact such as a fall from a standing position, neurapraxia will be the likely result. In contrast, if there is high impact as in extreme sports or high-speed motor vehicle accidents, then the likely injury is axonotmesis or neuronotmesis. This classification of nerve injury described by Seddon and associates in 19434 and expanded by Sunderland and colleagues in 19515 (Fig. 32.2) remains useful today, although most injuries occur along the continuum of pure grades.
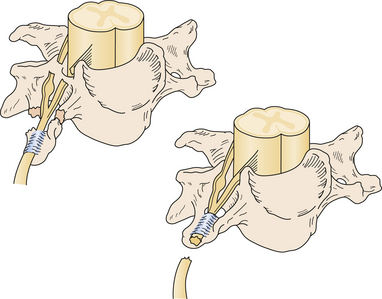
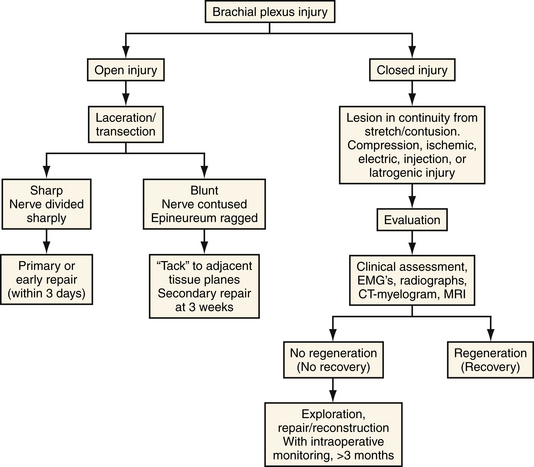
Nerve injuries can also be classified as preganglionic or postganglionic, with profound surgical implications (Fig. 32.3). A preganglionic injury implies injury proximal to the dorsal root ganglion with permanent paralysis of the muscles innervated by the avulsed roots, complete sensory loss of the corresponding dermatomes, and most importantly, the preclusion of spontaneous recovery. A postganglionic injury potentially retains function of the cell body within the ventral horn of the spinal cord and these neurons may regenerate axons in the appropriate conditions. Acute nerve injuries can be classified as open or closed. Open injuries include sharp lacerations and missile wounds whereas closed injuries include traction or compressive injuries. Current guidelines recommend early repair (days) of clean, sharp nerve lacerations, and the delayed (weeks) repair of ragged or dirty nerve lacerations. Missile wounds with vascular injury should be explored acutely. Early intervention is generally not recommended in closed injuries because of the potential for neurapraxic injury. Spontaneous regeneration, when it occurs, generally yields superior functional outcomes when compared to iatrogenic repair or reconstruction. Consideration of the clinical implications of these issues led to an algorithm for the surgical management of peripheral nerve and brachial plexus injuries6 (Fig. 32.4).
Directed Physical Examination
The directed physical examination can be divided arbitrarily into several parts (Box 32.1). Inspection and observation initiate the examination. The presence of bruises, abrasions, and obvious lacerations can lead the examiner to the site of nerve injury: for example, road rash over the shoulder implies an upper brachial plexus injury, whereas road rash in the axilla extending along the chest wall implies a lower brachial plexus injury. Likewise, Horner’s sign (ptosis, meiosis, anhydrosis) is indicative of a potential proximal T1/lower trunk brachial plexus lesion. Significant swelling can imply musculoskeletal injury such as a ruptured muscle/tendon in addition to the nerve injury in the absence of motor function. As described earlier, the observation of fasciculations and atrophy with time are also consistent with peripheral nerve injury.
An assessment of the relevant vasculature is a critical part of the directed physical examination because of the adjacent course of nerves and vessels. For example, the cords of the brachial plexus surround the axillary artery, and the median nerve courses adjacent to the brachial artery. Injury to the vessel can indicate simultaneous injury to the nerve, and the integrity of both should be assessed in the acute situation.
The motor examination should be conducted in the context of range of motion. If the range of motion is acutely compromised, musculoskeletal injury should be considered and motor power can be difficult to assess. To assess nerve function, there is no substitute for a thorough motor examination; the complete description of the motor examination lies outside the scope of this chapter but can be found in Aids to the Examination of the Peripheral Nervous System,7 among other texts.8 The importance of a thorough assessment of relevant muscles cannot be overemphasized because the standard cursory trauma examination can be misleading. Muscles in the distribution of noninjured nerves can simulate the motor function of the injured nerve to the inexperienced examiner: abduction of the fingers (ulnar nerve) can be mistaken for extension of the fingers (radial nerve), leading to the incorrect conclusion that the radial nerve is intact. A systematic examination of all muscles supplied by the relevant nerve(s) can preclude such errors.
Ancillary Studies
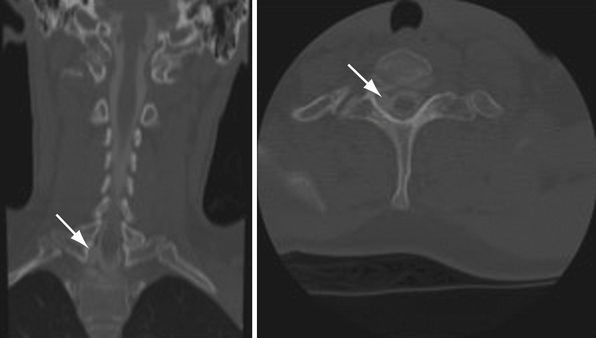
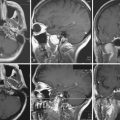
Radiographic studies may include long bone, cervical spine, and chest radiographs, which can reveal associated fractures, and in the case of brachial plexus injury, can demonstrate an elevated hemidiaphragm (phrenic nerve injury). Postmyelogram computed tomography (CT) and magnetic resonance imaging (MRI) can be used to detect pseudomeningoceles (Fig. 32.5), which are often associated with preganglionic injuries. The present resolution provided by standard imaging techniques is yet unable to demonstrate direct evidence of the site and severity of nerve injury.
Surgical Nerve Repair/Reconstruction
Surgical Options
Neurolysis is the removal of scar tissue from around the nerve (external neurolysis) or between fascicles (internal neurolysis) if the nerve is injured asymmetrically. Dissection is started with proximal and distal uninjured nerve ends toward the involved injured segment. Intraoperative EDS can determine if the injured segment can conduct electric signal. The presence of nerve action potentials (NAPs) across an involved segment implies that neurolysis alone is adequate for neural recovery (Fig. 32.6).9
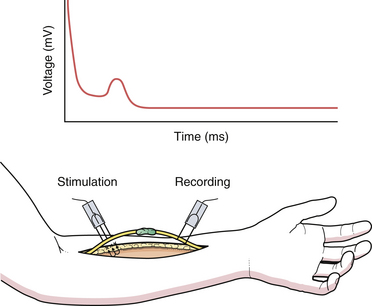
FIGURE 32.6 Nerve action potential electrodiagnostic tracing.
(From Spinner RJ, Kline DG. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve 2000;23(5):680-695.)
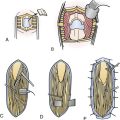
Graft (indirect repair) is needed when the gap that exists after resection of the nonfunctioning neural segment is too large for direct approximation without tension (Fig. 32.7). The length of the graft should be the length of the gap plus 10%. Autograft is usually used and derived from sural nerve, medial antebrachial cutaneous nerve, or superficial radial nerve because these small-caliber grafts achieve better results (faster revascularization) than larger-caliber grafts.10 Coaptation of the ends of the graft to the proximal and distal stumps is similar to that used for the direct repair. Artificial grafts are under active investigation but are not yet routinely used for repair of major nerves.
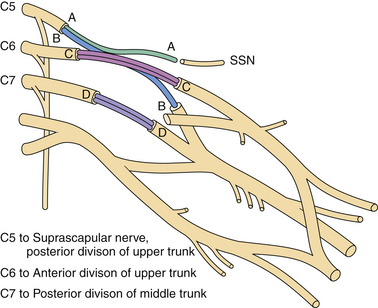
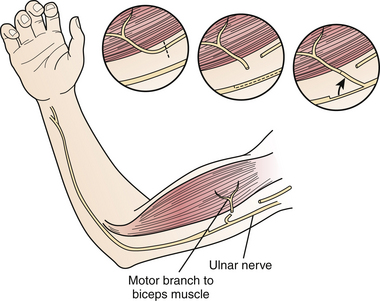
Nerve transfers/neurotization have augmented the surgical arena for the treatment of peripheral nerve injuries, particularly of brachial plexus injuries, especially in the context of avulsion (preganglionic) injury.11,12 Nerve transfers have changed the outlook for the “unrepairable” injuries to “reconstructable” injuries by coapting a proximal functional nerve donor with a distal denervated nerve to reinnervate the latter with healthy donor axons. Popularized nerve transfer techniques include neurotization of the spinal accessory to the suprascapular nerve13–16 or radial branch to axillary nerve17,18 to achieve shoulder abduction and intercostal,19–21 medial pectoral,22,23 phrenic,24,25 or ulnar fascicular nerve transfer to the musculocutaneous nerve (Fig. 32.8)26,27 for elbow flexion. Recovery of the translated function appears to rely upon cortical plasticity.28
Outcomes After Nerve Surgery
Outcomes for functional repair of sharp lacerations of the median and radial nerves have been reported to be as high as 91% in contrast to 73% for ulnar nerves; similarly, for graft repair, functional outcomes were better for the median (68%) and radial nerves (67%) than for ulnar nerves (56%).29 Outcomes for ulnar fascicular transfer to the musculocutaneous branch to biceps has been reported at 75% for antigravity elbow flexion with an average time for reinnervation at 5 months.27
1. Ducker T.B., Garrison W.B. Surgical aspects of peripheral nerve trauma. Curr Probl Surg. 1974:1-62. Sep
2. Kline D.G. Physiological and clinical factors contributing to the timing of nerve repair. Clin Neurosurg. 1977;24:425-455.
3. Burnett M.G., Zager E.L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16(5):E1.
4. Seddon H.J., Medawar P.B., Smith H. Rate of regeneration of peripheral nerves in man. J Physiol. 1943;102(2):191-215.
5. Sunderland D.A., Smith W.E., Sugiura K. The pathology and growth behavior of experimental tumors induced by certain petroleum products. Cancer. 1951;4(6):1232-1245.
6. Dubuisson A., Kline D.G. Indications for peripheral nerve and brachial plexus surgery. Neurol Clin. 1992;10(4):935-951.
7. O’Brien M. On behalf of the Guarantors of Brain. Aids to the Examination of the Peripheral Nervous System, 5th ed. Edinburgh: Saunders Elsevier; 2010.
8. Russell S.M. Examination of Peripheral Nerve Injuries: An Anatomical Approach. New York: Thieme; 2006.
9. Spinner R.J., Kline D.G. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23(5):680-695.
10. Millesi H. Reappraisal of nerve repair. Surg Clin North Am. 1981;61(2):321-340.
11. Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16(5):E5.
12. McGillicuddy J.E. Clinical decision making in brachial plexus injuries. Neurosurg Clin North Am. 1991;2(1):137-150.
13. Hattori Y., Doi K., Fuchigami Y., et al. Experimental study on donor nerves for brachial plexus injury: comparison between the spinal accessory nerve and the intercostal nerve. Plast Reconstr Surg. 1997;100(4):900-906.
14. Samardzic M., Grujicic D., Antunovic V., Joksimovic M. Reinnervation of avulsed brachial plexus using the spinal accessory nerve. Surg Neurol. 1990;33(1):7-11.
15. Merrell G.A., Barrie K.A., Katz D.L., Wolfe S.W. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am. 2001;26(2):303-314.
16. Malessy M.J., de Ruiter G.C., de Boer K.S., Thomeer R.T. Evaluation of suprascapular nerve neurotization after nerve graft or transfer in the treatment of brachial plexus traction lesions. J Neurosurg. 2004;101(3):377-389.
17. Witoonchart K., Leechavengvongs S., Uerpairojkit C., et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part I: an anatomic feasibility study. J Hand Surg Am. 2003;28(4):628-632.
18. Kawai H., Akita S. Shoulder muscle reconstruction in the upper type of the brachial plexus injury by partial radial nerve transfer to the axillary nerve. Tech Hand Up Extrem Surg. 2004;8(1):51-55.
19. Friedman A.H., Nunley J.A.2nd, Goldner R.D., et al. Nerve transposition for the restoration of elbow flexion following brachial plexus avulsion injuries. J Neurosurg. 1990;72(1):59-64.
20. Malessy M.J., Thomeer R.T. Evaluation of intercostal to musculocutaneous nerve transfer in reconstructive brachial plexus surgery. J Neurosurg. 1998;88(2):266-271.
21. Waikakul S., Wongtragul S., Vanadurongwan V. Restoration of elbow flexion in brachial plexus avulsion injury: comparing spinal accessory nerve transfer with intercostal nerve transfer. J Hand Surg Am. 1999;24(3):571-577.
22. Blaauw G., Slooff A.C. Transfer of pectoral nerves to the musculocutaneous nerve in obstetric upper brachial plexus palsy. Neurosurgery. 2003;53(2):338-341. discussion 341-342
23. Samardzic M., Grujicic D., Rasulic L., Bacetic D. Transfer of the medial pectoral nerve: myth or reality? Neurosurgery. 2002;50(6):1277-1282.
24. Songcharoen P. Brachial plexus injury in Thailand: a report of 520 cases. Microsurgery. 1995;16(1):35-39.
25. Luedemann W., Hamm M., Blomer U., et al. Brachial plexus neurotization with donor phrenic nerves and its effect on pulmonary function. J Neurosurg. 2002;96(3):523-526.
26. Oberlin C., Ameur N.E., Teboul F., et al. Restoration of elbow flexion in brachial plexus injury by transfer of ulnar nerve fascicles to the nerve to the biceps muscle. Tech Hand Up Extrem Surg. 2002;6(2):86-90.
27. Teboul F., Kakkar R., Ameur N., et al. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg Am. 2004;86-A(7):1485-1490.
28. Malessy M.J., Thomeer R.T., van Dijk J.G. Changing central nervous system control following intercostal nerve transfer. J Neurosurg. 1998;89(4):568-574.
29. Murovic J.A. Upper-extremity peripheral nerve injuries: a Louisiana State University Health Sciences Center literature review with comparison of the operative outcomes of 1837 Louisiana State University Health Sciences Center median, radial, and ulnar nerve lesions. Neurosurgery. 2009;65(Suppl 4):A11-A17.