CHAPTER 344 Acute Medical Management of Ischemic/Hemorrhagic Stroke
Stroke is the second most common cause of mortality in people 60 years and older worldwide.1 Each year, 5.54 million die of stroke worldwide. In the United States each year, 780,000 people have a new or recurrent stroke, and on average, every 40 seconds someone in the United States has a stroke.
Therapeutic interventions after acute stroke must be initiated early because irreversible neuronal damage can occur within minutes.2,3 The extent of ischemic injury is dependent on the specific neuronal vulnerability and residual CBF through the collateral circulation.4 Early reperfusion of the infarcted area can reduce the degree of ischemia and improve neurological outcome. Late reperfusion has been associated with increased hemorrhage inside the ischemic area (hemorrhagic conversion) and increased edema (reperfusion injury).
Currently, the only approved reperfusion therapy after ischemic stroke is tissue plasminogen activator (t-PA) administered within 3 hours of stroke onset.5 In addition, a recently published report showed improved outcome in selected patients when t-PA was administered within 4.5 hours.6 Other proven interventions after hemorrhagic and ischemic stroke include blood pressure management and supportive therapies such as glucose and temperature control.
Pathophysiology of Ischemic Stroke
Most acute focal ischemic events in the brain are due to embolism or in situ thrombosis.7 Thrombogenesis is dependent on a series of complex events that promote the formation of a stable clot. Platelet aggregation, endothelial injury, and fibrin formation are key components. Angiographic studies have revealed several preferential sites for atherothrombotic lesions. Lesions in the proximal internal carotid artery and common carotid artery bifurcation are found in 50% to 80% of ischemic stroke patients, and a cardioembolic source is suspected in approximately 15% to 20% of all ischemic strokes. Although any cardiac disease is a potential source of embolization, the clinical conditions most often associated are nonvalvular atrial fibrillation, prosthetic heart valves, acute myocardial infarction, rheumatic heart disease, and ventricular aneurysm. Hereditary or acquired hemostatic disorders associated with thrombotic conditions generally involve the venous rather than the arterial circulation and are frequently seen in young adult stroke patients (4% to 5%).
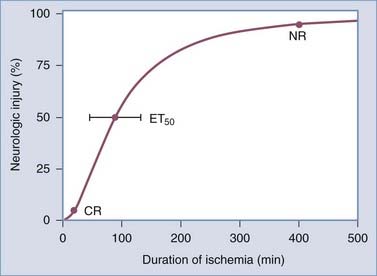
Data obtained from animal studies and clinical observations suggest that the amount of time before ischemia produces irreversible injury is relatively brief. Irreversible focal damage begins within several minutes after a significant reduction in CBF and is complete within approximately 6 hours. Figure 344-1 depicts the relationship between the duration of ischemia and the severity of the resulting neurological deficit.3 As the duration of ischemia is extended, there is a brief period during which the neurological injury is completely reversible if blood flow is restored. This type of injury is termed a transient ischemic attack. In a short amount of time, however, an ischemic event can result in irreversible injury, and as the ischemic period increases, the degree of the resulting neurological deficit increases. Ultimately, a point is reached where the injury produced becomes permanent and no intervention will result in tissue recovery.
In most cases the ischemic area is dynamic. Some regions supplied by the affected blood vessel are densely ischemic and require prompt restoration of blood flow to prevent irreversible damage. Other areas still receive some perfusion and are potentially salvageable if the cascade of cellular events resulting in neuronal death is blocked. In the past, this was simplistically viewed as an ischemic core surrounded by a region of incomplete ischemia. The latter has been referred to as the ischemic penumbra.8 The size and duration of the penumbra are unknown for any individual patient, and the topographic relationship between the ischemic core and penumbra is much more complex than initially thought based on recent preclinical and brain imaging studies.9
Pathophysiology of Hemorrhagic Stroke
Hemorrhagic stroke is caused by rupture of an intracranial blood vessel. In Western countries, 10% to 20% of strokes are caused by intracranial hemorrhage (ICH).10 Thirty-day mortality is higher than with ischemic stroke (50% versus 19%) and is affected by hematoma volume, patient age, admission Glasgow Coma Scale score, location of the hematoma above or below the tentorium, and the presence of intraventicular hematoma.11 The major risk factor for ICH is chronic arterial hypertension. Most of these hemorrhages occur in the thalamus, basal ganglia, cerebellum, or pons. In the elderly, especially those suffering from Alzheimer’s dementia, amyloid angiopathy is a frequent cause of ICH. Hemorrhage from this condition affects mostly the cortical areas. Another major cause of ICH is the use of anticoagulants. Warfarin is most often used after deep venous thrombosis or in patients with atrial fibrillation for the prevention of cardioembolic stroke.12
Patient Evaluation
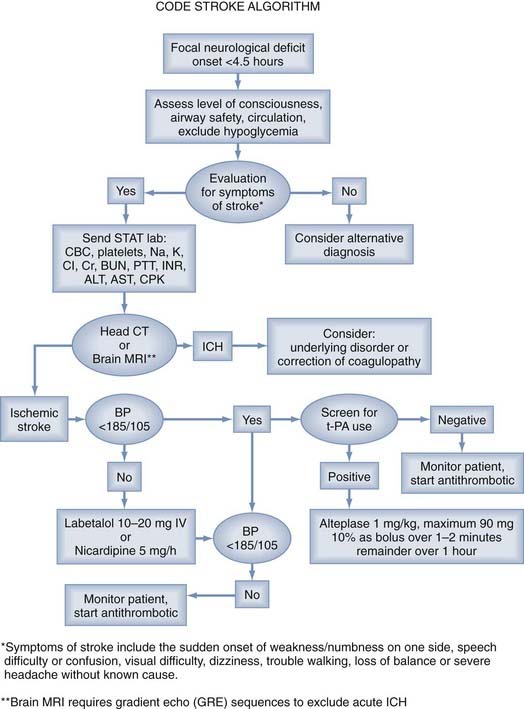
All patients with acute focal neurological symptoms should be evaluated immediately13 (Fig. 344-2). The evaluation must be brief and is similar to that for other critically ill patients. Special attention should be paid to the vital signs, including serial blood pressure measurements and level of consciousness. The history should include the time of symptom onset and any occurrence of similar neurological events, including the existence of stroke risk factors (arterial hypertension, diabetes, cardiac arrhythmia). Disease processes that can mimic stroke symptoms (migraine, hypoglycemia, postictal paralysis) should be excluded as causes if possible. In addition, the presence of serious coexisting illnesses and the recent use of oral anticoagulants should be determined.
During the brief initial evaluation, physical examination may be enhanced by the use of formal stroke scores, such as the National Institutes of Health Stroke Scale (NIHSS) (Table 344-1). The score helps ensure that the core elements of the neurological examination are assessed in timely manner, aids in communication with other health care providers, helps identify the location and extent of the neurological deficit, and provides early measures for prognosis.
Even though CT has been in routine clinical use for more than 30 years and has high sensitivity for the detection of acute ICH,14 MRI is being used increasingly frequently for the acute evaluation of stroke. Initially, MRI was inferior to CT in the detection of hyperacute blood,15 but since the introduction of gradient echo sequencing, higher sensitivity has been reported.16 Whether MRI will be as useful as CT in guiding treatment remains to be seen.
Other diagnostic tests for immediate evaluation after stroke are summarized in Table 344-2. These tests must include blood glucose, serum electrolytes, renal function, complete blood count with platelets, and coagulation studies. Markers of cardiac ischemia and an electrocardiogram should be assessed to evaluate for the possibility of myocardial infarction.
TABLE 344-2 Immediate Diagnostic Studies: Evaluation of a Patient with Suspected Acute Ischemic Stroke
CT, computed tomography; INR, international normalized ratio; ECG, electrocardiography; MRI, magnetic resonance imaging.
* Although it is desirable to know the results of these tests before giving recombinant tissue plasminogen activator, thrombolytic therapy should not be delayed while awaiting the results unless (1) there is clinical suspicion of a bleeding abnormality or thrombocytopenia, (2) the patient has received heparin or warfarin, or (3) the use of anticoagulants is not known.
From Adams HP Jr, del Zoppo G, Alberts MJ, et al. American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
Reperfusion
Intravenous Thrombolysis
Intravenous recombinant t-PA (Alteplase) administered within 3 hours of the onset of symptoms in patients with ischemic stroke is effective and a proven therapy in patients older than 18 years. Before t-PA can be used, a brief but thorough evaluation must exclude ICH, coagulopathies, low platelet count, and other factors precluding the safe use of systemic thrombolysis. To exclude ICH, all patients with acute neurological deficits should undergo (1) a detailed history and physical evaluation to establish the precise time of symptom onset or when the patient was last well, extent of the deficit, comorbid conditions, and medication use; (2) emergency laboratory evaluation; and (3) neuroimaging with cranial CT or MRI with a gradient echo sequence (Table 344-2). A reliable time of onset must be ascertained before administration of the drug. If the time of onset cannot be established or the patient awoke with symptoms, the time that the patient was last known to be well is used.
Use of t-PA within 3 hours of stroke onset is well proven to improve patient outcome. Thirty-nine percent of patients treated with t-PA had a good outcome (modified Rankin scale [mRs] score of 0 or 1) versus 26% of control patients. This is a 13% absolute treatment benefit and leads to a number needed to treat (NNT) of just 8. Pooled analysis of 2775 patients from the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS), European Cooperative Acute Stroke Study (ECASS), and National Institute of Neurological Disorders and Stroke (NINDS) trials treated with t-PA suggests a benefit up to 4.5 hours; however, the odds ratio was low (1.4; 95% confidence interval, 1.1 to 1.9), with the confidence approaching 1.0.17 A recent study by Hacke and colleagues showed that in selected patients, t-PA improves outcome when used up to 4.5 hours.6 Treating patients younger than 80 years without previous stroke and diabetes between 3 and 4.5 hours after onset led to a favorable outcome in 52.4% of all treated patients versus 45.2% of those untreated. The risk for symptomatic ICH was 2.4% versus 0.2%.6 Use of thrombolytic agents beyond 4.5 hours should be restricted to a selected few patients, and further randomized trials are needed to establish its safety and efficacy.
A large meta-analysis of 15 published studies of intravenous administration of t-PA after stroke revealed a symptomatic ICH rate of 5.2% and a favorable outcome in 37.1%.18
There are several contraindications and warnings related to the intravenous administration of t-PA. Some of the exclusion criteria include evidence of ICH on CT, elevated blood pressure (systolic >185 mm Hg or diastolic >110 mm Hg) on repeated measurements despite the administration of antihypertensive agents, and known platelet diathesis and recent use of an anticoagulant with an international normalized ratio (INR) greater than 1.4 (Table 344-3).
TABLE 344-3 Characteristics of Patients with Ischemic Stroke Who Could Be Treated with Tissue Plasminogen Activator
aPTT, activated partial thromboplastin time; CT, computed tomography; INR, international normalized ratio.
From Adams HP Jr, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056-1083.
Other thrombolytic agents that have been tested in patients with stroke are streptokinase, which showed a high symptomatic ICH rate, and reteplase, tenecteplase, desmoteplase, prourokinase, and urokinase. Although prourokinase was used in one successful trial of intra-arterial thrombolysis, none of these agents have been approved for use after stroke. Defibrinogenating enzymes such as ancrod, which is derived from snake venom, are currently being investigated for use after ischemic stroke.19
Intra-arterial Thrombolysis
Intra-arterial thrombolysis with prourokinase in patients with middle cerebral artery (MCA)-associated stroke of less than 6 hours’ duration led to a good outcome, as defined by an mRs score of 0 to 2, in 40% of treated versus 25% of control patients.20 Recanalization was achieved in 66% of prourokinase-treated patients, and symptomatic ICH occurred in 10%. No difference in death rate was seen, but the FDA has not approved the drug for the treatment of stroke. Despite the lack of FDA approval, intra-arterial thrombolysis was used in at least 1314 patients in the United States between 1999 and 2001.21 The uncontrolled observation that the rate of recanalization may be higher than in intravenously treated patients is offset by the delay in initiation of treatment and concern for hemorrhagic complications.
Mechanical Clot Extraction
In the Mechanical Embolus Removal in Cerebral Embolism (MERCI) trial, blood vessels were opened with a device that removed the thrombus from an intracranial artery.22 The device was able to open the target vessel in 57.3% of patients. When adjunctive treatment (t-PA or other mechanical devices) was included, 69.5% of target vessels were opened. The 90-day mortality rate, however, was high at 34%. The trial led to FDA approval of the device to remove blood clots, but not for the treatment of stroke. This technicality enables approval despite the absence of data showing improved patient outcomes. Ongoing studies have included various clot removal devices with or without thrombolysis, but data are not available to support the routine use of clot removal devices in stroke therapy.
Other
Ultrasound-enhanced thrombolysis has received attention after a publication by Alexandrov and associates in 2004.23 In this study, 49% of patients who received standard intravenous t-PA plus transcranial ultrasound insonation achieved a good clinical outcome versus 30% of patients treated by t-PA alone. The technique is not widely available and dependent on the operator, and safety concerns of increased ICH in some studies using ultrasound have to be addressed.
The combination of intravenous and intra-arterial thrombolysis is currently being investigated, and preliminary data from the Interventional Management of Stroke (IMS) trial group are encouraging.24
Very limited data exist about the safety of antiplatelet agents in combination with thrombolysis. Even though this approach is used routinely for coronary ischemia, safety concerns of ICH have limited its use for cerebral ischemia.25
Stroke Management
Blood Pressure
All stroke patients should be admitted to a hospital with expertise in stroke management. After the administration of thrombolytic therapy, the patient should be transferred to a monitored setting for close observation. Current guidelines mandate monitoring of neurological status, heart rate, and blood pressure every 15 minutes after t-PA administration for the first 2 hours, every 30 minutes for the next 6 hours, and then every hour for 18 hours.13 The goal in these patients is a blood pressure lower than 180/105 mm Hg for 24 hours after the administration of t-PA.26 No heparin, aspirin, or warfarin is allowed for the first 24 hours.
Many stroke patients suffer from chronic arterial hypertension, and elevated blood pressure is frequently noted in the acute period after stroke.27 This is often followed by a gradual reduction to baseline over a 14-day period. Although a pretreatment blood pressure higher than 185/110 mm Hg is an exclusion for the use of t-PA and blood pressure after t-PA use must be maintained below 180/105 mm Hg, reduction of blood pressure by more than 15% within the first 24 hours may lead to expansion of stroke. Rapid lowering of blood pressure after ischemic stroke is associated with death and poor neurological outcome. Therefore, treatment of blood pressure after stroke when t-PA is not given can be more conservative and should, in general, lead to a reduction in blood pressure of about 15% over a period of 24 hours. The timing for reinstitution of previously used antihypertensive medications and selection of therapies depend largely on the patient’s neurological status, concomitant diseases, and stroke risk factors.
Glucose Control
Glycemic changes can cause focal neurological deficits and need to be considered in the differential diagnosis during the initial evaluation of a stroke patient. Hypoglycemia after stroke can lead to worsening of the neurological deficit, but more commonly hyperglycemia is associated with poor outcome after stroke. Baird and coworkers found that blood glucose levels persistently elevated to higher than 200 mg/dL during the first 24 hours after stroke predict stroke expansion and poor neurological outcome.28 Several studies have shown improved outcome in critically ill patients when glucose was maintained between 80 and 140 mg/dL.29 A recent British study aiming to control blood glucose at between 80 and 140 mg/dL after stroke failed to show benefit.30 Close glucose control may result in an increased risk for hypoglycemia and hypokalemia.31
Temperature
Increased body temperature in the setting of acute stroke is associated with poor neurological outcome.32 The source of any fever should be investigated and the elevated temperature aggressively treated. In turn, induced hypothermia has been shown to be neuroprotective in clinical studies of global ischemia and stroke.33,34
Nutrition and Hydration
Dehydration is common after stroke and can possibly worsen CBF and stroke outcome. In addition, it is a major contributor to deep venous thrombosis. Oral food and liquid intake can be limited in stroke patients. Many patients suffer from dysphagia and carry a high risk for aspiration. Assessment of safe swallowing after stroke is important in preventing aspiration pneumonia.35 Most patients are initially treated with intravenous hydration. When necessary, nasogastric tubes are inserted to provide nutritional intake and administration of medications. Placement of a percutaneous endoscopic gastrostomy (PEG) tube should be instituted in patients who require prolonged tube feedings. The Feed or Ordinary Diet (FOOD) trial investigated the effect of nutritional supplements on stroke outcome in patients without impaired swallowing and the effect of early versus later initiation of nasogastric feeding in patients with impaired swallowing and the use of PEG over nasogastric tube feeding. The trial failed to show a benefit with nutritional supplements, earlier tube feeling, or the use of PEG tubes.36
Cardiac Monitoring
All patients should undergo cardiac monitoring after stroke to evaluate for cardiac ischemia or any transient electrocardiographic (ECG) changes that may occur, especially after right MCA–associated stroke affecting the insular cortex.37 In addition, ECG recordings can be used to diagnose arrhythmia, which can complicate care after stroke, and aid in the diagnosis of possible cardioembolic causes of stroke.38 In selected patients, echocardiography may be indicated to evaluate for cardioembolic sources such as valve disease or endocarditis.
Aspirin
Administration of aspirin within 48 hours of ischemic stroke has resulted in a reduction in 14-day morbidity and mortality.39,40 Routine use of aspirin is recommended by the American Heart Association (AHA) and is monitored by regulatory agencies in the United States that overlook specialized stroke center accreditation.41
Anticoagulants
Heparin is widely used after stroke, but no proof of improved stroke outcome with heparin exists.42 Current AHA guidelines do not recommend the urgent use of anticoagulation for the treatment of stroke, and heparin is contraindicated within 24 hours after t-PA. Anticoagulation with heparin, heparinoids, or warfarin is useful in selected patients to prevent stroke recurrence (secondary prevention), but its use after stroke must be weighed against the concern for an increased incidence of ICH.
Seizures
Epileptic seizures occur in 2% to 23% of all stroke patients. Originally, patients who suffered a seizure at or around symptom onset were excluded from t-PA treatment because of concern that the initial deficit may be caused by postictal paralysis. This was recently revised (AHA guidelines), and t-PA should be considered if the treating physician judges the deficit to probably be due to an ischemic stroke.13
Surgical Intervention
Patients with a large hemispheric stroke involving more than two thirds of the territory of the MCA have a poor prognosis.43 Edema formation can lead to swelling of the affected area and cause a mass effect and herniation, a condition called malignant cerebral infarction.44 It can result from occlusion of the distal internal carotid artery or proximal MCA. Early reperfusion therapy offers the best odds for full neurological recovery. Once ischemia develops, however, treatment options become limited. In the early days after stroke, frequent examination of the patient’s vital signs, neurological function, and level of consciousness is important. In particular, changes in level of consciousness are early indicators of malignant MCA infarction syndrome. The spontaneous case fatality rate is between 70% and 80%.45
Conventional therapies to reduce increased intracranial pressure from brain edema, such as hyperventilation and osmotherapy with mannitol or hypertonic saline, have not produced any effect on survival and neurological outcome after stroke. Decompressive craniectomy, however, leads to improved survival and neurological outcome, even in patients treated after stroke affecting the dominant hemisphere. Three smaller European studies were pooled and showed that patients between the ages of 18 and 60, when treated within 36 hours after stroke, benefited from craniotomy.46 Mortality was reduced from 78% to 29%. Good neurological outcome, defined as an mRs score of 3 or less, occurred in 48% of treated versus 21% of untreated patients. These findings are highly significant. Whether surgery improves neurological function in patients older than 60 years is not known. In the elderly, prevention of mortality carries a considerable risk of inducing severe disability.
In stroke involving the posterior circulation, large areas of cerebellar ischemia can lead to brainstem compression and herniation. Resection of the affected cerebellum has long been thought to improve survival and neurological outcome, although this has never been proved. In contrast to hemispheric craniotomy, which almost always leads to contralateral hemiparesis and persistent focal neurological dysfunction, cerebellar decompressive surgery, when preformed early to avoid lasting brainstem dysfunction, is not associated with significant focal neurological impairment and should be considered early.47
The role of surgery in children with ischemic stroke has not been systematically investigated, but many report successful craniotomies in children after stroke.48
Carotid endarterectomy (CEA) has proven efficacy in preventing stroke recurrence in patients with carotid artery stenosis greater than 70% and an ipsilateral stroke or transient ischemic stroke.49 The benefit of CEA increases with the degree of carotid artery stenosis. Six patients with greater than 70% stenosis need to undergo surgery to prevent one stroke (NNT = 6). For a stenosis grade of 50% to 69%, the NNT is 24.50 Little information exists about its usefulness in acute stroke therapy. Recent analysis of CEA trials, however, has shown that patients who were treated within 2 weeks of the stroke had a better outcome than did patients who underwent surgery at a later time point.51 Patients with intraluminal thrombus associated with arthrosclerotic plaque in the carotid artery may be candidates for acute CEA. In the past, investigators argued that delaying endarterectomy after stroke prevents ICH and other complications. This assumption was not founded on good clinical data and predates the routine use of CT, so patients who had primary ICH were erroneously included in the analysis.52,53 By performing current neurological evaluations and excluding patients with ICH, the risk for recurrent stroke within the delayed period clearly outweighs the risk potentially associated with early surgery.54,55
Angioplasty with stenting is technically feasible in the vertebrobasilar arteries, the carotid, and the proximal MCA. Only case series are available about its use in acute stroke victims. Nedeltchev and associates reported on 25 patients who underwent intra-arterial stenting and thrombolysis for acute carotid artery occlusion and found favorable outcomes in 56% as opposed to 26% in a medically treated cohort.55a Other series have reported successful use of angioplasty for vertebrobasilar stroke with or without adjuvant thrombolysis. Although no prospective or randomized trial has investigated angioplasty in acute stroke care, it is frequently considered in addition to intra-arterial thrombolysis in patients with severe brainstem ischemia. Eckstein and coauthors reported the results of a large randomized trial of carotid angioplasty and stent placement versus CEA. Although the treatment did not target the acute stroke, the procedure was performed with a mean delay of just 4 to 5 days.56 A total of 1136 patients were monitored for 2 years, and outcome did not differ between groups.
Management after Intracranial Hemorrhage
The outcome of ICH depends largely on patient age, hematoma size, location, presence of ventricular hemorrhage, and Glasgow Coma Scale score.11 ICH is a dynamic process, and 38% of patients undergo ultra-early hematoma expansion.57 Hemostatic therapy with recombinant factor VIIa was used in the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial but did not show a better outcome in the treated group; moreover, thromboembolic complications were increased in comparison to the placebo-treated patients.58
Surgical treatment of ICH has not proved to be better than medical therapy. The Surgical Trial in Intracerebral Haemorrhage (STICH) investigated early surgical removal of hematoma versus medical therapy and failed to show benefit in the surgical arm.59 Ongoing research is investigating whether subgroups of patients with larger and superficial hemorrhage could benefit from surgical clot removal. In contrast to supratentorial hemorrhage, most clinicians agree to operate on large cerebellar hematomas when a mass effect on the fourth ventricle or posterior fossa cisterns occurs.60
Endovascular approaches with or without thrombolytic therapy to dissolve the blood clot are still under investigation.61 These techniques involve stereotactic blood clot removal after liquefaction with thrombolytic medications. A study comparing administration of t-PA into the clot cavity versus conventional medical treatment has recently been funded by the National Institutes of Health.62
Endovascular therapy has been used successfully for the treatment of intraventicular hemorrhage and is particularly helpful in preventing hydrocephalus.63
Blood pressure management after ICH is controversial, with most recommending maintenance of systolic blood pressure below 180 mm Hg and mean arterial pressure below 130 mm Hg.62 When invasive intracranial pressure monitoring is used, intracranial perfusion pressure should be maintained above 60 mm Hg.64
Medical therapy focuses on correction of any underlying coagulopathies, such as correction of the INR in patients with warfarin-induced ICH and blood pressure management after hemorrhage. In many patients with ICH, the underlying condition is arterial hypertension. Alternatively, increases in arterial blood pressure are often found in the acute phase after hemorrhagic and ischemic stroke. The best blood pressure range after ICH is not known, but most centers aim for a mean arterial pressure lower than 130 mm Hg.62
The use of mannitol did not improve outcome and showed a trend toward worsening.65 Steroids likewise did not improve outcome.66
Adams HPJr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the american Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102-3109.
Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723-725.
Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001-2023.
CAST. Randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Cast (Chinese Acute Stroke Trial) collaborative group. Lancet. 1997;349:1641-1649.
Clinical alert. Benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (Nascet) investigators. Stroke. 1991;22:816-817.
Ferro JM. Update on intracerebral haemorrhage. J Neurol. 2006;253:985-999.
Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005;293:2391-2402.
Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768-774.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773-782.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet. 2005;365:387-397.
Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915-924.
Schwab S, Schwarz S, Spranger M, et al. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461-2466.
Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933-944.
The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556.
The International Stroke Trial (IST). A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
, 1995 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215-222.
1 World Health Organization (WHO). The World Health Report 2004. 2004.
2 Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773-782.
3 Zivin JA. Factors determining the therapeutic window for stroke. Neurology. 1998;50:599-603.
4 Ginsberg MD, Pulsinelli WA. The ischemic penumbra, injury thresholds, and the therapeutic window for acute stroke. Ann Neurol. 1994;36:553-554.
5 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
6 Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
7 Mohr JP, Caplan LR, Melski JW, et al. The Harvard Cooperative Stroke Registry: A prospective registry. Neurology. 1978;28:754-762.
8 Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723-725.
9 Kane I, Carpenter T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: Effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38:3158-3164.
10 Ferro JM. Update on intracerebral haemorrhage. J Neurol. 2006;253:985-999.
11 Hemphill JC3rd, Bonovich DC, Besmertis L, et al. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891-897.
12 Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116-121.
13 Adams HPJr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
14 Paxton R, Ambrose J. The EMI scanner. A brief review of the first 650 patients. Br J Radiol. 1974;47:530-565.
15 Bryan RN, Levy LM, Whitlow WD, et al. Diagnosis of acute cerebral infarction: Comparison of CT and MR imaging. AJNR Am J Neuroradiol. 1991;12:611-620.
16 Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet. 2007;369:293-298.
17 Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768-774.
18 Graham GD. Tissue plasminogen activator for acute ischemic stroke in clinical practice: A meta-analysis of safety data. Stroke. 2003;34:2847-2850.
19 Sherman DG, Atkinson RP, Chippendale T, et al. Intravenous ancrod for treatment of acute ischemic stroke: The STAT Study: A randomized controlled trial. Stroke Treatment with Ancrod Trial. JAMA. 2000;283:2395-2403.
20 Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. PROLYSE in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
21 Qureshi AI, Suri MF, Nasar A, et al. Thrombolysis for ischemic stroke in the United States: Data from National Hospital Discharge Survey 1999-2001. Neurosurgery. 2005;57:647-654.
22 Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the Multi MERCI Trial. Stroke. 2008;39:1205-1212.
23 Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170-2178.
24 Khatri P, Hill MD, Palesch YY, et al. Methodology of the interventional management of Stroke III Trial. Int J. Stroke. 2008;3:130-137.
25 Adams HPJr, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: Results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (ABESTT-II). Stroke. 2008;39:87-99.
26 Brott T, Lu M, Kothari R, Fagan SC, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29:1504-1509.
27 Castillo J, Leira R, Garcia MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520-526.
28 Baird TA, Parsons MW, Phanh T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208-2214.
29 Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461.
30 Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: The UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007;6:397-406.
31 Soylemez Wiener R, Wiener DC, et al. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933-944.
32 Jorgensen HS, Reith J, Pedersen PM, et al. Body temperature and outcome in stroke patients. Lancet. 1996;348:193.
33 The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556.
34 Schwab S, Schwarz S, Spranger M, et al. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461-2466.
35 Hinchey JA, Shephard T, Furie K, et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36:1972-1976.
36 Dennis M, Lewis S, Cranswick G, et al. FOOD: A multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol Assess. 2006;10:iii-iv. ix-x, 1-120
37 Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005;293:2391-2402.
38 Afsar N, Fak AS, Metzger JT, et al. Acute stroke increases qt dispersion in patients without known cardiac diseases. Arch Neurol. 2003;60:346-350.
39 The International Stroke Trial (IST). A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
40 CAST. Randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
41 Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102-3109.
42 Al-Sadat A, Sunbulli M, Chaturvedi S. Use of intravenous heparin by North American neurologists: Do the data matter? Stroke. 2002;33:1574-1577.
43 Qureshi AI, Kirmani JF, Sayed MA, et al. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology. 2005;64:2115-2120.
44 Hacke W, Schwab S, Horn M, et al. “Malignant” middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996;53:309-315.
45 Berrouschot J, Sterker M, Bettin S, et al. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620-623.
46 Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215-222.
47 Chen HJ, Lee TC, Wei CP. Treatment of cerebellar infarction by decompressive suboccipital craniectomy. Stroke. 1992;23:957-961.
48 Simma B, Tscharre A, Hejazi N, et al. Neurologic outcome after decompressive craniectomy in children. Intensive Care Med. 2002;28:1000.
49 Clinical alert. Benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators. Stroke. 1991;22:816-817.
50 Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107-116.
51 Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915-924.
52 Bruetman ME, Fields WS, Crawford ES, et al. Cerebral hemorrhage in carotid artery surgery. Arch Neurol. 1963;9:458-467.
53 Wylie EJ, Hein MF, Adams JE. Intracranial hemorrhage following surgical revascularization for treatment of acute strokes. J Neurosurg. 1964;21:212-215.
54 Ballotta E, Da Giau G, Baracchini C, et al. Early versus delayed carotid endarterectomy after a nondisabling ischemic stroke: A prospective randomized study. Surgery. 2002;131:287-293.
55 Eckstein HH, Ringleb P, Dorfler A, et al. The carotid surgery for ischemic stroke trial: A prospective observational study on carotid endarterectomy in the early period after ischemic stroke. J Vasc Surg. 2002;36:997-1004.
55a Nedeltchev K, Brekenfeld C, Remonda L, et al. Internal carotid artery stent implantation in 25 patients with acute stroke: Preliminary results. Radiology. 2005;237:1029-1037.
56 Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893-902.
57 Broderick JP, Brott TG, Tomsick T, et al. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195-199.
58 Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127-2137.
59 Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet. 2005;365:387-397.
60 Hankey GJ. Evacuation of intracerebral hematoma is likely to be beneficial against. Stroke. 2003;34:1568-1569.
61 Teernstra OP, Evers SM, Lodder J, et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: A multicenter randomized controlled trial (SICHPA). Stroke. 2003;34:968-974.
62 Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001-2023.
63 Naff NJ, Carhuapoma JR, Williams MA, et al. Treatment of intraventricular hemorrhage with urokinase: Effects on 30-day survival. Stroke. 2000;31:841-847.
64 Fernandes HM, Siddique S, Banister K, et al. Continuous monitoring of ICP and CPP following ICH and its relationship to clinical, radiological and surgical parameters. Acta Neurochir Suppl. 2000;76:463-466.
65 Misra UK, Kalita J, Ranjan P, et al. Mannitol in intracerebral hemorrhage: A randomized controlled study. J Neurol Sci. 2005;234:41-45.
66 Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316:1229-1233.


 of the cerebral hemisphere)
of the cerebral hemisphere)





