58 Acute Lung Injury and Acute Respiratory Distress Syndrome
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common problems in the intensive care unit (ICU) and can complicate a wide spectrum of critical illnesses. First described by Ashbaugh in 1967,1 the syndrome was initially termed adult respiratory distress syndrome to distinguish it from the respiratory distress syndrome of neonates. However, with the recognition that ALI/ARDS can occur in children, the term acute has replaced adult in the nomenclature in recognition of the typical acute onset that defines the syndrome. Although specific treatments for ALI/ARDS have been slow to emerge, the recent development of new strategies for mechanical ventilation that improve mortality, and fluid management strategies that reduce the length of mechanical ventilation, emphasizes the importance of identifying and appropriately treating all patients with ALI/ARDS. Although this point would seem to be straightforward, in practice, ALI/ARDS remains largely underdiagnosed,2,3 and often expert practitioners disagree on the diagnosis,4 which perpetuates inappropriate or inadequate treatment.
 Epidemiology
Epidemiology
The exact incidence of ALI/ARDS has been difficult to estimate for a variety of reasons. In the past, variable definitions of the syndrome were used.5 The wide variety of causes and coexisting disease processes has also made identification of cases difficult, both at the clinical and administrative coding level.6 The National Institutes of Health first estimated the incidence at 75 per 100,000 population in 1977,7 but a number of studies since then have reported lower incidences.6 Two prospective studies confirmed the higher original National Institutes of Health Estimate. The first utilized enrollment logs from the National Heart, Lung and Blood Institute–sponsored ARDS Network of 20 hospitals and estimated that the incidence could be as high as 64 cases per 100,000 population. This dataset has the advantage of being prospectively collected from a large number of academic medical centers. The second was a large prospective study of residents of King County, Washington. In that study, the crude incidence of ALI/ARDS in adults was 78.9 per 100,000 patient years.8 A large prospective European study of the incidence of ARDS found that ALI occurred in 7.1% of all hospital admissions.9 A third of these patients presented with only mild ALI, but of these, half progressed rapidly to ARDS. Some studies suggest a decline in the incidence of ARDS over time. A large prospective cohort of trauma patients at risk for ARDS and multisystem organ failure collected over time showed that the incidence of ARDS decreased from 43% in 1997 to 12% in 2004, a finding that may reflect advances in posttrauma critical care.10 Regardless of the exact incidence, it is clear that ALI/ARDS is a major public health problem that will be encountered frequently by all physicians who care for critically ill patients.
 Risk Factors
Risk Factors
ALI/ARDS can occur as a result of either direct or indirect injury to the lungs (Table 58-1) in patients with a predisposing risk factor. The commonly associated clinical disorders can be separated into those that directly injure the lung and those that indirectly injure the lung. Although it is not always feasible to determine the exact cause of ALI/ARDS in a given patient, direct causes appear to account for approximately half of all cases of ALI/ARDS.11 It is not clear whether the distinction between direct and indirect lung injury is clinically useful.12 Some investigators have demonstrated reduced respiratory system compliance in patients with ARDS due to direct pulmonary injury compared to indirect causes,13 although total respiratory system compliance (including the chest wall) is similar.14 Patients with direct lung injury may be more likely to have improved lung mechanics with the application of PEEP. However, in the largest cohort of patients studied to date, there was no difference in mortality between those with direct (pulmonary) and indirect (extrapulmonary) causes of lung injury.11 Regardless of the underlying cause of ALI/ARDS, most patients with ALI/ARDS appear to have a systemic illness with inflammation and organ dysfunction not confined to the lung.15
TABLE 58-1 Risk Factors Associated with Development of Acute Lung Injury and Acute Respiratory Distress Syndrome
| Direct Lung Injury | Indirect Lung Injury |
|---|---|
| Pneumonia | Sepsis |
| Aspiration of gastric contents | Multiple trauma |
| Pulmonary contusion | Cardiopulmonary bypass |
| Fat, amniotic fluid, or air emboli | Drug overdose |
| Near-drowning | Acute pancreatitis |
| Inhalational injury | Transfusion of blood products |
| Reperfusion pulmonary edema |
Sepsis is the most common cause of indirect lung injury, with an overall risk of progression to ALI or ARDS of approximately 30% to 40%.16–19 In a more recent prospective study of hospitalized patients with a risk factor for acute lung injury (e.g., sepsis, pneumonia) 6.5% of patients developed ALI, and 4% met criteria for ARDS; the risk was higher with multiple risk factors.20 In addition to sepsis itself being a risk factor for development of ARDS, the site of infection may also influence the risk of lung injury. In patients with sepsis admitted to an ICU, patients who had pneumonia as the source of sepsis had an increased risk of ARDS compared to those with infections at other sites such as the abdomen, skin, or soft tissue.21 Severe trauma with shock and multiple transfusions also can cause indirect lung injury. Although the other causes of indirect lung injury are less common, many, such as blood transfusions, are commonplace events in the ICU setting. The most common cause of direct lung injury is pneumonia, which may be of bacterial, viral, or fungal origin. The risk of developing ALI/ARDS increases substantially in the presence of multiple predisposing disorders.19 Secondary factors may also increase the risk. Such factors include chronic lung disease,18 chronic or acute alcohol abuse,22,23 increasing age,24 transfusion of blood products,25–27 lung resection,28 and obesity.24 Emerging evidence has suggested that some at-risk patients may actually be protected from the development of ARDS. Several studies have shown that patients with diabetes are less likely to develop ARDS.29–31 To some extent, every patient in the ICU is at risk for developing ALI/ARDS, and vigilance is required to recognize the diagnosis and treat appropriately.
 Pathophysiology
Pathophysiology
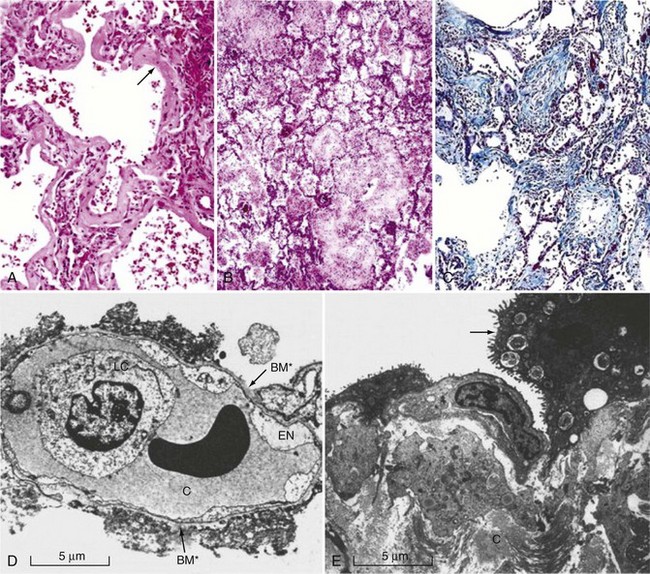
The pathophysiology of ALI/ARDS is complex and remains incompletely understood. Microscopically, lungs from afflicted individuals in the early stages show diffuse alveolar damage with alveolar flooding by proteinaceous fluid, neutrophil influx into the alveolar space, loss of alveolar epithelial cells, deposition of hyaline membranes on the denuded basement membrane, and formation of microthrombi (Figure 58-1).32 Alveolar flooding occurs as a result of injury to the alveolar-capillary barrier and is a major determinant of the hypoxemia and altered lung mechanics that characterize early ALI/ARDS.
The alveolar-capillary barrier is formed of two separate cell layers, the microvascular endothelium and the alveolar epithelium. Injury to the alveolar epithelium is a prominent histologic feature, with loss of alveolar epithelial barrier integrity and sloughing of alveolar epithelial type I cells. Alveolar epithelial apoptosis is widespread and likely contributes to the loss of epithelium seen ultrastructurally.33,34 Although endothelial injury is less obvious at the microscopic level, ultrastructural studies reveal that it is widespread.35,36 Endothelial injury allows leakage of plasma from the capillaries into the interstitium and airspaces. Alveolar flooding in ALI/ARDS is characteristically with a protein-rich edema fluid, owing to the increased permeability of the alveolar capillary barrier, in contrast to the low-protein pulmonary edema that results from hydrostatic causes such as congestive heart failure or acute myocardial infarction.37–40
Neutrophils play an important role41 in the initial inflammatory response in ARDS. Early ALI/ARDS is characterized by migration of neutrophils into the alveolar compartment.35,36 Neutrophils can release a variety of injurious substances, including proteases such as neutrophil elastase, collagenase, and gelatinases A and B, as well as reactive nitrogen and oxygen species. In addition, they can elaborate proinflammatory cytokines and chemokines which amplify the inflammatory response in the lung. Resident alveolar macrophages are also involved in initiating and sustaining a proinflammatory cytokine cascade that leads to recruitment of neutrophils into the lung.
In addition to acute neutrophilic inflammation and elaboration of a proinflammatory cytokine cascade, a variety of other abnormalities contribute to the pathogenesis of ALI/ARDS. Surfactant dysfunction is characteristic, with abnormalities in both the protein and lipid components.42–45 This likely results from disruption of normal surfactant activity secondary to the influx of plasma proteins into the airspaces, intraalveolar proteolysis, and injury to the alveolar epithelial type II cells. Surfactant dysfunction may have important implications both for lung mechanics and host defense.46 Activation of the coagulation cascade and impaired fibrinolysis are also apparent in patients with ALI/ARDS,47,48 both in the lung49–51 and systemically.52,53 Alteration in the balance of endogenous oxidants and antioxidants, with a decrease in endogenous antioxidants54 despite the increased oxidant production, has also been observed.55
The contribution of ventilator-associated lung injury to the pathogenesis of ALI/ARDS has been recognized. There are several mechanisms by which mechanical ventilation can injure the lung. Ventilation at very high volumes and pressures can injure even the normal lung, leading to increased permeability pulmonary edema due to capillary stress failure56 and sustained elevations of circulating plasma cytokines.57 In the injured lung, even tidal volumes that are well tolerated in the normal lung can lead to alveolar overdistension in relatively uninjured areas because the lung available for distribution of the administered tidal volume is greatly reduced and because of uneven distribution of inspired gas.58,59 In addition to alveolar overdistension, cyclic opening and closing of atelectatic alveoli can cause lung injury even in the absence of alveolar overdistension. The combination of alveolar overdistension with cyclic opening and closing of alveoli is particularly harmful and can initiate a proinflammatory cascade.60
A ventilatory strategy that was designed to minimize alveolar overdistension and maximize alveolar recruitment ameliorated proinflammatory cytokine release.61 This fundamental insight into the pathogenesis of clinical ALI/ARDS has led to multiple clinical trials of novel ventilatory strategies for patients with ALI/ARDS, including the landmark ARDS Network trial of 6 mL/kg versus 12 mL/kg tidal volume ventilation62 (see Treatment section).
 Diagnosis
Diagnosis
In 1994, the American-European Consensus Conference published new clinical definitions for ALI and ARDS.5 Prior to this time, a variety of definitions were used clinically, including the Murray Lung Injury Score.63 To meet the Consensus diagnostic criteria for either ALI/ARDS, the acute onset of bilateral radiographic infiltrates is required. There should be no clinical evidence of left atrial hypertension, with a pulmonary artery occlusion pressure (PAOP) ≤ 18 mm Hg if measured. Although not strictly part of these definitions, an underlying cause of lung injury should be sought. In the absence of an identifiable underlying cause (see Table 58-1), particular attention should be given to the possibility of other causes of pulmonary infiltrates and hypoxemia, such as hydrostatic pulmonary edema. One potential limitation of the consensus definition is the need for arterial blood gas sampling to calculate a PaO2/FIO2 ratio. Recent work has shown good correlation between the SpO2/FIO2 ratio (measured by pulse oximetry) and the PaO2/FIO2 ratio,64,65 with an SpO2/FIO2 ratio of 235 corresponding to a PaO2/FIO2 ratio of 200, and an SpO2/FIO2 ratio of 315 correlating to a PaO2/FIO2 ratio of 300. These calculations are valid only when the SpO2 is less than 98%, because the oxyhemoglobin dissociation curve is flat above this level. Oxygen saturation is a noninvasive, continuously available measurement; use of the SpO2/FIO2 ratio may improve the ability of clinicians to diagnose ARDS.
Differentiating ARDS from hydrostatic edema can be difficult, and there may be significant overlap in the syndromes (Figure 58-2).66 A recent multicenter trial on intravenous catheter directed fluid management strategies in patients with ARDS showed that 29% of patients with clinically defined ARDS had a PAOP greater than 18 mm Hg at the time of pulmonary artery catheter insertion, but that 97% of patients had a normal or elevated cardiac index, suggesting they did not have clinical heart failure.67 Other studies have shown similar rates of elevated PAOP in patients with ARDS.68
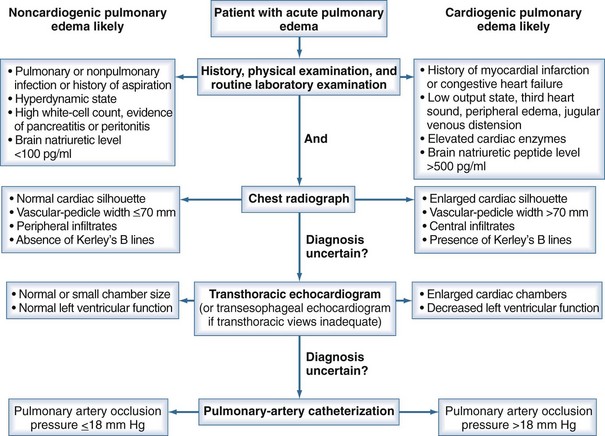
Figure 58-2 Algorithm for differentiating between cardiogenic and noncardiogenic pulmonary edema.
(With permission from Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353[26]:2788-2796.)
There are no specific clinical or laboratory studies that can reliably distinguish between ARDS and hydrostatic edema. A study examining the diagnostic utility of serum levels of B-type natriuretic peptide (BNP) showed that BNP measured at admission could not reliably differentiate between hydrostatic edema and ARDS. Furthermore, BNP levels in these patients did not correlate with invasive hemodynamic measurements.69
The standardization of definitions for ALI and ARDS has been helpful from several perspectives. For clinical research, it has been valuable in allowing the comparison of different studies and the rapid identification of patients for enrollment in clinical trials. Clinically, the new definitions are easy to apply and facilitate rapid identification and appropriate treatment of patients with ALI/ARDS. However, it should be noted the nature of ALI/ARDS is such that any definition will have significant shortcomings. First, the definitions must be based solely on clinical criteria, because currently there is no laboratory test that allows clinical assessment of the presence or absence of ALI/ARDS. Second, there is no reference to pathogenesis or underlying cause. This is because the list of potential causes of ALI/ARDS is so long, diverse, and common in the critically ill. Third, the presence or absence of multiorgan dysfunction, an important determinant of outcome, is not specified. Finally, though the presence of bilateral infiltrates has major prognostic significance and is clearly a hallmark of the syndrome, the radiographic findings are not specific for ALI/ARDS.4,70 Diagnostic uncertainty in ALI/ARDS is a major barrier to initiation of appropriate therapy and one of the main reasons why clinicians fail to initiate lung-protective ventilation in clinically appropriate patients.71
Recent work has focused on alternative methods to increase sensitivity and specificity of the clinical definitions for ALI/ARDS. The pulmonary edema fluid–to–plasma protein ratio can reliably distinguish between low-permeability (hydrostatic edema) and high-permeability (ARDS) pulmonary edema if measured early after endotracheal intubation,72 but prospective validation is still needed. Alternatively, circulating biomarkers may prove useful for the diagnosis of ALI/ARDS.73 Despite its shortcomings, the current clinical definition of ALI/ARDS based on consensus criteria should be used to rapidly identify patients with ALI/ARDS so appropriate therapy can be initiated promptly.
In the majority of patients, the initial diagnosis of ALI/ARDS is made clinically. Invasive techniques for diagnosis are of limited clinical utility, and the benefits rarely outweigh the risks. Bronchoscopy may be indicated in the early phases of ALI/ARDS in patients in whom there is no identifiable predisposing risk factor. Rarely, an alternate treatable diagnosis is found, such as acute eosinophilic pneumonia, pulmonary alveolar proteinosis, diffuse alveolar hemorrhage, or unsuspected infection. Bronchoalveolar lavage for cultures and cytologic examination can identify the cause of pneumonia, and is particularly useful in the diagnosis of opportunistic infections. Lavage fluid usually has a predominance of neutrophils, and there may be evidence of diffuse alveolar hemorrhage. Cytologic examination can be used to confirm the presence of diffuse alveolar damage.74
In the past, open lung biopsy was obtained more frequently for diagnosis in patients with suspected ARDS. Interestingly, the degree of histologic abnormality on lung biopsy does not correlate with ultimate outcome as measured by pulmonary function.75 Open or thoracoscopic lung biopsy may still be useful in some cases where the diagnosis is uncertain and the underlying cause is not apparent. Although open lung biopsy can provide findings that lead to a change in treatment, postoperative complications can occur in 20% of patients.76 Several pathologic studies have shown that biopsy or autopsy can identify unsuspected diagnoses requiring specific therapy (e.g., miliary tuberculosis, pulmonary blastomycosis, aspergillosis, bronchiolitis obliterans organizing pneumonia) in 40% to 60% of cases,76–78 although the general applicability of these studies may be limited by the fact that they were retrospective case series.
 Clinical Course
Clinical Course
Early Ali/Ards
The Consensus definitions are designed to identify ALI/ARDS patients early in their course, in the acute or exudative phase. Clinically, the acute phase is manifested by the acute onset of radiographic infiltrates consistent with pulmonary edema, hypoxemia, and increased work of breathing. Radiographic infiltrates are bilateral (by definition), but may be patchy or diffuse, fluffy or dense (Figure 58-3), and pleural effusions may occur.79 Chest computed tomographic (CT) imaging, though rarely of use clinically, has been employed extensively as an investigative tool to better define the nature of the infiltrates in patients with ALI/ARDS. The distribution of infiltrates by CT is surprisingly patchy; areas of alveolar filling and consolidation occur predominantly in dependent zones, while non-dependent regions can appear relatively spared.80–82 Even areas that appear spared in conventional radiographic images may have substantial inflammation when sampled using bronchoalveolar lavage83 or using FDG-PET scanning.84
The hypoxemia that characterizes early ALI/ARDS is usually relatively refractory to supplemental oxygen. The increased work of breathing in the acute phase of ALI/ARDS is due to decreased lung compliance as a result of alveolar and interstitial edema combined with increased airflow resistance85 and increased respiratory drive.86 The combination of hypoxemia and increased work of breathing usually necessitates endotracheal intubation and mechanical ventilation, although occasionally patients can be managed with noninvasive ventilation (see Treatment section).
In addition to hypoxemia and increased work of breathing, many patients with ARDS also develop evidence of increased pulmonary vascular resistance leading to pulmonary hypertension and RV failure. The prevalence of pulmonary hypertension in patients presenting to the hospital with ARDS may be as high as 92%,87 and as many as 10% of patients with ARDS may have right ventricular (RV) failure defined by hemodynamic measurements.88 Nevertheless, the presence of RV failure does not impact mortality. Attempts to reverse pulmonary hypertension and RV failure with pulmonary vasodilators such as sildenafil have led to decreased pulmonary artery pressure with treatment, as well as concomitant increases in shunt fraction and decreases in oxygenation.89 These findings suggest that although patients with ARDS have evidence of pulmonary hypertension, it may in some cases be a beneficial physiologic response to reduce blood flow to areas of severely compromised lung.
Late Fibroproliferative Ali/Ards
In most patients, ALI/ARDS will substantially resolve after the acute phase. However, in others, the syndrome progresses to a fibrosing alveolitis. Fibrosing alveolitis usually becomes clinically apparent after 7 to 10 days, although evidence of deposition of extracellular matrix has been identified in alveolar lining fluid from patients as early as the first day after intubation.90 Radiographically, linear opacities develop, consistent with the evolving fibrosis. Histologically, pulmonary edema and neutrophilic inflammation are less prominent. A severe fibroproliferative process fills the airspaces with granulation tissue that contains extracellular matrix rich in collagen and fibrin, as well as new blood vessels and proliferating mesenchymal cells.91,92
Clinically, the late fibroproliferative phase of ALI/ARDS is characterized by continued need for mechanical ventilation, often with persistently high levels of PEEP and FIO2. Lung compliance may fall even further, and pulmonary dead space is elevated. If it has not developed in the acute phase, pulmonary hypertension may occur now owing to obliteration of the pulmonary capillary bed, and right ventricular failure may appear.93 This phase of the illness can be prolonged, lasting weeks, and can be very frustrating for the clinician, patient, and family; small gains in pulmonary function are frequently offset by new problems such as hospital-acquired infections, organ failures, or barotrauma. Progressive deconditioning can make eventual weaning from mechanical ventilation difficult if the fibrosing alveolitis stage is prolonged. Based on improvement in number of ventilator-free days through use of lower tidal volumes, it seems likely the incidence of fibrosing alveolitis will fall.
Resolution of Ali/Ards
Lung biopsies from ALI/ARDS survivors typically show normal or near-normal lung histology. For such histologically complete resolution of ALI/ARDS to occur, a variety of processes must be reversed. Alveolar edema is actively reabsorbed by the vectorial transport of sodium and chloride from the distal airway and alveolar spaces into the lung interstitium.94 Water is passively absorbed along the osmotic gradient, probably through water channels, the aquaporins.95 The majority of patients with early ALI/ARDS have impaired alveolar fluid transport, but in those with intact alveolar fluid transport, faster rates of alveolar epithelial fluid transport are associated with better outcomes.37 Soluble and insoluble protein must also be cleared from the airspaces. Soluble protein probably diffuses by a paracellular route into the interstitium, where it is cleared by lymphatics. Insoluble protein probably is cleared by macrophage phagocytosis or alveolar epithelial cell endocytosis and transcytosis.96
The denuded alveolar epithelium in ALI/ARDS must be repaired. The alveolar epithelial type II cell serves as the progenitor cell for repopulating the alveolar epithelium. Type II cells proliferate, migrate, and differentiate to reconstitute a tight alveolar epithelial type I cell barrier. The inflammatory cell infiltrate must also resolve, but here the mechanisms are less clear. Resolution of neutrophilic inflammation may be predominantly via neutrophil apoptosis and phagocytosis by macrophages. However, one report suggests that neutrophil apoptosis is impaired in the lungs of patients with ALI/ARDS.97 The resolution of fibrotic changes is also not well understood. Clearly, however, substantial remodeling is necessary to restore a normal or near-normal alveolar architecture. In patients with advanced fibrosis, this process likely takes place over many months; pulmonary function abnormalities continue to improve, sometimes remarkably so, out to the first year in survivors of ALI/ARDS (see later discussion).98
 Treatment
Treatment
Standard Supportive Therapy
Treatment of Predisposing Factors
First and foremost, a search for the underlying cause of ALI/ARDS should be undertaken. Appropriate treatment for any precipitating infection such as pneumonia is critical to enhance the chance of survival. In the immunocompromised host or patients without predisposing risk factors, invasive diagnostic evaluation including bronchoscopy may be warranted to look for evidence of opportunistic infections or alternative specific causes of ARDS. In a patient with sepsis and ALI/ARDS of unknown source, an intraabdominal process should be considered. Timely surgical management of intraabdominal sepsis is associated with better outcomes.99 In some patients, the cause of lung injury will not be specifically treatable (such as aspiration of gastric contents) or will not be readily identifiable.
Fluid and Hemodynamic Management
There are data supporting the use of early goal-directed therapy to support cardiac output and oxygen delivery within a set range with fluids, inotropes, and blood transfusions using central venous oxygen saturation as a therapeutic driver in patients who have severe sepsis and septic shock,100 many of whom develop ALI/ARDS. But this approach has not been specifically studied in ALI/ARDS. Historically, patients with critical illness and ALI/ARDS received a pulmonary artery catheter (PAC) to manage fluid and hemodynamic status. A large, randomized European trial of PAC use versus no PAC use in all patients admitted with ARDS101 showed no difference in clinical outcomes in either group, suggesting that routine PAC use in ARDS is not beneficial. The ARDS Clinical Trials Network tested the value of pulmonary artery catheterization in the context of specific fluid-management protocols and was unable to demonstrate improved outcomes through use of the PAC.67 Some investigators have proposed that clinical outcomes in ALI/ARDS can be improved by delivery of supranormal levels of oxygen to the tissues using vigorous volume resuscitation and positive inotropes. However, no benefit to supranormal levels of oxygen delivery has been demonstrated in patients with ALI/ARDS.102,103
For decades there was disagreement as to the best fluid-management strategy in patients with ARDS. Proponents of a liberal fluid strategy reasoned that increased circulating volume would preserve end-organ perfusion and protect patients from the development of non-pulmonary organ failures. Reductions in intravascular volume can have adverse effects on cardiac output and tissue perfusion, factors that could contribute to multisystem organ failure. This is a legitimate concern, since mortality in ALI/ARDS is usually from non-pulmonary causes including other organ failures. Others supported a conservative fluid strategy in an attempt to reduce circulating volume, thereby reducing the driving force for pulmonary edema formation. In experimental lung injury, lower left atrial pressures are associated with less formation of pulmonary edema.93,104 There is some clinical evidence to support this approach.105–108 Given the equipoise with the approach to fluids in ALI/ARDS, the ARDS Network conducted a large, multicenter, randomized controlled trial of catheter-driven (central venous catheter versus PAC) fluid management in patients with ALI.109 Once patients were out of shock, they were randomized to a liberal fluid treatment strategy that resulted in an average of 1 liter of fluid accumulation per day or to a conservative fluid treatment strategy with aggressive use of diuretics to achieve a goal central venous pressure (CVP) below 4 or a goal PAOP below 8, an approach that resulted in an average of zero net fluid accumulation by day 7. Although there was no difference in mortality at 60 days (the primary outcome of the study), patients in the conservative group had improved oxygenation and significantly more ventilator-free days without the development of additional organ failures. In this study, it did not matter whether treatment was guided by CVP measurements (derived from a central venous line) or from PAOP measurements (derived from a PAC).110
Despite the findings in support of conservative fluid management strategy in patients with ARDS, there continues to be a great deal of uncertainty about appropriate goals for hemodynamic therapy in ALI/ARDS. Currently, the recommended strategy is to aim to achieve the lowest intravascular volume that maintains adequate tissue perfusion as measured by urine output, other organ perfusion, and metabolic acid-base status, using CVP monitoring to direct therapy. If organ perfusion cannot be maintained in the setting of adequate intravascular volume, administration of vasopressors and/or inotropes should be used to restore end-organ perfusion.93 Available evidence does not support the use of one particular vasopressor or combination of vasopressors. Once shock has resolved, patients should be managed with a conservative fluid strategy, with the goal of driving the CVP below 4 to keep each patient’s fluid balance net zero over their ICU stay.
Nutrition
Standard supportive care for the patient with ALI/ARDS includes providing adequate nutrition. The NIH NHLBI ARDS Network is currently conducting a randomized trial of trophic (10 mL/h, well below caloric requirements) versus full-calorie enteral feeds in patients with ALI/ARDS. The enteral route is preferred to the parenteral route and is associated with fewer infectious complications.111 Enteral feeding may also have other beneficial effects. Experimentally, lack of enteral feeding promoted translocation of bacteria from the intestine.112 In normal volunteers, administration of parenteral nutrition with bowel rest increased circulating levels of tumor necrosis factor alpha (TNF-α), glucagon, and epinephrine, and increased febrile responses compared to volunteers who received enteral nutrition.113
Until the results of the ARDS Network study become available, the goals of nutritional support in any critically ill patient include providing adequate nutrients for the patient’s level of metabolism and treating and preventing any deficiencies in micro- or macronutrients.114 Whether a particular dietary composition is beneficial in patients with ALI/ARDS is unclear. Immunomodulation via dietary manipulation has been attempted in critically ill patients, using various combinations of omega-3 fatty acids, ribonucleotides, arginine, and glutamine. A meta-analysis of these trials suggested a beneficial effect on infection rate but not on overall mortality.115 The ARDS Network recently conducted a large, multicenter, randomized placebo-controlled study of omega-3 fatty acid and antioxidant supplementation in patients with ALI/ARDS. This study was stopped early by the data safety monitoring board for a trend towards excess mortality in patients receiving the omega-3 fatty acid supplement (personal communication from Dr. Art Wheeler and Dr. Todd Rice). One other randomized controlled trial in ALI/ARDS studied the effects of an immunomodulatory nutritional formula.116 In that trial, a diet rich in fish oil, γ-linoleic acid, and antioxidants was associated with a shorter duration of mechanical ventilation and fewer organ failures, but no difference in mortality. Using a different approach, a high-fat, low-carbohydrate diet reduced the duration of mechanical ventilation in patients with acute respiratory failure.117 Although the mechanism of this beneficial effect was postulated to be due to reduction of the respiratory quotient and a resultant fall in carbon dioxide production, the most common cause of a high respiratory quotient in critically ill patients is not dietary composition but simply overfeeding.114 Overall, there is still no compelling evidence to support the use of anything other than standard enteral nutritional support, with avoidance of overfeeding, in patients with ALI/ARDS. There is evidence from one large study to suggest that omega-3 fatty acid and antioxidant supplementation may be deleterious, so this regimen is not recommended at present. How early to attempt institution of feeding remains an unanswered question.
Mechanical Ventilation
Lung-Protective Ventilation
Although historically a tidal volume of 12 to 15 mL/kg was recommended in patients with ALI/ARDS, it is now clear that a low-tidal-volume, protective ventilatory strategy reduces mortality. In 2000, the NIH ARDS Network published the findings of their first randomized, controlled, multicenter clinical trial in 861 patients.62 The trial was designed to compare a lower-tidal-volume ventilatory strategy (6 mL/kg predicted body weight, plateau pressure < 30 cm H2O) with a higher tidal volume (12 mL/kg predicted body weight, plateau pressure < 50 cm H2O). The rationale for the clinical trial was the growing body of clinical and experimental evidence suggesting that ventilation with high tidal volumes and high plateau pressures might be harmful to the injured lung (see earlier Pathophysiology section). In this trial, the in-hospital mortality rate was 40% in the 12 mL/kg group and 31% in the 6 mL/kg—a 22% reduction. Ventilator-free days and organ failure–free days were also significantly improved in the low-tidal-volume group. These findings were truly remarkable, since no prior large randomized clinical trial of any specific therapy for ALI/ARDS has ever demonstrated a mortality benefit.
The current recommended treatment strategy for patients with ARDS is summarized in Table 58-2. Predicted body weight is calculated based on measured height, using the equations provided. This is a key point often overlooked by clinicians; use of actual rather than predicted body weight can result in the use of erroneously high and potentially injurious tidal volumes. The tidal volume should initially be set at 6 mL/kg predicted body weight. Interestingly, a tidal volume of 6 mL/kg predicted body weight is similar to normal tidal volumes in spontaneously breathing adults at rest. So, although this size tidal volume is often referred to as low tidal volume, it is really normal tidal volume ventilation. However, if end-inspiratory plateau pressure (measured during a 0.5-second pause) is still above 30 cm H2O, then tidal volume must be reduced in a stepwise fashion by 1 mL/kg to a minimum of 4 mL/kg. Ventilation with this size tidal volume is generally well tolerated. Some patients may have breath stacking or significant dyssynchrony with the ventilator. Increasing the inspiratory flow rate and, if necessary, the level of sedation is usually sufficient to manage these problems. Several studies have shown that on average, patients receiving lower-tidal-volume ventilation do not require increases in dose or duration of sedatives compared to patients receiving higher-tidal-volume ventilation.118,119 As with any mode of ventilation in ALI/ARDS, occasionally patients will require neuromuscular blockade, but this should be used only as a last resort in patients with refractory hypoxemia, since use of paralytics may increase the risk of critical illness, polyneuropathy, and myopathy. Respiratory acidosis may develop but is usually not symptomatic. Increasing the respiratory rate is usually sufficient to compensate for the decreased tidal volume; a rate as high as 35 was used in the ARDSNet clinical trial.
In the ARDS Network protocol, the level of PEEP and FIO2 was titrated according to a set of predetermined values (see Table 58-2). The optimal level of PEEP in ALI/ARDS has been controversial and is not yet established. Higher levels of PEEP may be beneficial in preventing alveolar collapse and minimizing injurious repeated opening and closing of alveoli. On the other hand, higher PEEP may overdistend and injure more complaint areas of the lung. Several studies have investigated the effects of different levels of PEEP in patients with ALI/ARDS.120 One large multicenter trial conducted by the ARDS Network randomized patients with ARDS ventilated with low-tidal-volume ventilation to receive lower (mean PEEP levels on days 1 to 4 were 8.3 ± 3.2) versus higher levels of PEEP (mean PEEP levels on days 1 to 4 were 13.2 ± 3.5).121 In this study, there were no differences between the groups in clinical outcomes, including ventilator-free days and mortality. Two other studies of the effects of PEEP in ARDS had similar results,122,123 although one of the studies did show an increase in the number of ventilator-free days and organ failure–free days with application of higher PEEP.123 None of these trials have shown significant increases in barotrauma related to higher PEEP levels. Although these three large studies have not shown beneficial effects of higher PEEP in all patients with ALI/ARDS, there may be a subset of patients who would benefit from higher PEEP. In a small trial, one investigator reported that a ventilator strategy that incorporated low tidal volume and titration of the PEEP level to above the lower inflection point on each individual patient’s pressure volume curve improved mortality in ARDS.124 However, measurement of the pressure-volume curve in any given patient is not practical clinically. Given the lack of compelling data favoring either a high PEEP or low PEEP strategy, current recommendations are to adjust the level of PEEP within an acceptable range (see Table 58-2) to achieve adequate oxygenation at a given FIO2.
Noninvasive Ventilation
Noninvasive positive-pressure ventilation (NIV) delivered by nasal or full face mask has been highly successful in avoidance of intubation in patients with acute exacerbation of COPD.125 NIV is commonly used in pediatric patients with ALI/ARDS,126 but there is only one small randomized trial of 50 patients which showed that NIV improved oxygenation and prevented the need for endotracheal intubation in children admitted with acute respiratory failure. The role for NIV in adults with ALI/ARDS is still unclear. A growing number of small studies suggest that bilevel NIV with pressure-support ventilation and PEEP may reduce the need for intubation and improve outcomes in selected patients with ALI/ARDS.127,128 However, data from large randomized controlled trials is still lacking. Furthermore, it seems likely that the majority of patients with ALI/ARDS will still require invasive mechanical ventilation. In one large multicenter study of 354 of 2770 patients with acute hypoxemic respiratory failure who were not already intubated, NIV failed in 30% of patients but failed in 51% of patients with ARDS.129
One group of patients in whom NIV is particularly appealing is those patients who are immunosuppressed for various reasons and are at highest risk for nosocomial infections. Encouraging results have now been reported in a variety of patients with acute respiratory failure and immunosuppression.130–132 Pending data from larger randomized clinical trials, a trial of noninvasive mechanical ventilation can be considered in a patient with ALI/ARDS who does not have a severe oxygenation defect, hemodynamic instability, or altered mental status, so long as the patient can be closely observed and readily intubated if NIV fails.
Pharmacologic Therapy
There is no specific pharmacologic therapy for ALI/ARDS. A variety of treatment strategies have been investigated in large randomized trials, with a predominant focus on antiinflammatory strategies. Agents that appeared promising in experimental and early clinical studies but failed in large randomized trials include early glucocorticoids,133,134 alprostadil,135–137 surfactant,138–140 ketoconazole,141 N-acetylcysteine,142 procysteine,142 lisofylline,143 and site-inactivated recombinant factor VIIa.144 Some investigators have suggested that glucocorticoid therapy, although not helpful for the acute phase of ALI/ARDS, might hasten the resolution of late fibroproliferative ALI/ARDS. In one very small randomized study (plagued by crossovers such that only four patients remained in the placebo arm) there was a suggestion that glucocorticoid therapy might be beneficial in late ARDS.145 This question was addressed in a randomized multicenter study conducted by the ARDS Network of 14 days of methylprednisolone in patients who had persistent ARDS for at least 7 days.146 Compared to patients treated with placebo, those treated with methylprednisolone had an increase in the number of shock-free days and ventilator-free days by day 28, as well as improvements in oxygenation; but they did not have improved survival and had higher rates of reintubation, perhaps due to neuromuscular weakness. Given the serious concern about safety of high-dose glucocorticoids in critically ill patients, including the possibility of increasing the risk of nosocomial infections or critical illness polyneuropathy/myopathy, as well as the lack of improvement in mortality, routine use of glucocorticoids in ARDS cannot be recommended.
Despite the dismal findings in the numerous studies of pharmacologic therapy for ALI/ARDS to date, new therapeutic strategies are under investigation and may yet be beneficial. One area that has been largely ignored in the therapeutic realm is modulation of coagulation. There is mounting evidence that like sepsis, ALI/ARDS is a procoagulant, antifibrinolytic state47,48 and that coagulation is activated and modulated locally in the airspace.49,51 Modulation of coagulation by administration of recombinant human activated protein C (rhAPC; drotrecogin alfa) significantly reduces mortality in patients with severe sepsis, many of whom also had ALI/ARDS,147 but the same therapy in patients with nonseptic ALI/ARDS did not show a mortality difference.148 Likewise, a randomized trial of site-inactivated recombinant factor VIIa did not show benefit in patients with ALI/ARDS.144 Another ongoing area of research involves the use of HMG-CoA reductase inhibitors (statins) in patients with ALI/ARDS. Some studies have shown that patients admitted to the hospital on statins have a lower mortality if they develop ALI/ARDS,149 although other studies have not found the same association.150 Another promising area of research is in modulating peroxisome proliferator–activated receptors using the glitazone class of diabetes medications in patients at risk for ALI/ARDS.151 Clinical studies have shown a decreased incidence of ALI/ARDS in patients with diabetes,29–31 but it is unclear whether this protection is a result of the diabetes itself or an effect of treatment. Another area that is actively under investigation involves strategies to hasten or facilitate the resolution of ALI/ARDS. Such therapies might be targeted at enhancing the rate of alveolar fluid clearance or modulating alveolar repair.
Rescue Therapies
Despite appropriate treatment, some patients with ARDS will have profound and refractory hypoxemia. Initial management of these patients includes increased sedation and occasionally neuromuscular paralysis to maintain adequate oxygenation. In patients who do not respond to conventional treatment with low-tidal-volume ventilation and remain persistently hypoxemic, there are several unproven rescue therapies that may be tried to improve oxygenation in the acute setting (summarized in Table 58-3). Extracorporeal membrane oxygenation (ECMO) has been used in patients with ARDS and severe hypoxemia. In specialized centers, ECMO has been used successfully to treat patients with severe ARDS,152–154 but it has not proven effective at reducing mortality in small randomized trials.155,156 One large trial randomized 180 patients with severe ARDS to ECMO versus conventional management and showed reduced mortality in patients treated with ECMO.157 In this study, patients randomized to ECMO were transferred to a specialty center to receive therapy. Upon arrival, only 75% of patients in the ECMO group were actually treated with ECMO. Because of the study design, it is difficult to determine whether it was transfer to a specialty center for care or ECMO itself that conferred benefit. Although the results of this study are encouraging, the need for transfer to a specialty center and the dropout rate of 25% upon transfer limit the widespread use of ECMO in severe ARDS.
TABLE 58-3 Summary of Rescue Therapies for Acute Lung Injury and Acute Respiratory Distress Syndrome
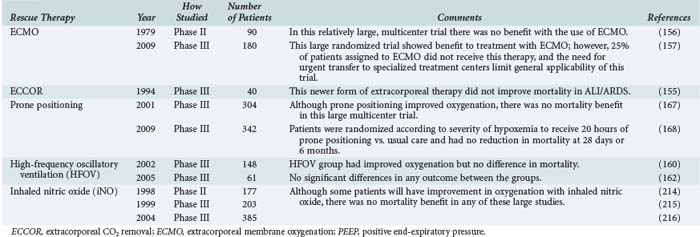
High-frequency oscillatory ventilation (HFVO) has been studied in several small randomized trials in patients with ARDS158–162 and did improve oxygenation but not mortality in these studies. Likewise, prone positioning has been studied in several small163–165 and three large trials166–168 and has been associated with improvements in oxygenation but no reduction in mortality. Other rescue therapies include the use of a pulmonary vasodilator, such as inhaled nitric oxide (iNO) or inhaled prostacyclin. One clinical study showed that higher urinary NO excretion, a surrogate for endogenous NO activity, was associated with improved clinical outcomes in patients with ALI.169 There have been several small, randomized clinical trials of iNO in ARDS, and although none have shown improved mortality, its use has been associated with improvements in oxygenation.170 Inhaled prostacyclin is another pulmonary vasodilator that may be used as rescue therapy in severe refractory ARDS, although there are no randomized trials showing a mortality benefit.171–173
 Complications
Complications
Barotrauma
Barotrauma occurs when air dissects out of the airways or alveolar space into surrounding tissues, leading to pneumothorax, pneumomediastinum, pneumatocele, or subcutaneous emphysema (Figure 58-4). The exact incidence of pulmonary barotrauma in ALI/ARDS is unclear but appears to be declining. Data from two recent large randomized trials of protective ventilatory strategies suggest an incidence of early pneumothorax of 12% to 13%.62,174 Higher incidences have been reported in the past, a finding that may have been the result of the use of mechanical ventilation with high tidal volumes and very high inspiratory plateau pressures.175 In 861 patients enrolled in the NIH ARDS Network trial, approximately 10% of patients developed some form of barotrauma regardless of whether they were in the 6 or 12 mL/kg tidal volume arm. Further, PEEP level was the only factor that predicted the development of barotrauma in a multivariate analysis.176
Treatment of barotrauma depends on the location of the extravasated air. Pneumothorax can be life threatening, particularly if it is under tension; immediate diagnosis and tube thoracostomy are essential. Pneumothorax should be considered in any mechanically ventilated patient with ALI/ARDS who develops sudden unexplained worsening of hypoxemia, respiratory distress, or hemodynamic instability. A chest radiograph (preferably upright) is usually sufficient to make the diagnosis, but in many cases there may not be time to obtain one. Pneumomediastinum and subcutaneous emphysema can be painful, but other than analgesia, they do not require specific therapy. Air embolus is a potentially fatal complication of positive-pressure mechanical ventilation that has been reported occasionally in patients with ALI/ARDS155,177,178 and usually occurs in conjunction with other evidence of pulmonary barotraumas, many times simultaneously.
Nosocomial Pneumonia
The incidence of nosocomial pneumonia in patients with ALI/ARDS is difficult to quantify. Depending on the diagnostic definition and/or strategy employed, estimates range from 15% to 60% of patients.179,180 There is yet no consensus regarding the appropriate way to diagnose nosocomial pneumonia in the mechanically ventilated patient. Since patients with ALI/ARDS frequently die from uncontrolled infection, recognition (though notably difficult) and treatment of nosocomial pneumonia is an important part of caring for the ALI/ARDS patient. Clinical criteria commonly used in the diagnosis include fever, elevated white blood cell count, purulent secretions, and pulmonary infiltrates. However, these signs are often present in patients with ALI/ARDS even in the absence of nosocomial pneumonia.181 Autopsy studies of patients dying with ALI/ARDS show a high incidence of unsuspected pneumonia.182–184 Regardless of the methods used for diagnosis, early, appropriate, empirical therapy is the mainstay of treatment for nosocomial pneumonia. The adequacy and timeliness of initial empirical therapy are important determinants of outcome. Knowledge of local bacterial resistance patterns is crucial, and a high index of suspicion is required.
Multisystem Organ Dysfunction
Although ALI/ARDS is often thought of as a primary pulmonary disorder, evidence is accumulating to suggest that ALI/ARDS is a systemic disorder with many similarities to sepsis or SIRS. Multisystem organ dysfunction is a common complication in ALI/ARDS. Organ dysfunction may result from the underlying cause of ALI/ARDS, such as sepsis, or occur independently. The exact incidence of multisystem organ dysfunction in ALI/ARDS is difficult to quantify. In the recent ARDS Network trial of low-tidal-volume ventilation, the mean number of non-pulmonary organ system failures per patient was 1.8.62 Given the simultaneous occurrence of multiple organ failures, it is often difficult to determine the exact cause of death in ALI/ARDS patients, and survival ultimately depends on the successful support of the failing organs.
Neuromuscular Weakness
Patients with ALI/ARDS are at high risk for developing prolonged muscle weakness that persists after resolution of pulmonary infiltrates and can complicate weaning from mechanical ventilation and rehabilitation. This clinical syndrome is commonly called critical illness polyneuropathy, but it actually has components of neuropathy and myopathy which can coexist or occur separately.185 Although little prospective data are available, one study suggests that neuromuscular abnormalities are persistent in many survivors of critical illness, even when studied up to 5 years after ICU discharge.186 Prolonged muscle weakness is most common in critically ill patients who are treated with glucocorticoids. In one study, use of corticosteroids was shown to be the best independent predictor of ICU-acquired paresis (odds ratio 14.9, 95% CI 3.2–69.8).187 Neuromuscular blockade has also been implicated, and for this reason, the use of neuromuscular blockade should be reserved for those patients who are unable to be adequately oxygenated or who have problematic dyssynchrony with the mechanical ventilator despite deep sedation. In the absence of a compelling clinical indication, such as underlying connective tissue disease, the use of glucocorticoids should not be routine unless new clinical evidence in support of their clinical utility in ALI/ARDS becomes available.
 Clinical Outcomes and Prognosis
Clinical Outcomes and Prognosis
Once a patient develops ALI/ARDS, there are several prognostic factors that can help clinicians predict outcome. Elevated pulmonary dead space fraction in ALI/ARDS is a reflection of extensive injury to the lung microcirculation, lung microvascular thrombi, and regional differences in pulmonary blood flow and is a predictor of death in patients with ARDS.188,189 Although dead space fraction may predict mortality, it is not routinely measured in the ICU. For this reason, predictive models that use readily available clinical variables have been developed.190 In addition to dead space fraction, a positive cumulative fluid balance at day 4 in patients with ARDS predicted increased mortality,191 further supporting the use of a conservative fluid strategy.109
Mortality from ALI/ARDS appears to be gradually declining,192 although this finding has not been consistent among retrospective studies.193 Prior to the 1990s, mortality in clinical trials was approximately 40% to 60%.194 Several recent single-center studies suggest that mortality rates measured in the same centers had declined over time.195–198 In the ARDS Network study of 861 patients with ALI/ARDS, aggregate mortality to hospital discharge was 31% in the 6 mL/kg tidal volume arm and 40% in the 12 mL/kg tidal volume arm. However, mortality data from this study may significantly underestimate overall ALI/ARDS mortality, since many severely ill patients were excluded, including those with advanced liver disease, bone marrow transplantation, severe chronic respiratory disease, burns greater than 30% body surface area, or any other underlying condition with a likelihood of death greater than 50% within 6 months. As has previously been observed in other studies, in this study risk of in hospital mortality was highest in patients with sepsis (43%), intermediate in those with pneumonia (36%) or aspiration (37%), and lowest in those with multiple trauma (11%).11 The low-tidal-volume strategy was effective at reducing mortality across all causes of ALI/ARDS.11 Another study has shown that implementation of the ARDSNet low-tidal-volume ventilator strategy was associated with reducing hospital mortality compared to historical controls.199
Several recent multicenter studies in France,200 Sweden,201 Australia,202 and Argentina203 attempted to define mortality and prognostic variables in observational population-based studies rather than from clinical trial participants. In these studies, mortality was variable, ranging from 32% for ALI to 58% to 60% for ARDS. The highest mortality observed in patients who met Consensus definitions of ARDS was reported from the French study (60%). Factors that were independently associated with mortality from ALI/ARDS varied from study to study and included age, Acute Physiology Score, PaO2/FIO2 ratio, organ failures or septic shock, immunosuppression, cardiovascular failure, and chronic liver disease.200–204 Two other U.S. studies of patients with ALI/ARDS predominantly from medical ICUs reported high overall mortality rates (58%).205,206 Mortality was associated with chronic liver disease and other underlying diseases, including HIV infection or cancer. In summary, these studies suggest that while some improvements in ALI/ARDS mortality have been made, mortality remains quite high in population-based studies.
ALI/ARDS survivors frequently have long-term functional disability, cognitive dysfunction, and psychosocial problems.207 Interestingly, pulmonary function frequently returns to normal or near normal in survivors. In a report of 1-year follow-up in 109 survivors from ARDS,98 lung volumes and spirometry had returned to normal by 6 months. However, carbon monoxide diffusing capacity was persistently low at 12 months. Six-minute walk distances were persistently low at 12 months, largely due to muscle wasting and weakness rather than pulmonary function abnormalities.98 Treatment with any systemic corticosteroid, the presence of illness acquired during the ICU stay, and the rate of resolution of the lung injury and multiorgan dysfunction during the ICU stay were the most important determinants of the 6-minute walk distance during the first year of follow-up. In other studies, patients who survive ALI/ARDS have been reported to have reduced health-related quality of life208 and pulmonary disease–specific health-related quality of life,209–211 as well as functional impairment that persist 2 years after ICU discharge.212 In addition to physical and social difficulties after ARDS, survivors have high rates of depression and anxiety.213
Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818-824.
Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683-693.
Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003;31(4 Suppl):S276-S284.
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
Ware LB, Matthay MA. Medical progress: the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334-1349.
Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788-2796.
The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213-2224.
The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575.
Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007 Aug;132(2):410-417.
1 Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967 Aug 12;2(7511):319-323.
2 Mahoney AM, Caldwell E, Hudson LD, Rubenfeld GD. Barriers to physician recognition of acute lung injury. Am J Respir Crit Care Med. 2003;167:A738.
3 Howard AE, Courtney-Shapiro C, Kelso LA, Goltz M, Morris PE. Comparison of 3 methods of detecting acute respiratory distress syndrome: clinical screening, chart review, and diagnostic coding. Am J Crit Care. 2004 Jan;13(1):59-64.
4 Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116:1347-1353.
5 Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818-824.
6 Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003 Apr;31(4 Suppl):S276-S284.
7 Murray JF, and the staff of the Division of Lung Diseases National Heart Lung and Blood Institute. Mechanisms of acute respiratory failure. Am Rev Respir Dis. 1977;115:1071-1078.
8 Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685-1693.
9 Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004 Jan;30(1):51-61.
10 Ciesla DJ, Moore EE, Johnson JL, et al. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery. 2006 Oct;140(4):640-647. discussion 7-8
11 Eisner M, Thompson T, Hudson L, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231-236.
12 Matthew EJ, Callister MA, Evans TW. Pulmonary versus extrapulmonary acute respiratory distress syndrome: different diseases or just a useful concept? Curr Opin Crit Care. 2002;8:21-25.
13 Rouby JJ, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P. Regional distribution of gas and tissue in acute respiratory distress syndrome. II. Physiological correlations and definition of an ARDS severity score. CT Scan ARDS Study Group. Intensive Care Med. 2000;26:1046-1056.
14 Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158:3-11.
15 Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003 Apr 1;167(7):1027-1035.
16 Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
17 Bernard G, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336:912-918.
18 Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995 Feb;151(2 Pt 1):293-301.
19 Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124-130.
20 Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96.
21 Sheu CC, Gong MN, Zhai R, et al. The influence of infection sites on development and mortality of ARDS. Intensive Care Med 2010;36(6):963-70.
22 Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50-54.
23 Thakur L, Kojicic M, Thakur SJ, et al. Alcohol consumption and development of acute respiratory distress syndrome: a population-based study. Int J Environ Res Public Health. 2009 Sep;6(9):2426-2435.
24 Towfigh S, Peralta MV, Martin MJ, et al. Acute respiratory distress syndrome in nontrauma surgical patients: a 6-year study. J Trauma. 2009 Dec;67(6):1239-1243.
25 Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009 Aug;67(2):221-227. discussion 8-30
26 Zilberberg MD, Carter C, Lefebvre P, et al. Red blood cell transfusions and the risk of acute respiratory distress syndrome among the critically ill: a cohort study. Crit Care. 2007;11(3):R63.
27 Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007 May;131(5):1308-1314.
28 Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care. 2009 Jan;37(1):14-19.
29 Frank JA, Nuckton TJ, Matthay MA. Diabetes mellitus: a negative predictor for the development of acute respiratory distress syndrome from septic shock. Crit Care Med. 2000 Jul;28(7):2645-2646.
30 Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005 Jun;33(6):1191-1198.
31 Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000 Jul;28(7):2187-2192.
32 Tomashefski JF. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435-466.
33 Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002 Nov;161(5):1783-1796.
34 Lee KS, Choi YH, Kim YS, et al. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir Med. 2008 Mar;102(3):464-469.
35 Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982 Jan;3(1):35-56.
36 Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589-615.
37 Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001 May;163(6):1376-1383.
38 Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1250-1257.
39 Sprung C, Rackow E, Fein I, Jacob A, Isikoff S. The spectrum of pulmonary edema: differentiation of cardiogenic intermediate and noncardiogenic forms of pulmonary edema. Am Rev Respir Dis. 1981;124:718-722.
40 Fein A, Grossman RF, Jones JG, et al. The value of edema protein measurements in patients with pulmonary edema. Am J Med. 1979;67:32-39.
41 Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1-7.
42 Gregory TJ, Longmore WJ, Moxley MA, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88:1976-1981.
43 Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147:218-233.
44 Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med. 2003 Jan;31(1):20-27.
45 Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999 Dec;160(6):1843-1850.
46 Gunther A, Ruppert C, Schmidt R, et al. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res. 2001;2:353-364.
47 Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000 Apr;22(4):401-404.
48 Idell S. Anticoagulants for acute respiratory distress syndrome: can they work? Am J Respir Crit Care Med. 2001 Aug 15;164(4):517-520.
49 Bastarache J, Wang L, Geiser T, et al. The Alveolar Epithelium can Initiate the Extrinsic Coagulation Cascade through Expression of Tissue Factor. Thorax. 2007 July;62(7):608-616.
50 Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009 Dec;297(6):L1035-L1041.
51 Wang L, Bastarache JA, Wickersham N, Fang X, Matthay MA, Ware LB. Novel role of the human alveolar epithelium in regulating intra-alveolar coagulation. Am J Respir Cell Mol Biol. 2007 Apr;36(4):497-503.
52 Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003 Sep;285(3):L514-L521.
53 Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003 Jul;285(1):L20-L28.
54 Bowler RP, Velsor LW, Duda B, et al. Pulmonary edema fluid antioxidants are depressed in acute lung injury. Crit Care Med. 2003 Sep;31(9):2309-2315.
55 Chabot F, Mitchell JA, Gutteridge JMC, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745-757.
56 Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end expiratory pressure. Am Rev Respir Dis. 1974;110:556-565.
57 Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010 Jan 7;14(1):R1.
58 Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159-1164.
59 Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressure. J Appl Physiol. 1984;57:1809-1816.
60 Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor. Am J Respir Crit Care Med. 1998;157:1721-1725.
61 Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. JAMA. 1999;282:54-61.
62 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000 May 4;342(18):1301-1308.
63 Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988 Sep;138(3):720-723.
64 Pandharipande PP, Shintani AK, Hagerman HE, et al. Derivation and validation of SpO2/FIO2 ratio to impute for PaO2/FIO2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med. 2009 Apr;37(4):1317-1321.
65 Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007 Aug;132(2):410-417.
66 Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005 Dec 29;353(26):2788-2796.
67 Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006 May 25;354(21):2213-2224.
68 Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002 Aug;28(8):1073-1077.
69 Levitt JE, Vinayak AG, Gehlbach BK, et al. Diagnostic utility of B-type natriuretic peptide in critically ill patients with pulmonary edema: a prospective cohort study. Crit Care. 2008;12(1):R3.
70 Meade MO, Cook RJ, Guyatt GH, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85-90.
71 Mikkelsen ME, Dedhiya PM, Kalhan R, Gallop RJ, Lanken PN, Fuchs BD. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respir Care. 2008 Apr;53(4):455-461.
72 Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the etiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2009 Sep 9.
73 Fremont RD, Koyama T, Calfee CS, et al. Acute Lung Injury in Patients With Traumatic Injuries: Utility of a Panel of Biomarkers for Diagnosis and Pathogenesis. J Trauma. 2009 Dec 24.
74 Beskow CO, Drachenberg CB, Bourquin PM, et al. Diffuse alveolar damage. Morphologic features in bronchoalveolar lavage fluid. Acta Cytol. 2000;44:640-646.
75 Suchyta MR, Elliott CG, Colby T, Rasmusson BY, Morris AH, Jensen RL. Open lung biopsy does not correlate with pulmonary function after the adult respiratory distress syndrome. Chest. 1991;99:1232-1237.
76 Kao KC, Tsai YH, Wu YK, et al. Open lung biopsy in early-stage acute respiratory distress syndrome. Crit Care. 2006;10(4):R106.
77 de Hemptinne Q, Remmelink M, Brimioulle S, Salmon I, Vincent JL. ARDS: A Clinicopathologic Confrontation. Chest. 2008 Dec 31.
78 Patel SR, Karmpaliotis D, Ayas NT, et al. The role of open-lung biopsy in ARDS. Chest. 2004 Jan;125(1):197-202.
79 Wiener-Kronish JP, Matthay MA. Pleural effusions associated with hydrostatic and increased permeability pulmonary edema. Chest. 1988;93:852-858.
80 Gattinoni L, Bombino M, Pelosi P, et al. Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA. 1994;271:1772-1779.
81 Goodman LR. Congestive heart failure and adult respiratory distress syndrome. New insights using computed tomography. Radiol Clin North Am. 1996;34:33-46.
82 Maunder RJ, Shuman WP, McHugh JW, Marglin SI, Butler J. Preservation of normal lung regions in the adult respiratory distress syndrome. Analysis by computed tomography. JAMA. 1986 May 9;255(18):2463-2465.
83 Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997 Apr;155(4):1187-1205.
84 Rodrigues RS, Miller PR, Bozza FA, et al. FDG-PET in patients at risk for acute respiratory distress syndrome: a preliminary report. Intensive Care Med. 2008 Dec;34(12):2273-2278.
85 Wright PE, Bernard GR. The role of airflow resistance in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139:1169-1174.
86 Kallet RH, Hemphill JC3rd, Dicker RA, et al. The spontaneous breathing pattern and work of breathing of patients with acute respiratory distress syndrome and acute lung injury. Respir Care. 2007 Aug;52(8):989-995.
87 Beiderlinden M, Kuehl H, Boes T, Peters J. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: predictive value of computed tomography. Intensive Care Med. 2006 Jun;32(6):852-857.
88 Osman D, Monnet X, Castelain V, et al. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009 Jan;35(1):69-76.
89 Cornet AD, Hofstra JJ, Swart EL, Girbes AR, Juffermans NP. Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med. 2010 May;36(5):758-764.
90 Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Am J Respir Crit Care Med. 1997;156:840-845.
91 Raghu G, Striker LJ, Hudson LD, Striker GE. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis. 1985;131:281-289.
92 Bitterman PB. Pathogenesis of fibrosis in acute lung injury. Am J Med. 1992;92:39S-43S.
93 Matthay MA, Broaddus VC. Fluid and hemodynamic management in acute lung injury. Semin Respir Crit Care Med. 1994;15:271-288.
94 Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002 Jul;82(3):569-600.
95 Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci. 1998;95:2991-2996.
96 Folkesson HG, Matthay MA, Westrom BR, Kim KJ, Karlsson BW, Hastings RH. Alveolar epithelial clearance of protein. J Appl Physiol. 1996;80:1431-1445.
97 Matute-Bello G, Liles WC, Radella IIF, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969-1977.
98 Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683-693.
99 Anderson ID, Fearon KC, Grant IS. Laparotomy for abdominal sepsis in the critically ill. Br J Surg. 1996;83:535-539.
100 Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-1377.
101 Richard C, Warszawski J, Anguel N, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003 Nov 26;290(20):2713-2720.
102 Hayes MA, Timmins AC, Yau EHS, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717-1722.
103 Yu M, Levy MM, Smith P, Takiguchi SA, Miyasaki A. Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: a prospective, randomized, controlled study. Crit Care Med. 1993;21:830-838.
104 Prewitt RM, McCarthy J, Wood LDH. Treatment of acute low pressure pulmonary edema in dogs. J Clin Invest. 1981;67:409-418.
105 Martin GS, Mangialardi RJ, Wheeler AP, Dupont WD, Morris JA, Bernard GR. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit Care Med. 2002 Oct;30(10):2175-2182.
106 Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990-998.
107 Humphrey H, Hall J, Sznajder I, Silverstein M, Wood L. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97:1176-1180.
108 Simmons RS, Berdine GG, Seidenfeld JJ, et al. Fluid balance and the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:924-929.
109 Network TARDS. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575. 2006 June 15
110 Network TARDS. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213-2224. 2006 May 25
111 Heyland DK, Cook DJ, Guyatt GH. Enteral nutrition in the critically ill patients: a critical review of the evidence. Intens Care Med. 1993;19:435-442.
112 Alverdy JC, Aoys E, Moss GS. TPN promotes bacterial translocation from the gut. Surgery. 1988;104:185-190.
113 Fong Y, Marano MA, Barber A. TPN and bowel rest modify the metabolic response to endotoxins in humans. Ann Surg. 1989;210:449-457.
114 Cerra FB, Benitez MR, Blackburn GL, et al. Applied nutrition in ICU patients: a consensus statement of the American College of Chest Physicians. Chest. 1997;111:769-778.
115 Heys SD, Walker LG, Smith I, et al. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229:467-477.
116 Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999 Aug;27(8):1409-1420.
117 al-Saady NM, Blackmore CM, Bennett ED. High fat, low carbohydrate, enteral feeding lowers PaCO2 and reduces the period of ventilation in artificially ventilated patients. Intensive Care Med. 1989;15:290-295.
118 Kahn JM, Andersson L, Karir V, Polissar NL, Neff MJ, Rubenfeld GD. Low tidal volume ventilation does not increase sedation use in patients with acute lung injury. Crit Care Med. 2005 Apr;33(4):766-771.
119 Wolthuis EK, Veelo DP, Choi G, et al. Mechanical ventilation with lower tidal volumes does not influence the prescription of opioids or sedatives. Crit Care. 2007;11(4):R77.
120 Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010 Mar 3;303(9):865-873.
121 Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004 Jul 22;351(4):327-336.
122 Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008 Feb 13;299(6):637-645.
123 Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008 Feb 13;299(6):646-655.
124 Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347-354.
125 Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817-822.
126 Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009 Aug;37(8):2448-2454.
127 Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA. 2002;288:932-935.
128 Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007 Jan;35(1):18-25.
129 Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27:1718-1728.
130 Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation. JAMA. 2000;283:235-241.
131 Hilbert G, Gruson D, Vargas F, et al. Noninvasive continuous positive airway pressure in neutropenic patients with acute respiratory failure requiring intensive care unit admission. Crit Care Med. 2000;28:3185-3190.
132 Hilbert G, Gruson D, Vargas F, et al. Non-invasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481-487.
133 Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988 Jul;138(1):62-68.
134 Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987 Dec 17;317(25):1565-1570.
135 Abraham E, Baughman R, Fletcher E, et al. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med. 1999;27:1478-1485.
136 Bone RC, Slotman G, Maunder R, Silverman H, Hyers TM, Kerstein M. Randomized double-blinded, multicenter study of prostaglandin E1 in patients with adult respiratory distress syndrome. Chest. 1989;96:114-119.
137 Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007 Apr;131(4):954-963.
138 Spragg RG, Lewis JF, Wurst W, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562-1566.
139 Anzueto A, Baughman RP, Guntupalli KK, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. N Engl J Med. 1996;334:1417-1421.
140 Spragg RG, Lewis JF, Walmrath HD, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004 Aug 26;351(9):884-892.
141 Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA. 2000 Apr 19;283(15):1995-2002.
142 Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. Chest. 1997;112:164-172.
143 The ARDS Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1-6.
144 Vincent JL, Artigas A, Petersen LC, Meyer C. A multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial assessing safety and efficacy of active site inactivated recombinant factor VIIa in subjects with acute lung injury or acute respiratory distress syndrome. Crit Care Med. 2009 Jun;37(6):1874-1880.
145 Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998 Jul 8;280(2):159-165.
146 Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006 Apr 20;354(16):1671-1684.
147 Bernard G, Vincent J-L, Laterre P-F, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
148 Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008 Sep 15;178(6):618-623.
149 Acute lung injury and the acute respiratory distress syndrome in Ireland: a prospective audit of epidemiology and management. Crit Care. 2008;12(1):R30.
150 Kor DJ, Iscimen R, Yilmaz M, Brown MJ, Brown DR, Gajic O. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009 Jun;35(6):1039-1046.
151 Paola RD, Cuzzocrea S. Peroxisome proliferator-activated receptors and acute lung injury. PPAR Res. 2007;2007:63745.
152 Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004 Oct;240(4):595-605. discussion -7
153 Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997 Sep;112(3):759-764.
154 Lewandowski K, Rossaint R, Pappert D, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med. 1997 Aug;23(8):819-835.
155 Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295-305.
156 Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. JAMA. 1979;242:2193-2196.
157 Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 Oct 17;374(9698):1351-1363.
158 Mehta S, Lapinsky SE, Hallett DC, et al. Prospective trial of high-frequency oscillation in adults with acute respiratory distress syndrome. Crit Care Med. 2001 Jul;29(7):1360-1369.
159 Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med. 2005 Mar;33(3):479-486.
160 Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002 Sep 15;166(6):801-808.
161 David M, Weiler N, Heinrichs W, et al. High-frequency oscillatory ventilation in adult acute respiratory distress syndrome. Intensive Care Med. 2003 Oct;29(10):1656-1665.
162 Bollen CW, van Well GT, Sherry T, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care. 2005 Aug;9(4):R430-R439.
163 Voggenreiter G, Aufmkolk M, Stiletto RJ, et al. Prone positioning improves oxygenation in post-traumatic lung injury–a prospective randomized trial. J Trauma. 2005 Aug;59(2):333-341. discussion 41-3
164 Beuret P, Carton MJ, Nourdine K, Kaaki M, Tramoni G, Ducreux JC. Prone position as prevention of lung injury in comatose patients: a prospective, randomized, controlled study. Intensive Care Med. 2002 May;28(5):564-569.
165 Mancebo J, Fernandez R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006 Jun 1;173(11):1233-1239.
166 Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004 Nov 17;292(19):2379-2387.
167 Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568-573.
168 Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009 Nov 11;302(18):1977-1984.
169 McClintock DE, Ware LB, Eisner MD, Wickersham N, Thompson BT, Matthay MA. Higher urine nitric oxide is associated with improved outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2007 Feb 1;175(3):256-262.
170 Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007 Apr 14;334(7597):779.
171 Zwissler B, Kemming G, Habler O, et al. Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1996 Dec;154(6 Pt 1):1671-1677.
172 Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996 Mar;153(3):991-996.
173 Van Heerden PV, Blythe D, Webb SA. Inhaled aerosolized prostacyclin and nitric oxide as selective pulmonary vasodilators in ARDS–a pilot study. Anaesth Intensive Care. 1996 Oct;24(5):564-568.
174 Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:1831-1838.
175 Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28:406-413.
176 Eisner MD, Thompson BT, Schoenfeld D, Anzueto A, Matthay MA, the Acute Respiratory Distress Syndrome Network. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:978-982.
177 Weaver LK, Morris A. Venous and arterial gas embolism associated with positive pressure ventilation. Chest. 1998;113:1132-1134.
178 Marini JJ, Culver BH. Sysemic gas embolism complicating mechanical ventilation in the adult repiratory distress syndrome. Ann Intern Med. 1989;110:699-703.
179 Chastre J, Trouillet JL, Vuagnat A, et al. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1165-1172.
180 Sutherland KR, Steinberg KP, Maunder RJ, Milberg JA, Allen DL, Hudson LD. Pulmonary infection during the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:550-556.
181 Meduri G, Mauldin G, Wunderink R, et al. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994;106:221-235.
182 Seidenfeld JJ, Pohl DF, Bell RC, Harris GD, Johanson WGJ. Incidence, site and outcome of infections in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1986;134:12-16.
183 Bell RC, Coalson JJ, Smith JD, Johanson WG. Multiple organ system failure and infection in adult respiratory distress syndrome. Ann Intern Med. 1983;99:293-298.
184 Rouby JJ, Martin De Lassale E, Poete P, et al. Nosocomial bronchopneumonia in the critically ill. Histologic and bacteriologic aspects. Am Rev Respir Dis. 1992;146:1059-1066.
185 Hudson LD, Lee CM. Neuromuscular sequelae of critical illness. N Engl J Med. 2003;348:745-747.
186 Fletcher SN, Kennedy DD, Ghosh IR, et al. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012-1016.
187 De Jonghe B, Sharshar T, Lefaucheur J-P, et al. Paresis acquired in the intensive care unit. A prospective multicenter study. JAMA. 2002;288:2859-2867.
188 Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002 Apr 25;346(17):1281-1286.
189 Raurich JM, Vilar M, Colomar A, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care. 2010 Mar;55(3):282-287.
190 Cooke CR, Shah CV, Gallop R, et al. A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med. 2009 Jun;37(6):1913-1920.
191 Rosenberg AL, Dechert RE, Park PK, Bartlett RH. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009 Jan-Feb;24(1):35-46.
192 Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008 May;133(5):1120-1127.
193 Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009 Feb 1;179(3):220-227.
194 Ware LB, Matthay MA. Medical progress: The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-1349.
195 Navarrete-Navarro P, Rodriguez A, Reynolds N, et al. Acute respiratory distress syndrome among trauma patients: trends in ICU mortality, risk factors, complications and resource utilization. Intensive Care Med. 2001;27:1133-1140.
196 Rocco TRJr, Reinert SE, Cioffi W, Harrington D, Buczko G, Simms HH. A 9-year, single-institution, retrospective review of death rate and prognostic factors in adult respiratory distress syndrome. Ann Surg. 2001;233:414-422.
197 Abel SJC, Finney SJ, Brett SJ, Keogh BF, Morgan CJ, Evans TW. Reduced mortality in association with the acute respiratory distress syndrome. Thorax. 1998;53:292-294.
198 Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA. 1995;273:306-309.
199 Kallet RH, Jasmer RM, Pittet JF, et al. Clinical implementation of the ARDS network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med. 2005 May;33(5):925-929.
200 Roupie E, Lepage E, Wysocki M, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Society de Reanimation de Langue Francaise. Intensive Care Med. 1999;25:920-929.
201 Luhr OR, Karlsson M, Thorsteinsson A, Rylander C, Frostell CG. The impact of respiratory variables on mortality in non-ARDS and ARDS patients requiring mechanical ventilation. Intensive Care Med. 2000;26:508-517.
202 Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002 Feb 15;165(4):443-448.
203 Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450-2456.
204 Gajic O, Afessa B, Thompson BT, et al. Prediction of death and prolonged mechanical ventilation in acute lung injury. Crit Care. 2007;11(3):R53.
205 Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU. Comorbid conditions, age, etiology and hospital outcome. Am J Respir Crit Care Med. 1998;157:1159-1164.
206 Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995 Dec;152(6 Pt 1):1818-1824.
207 Wilcox ME, Herridge MS. Long-term outcomes in patients surviving acute respiratory distress syndrome. Semin Respir Crit Care Med. 2010 Feb;31(1):55-65.
208 Deja M, Denke C, Weber-Carstens S, et al. Social support during intensive care unit stay might improve mental impairment and consequently health-related quality of life in survivors of severe acute respiratory distress syndrome. Crit Care. 2006;10(5):R147.
209 Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354-360.
210 Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156:1120-1128.
211 McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:90-94.
212 Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006 Sep 1;174(5):538-544.
213 Hopkins RO, Key CW, Suchyta MR, Weaver LK, Orme JFJr. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. Gen Hosp Psychiatry. 2010 Mar-Apr;32(2):147-155.
214 Dellinger RP, Zimmerman JL, Taylor RW. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: Results of a randomized phase II trial. Crit Care Med. 1998;26:15-23.
215 Payen D, Vallet B, the Genoa Group. Results of the French prospective multicentric randomized double-blind placebo-controlled trial on inhaled nitric oxide in ARDS. Intensive Care Med. 1999;25:S166.
216 Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004 Apr 7;291(13):1603-1609.