Chapter 54 Acute calcium disorders
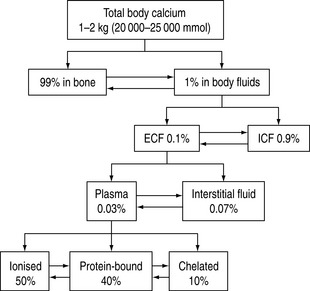
Calcium is an important cation and the principal electrolyte of the body. A total of 1–2 kg is present in the average adult, of which 99% is found in bone. Of the remaining 1%, nine-tenths is present in the cells and only a tenth in the extracellular fluid. In plasma, 50% of the calcium is ionised, 40% bound to plasma proteins, mainly to albumin, and the remaining 10% is chelated to anions such as citrate, bicarbonate, lactate, sulphate phosphate and ketones.1 The chelated fraction is usually of little clinical importance, but is increased in conditions where some of these anionic concentrations might be elevated, as in renal failure. Whilst most calcium inside the cell is in the form of insoluble complexes, the concentration of intracellular ionised calcium is about 0.1 μmol/l, creating a gradient of 10 000:1 between plasma and intracellular fluid levels of ionised calcium.2 A schematic illustration of calcium distribution within the various body compartments is shown in Figure 54.1.
Because ionised calcium is the biologically active component of extracellular fluid calcium with respect to physiological functions (Table 54.1) and is also the reference variable for endocrine regulation of calcium homeostasis, its measurement is recognised as being one of prime importance in the management of disorders of calcium homeostasis.
Table 54.1 Functions of calcium1,15
| Excitation – contraction coupling in cardiac, skeletal and smooth muscle |
| Cardiac action potentials and pacemaker activity |
| Release of neurotransmitters |
| Coagulation of blood |
| Bone formation and metabolism |
| Hormone release |
| Ciliary motility |
| Catecholamine responsiveness at the receptor site7 |
| Role as a strong cation |
| Regulation of cell growth and apoptosis |
METABOLIC FACTORS INFLUENCING CALCIUM HOMEOSTASIS
Changes in pH alter calcium protein binding. An increase in pH by 0.1 pH units results in a decrease in ionised calcium by approximately 0.1 mmol/l.4
As magnesium is required for PTH secretion and end-organ responsiveness, alterations in serum magnesium have an impact on serum calcium concentration.
Turnover of calcium in the bone is predominantly under the control of PTH and calcitriol, although prostaglandins and some of the cytokines also play a role in it. Bone resorption is mediated by osteoclasts, whereas osteoblasts are involved in bone formation. The daily calcium balance is summarised in Table 54.2.
| Gastrointestinal tract | |
| Diet | 600–1200 mg/day |
| Absorbed | 200–400 mg/day |
| Secreted | 150–800 mg/day |
| Renal | |
| Filtered | 11 000 mg/day |
| Reabsorbed (97% in the proximal convoluted tubule) | 10 800 mg/day |
| Urinary calcium | 200 mg/day |
| Bone | |
| Turnover | 600–800 mg/day |
MEASUREMENT OF SERUM CALCIUM
Most hospital laboratories measure total serum calcium. The normal plasma concentration is 2.2–2.6 mmol/l. However, the ionised form (1.1–1.3 mmol/l) is the active fraction and its measurement is not routine in many laboratories, although most state-of-the-art blood gas analysers can measure serum ionised calcium concentrations. Estimation of ionised calcium from total serum calcium concentration using mathematical algorithms is unreliable in critically ill patients.5–7 Heparin forms complexes with calcium and decreases ionised calcium.8 A heparin concentration of < 15 units/ml of whole blood is therefore recommended for the measurement of ionised calcium.9 Anaerobic collection of the specimen is recommended, as CO2 loss from the specimen may result in alkalosis and reduction in ionised calcium concentration. Calcium levels are also reduced by a concomitant lactic acidosis owing to chelation by lactate ion.10 Free fatty acids (FFAs) increase calcium binding to albumin and may form a portion of the calcium-binding site.11 Increases in FFAs may be seen in relation to stress, use of steroids, catecholamines and heparin. The impact of pH on calcium measurements has been described above. The normal reference levels of serum calcium are reduced in pregnancy and in the early neonatal period.12
HYPERCALCAEMIA IN CRITICALLY ILL PATIENTS
The frequency of hypercalcaemia in critically ill patients is not well established, although it is not as common as hypocalcaemia. Depending on the patient population, the reported incidence ranges from 3–5% to as high as 32%.13,14 Admission to the intensive care unit (ICU) with a primary diagnosis of a hypercalcaemic crisis is uncommon. Although a number of aetiologies has been described (Table 54.3), in the critical care setting it is usually due to malignancy-related hypercalcaemia, renal failure or posthypocalcaemic hypercalcaemia.15 Before undertaking a work-up for hypercalcaemia, it is important to exclude false-positive measurements. This is usually the result of inadvertent haemoconcentration during venepuncture and elevation in serum protein, although ionised calcium levels are not reported to be affected by haemoconcentration.16Pseudohypercalcaemia has also been described in the setting of essential thrombocythaemia. The erroneous result is thought to be due to in vitro release of calcium from platelets, analogous to the pseudohyperkalaemia seen in the same condition.17
| Common causes of hypercalcaemia in the critically ill patient |
| Complication of malignancy |
| Bony metastases |
| Humoral hypercalcaemia of malignancy |
| Posthypocalcaemic hypercalcaemia |
| Recovery from pancreatitis15 |
| Recovery from acute renal failure following rhabdomyolysis37–41 |
| Primary hyperparathyroidism |
| Adrenal insufficiency23,24 |
| Prolonged immobilisation18–21 |
| Disorders of magnesium metabolism |
| Use of total parenteral nutrition42 |
| Hypovolaemia |
| Iatrogenic calcium administration |
| Less common causes of hypercalcaemia in the critically ill patient |
| Granulomatous diseases – sarcoidosis, tuberculosis, berylliosis |
| Vitamin A and D intoxication |
| Multiple myeloma |
| Endocrine |
| Thyrotoxicosis |
| Acromegaly |
| Phaeochromocytoma |
| Lithium – chronic therapy |
| Rare association between drugs and hypercalcaemia |
| Theophylline, omeprazole and growth hormone therapy |
From a pathophysiological standpoint, hypercalcaemia may be due to an elevation in PTH, in which case the homeostatic regulatory and feedback mechanisms are preserved, and this is termed equilibrium hypercalcaemia. Alternatively, it could be a non-parathyroid-mediated hypercalcaemia with associated breakdown of homeostatic mechanisms, and this situation is termed dysequilibrium hypercalcaemia.
MECHANISMS OF HYPERCALCAEMIA
Posthypocalcaemic hypercalcaemia is a transient phenomenon seen in patients following a period of hypocalcaemia.15 This has been attributed to a parathyroid hyperplasia which develops during the period of hypocalcaemia, resulting in a rebound hypercalcaemia following resolution of the underlying hypocalcaemic disorder.
Immobilisation hypercalcaemia results from an alteration in balance between bone formation and resorption.18–21 This leads to loss of bone minerals, hypercalcaemia, hypercalciuria and increased risk of renal failure. In patients with normal bone turnover, immobilisation rarely causes significant hypercalcaemia. However, in patients with rapid turnover of bone (children, postfracture patients, hyperparathyroidism, Paget’s disease, spinal injuries and Guillain–Barré syndrome), this may result in severe hypercalcaemia.
Extrarenal production of calcitriol by lymphocytes in granulomata is thought to be the predominant mechanism of hypercalcaemia in granulomatous diseases.22
Only 10–20% of patients with adrenal insufficiency develop hypercalcaemia.23,24 The aetiology of this is thought to be multifactorial: intravascular volume depletion, haemoconcentration of plasma proteins and the loss of antivitamin D effects of glucocorticoids.
For rarer causes of hypercalcaemia, the reader is referred to a recent review.25
MANIFESTATIONS OF HYPERCALCAEMIA
The clinical manifestations of hypercalcaemia (commonly encountered when total serum calcium exceeds 3 mmol/l) are outlined in Table 54.4. Hypercalcaemic crisis is defined as severe hypercalcaemia (total serum Ca > 3.5 mmol/l) associated with acute symptoms and signs.
| Cardiovascular |
| Hypertension |
| Arrhythmias |
| Digitalis sensitivity |
| Catecholamine resistance |
| Urinary system |
| Nephrocalcinosis |
| Nephrolithiasis |
| Tubular dysfunction |
| Renal failure |
| Gastrointestinal |
| Anorexia/nausea/vomiting |
| Constipation |
| Peptic ulcer |
| Pancreatitis |
| Neuromuscular |
| Weakness |
| Neuropsychiatric |
| Depression |
| Disorientation |
| Psychosis |
| Coma |
| Seizures |
* Ectopic calcification is usually seen with chronic hypercalcaemia.
THERAPY OF HYPERCALCAEMIA AND HYPERCALCAEMIC CRISIS
The management of hypercalcaemic crisis consists of two principal components:
INCREASING URINARY EXCRETION OF CALCIUM
As almost all patients with hypercalcaemia are volume-depleted, the initial therapy consists of rehydration with normal saline followed by diuresis with furosemide. Rehydration with normal saline improves intravascular volume, reduces serum calcium by extracellular dilution and saliuresis promotes calcium loss in the urine. Volume expansion should be titrated to clinical endpoints and central venous pressure monitoring. A urine output of 4–5 litres should be aimed for in these patients to promote calciuresis. In many patients, these measures would achieve a reduction in serum calcium by about 0.4–0.5 mmol/l. Hypokalaemia, hypomagnesaemia and calcium stone formation in the urine are potential side-effects of this mode of treatment.
REDUCING BONE RESORPTION
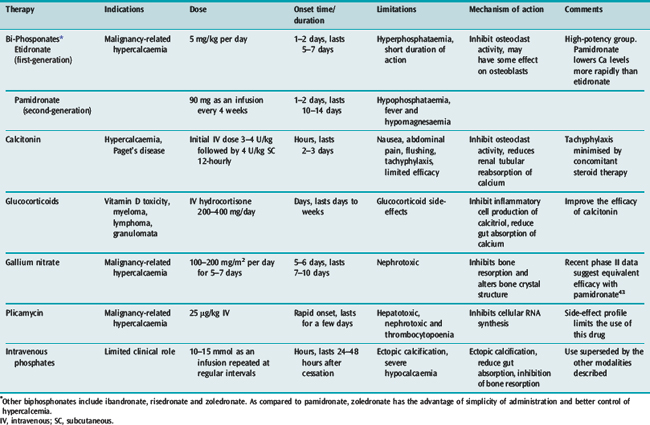
Measures to increase urinary excretion of calcium should be followed up with administration of agents minimising bone resorption. A number of agents are available and these are listed in Table 54.5.
Disodium ethylenediaminetetraacetic acid (EDTA) at a dose of 15–50 mg/kg intravenously rapidly lowers serum calcium. However, its propensity to reduce serum calcium rapidly coupled with its nephrotoxic effects limits its usefulness in life-threatening hypercalcaemia. Other therapeutic modalities include the use of non-steroidal anti-inflammatory drugs and parathyroidectomy. Calcimimetics are agents which increase the activation of the calcium receptor, thus reducing serum PTH concentrations. Cinacalcet is a first-generation calcimimetic which has shown promise in early randomised trials for the management of hypercalcaemia. However, their role in the management of acute hypercalcaemia needs to be established.26
Other drugs that have been used in the management of hypercalcaemia include prostaglandin inhibitors for cancer-related hypercalcaemia, ketoconazole and chloroquine (for sarcoid-induced hypercalcaemia).27
HYPOCALCAEMIA
Hypocalcaemia is more common than hypercalcaemia in critically ill patients, with an estimated incidence of around 70–90%.5 As the ionised calcium is the biologically active moiety, it is important to look at ionised hypocalcaemia. The frequency of this is far more varied, ranging from 15 to 70%.5,28,29 Spurious hypocalcaemia may be seen in the following circumstances: a) prolonged storage of specimens prior to analysis (resulting in CO2 loss from specimens) or b) if the blood sample is drawn with a syringe containing large doses of heparin as an anticoagulant or c) inadvertent collection of the blood sample into EDTA containing tubes (due to calcium chelation). Gadolinium agents (used as contrast for MRI) may also cause a pseudohypocalcaemia if a colorimetric assay is used for the measurement of calcium.
AETIOLOGIES
The aetiology of ionised hypocalcaemia based on the predominant pathophysiological mechanism is listed in Table 54.6. The other contributory mechanisms of hypocalcaemia in each of the conditions are shown in brackets.
| Calcium chelation |
| Alkalosis (increased binding of calcium by albumin) |
| Citrate toxicity (calcium chelation) |
| Hyperphosphataemia (calcium chelation, ectopic calcification, reduced vitamin D3 activity) |
| Pancreatitis (calcium soap formation, reduced parathyroid secretion) |
| Tumour lysis syndrome (hyperphosphataemia) |
| Rhabdomyolysis (hyperphosphataemia and reduced levels of calcitriol) |
| Hypoparathyroidism |
| Hypo- and hypermagnesaemia |
| Sepsis (decreased parathyroid hormone secretion, calcitriol resistance, intracellular shift of calcium) |
| Burns (decrease in parathyroid hormone secretion) |
| Neck surgery (removal of parathyroid gland, calcitonin release during thyroid surgery and ‘hungry-bone’ syndrome postparathyroidectomy) |
| Hypovitaminosis D |
| Inadequate intake |
| Malabsorption |
| Liver disease (impaired 25-hydroxylation of cholecalciferol) |
| Renal failure (impaired 1-hydroxylation of cholecalciferol, hyperphosphataemia) |
| Reduced bone turnover |
| Osteoporosis |
| Elderly |
| Cachexia |
| Drug-induced |
| Phenytoin (accelerated metabolism of vitamin D3) |
| Diphosphonates (see under hypercalcaemia) |
| Propofol |
| Ethylenediaminetetraacetic acid (EDTA: calcium chelation) |
| Ethylene glycol (formation of calcium oxalate crystals in the urine) |
| Cis-platinum (renal tubular damage leading to hypermagnesuria) |
| Protamine |
| Gentamicin (hypermagnesuria leading to hypomagnesaemia and therefore hypocalcaemia) |
Although a long list of causes exists for hypocalcaemia, calcium chelation and hypoparathyroidism constitute the common mechanisms of ionised hypocalcaemia in intensive care. Frequently, hypocalcaemia is accompanied by a number of other biochemical abnormalities; thus a pattern recognition approach towards the cause of hypocalcaemia will point to its aetiology and save a considerable amount of investigations for the patient. Common diagnostic patterns are listed in Table 54.7.
Table 54.7 Pattern recognition in the diagnosis of common causes of hypocalcaemia
| Aetiology of hypocalcaemia | Clinical/biochemical patterns |
|---|---|
| Low serum albumin | Reduced total calcium, normal ionised calcium |
| Alkalosis | Normal total calcium, reduced ionised calcium |
| Hypomagnesaemia | Reduced ionised calcium and hypokalaemia |
| Pancreatitis | Hypocalcaemia, elevated serum lipase and glucose |
| Renal failure | Elevated blood urea nitrogen, elevated phosphate |
| Rhabdomyolysis | Hypocalcaemia, elevated phosphate, creatine kinase and urinary myoglobin |
| Tumour lysis syndrome | Hypocalcaemia, elevated phosphate, potassium and urate |
Whilst alkalosis is frequently associated with ionised hypocalcaemia, the presence of a metabolic acidosis in the face of a low serum ionised calcium narrows the differential diagnosis even further (Table 54.8).
Table 54.8 Hypocalcaemia with metabolic acidosis
| Acute renal failure |
| Tumour lysis |
| Rhabdomyolysis |
| Pancreatitis |
| Ethylene glycol poisoning |
| Hydrofluoric acid intoxication |
CLINICAL MANIFESTATIONS OF HYPOCALCAEMIA
Mild degrees of hypocalcaemia are usually asymptomatic. Ionised calcium levels less than 0.8 mmol/l may cause neuromuscular irritability and result in clinical symptoms. The clinical manifestations of hypocalcaemia are summarised in Table 54.9. The manifestations listed in Table 54.9 are by no means a comprehensive list of all the clinical features, but include the ones most commonly seen in the critical care setting.
| Central nervous system |
| Circumoral and peripheral paraesthesia |
| Muscle cramps |
| Tetany |
| Seizures |
| Extrapyramidal manifestations: tremor, ataxia, dystonia |
| Proximal myopathy |
| Depression, anxiety, psychosis |
| Cardiovascular |
| Arrhythmias |
| Hypotension, inotrope unresponsiveness |
| Prolonged QT intervals, T-wave inversion |
| Loss of digitalis effect |
| Respiratory |
| Apnoea |
| Laryngospasm |
| Bronchospasm |
When eliciting tetany, Trousseau’s sign (carpopedal spasm) is more specific for hypocalcaemia than Chvostek’s sign (facial twitch in response to facial nerve stimulus – present in 10–30% of the normal population). Electrocardiogram changes do not correlate well with the degree of hypocalcaemia. The symptoms of hypocalcaemia are exacerbated by a coexisting hypokalaemia and hypomagnesaemia.
APPROACH TO THE TREATMENT OF ASYMPTOMATIC AND SYMPTOMATIC HYPOCALCAEMIA
ARGUMENTS FOR AND AGAINST CORRECTION OF ASYMPTOMATIC HYPOCALCAEMIA
As stated before, it is not clear if asymptomatic hypocalcaemia needs correction. Based on published data which suggest that critical care hypocalcaemia is associated with a higher mortality and increased length of stay in intensive care,30–32 it is advocated that ionised hypocalcaemia be corrected routinely, irrespective of the level. However, arguments exist against the routine correction of asymptomatic ionised hypocalcaemia. Increases in cytosolic calcium lead to disruption of intracellular processes and activation of proteases and can lead to ischaemia and reperfusion injury.33 Also, there are data suggesting that ionised calcium is an important participant in the pathogenesis of coronary and cerebral vasospasm.34 In rodent models of endotoxic shock, there are also data demonstrating an increased mortality when these rats were administered intravenous calcium.35 Most clinicians agree that an ionised calcium level of < 0.8 mmol/l needs correction, even if asymptomatic.
MANAGEMENT OF ACUTE SYMPTOMATIC HYPOCALCAEMIA
Acute symptomatic hypocalcaemia is a medical emergency that requires immediate therapy. In addition to treatment of underlying cause and support of airway, breathing and circulation, the definitive treatment includes administration of intravenous calcium. Intravenous calcium is available as a calcium salt of chloride or gluconate or acetate. The main difference between these formulations is the amount of elemental calcium available at equivalent volumes of drug (Table 54.10). The dose of calcium required should be based on the elemental calcium.36 Intravenous calcium can be administered as a bolus or as an infusion. Rapid administration of calcium may cause nausea, flushing, headache and arrhythmias. Digitalis toxicity may be precipitated. Extravasation of calcium may lead to tissue irritation, particularly with the chloride salt.
| Preparation | Dosage | Elemental calcium/gram |
|---|---|---|
| Calcium gluconate | 10 ml | 93 mg (2.3 mmol) |
| Calcium chloride | 10 ml | 272 mg (6.8 mmol) |
Calcium chloride may be better than calcium gluconate for the management of hypocalcaemia, if there is concomitant alkalosis. Following an initial bolus, an infusion may be commenced at a rate of 1–2 mg/kg per hour of elemental calcium to maintain target levels of ionised calcium. With correction of the underlying disorder and restoration of calcium to normal levels, the infusion can be tapered and stopped. Adequacy of calcium therapy can be monitored clinically and by performing serial determinations of ionised calcium. Failure of ionised calcium to increase after commencement of intravenous calcium may indicate an underlying magnesium deficiency. This can be corrected by administration of 10 mmol of intravenous magnesium over 20 minutes. Administration of calcium in the setting of hyperphosphataemia may result in calcium precipitation in the tissues. Calcium salts should not be administered with bicarbonate since the two precipitate. The other indications for calcium administration are listed in Table 54.11. Other therapy for hypocalcaemia consists of oral calcium supplements and calcitriol administration, although these are usually used in the management of chronic hypocalcaemia.
Table 54.11 Indications for calcium administration
| Absolute |
| Symptomatic hypocalcaemia |
| Ionised Ca < 0.8 mmol/l |
| Hyperkalaemia |
| Calcium channel blocker overdose |
| Relative |
| β-blocker overdose |
| Hypermagnesaemia |
| Hypocalcaemia in the face of high inotrope requirement |
| Massive blood transfusion post cardiopulmonary bypass to augment cardiac contractility |
1 Bourdeau J, Attie M. Calcium metabolism. In: Narins R, editor. Clinical Disorders of Fluid and Electrolyte Metabolism. 5th edn. New York: McGraw-Hill; 1994:243-250.
2 Zaloga GP. Hypocalcemia in critically ill patients. Crit Care Med. 1992;20:251-262.
3 Holick M, Krane S, Potts J. Calcium, phosphorus and bone metabolism: calcium-regulating hormones. In: Fauci AS, editor. Principles of Internal Medicine. New York: McGraw Hill, 1998.
4 Watchko J, Bifano EM, Bergstrom WH. Effect of hyperventilation on total calcium, ionized calcium, and serum phosphorus in neonates. Crit Care Med. 1984;12:1055-1056.
5 Zaloga GP, Chernow B, Cook D, et al. Assessment of calcium homeostasis in the critically ill surgical patient. The diagnostic pitfalls of the McLean–Hastings nomogram. Ann Surg. 1985;202:587-594.
6 Vincent JL, Jankowski S. Why should ionized calcium be determined in acutely ill patients? Acta Anaesthesiol Scand. 1995;107(suppl.):281-286.
7 Toffaletti J. Physiology and regulation. Ionized calcium, magnesium and lactate measurements in critical care settings. Am J Clin Pathol. 1995;104(Suppl. 1):88-94.
8 Landt M, Hortin GL, Smith CH, et al. Interference in ionized calcium measurements by heparin salts. Clin Chem. 1994;40:565-570.
9 Sachs C, Rabouine P, Chaneac M, et al. In vitro evaluation of a heparinized blood sampler for ionized calcium measurement. Ann Clin Biochem. 1991;28:240-244.
10 Toffaletti J, Abrams B. Effects of in vivo and in vitro production of lactic acid on ionized, protein-bound, and complex-bound calcium in blood. Clin Chem. 1989;35:935-938.
11 Zaloga GP, Willey S, Tomasic P, et al. Free fatty acids alter calcium binding: a cause for misinterpretation of serum calcium values and hypocalcemia in critical illness. J Clin Endocrinol Metab. 1987;64:1010-1014.
12 Aggarwal R, Upadhyaya M, Deorari AK, et al. Hypocalcemia in the new born. Ind J Pediatr. 2001;68:973-975.
13 Forster J, Querusio L, Burchard KW, et al. Hypercalcemia in critically ill surgical patients. Ann Surg. 1985;202:512-518.
14 Lind L, Ljunghall S. Critical care hypercalcemia – a hyperparathyroid state. Exp Clin Endocrinol. 1992;100:148-151.
15 Zaloga GP. Calcium homeostasis in the critically ill patient. Magnesium. 1989;8:190-200.
16 McMullan AD, Burns J, Paterson CR. Venepuncture for calcium assays: should we still avoid the tourniquet? Postgrad Med J. 1990;66:547-548.
17 Howard MR, Ashwell S, Bond LR, et al. Artefactual serum hyperkalemia and hypercalcemia in essential thrombocythaemia. J Clin Pathol. 2000;53:105-109.
18 Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999;80:998-1000.
19 Sato Y, Fujimatsu Y, Kikuyama M, et al. Influence of immobilization on bone mass and bone metabolism in hemiplegic elderly patients with a long-standing stroke. J Neurol Sci. 1998;156:205-210.
20 Kedlaya D, Brandstater ME, Lee JK. Immobilization hypercalcemia in incomplete paraplegia: successful treatment with pamidronate. Arch Phys Med Rehabil. 1998;79:222-225.
21 Evans RA, Lawrence PJ, Thanakrishnan G, et al. Immobilization hypercalcaemia due to low bone formation and responding to intravenous sodium sulphate. Postgrad Med J. 1986;62:395-398.
22 Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med. 2000;6:442-447.
23 Miell J, Wassif W, McGregor A, et al. Life-threatening hypercalcaemia in association with Addisonian crisis. Postgrad Med J. 1991;67:770-772.
24 Vasikaran SD, Tallis GA, Braund WJ. Secondary hypoadrenalism presenting with hypercalcaemia. Clin Endocrinol (Oxf). 1994;41:261-264.
25 Jacobs TP, Bilezikian JP. Clinical review. Rare causes of hypercalcemia. J Clin Endocrinol Metab. 2005;90:6316-6322.
26 Steddon SJ, Cunningham J. Calcimimetics and calcilytics – fooling the calcium receptor. Lancet. 2005;365:2237-2239.
27 Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med. 2000;6:442-447.
28 Zaloga GP, Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone–vitamin D axis. Ann Intern Med. 1987;107:36-41.
29 Desai TK, Carlson RW, Geheb MA. Prevalence and clinical implications of hypocalcemia in acutely ill patients in a medical intensive care setting. Am J Med. 1988;84:209-214.
30 Chernow B, Zaloga G, McFadden E, et al. Hypocalcemia in critically ill patients. Crit Care Med. 1982;10:848-851.
31 Desai TK, Carlson RW, Thill-Baharozian M, et al. A direct relationship between ionized calcium and arterial pressure among patients in an intensive care unit. Crit Care Med. 1988;16:578-582.
32 Broner CW, Stidham GL, Westenkirchner DF, et al. Hypermagnesemia and hypocalcemia as predictors of high mortality in critically ill pediatric patients. Crit Care Med. 1990;18:921-928.
33 Cheung JY, Bonventre JV, Malis CD, et al. Calcium and ischemic injury. N Engl J Med. 1986;314:1670-1676.
34 Lemmer JHJr, Kirsh MM. Coronary artery spasm following coronary artery surgery. Ann Thorac Surg. 1988;46:108-115.
35 Zaloga GP, Sager A, Black KW, et al. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock. 1992;37:226-229.
36 Stratta P, Soragna G, Morellini V, et al. The patient whose hypocalcaemia worsened after prompt intravenous calcium replacement therapy. Lancet. 2006;367:273.
37 Meneghini LF, Oster JR, Camacho JR, et al. Hypercalcemia in association with acute renal failure and rhabdomyolysis. Case report and literature review. Miner Electrolyte Metab. 1993;19:1-16.
38 Akmal M, Bishop JE, Telfer N, et al. Hypocalcemia and hypercalcemia in patients with rhabdomyolysis with and without acute renal failure. J Clin Endocrinol Metab. 1986;63:137-142.
39 Leonard CD, Eichner ER. Acute renal failure and transient hypercalcemia in idiopathic rhabdomyolysis. JAMA. 1970;211:1539-1540.
40 Prince RL, Hutchison BG, Bhagat CI. Hypercalcemia during resolution of acute renal failure associated with rhabdomyolysis: evidence for suppression of parathyroid hormone and calcitriol. Aust NZ J Med. 1986;16:506-508.
41 Sperling LS, Tumlin JA. Case report: delayed hypercalcemia after rhabdomyolysis-induced acute renal failure. Am J Med Sci. 1996;311:186-188.
42 Izsak EM, Shike M, Roulet M, et al. Pancreatitis in association with hypercalcemia in patients receiving total parenteral nutrition. Gastroenterology. 1980;79:555-558.
43 Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12:47-53.