Chapter 20 Acute Bacterial Pneumonia
1 Define severe community-acquired pneumonia (CAP)
Patients with severe CAP have a number of characteristics:
 They generally require intensive care unit (ICU) management.
They generally require intensive care unit (ICU) management.
 They have a higher mortality rate than do patients with nonsevere CAP.
They have a higher mortality rate than do patients with nonsevere CAP.
 Empiric antibiotic therapy in this group differs from that in patients with nonsevere CAP.
Empiric antibiotic therapy in this group differs from that in patients with nonsevere CAP.
Unfortunately, it is challenging to prospectively identify this cohort of patients. Of particular concern are patients who are initially triaged as having nonsevere CAP but subsequently need ICU admission (up to 50% of ICU admissions fall under this category in some studies). Such patients tend to have a higher mortality than equally sick patients who have been directly admitted to an ICU. A number of severity of illness scores have been developed to help define severe CAP, a popular one being derived from the joint Infectious Diseases Society of America–American Thoracic Society guidelines for the management of CAP in adults (Box 20-1), which incorporates elements of the confusion, urea, respiratory rate, and blood pressure (CURB) score. By this definition, patients with one major criterion or three minor criteria are designated as having severe CAP. Another widely used score is the Pneumonia Severity Index (PSI). However, none of these scores has been prospectively validated for individual patients. Clinical judgment remains critical; do not blindly follow scores! In recent years other approaches have been explored to identify patients with severe CAP; some of these are discussed below (see answer 9 on recent developments in CAP).
Box 20-1 Criteria for Severe CAP
Minor Criteria
Modified from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(2 Suppl):S27-S72, 2007.
2 Which pathogens most commonly cause severe CAP?
The most common causes of severe CAP in ICU patients are (in order of decreasing incidence):
4 What determines the selection of empiric antimicrobial therapy for patients with severe CAP?
The initial empiric antibiotic regimen for patients in the ICU with severe CAP is outlined in Box 20-2. Broadly speaking, the general principles of antibiotic therapy are as follows:
 Empiric treatment should cover the three most common pathogens causing severe CAP (see earlier), all atypical pathogens, and most relevant Enterobacteriaceae species. Broader coverage may be considered depending on epidemiologic considerations (see later).
Empiric treatment should cover the three most common pathogens causing severe CAP (see earlier), all atypical pathogens, and most relevant Enterobacteriaceae species. Broader coverage may be considered depending on epidemiologic considerations (see later).
 Combination therapy is better than monotherapy.
Combination therapy is better than monotherapy.
 Recent data strongly suggest that benefits of combination therapy are maximal when one of the agents is a macrolide. Therefore a macrolide should be included in all regimens unless a compelling reason exists not to do so.
Recent data strongly suggest that benefits of combination therapy are maximal when one of the agents is a macrolide. Therefore a macrolide should be included in all regimens unless a compelling reason exists not to do so.
Box 20-2 Recommended Empiric Antibiotics for Severe CAP in the ICU
A β-lactam (cefotaxime, ceftriaxone, or ampicillin-sulbactam)
A respiratory fluoroquinolone (levofloxacin [750 mg], moxifloxacin, or gemifloxacin)
If Pseudomonas is a consideration:
An antipneumococcal, antipseudomonal β-lactam (piperacillin-tazobactam, cefepime, imipenem, or meropenem)
Either ciprofloxacin or levofloxacin (750 mg)
The previously mentioned β-lactam plus an aminoglycoside and azithromycin
The previously mentioned β-lactam plus an aminoglycoside and an antipneumococcal fluoroquinolone (for penicillin-allergic patients, substitute aztreonam for previously mentioned β-lactam)
If CA-MRSA is a consideration: Add vancomycin or linezolid.
Penicillin allergy: Substitute aztreonam for the previously mentioned β-lactams.
Modified from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(2 Suppl):S27-S72, 2007.
5 What risk factors would prompt broader coverage?
 Pseudomonas: Long-term oral steroids (> 10 mg prednisone per day), underlying bronchopulmonary disease (bronchiectasis), severe chronic obstructive pulmonary disease, alcoholism, frequent antibiotic use. Note that the strongest justification for beginning antipseudomonal coverage is the presence of a consistent Gram stain of blood or sputum.
Pseudomonas: Long-term oral steroids (> 10 mg prednisone per day), underlying bronchopulmonary disease (bronchiectasis), severe chronic obstructive pulmonary disease, alcoholism, frequent antibiotic use. Note that the strongest justification for beginning antipseudomonal coverage is the presence of a consistent Gram stain of blood or sputum.
 Community-acquired methicillin-resistant S. aureus (CA-MRSA): Patients with cavitary lesions, patients who have had influenza, patients receiving long-term dialysis, intravenous (IV) drug abusers, and patients who have had recent antibiotic treatment (particularly with fluoroquinolones). Although a consistent sputum Gram stain is a strong reason to cover for S. aureus, a blood Gram stain may be falsely positive because of contamination.
Community-acquired methicillin-resistant S. aureus (CA-MRSA): Patients with cavitary lesions, patients who have had influenza, patients receiving long-term dialysis, intravenous (IV) drug abusers, and patients who have had recent antibiotic treatment (particularly with fluoroquinolones). Although a consistent sputum Gram stain is a strong reason to cover for S. aureus, a blood Gram stain may be falsely positive because of contamination.
 Anaerobes: Aspiration in the setting of alcohol or drug intoxication or in the presence of gingival disease or esophageal dysmotility.
Anaerobes: Aspiration in the setting of alcohol or drug intoxication or in the presence of gingival disease or esophageal dysmotility.
 Drug-resistant S. pneumoniae (DRSP): Age > 65 years, alcoholism, immunosuppression, exposure to antibiotics in the last 3 months (class-specific resistance), comorbidities, and exposure to children attending day care. In most cases, typical empiric therapy for CAP in the ICU (Box 20-2) should cover DRSP.
Drug-resistant S. pneumoniae (DRSP): Age > 65 years, alcoholism, immunosuppression, exposure to antibiotics in the last 3 months (class-specific resistance), comorbidities, and exposure to children attending day care. In most cases, typical empiric therapy for CAP in the ICU (Box 20-2) should cover DRSP.
6 When should antibiotics be initiated, and what is the optimal duration of treatment?
Patients with CAP should be treated for a minimum of 5 days, should be afebrile for 48 to 72 hours, and should not have more than one CAP-associated sign of clinical instability (Box 20-3) before stopping treatment.
Box 20-3 Criteria for Clinical Stability in Resolving CAP
Respiratory rate < 24 breaths/min
Systolic blood pressure > 90 mm Hg
Arterial oxygen saturation > 90% or PO2 > 60 mm Hg with room air
Modified from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(2 Suppl):S27-S72, 2007.
8 Discuss CA-MRSA infections
 CA-MRSA infections have reached epidemic proportions in the United States and are now the most common cause of infections in patients coming to EDs. The majority of infections are skin and soft tissue infections; approximately 2% of CA-MRSA infections present as CAP.
CA-MRSA infections have reached epidemic proportions in the United States and are now the most common cause of infections in patients coming to EDs. The majority of infections are skin and soft tissue infections; approximately 2% of CA-MRSA infections present as CAP.
 CAP caused by CA-MRSA tends to be severe, with a high incidence of necrotizing pneumonia, shock, respiratory failure, lung abscess, and empyema.
CAP caused by CA-MRSA tends to be severe, with a high incidence of necrotizing pneumonia, shock, respiratory failure, lung abscess, and empyema.
 CA-MRSA–induced pneumonia has typically been more common in children but is being increasingly seen in adults. Risk factors that predispose to CA-MRSA were mentioned earlier.
CA-MRSA–induced pneumonia has typically been more common in children but is being increasingly seen in adults. Risk factors that predispose to CA-MRSA were mentioned earlier.
 CA-MRSA differs from the more typical health care–associated MRSA (HA-MRSA) at the genomic, phenotypic, and epidemiologic levels. However, CA-MRSA strains are beginning to be increasingly represented in nosocomial infections, and the distinctions between them may be blurring.
CA-MRSA differs from the more typical health care–associated MRSA (HA-MRSA) at the genomic, phenotypic, and epidemiologic levels. However, CA-MRSA strains are beginning to be increasingly represented in nosocomial infections, and the distinctions between them may be blurring.
 Two key features that distinguish CA-MRSA from HA-MRSA are the production of more virulence factors, including the Panton-Valentine leukocidin (PVL) toxin, and a greater susceptibility to non–β-lactam antibiotics in vitro. However, the role of PVL in human disease is unclear.
Two key features that distinguish CA-MRSA from HA-MRSA are the production of more virulence factors, including the Panton-Valentine leukocidin (PVL) toxin, and a greater susceptibility to non–β-lactam antibiotics in vitro. However, the role of PVL in human disease is unclear.
 First-line treatment for CA-MRSA CAP remains vancomycin. Alternatives may include linezolid or clindamycin, which have the theoretic advantage of having some efficacy against CA-MRSA exotoxins.
First-line treatment for CA-MRSA CAP remains vancomycin. Alternatives may include linezolid or clindamycin, which have the theoretic advantage of having some efficacy against CA-MRSA exotoxins.
9 What are some recent developments in CAP?
 Biomarkers in CAP: The potential applications of biomarkers in CAP include stratifying patients accurately into high- and low-risk groups and guiding antibiotic therapy (both initiation and duration). Examples of biomarkers that have been studied include procalcitonin and proadrenomedullin. Some studies have shown that combining these markers with existing severity of illness scores such as the PSI or CRB-65 (a modified form of the CURB score) has resulted in improved predictive capacity. However, the data are not convincing enough for these biomarkers to have entered routine clinical practice.
Biomarkers in CAP: The potential applications of biomarkers in CAP include stratifying patients accurately into high- and low-risk groups and guiding antibiotic therapy (both initiation and duration). Examples of biomarkers that have been studied include procalcitonin and proadrenomedullin. Some studies have shown that combining these markers with existing severity of illness scores such as the PSI or CRB-65 (a modified form of the CURB score) has resulted in improved predictive capacity. However, the data are not convincing enough for these biomarkers to have entered routine clinical practice.
 Quantitative bacterial load: Recently some investigators have been studying the use of quantitative bacterial load in blood as a marker of severity of illness, analogous to the use of viral load in the management of diseases such as hepatitis C and human immunodeficiency virus (HIV). Quantification of S. pneumoniae DNA in blood with use of real-time polymerase chain reaction was shown to be a strong predictor of the risk for shock and the risk for death in pneumococcal pneumonia. This test is more sensitive than blood cultures, with a specificity approaching 100%. It is rapid (turnover time < 3 hours), is inexpensive, and can also determine susceptibility to penicillin. If validated by further studies, this test could have a major impact in the management of CAP.
Quantitative bacterial load: Recently some investigators have been studying the use of quantitative bacterial load in blood as a marker of severity of illness, analogous to the use of viral load in the management of diseases such as hepatitis C and human immunodeficiency virus (HIV). Quantification of S. pneumoniae DNA in blood with use of real-time polymerase chain reaction was shown to be a strong predictor of the risk for shock and the risk for death in pneumococcal pneumonia. This test is more sensitive than blood cultures, with a specificity approaching 100%. It is rapid (turnover time < 3 hours), is inexpensive, and can also determine susceptibility to penicillin. If validated by further studies, this test could have a major impact in the management of CAP.
 Long-term consequences of CAP: An important change in our understanding of the impact of CAP on patients has been the realization that the 2-year mortality of patients with an episode of CAP was significantly increased over that of controls, even in the absence of comorbid diseases. Although the cause of the increased mortality is not completely clear, some evidence suggests a predominantly cardiovascular cause. Epidemiologic data show a strong association between acute respiratory tract infections and subsequent acute myocardial infarctions. This gives rise to the possibility that the acute inflammatory and procoagulant state induced by CAP can destabilize atheromatous plaques and accelerate underlying cardiovascular disease. Further studies are needed to identify patients most at risk for delayed mortality, and potential treatments such as aspirin or 3-hydroxy-3-methyl-glutaryl (HMG) coenzyme A reductase inhibitors (such as statins) can perhaps be tried. It may therefore be helpful to view CAP as an acute illness with long-term health implications rather than a self-limiting process.
Long-term consequences of CAP: An important change in our understanding of the impact of CAP on patients has been the realization that the 2-year mortality of patients with an episode of CAP was significantly increased over that of controls, even in the absence of comorbid diseases. Although the cause of the increased mortality is not completely clear, some evidence suggests a predominantly cardiovascular cause. Epidemiologic data show a strong association between acute respiratory tract infections and subsequent acute myocardial infarctions. This gives rise to the possibility that the acute inflammatory and procoagulant state induced by CAP can destabilize atheromatous plaques and accelerate underlying cardiovascular disease. Further studies are needed to identify patients most at risk for delayed mortality, and potential treatments such as aspirin or 3-hydroxy-3-methyl-glutaryl (HMG) coenzyme A reductase inhibitors (such as statins) can perhaps be tried. It may therefore be helpful to view CAP as an acute illness with long-term health implications rather than a self-limiting process.
11 Discuss the potential reasons why a patient may not respond favorably to empiric therapy
1. Inappropriate antimicrobial therapy
14 What are hospital-acquired pneumonia (HAP), health care–associated pneumonia (HCAP), and ventilator-associated pneumonia (VAP)?
 HAP is defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission.
HAP is defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission.
 HCAP refers to pneumonia that develops in a patient who lives in a nursing home or long-term care facility; undergoes hemodialysis; has received IV antimicrobial therapy, chemotherapy, or wound care within the preceding 30 days; or has been hospitalized for at least 2 days within the preceding 90 days. The causative pathogens in these patients are similar to those responsible for HAP and VAP and are often multidrug resistant (MDR).
HCAP refers to pneumonia that develops in a patient who lives in a nursing home or long-term care facility; undergoes hemodialysis; has received IV antimicrobial therapy, chemotherapy, or wound care within the preceding 30 days; or has been hospitalized for at least 2 days within the preceding 90 days. The causative pathogens in these patients are similar to those responsible for HAP and VAP and are often multidrug resistant (MDR).
 VAP: Universally agreed-on diagnostic criteria for VAP do not exist; however, commonly used criteria include the presence of all of the following:
VAP: Universally agreed-on diagnostic criteria for VAP do not exist; however, commonly used criteria include the presence of all of the following:
The use of clinical criteria alone without microbiologic data tends to overdiagnose lung infection.
17 What initial empiric antibiotic therapy is recommended for HAP, HCAP, or VAP in patients with no known risk factors for MDR, early onset pneumonia development, and any disease severity?
18 What initial empiric antibiotic therapy is recommended for HAP, HCAP, or VAP in patients with known risk factors for MDR, late-onset disease development, and any disease severity?
Recommended combination antibiotic therapy includes an antipseudomonal cephalosporin (cefepime or ceftazidime), antipseudomonal carbapenems (imipenem or meropenem), or β-lactam–β-lactamase inhibitor (piperacillin-tazobactam) plus an antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin) or an aminoglycoside (amikacin, gentamicin, or tobramycin). Linezolid or vancomycin should be added if MRSA risk factors are present or there is a high incidence locally. Potential MDR pathogens include P. aeruginosa, K. pneumoniae, Acinetobacter species, and MRSA (Table 20-1).
Table 20-1 Initial Empiric Therapy for HAP, VAP, and HCAP: for Patients with Late-Onset Disease or Risk Factors for MDR Pathogens and All Disease Severity
| Potential pathogens | Combination antibiotic therapy |
|---|---|
| MDR pathogens | Antipseudomonal cephalosporin (cefepime, ceftazidime) |
| P. aeruginosa | or |
| K. pneumoniae (ESBL+)* | Antipseudomonal carbapenem (imipenem or meropenem) |
| Acinetobacter species* | or |
| β-Lactam/β-lactamase inhibitor (piperacillin–tazobactam) | |
| plus | |
| Antipseudomonal fluoroquinolone* (ciprofloxacin or levofloxacin) | |
| or | |
| Aminoglycoside (amikacin, gentamicin, or tobramycin) | |
| plus | |
| MRSA | Linezolid or vancomycin† |
| Legionella pneumophila* |
ESBL, Extended-spectrum β-lactamase.
* If an ESBL+ strain, such as K. pneumoniae, or an Acinetobacter species is suspected, a carbapenem is a reliable choice. If L. pneumophila is suspected, the combination antibiotic regimen should include a macrolide (e.g., azithromycin), or a fluoroquinolone (e.g., ciprofloxacin or levofloxacin) should be used rather than an aminoglycoside.
† If MRSA risk factors are present or there is a high incidence locally.
Modified from American Thoracic Society, Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388-416, 2005.
19 What are some specific treatment strategies for MDR Pseudomonas, Acinetobacter, and MRSA VAP?
 Combination therapy for P. aeruginosa pneumonia remains controversial. Resistance is mediated partly by multiple efflux pumps.
Combination therapy for P. aeruginosa pneumonia remains controversial. Resistance is mediated partly by multiple efflux pumps.
 Acinetobacter species are most sensitive to the carbapenems, sulbactam, colistin, and polymyxin. More than 85% of isolates are susceptible to carbapenems, but resistance is increasing because of either integral membrane protein (IMP)-type metalloenzymes or carbapenemases of the oxacillinase (OXA) type.
Acinetobacter species are most sensitive to the carbapenems, sulbactam, colistin, and polymyxin. More than 85% of isolates are susceptible to carbapenems, but resistance is increasing because of either integral membrane protein (IMP)-type metalloenzymes or carbapenemases of the oxacillinase (OXA) type.
 MRSA produces a penicillin-binding protein with reduced affinity for β-lactam antibiotics. Linezolid is an alternative to vancomycin for the treatment of MRSA VAP.
MRSA produces a penicillin-binding protein with reduced affinity for β-lactam antibiotics. Linezolid is an alternative to vancomycin for the treatment of MRSA VAP.
20 What measures can be taken to decrease the risk of VAP?
1. Avoid intubation when possible, and apply noninvasive positive-pressure ventilation when appropriate.
2. Use orotracheal tubes preferentially over nasotracheal tubes.
3. Minimize the duration of mechanical ventilation with the aid of weaning protocols.
4. Apply continuous aspiration of subglottic secretions.
5. Maintain an endotracheal tube cuff pressure > 20 cm H2O to prevent leakage of oropharyngeal secretions containing bacteria into the lungs.
6. Avoid unnecessary manipulation of the ventilator circuit.
7. Carefully discard contaminated condensate from the ventilator circuit.
8. Keep the head of the bed elevated by 30 degrees.
9. Avoid heavy sedation and paralytics because they impair the patient’s ability to cough.
10. It does not appear that sucralfate or therapies that decrease gastric acid increase the incidence of nosocomial pneumonia.
21 How do you decide when to continue, de-escalate, and discontinue the use of antibiotic treatment on the basis of clinical response and culture data?
When HAP, VAP, or HCAP is suspected, consider obtaining lower respiratory tract samples for culture (quantitative or semiquantitative) and microscopy. Unless there is both a low clinical suspicion for pneumonia and negative microscopy of the lower respiratory tract sample, begin empiric antimicrobial therapy. At day 2 and 3, check cultures and assess clinical response (temperature, white blood cell [WBC] count, chest radiograph, oxygenation, purulent sputum, hemodynamic changes, and organ function). If no clinical improvement is seen after 2 to 3 days with negative cultures, search for other pathogens, complications, diagnoses, or sites of infection. If no improvement is seen but cultures are positive, adjust antibiotic therapy but also broaden infectious search as you would with negative cultures. If clinical improvement is noted after 2 to 3 days but cultures are negative, consider stopping antibiotics. If clinical improvement is noted and cultures are positive, de-escalate antibiotics, and consider treating selected patients for 7 to 8 days and reassess (Fig. 20-1).
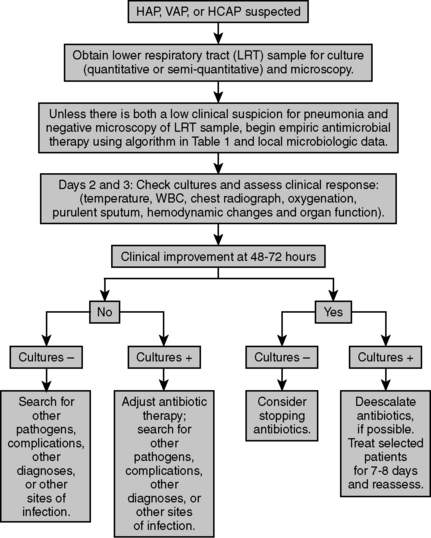
Figure 20-1 Algorithm for treatment of HAP, VAP, or HCAP.
Data from American Thoracic Society, Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388-416, 2005.
22 How long should you continue antibiotic management for HAP, HCAP, or VAP?
Key Points Initial Management of Acute Bacterial Pneumonia
1. Treat empirically if pneumonia is clinically suspected.
2. Select the initial empiric therapy on the basis of the current bacteriology and resistance patterns at each institution. Alternatively, published evidence-based practice guidelines may be used.
3. Obtain cultures of respiratory tract specimens to identify pathogen(s), preferably before initiation of antibiotics. However, the administration of antibiotic therapy should not be delayed for diagnostic testing.
4. Narrow the initial antibiotic regimen on the basis of quantitative culture results and clinical response (de-escalation).
5. Avoid excessive antibiotic use by de-escalating therapy when appropriate and prescribing the minimal duration of therapy required for efficacy.
1 American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
2 Chastre J., Wolff M., Fagon J.-Y., et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults. JAMA. 2003;290:2588–2598.
3 Chow J.W., Fine M.J., Shlaes D.M., et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590.
4 Kobayashi S.D., DeLeo F.R. An update on community-associated MRSA virulence. Curr Opin Pharmacol. 2009;9:545–551.
5 Kruger S., Santiago E., Sven G., et al. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia. Am J Respir Crit Care Med. 2010;182:1426–1434.
6 Mandell L.A., Wunderink R.G., Anzueto A., et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(2 Suppl):S27–S72.
7 Messori A., Trippoli S., Vaiani M., et al. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta-analysis of randomized controlled trials. BMJ. 2000;321:1–7.
8 Nordmann P., Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect. 2002;8:321–331.
9 Rello J., Lisboa T., Lujan M., et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136:832–840.
10 Waterer G.W., Rello J., Wunderink R.G. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med. 2011;183:157–164.
11 Wunderink R.G., Cammarata S.K., Oliphant T.H., et al. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther. 2003;25:980–992.
12 Wunderink R.G., Rello J., Cammarata S.K., et al. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797.







