CHAPTER 93 Acute Aortic Syndrome
Acute aortic syndrome describes a variety of potentially life-threatening aortic pathologic processes. These lesions include aortic dissection, intramural hematoma, penetrating atherosclerotic ulcer, aortic aneurysm leak and rupture, and traumatic aortic transection.1,2 The clinical presentation is often characterized by acute chest pain in patients with a history of hypertension. Chest pain is variously described as severe, tearing, or migratory. Symptoms can sometimes be confused with acute myocardial infarction or pulmonary embolism. Furthermore, the various types of acute aortic syndromes cannot be reliably differentiated by their clinical presentations.
Acute aortic syndrome is a clinical emergency. Accurate diagnosis and rapid treatment are essential to improve prognosis. Therefore, the aorta must be fully assessed by imaging when the syndrome is suspected. During the last 20 years, new imaging modalities have been developed that dramatically improve assessment of aortic disease. Computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography allow examination of aortic disease, providing more detail than that of chest radiography or catheter angiography.3
AORTIC DISSECTION
Prevalence and Epidemiology
Aortic dissection is the most common form of acute aortic syndrome. Incidence in the general population is reported from 2.9 to 3.5 cases per 100,000 person-years.4,5 Two thirds of patients are male. In women, aortic dissection typically presents at an older age (67 years versus 63 years in men).6
Etiology and Pathophysiology
The pathology of aortic dissection is a primary intimal tear that allows blood to enter the aortic media, extending proximally and distally into the aortic wall and displacing the intima inward. This entry tear typically occurs at sites of greatest wall tension, notably within a few centimeters of the aortic valve or close to the attachment site of the ligamentum arteriosum.2,7 The intima and inner part of the aortic media form the intimomedial flap, and the outer part of the aortic media and adventitia form the outer boundary of the false lumen (also known as a false channel; Fig. 93-1). The true lumen is directly connected to the lumen of the unaffected aorta and usually experiences high-velocity flow. The false lumen communicates with the true lumen through the intimal tear and experiences slower, turbulent blood flow. Re-entry tears are usually present in the intima, creating additional communication between the true and false lumens in the distal aorta.2
Most patients with aortic dissection have a history of systemic hypertension, which adds to the mechanical strains and shearing forces along the aortic wall. Long-standing hypertension is also associated with increased stiffness of the aortic media, which may introduce additional interlaminar shearing stresses and further contribute to the development of aortic dissection.2
Atherosclerotic disease may also be associated with aortic dissection, namely, through the development of a penetrating atherosclerotic ulcer. However, atherosclerosis is not a typical cause of aortic dissection. Dissection in patients with severe atherosclerosis tends to be limited by fibrosis and calcification, but the relationship between an atheroma and the location of aortic dissection is not clear in most patients.8 Other predisposing factors for aortic dissection include connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome, bicuspid aortic valve, aortitis, and aortic coarctation. Aortic dissection can also be caused iatrogenically by aortic surgery or percutaneous procedures such as catheterization and placement of intra-aortic balloon pumps.9,10
Manifestations of Disease
Clinical Presentation
Aortic dissection often presents as excruciating chest pain in a patient with a history of hypertension. The pain is usually described as severely intense, acute, searing or tearing, throbbing, and occasionally migratory. Involvement of the ascending aorta can cause anterior chest, neck, throat, and even jaw pain, whereas involvement of the descending aorta may cause back and abdominal pain.11 Ischemic symptoms usually are due to obstruction of the aortic branches by the dissection flap.
Aortic dissection is divided into acute and chronic forms according to the duration of symptoms. An acute form refers to dissection when the diagnosis is made within 2 weeks of symptom onset; in a chronic form, symptoms persist for more than 2 weeks. More than 60% of dissection-related mortality occurs in the first week of disease evolution and 74% within 2 weeks.12
Two classifications of aortic dissection are widely used according to the location and extent of involvement of the thoracic aorta. The DeBakey classification system uses three types (Fig. 93-2).13 Type I dissection involves the ascending aorta, the arch, and a variable length of the descending thoracic and abdominal aorta. Type II dissection is confined to the ascending aorta, and type III dissection may be confined to the descending thoracic aorta (type IIIa) or extend into the abdominal aorta and iliac arteries (type IIIb). The Stanford classification system divides aortic dissection into two types (see Fig. 93-2).14 Type A involves the ascending aorta, with or without involvement of the arch or the descending thoracic aorta. Type B dissection involves the descending thoracic aorta distal to the origin of the left subclavian artery.
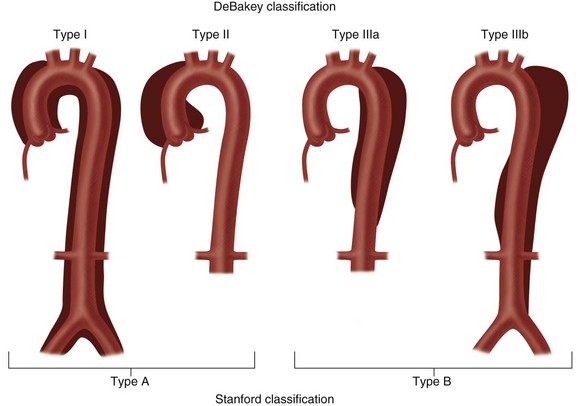
 FIGURE 93-2 The two most widely used classifications of aortic dissection. The DeBakey classification includes three types.14 Type I: the intimal tear usually originates in the proximal ascending aorta; the dissection propagates to the aortic arch and often beyond it distally. Type II: the dissection originates in and is confined to the ascending aorta. Type III: the dissection originates in the descending thoracic aorta (type IIIa) or extends distally down the aorta and iliac arteries (type IIIb); the dissection rarely extends proximally into the aortic arch and ascending aorta. The Stanford classification has two types.15 Type A: dissection involves the ascending aorta, with or without involvement of the aortic arch or the descending aorta. Type B: dissection does not involve the ascending aorta.
FIGURE 93-2 The two most widely used classifications of aortic dissection. The DeBakey classification includes three types.14 Type I: the intimal tear usually originates in the proximal ascending aorta; the dissection propagates to the aortic arch and often beyond it distally. Type II: the dissection originates in and is confined to the ascending aorta. Type III: the dissection originates in the descending thoracic aorta (type IIIa) or extends distally down the aorta and iliac arteries (type IIIb); the dissection rarely extends proximally into the aortic arch and ascending aorta. The Stanford classification has two types.15 Type A: dissection involves the ascending aorta, with or without involvement of the aortic arch or the descending aorta. Type B: dissection does not involve the ascending aorta.
Imaging Indications and Algorithm
Diagnostic imaging is essential for proper evaluation of patients with suspected aortic dissection. The primary goals of imaging are to depict the intimal flap and to delineate the extent of aortic involvement and extension into the arch vessels and coronary arteries. It is also important to recognize associated complications, including pericardial hemorrhage, which can lead to life-threatening cardiac tamponade, periaortic or mediastinal hematoma, and pleural hemorrhage.15
Imaging techniques for dissection, consisting primarily of radiography and catheter angiography in the past, have changed rapidly in recent years. Multidetector CT (MDCT) and, to a lesser extent, ultrasonography and MRI have taken precedence and relegated catheter angiography to the occasional instance in which noninvasive imaging is not definitive or therapeutic intervention is desired.15 A meta-analysis found the diagnostic accuracy of CT, transesophageal echocardiography, and MRI to be virtually the same (95% to 100%) for the diagnosis of thoracic aortic dissection.16
Imaging Techniques and Findings
Radiography
Chest radiography is commonly performed as an initial study in patients with chest pain but is less sensitive for the diagnosis of aortic dissection. Chest radiography, however, does remain important because it may suggest an underlying aortic pathologic process, and it is performed routinely in patients with suspected aortic disease.15 The most common chest radiographic findings in patients with aortic dissection are widening of the mediastinum and aortic contour, disparity in the size of the ascending and descending aorta, changes in aortic configuration on serial studies, and displacement of a calcified plaque by more than 10 mm (Fig. 93-3).17 Other findings may include tracheal deviation, inferior displacement of the left main bronchus and opacification of the aorta-pulmonary window, and widening of the left paraspinal line.
In practice, mediastinal widening is often difficult to evaluate on initial chest radiographs because patients are often examined in the supine position with portable radiography and are unable to hold the breath in full inspiration. Individual features suggestive of aortic dissection, such as displacement of aortic calcification, may be misdiagnosed because of the variable positions of the calcified plaque and the lateral aortic border.17 Proximal dissection is especially difficult to diagnose on chest radiographs. Serial studies can be helpful in identifying the changes between the prior and current studies.
Ultrasonography
Ultrasound techniques available for evaluation of aortic dissection include transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and intravascular ultrasound (IVUS).15 TTE is readily available, noninvasive, and portable but limited by a narrow acoustic window. It can be useful for evaluation of the aortic root, including aortic valve complications or cardiac and aortic wall motion abnormalities. TEE is a relatively invasive diagnostic procedure, but it can be performed at bedside and demonstrate true and false lumens, direction of blood flow, and complications such as aortic regurgitation on Doppler examination (Fig. 93-4). TEE typically provides broader and more accurate aortic visualization than does TTE for improved evaluation for aortic dissection; however, TEE cannot image the distal ascending aorta and proximal aortic arch completely as the airway may be positioned between the esophagus and the aorta.18 Catheter-based IVUS can provide imaging of the entire aorta with high sensitivity and specificity. However, it is limited in detecting the site of intimal tear, particularly in the ascending aorta.19 IVUS is rarely used in the initial screening or follow-up of patients with aortic dissection.
Diagnosis of aortic dissection by ultrasound examination requires demonstration of the true and false lumens separated by an intimal flap. To determine the therapeutic implications, one must evaluate the involvement of the ascending aorta, the coexistence of aortic insufficiency, the site of the original tear, the characteristics of blood flow and clot formation in the false lumen, the relative position of the coronary arteries, and the involvement of the aortic arch vessels, notably the carotid arteries. Ventricular function and the presence of pericardial effusion are also important for therapeutic strategy.3
The advantage of ultrasonography over MRI or CT is its portability, which makes it useful at the bedside or in the operating room. However, objective and reproducible thoracic aortic evaluation with ultrasonography remains problematic given operator dependence and restricted thoracic acoustic windows. Ultrasonography, moreover, is prone to artifacts and the detection of “pseudolesions,” such as reverberation artifacts, which can be misdiagnosed as aortic dissection and cause false-positive study results.15
Computed Tomography
CT is the most commonly used diagnostic tool in patients with suspected aortic dissection. A study from the International Registry of Acute Aortic Dissection reported that CT was used in 61% of cases as the primary diagnostic procedure for detection of aortic dissection. Ultrasonography was used in 33% of cases, followed by catheter angiography in 4.4% and MRI in 1.8%.6 The advantages of CT scans are faster scanning, high spatial resolution, and the ability with moving table bolus chase imaging to provide extended anatomic coverage for illustration of the entire thoracoabdominal aorta. The development of multidetector technology has allowed acquisition of submillimeter-thick axial images, which can provide isotropic imaging volumes for improved postprocessed viewing of complex arterial geometries and relationships. Compared with ultrasonography, MDCT benefits from being less operator dependent, having a relative ease of use, and providing a larger field of view for improved detection of both vascular and extravascular findings. The key disadvantages of MDCT include its use of ionizing radiation and iodine-based intravenous contrast agents, which are contraindicated in certain patients.15
CT is highly accurate, with a sensitivity and a specificity of more than 95% reported for the diagnosis of aortic dissection.3 Compared with ultrasonography and MRI, CT also provides an improved ability to evaluate for confounding differential diagnoses in the thorax and abdomen (Fig. 93-5).
Although major diagnostic clues are provided by contrast-enhanced CT scans, the importance of non–contrast-enhanced scans should not be overlooked. Non–contrast-enhanced CT scans can depict intimal calcifications and high-density lesions from leaking or thrombosed blood in the aortic wall, in periaortic regions, or in pleural and pericardial spaces.20
The main CT findings in aortic dissection include variable appearances of the true and false lumens, involvement of the ascending aorta, extension into arterial branches, and associated lesions.15,21,22 The intimal flap divides the aortic lumen into two channels, and the true lumen is typically more opacified than the false lumen because of the higher blood pressure and faster mixing of blood with contrast material in the true lumen on early arterial phase images (Fig. 93-6). True and false lumens can be differentiated on the basis of other characteristics. The true lumen is generally posterior and left posterolateral in the ascending aorta, caudal in the aortic arch, and anterior and right anterolateral in the descending aorta. The true lumen tends to be smaller than the false lumen because of inward displacement of the intimal flap. Thrombus is commonly found in the false lumen because of its slow flow. Multiphase contrast-enhanced CT scans may show that the false lumen enhances later and washes out in a delayed fashion.21
Demonstration of the intimal flap on CT images is accurate and precise in the descending aorta. In contrast, the dissecting flap in the ascending aorta can be difficult to properly identify as it may be confused with aortic valve cusps on axial images or mistaken for motion artifacts of the aortic root due to cardiac or respiratory movement (Fig. 93-7). However, these artifacts can be minimized by use of a rapid scan technique and cardiac synchronization (i.e., either prospective ECG triggering or retrospective ECG gating).23
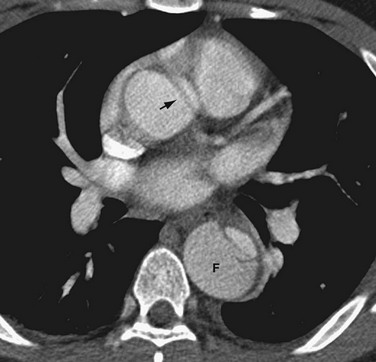
 FIGURE 93-7 Type B aortic dissection in which the false lumen (F) is noted on non–ECG-gated, contrast-enhanced CT to be larger than the true lumen in the descending thoracic aorta. An artifactual dark linear band (arrow) mimics an intimal tear in the ascending aorta. This artifact is secondary to through-plane motion of the geometrically complex and obliquely positioned aortic root and can be minimized by ECG-gated CT acquisition. Note that genuine intimal tears are more typically oriented in the right anterolateral aspect of the ascending aorta (see Fig. 93-6).
FIGURE 93-7 Type B aortic dissection in which the false lumen (F) is noted on non–ECG-gated, contrast-enhanced CT to be larger than the true lumen in the descending thoracic aorta. An artifactual dark linear band (arrow) mimics an intimal tear in the ascending aorta. This artifact is secondary to through-plane motion of the geometrically complex and obliquely positioned aortic root and can be minimized by ECG-gated CT acquisition. Note that genuine intimal tears are more typically oriented in the right anterolateral aspect of the ascending aorta (see Fig. 93-6).
Magnetic Resonance
Like CT, MRI has been reported to have a sensitivity and specificity of more than 95%3; thus, it is a suitable alternative to catheter angiography for the diagnosis and evaluation of aortic dissection. T1- and T2-weighted black blood spin-echo MR images provide high tissue contrast between the blood pool, the vascular wall, and the adjacent soft tissues. Cine bright blood imaging provides supplemental images for improved detection of the intimal flap and extent of the true and false lumens as well as functional information about blood flow associated with the aortic dissection. Moreover, cine MR enables evaluation of the aortic valve and its function.16
On black blood spin-echo images, the true and false lumens are depicted as regions of signal void, and the intimal flap is seen as a linear structure of isointense signal intensity that is outlined by the flow void in the dual lumens (Fig. 93-8). The false lumen can be differentiated from the true because the flow is slower and thrombus is more likely to form. If the false lumen contains thrombus, the intimal flap may not be seen because it is not outlined by moving blood on both sides. Although MRI is superior to CT in depicting the presence of blood flow, non–contrast-enhanced MRI is sometimes unable to differentiate slowly moving blood from thrombus in the false lumen.17
Aortic MR angiography (MRA) can be performed with time-of-flight and phase contrast techniques.24,25 These flow-based techniques may be helpful in differentiating the signal intensities between slow blood flow and intravascular thrombus. However, the disadvantage of these techniques is relatively long scan time, which restricts their use in hemodynamically unstable patients.
Aortic MRA is more routinely performed with use of gadolinium-enhanced three-dimensional MRA.26 Gadolinium-enhanced MRA has dramatically shortened total examination time in the diagnosis of aortic dissection and has replaced non–contrast-enhanced MRA techniques. Like contrast-enhanced CT angiography (CTA), gadolinium-enhanced MR provides arterial “luminograms” that can be postprocessed by an array of three-dimensional processing tools on an independent workstation. The advantages of gadolinium-enhanced MRA over contrast-enhanced CTA include its use of generally safer gadolinium-chelate contrast agents, lack of ionizing radiation, and production of high contrast images with fewer image sections. Furthermore, postprocessing is much faster with gadolinium-enhanced MRA than with CTA.27 Like contrast-enhanced CTA, gadolinium-enhanced MRA can differentiate the true and false lumens according to their signal intensity differences (Fig. 93-9). The intimal flap is visualized as a line of low signal intensity with a linear or curved shape. The true lumen often has higher signal intensity than the false because of a higher concentration of contrast material during the arterial phase. The acquisition of delayed images improves depiction of thrombosis of the false lumen, which can be easily depicted as lower signal intensity.
Despite these advantages of MR techniques, MRI is rarely used as the initial imaging modality for the evaluation of aortic dissection. Data from the International Registry of Acute Aortic Dissection revealed that only 1.8% of patients with aortic dissection underwent MRI as an initial imaging test, which was less often than catheter aortography (4.4%).6 This may be due to limited emergent availability of MRI, incompatibility with implanted metal devices such as pacemakers and aneurysm clips, or monitoring difficulties during examination. Furthermore, MRI is not suitable in patients with hemodynamic instability. The use of MRI may also be restricted in patients with claustrophobia15 or with renal insufficiency, in whom there is an increased risk for development of nephrogenic systemic fibrosis after the intravenous administration of gadolinium-chelate contrast agents.
Angiography
Angiographic signs of aortic dissection can be categorized as direct or indirect. Direct findings include visualization of the intimal flap or dual lumens (Fig. 93-10). Indirect findings include compression of the true lumen, thickening of the aortic wall, abnormalities of branch vessels, and aortic insufficiency.17 The major advantage of catheter angiography is its ability to evaluate the aortic valve and extent of involvement of major aortic branches. Sensitivity and specificity of catheter angiography for diagnosis of dissection are 88% and 94%.28
Although catheter angiography is sensitive for the diagnosis of aortic dissection, it has recently been replaced by noninvasive modalities such as CT, ultrasonography, and MRI.29 Proper diagnosis of aortic dissection may be difficult in situations in which the false lumen is not opacified or in which both lumens are equally opacified. The false lumen may not be opacified when it is thrombosed, when there is no intimal tear, or when the catheter tip is distal to the site of the intimal tear.17 In addition, because catheter angiography cannot directly depict the aortic wall, it cannot accurately evaluate the size of the aorta, intramural features of the aortic wall, or periaortic complications. The higher false-negative rate, therefore, results from its inability to identify extraluminal abnormalities such as an intramural hematoma, thrombosed false lumens, and periaortic fluid collections. Because of its invasiveness and time-consuming nature, the use of catheter angiography in the diagnosis of aortic disease has been dramatically reduced.15
Classic Signs
On cross-sectional imaging, the “beak sign” can be seen at the corner of the false lumen, forming an acute angle with the intimomedial flap and the outer wall of the aorta (Fig. 93-11).30 The “cobweb sign” of the medial layer sometimes persists between the intima and media, forming thin strands of medial tissue that can be seen crossing the false lumen (Fig. 93-12).31
Differential Diagnosis
Synopsis of Treatment Options
Medical
Aortic dissection requires immediate treatment with medical therapy or surgery, depending on the involved sites. The initial therapy is typically targeted at relieving chest pain and normalizing the systolic blood pressure to between 100 and 120 mm Hg, which may prevent further extension of the dissection or rupture of the false lumen.32 In most patients, these results are usually achieved with morphine sulfate and intravenous β blockers, respectively. Patients who have contraindications to β blockers can receive calcium channel blockers such as verapamil or intravenous diltiazem. Most patients with type B dissections can be treated with medications alone. In patients with type A dissections, these medications may be used to prepare a patient for surgery.
Surgical/Interventional
Type A (Ascending) Aortic Dissection
Medical treatment alone is ineffective in patients with acute aortic dissections involving the ascending thoracic aorta (Stanford type A or DeBakey type I or type II). Type A dissections require urgent surgical treatment, particularly when the patient presents within the first 48 hours and if there is cerebral or visceral ischemia.12 The mortality rate is up to 1% to 2% per hour during the first 24 to 48 hours after presentation. Patients with type A dissections may be at high risk of life-threatening complications, such as rupture of the false lumen, stroke, visceral ischemia, cardiac tamponade, and heart failure. In addition, when the dissection extends to the aortic annulus, obstruction of coronary blood flow and aortic regurgitation can occur abruptly. Therefore, the major surgical objective in type A dissection is to prevent rupture of the false lumen or development of pericardial effusion, which can lead to cardiac tamponade or death.32
Surgical technique varies according to involvement of the lesion in the aorta. David’s and Yacoub’s33 techniques represent repair of the aortic root and preservation of the aortic valve. However, in conditions with serious anatomic injury in the aortic root, surgeons prefer to carry out complete replacement with a valved conduit and coronary artery reimplantation. This technique may prevent a second intervention or operation, and it is generally applied in cases associated with Marfan syndrome or ectasia of the aortic root.34 When the dissection extends to the aortic arch or the descending thoracic aorta, the patient may require partial or total arch replacement because the intimal flap cannot be completely resected. A two-stage operation may be a useful technique when there is also aneurysmal dilation of the proximal descending thoracic aorta.34 A prosthetic tube graft is first placed in the proximal descending thoracic aorta and then connected distally in the second procedure.
Medical treatment after surgery focuses on blood pressure control, with a systolic blood pressure target not to exceed 100 to 110 mm Hg. Imaging follow-up is also important to screen for development of a new dissection or formation of aneurysms at other sites of the aorta. The recurrence rate is approximately 25%, and recurrence is frequently associated with complications such as aortic rupture and death from exsanguinaton.12
Type B (Descending) Aortic Dissection
Medical treatment is acceptable in type B dissection when the lesion is stable. The objective of immediate treatment in type B dissection is control of pain and lowering of systolic blood pressure to 110 to 120 mm Hg. However, some patients with type B aortic dissection may require surgery when the patients have hemodynamic instability, intractable pain, rapid expansion of aortic diameter, and mediastinal or periaortic hematoma as signs of imminent rupture of the aorta.12
With the development of percutaneous approaches for the treatment of vascular disease, catheter angiography is again playing an important role in the management of aortic dissection. Endovascular treatment has recently been introduced in the minimally invasive treatment of type B dissection.35–37 These catheter-based approaches have been used to stent across intimal tears, to treat branch vessel occlusion, and to fenestrate intimal flaps (Fig. 93-13). Exclusion of the false lumen can avoid later development of aneurysmal dilation and rupture. Fenestration of the intimal flap is helpful to restore perfusion to ischemic organs. This procedure is relatively safe and can yield better results than surgery in the treatment of dissection of the descending aorta.
INTRAMURAL HEMATOMA
Prevalence and Epidemiology
Intramural hematoma is reported to represent 10% to 30% of acute aortic syndromes.9,38 Intramural hematoma of the aorta generally occurs in older patients with a history of hypertension, and it is located in the descending aorta in about 60% of cases.39 Intramural hematoma can extend retrograde to involve the ascending aorta or anterograde to involve the descending aorta.
Etiology and Pathophysiology
The pathology of many cases of intramural hematoma is spontaneous rupture of the vasa vasorum, which results in bleeding into the aortic media. In some patients, intramural hematoma can be secondary to a penetrating atherosclerotic ulcer.1,2 Blunt chest trauma associated with aortic wall injury sometimes causes intramural hematoma, and other causes include connective tissue disorders, Marfan syndrome, Turner syndrome, coarctation of the aorta, aortic aneurysm, pregnancy, and cocaine abuse.40
The morphology of the involved aortic wall may change rapidly. Bleeding into the media may be self-limited but may lead to classic aortic dissection. The rate of aortic rupture is much higher (up to 35%) in intramural hematoma than in aortic dissection because intramural hematoma usually occurs closer to the adventitia.8 Intramural hematoma is frequently associated with mediastinal, pericardial, and pleural hemorrhage, which are related to increased permeability of the aortic wall.
Manifestations of Disease
Clinical Presentation
Symptoms in patients with intramural hematoma are similar to those of aortic dissection.
Imaging Techniques and Findings
Ultrasonography
TEE allows direct observation of the aortic intima. Diagnostic criteria for intramural hematoma include absence of an intimal flap, no communication between false and true lumens on Doppler examination, and regional crescentic thickening of the aortic wall above 0.7 cm.41 Recent improvements in diagnostic sensitivity of TEE enable evaluation of aortic wall thicknesses of more than 5 mm, which is sufficient for the diagnosis of intramural hematoma in patients with typical symptoms of acute aortic syndrome.
In intramural hematoma, a hypoechoic zone can sometimes be detected within the thickened aortic wall. This echolucent space represents the accumulation of blood from the vasa vasorum within the media (i.e., the intramural hematoma). However, an echolucent space is not a poor prognostic sign and is not associated with development of aortic dissection.42
Serial follow-up studies in patients with intramural hematoma are important for early detection of disease progression to dissection or development of complications such as mediastinal hemorrhage, pericardial effusion, and pleural effusion. Among these complications, cardiac tamponade is a clinical challenge because mortality is relatively high. Progressive accumulation of pleural effusion can occur in intramural hematoma, but this alone is not an indication for surgical intervention.43
Computed Tomography
CT is the most commonly used imaging technique for intramural hematoma because of its availability, accuracy, and speed. CT is beneficial for visualization of the entire aorta, diagnosis of periaortic bleeding, and evaluation of arterial branches. Intramural hematoma is visualized as crescentic or circumferential aortic wall thickening, which is manifested as high attenuation on non–contrast-enhanced CT images (Fig. 93-14A). Non–contrast-enhanced CT scans can also depict the critical complication of bleeding into the pericardium, mediastinum, or pleural spaces. Because high-attenuation areas (i.e., the intramural hematoma) are usually masked on the contrast-enhanced CT images (see Fig. 93-14B), the performance of CT before the administration of contrast material is recommended in patients with suspected acute aortic syndrome before contrast-enhanced CT.44 High-attenuation areas along the aortic wall tend to change over time, and this noncommunicating aortic hematoma can progress to a typical dissection, aortic rupture, or hemorrhage to the mediastinal, pleural, or pericardial spaces.
Magnetic Resonance
Although crescentic wall thickening of the aorta is easily detected by MRI, the use of MRI remains controversial in patients with suspected acute aortic syndrome; the patients are often hemodynamically unstable, and the typically longer examination times of MRI may unnecessarily delay proper diagnosis and impede immediate treatment. However, its use is justified in asymptomatic and stable patients when the diagnosis of intramural hematoma has not been established by other techniques. Bleeding of the vasa vasorum can be progressive in intramural hematoma. The signal intensity of the thickened aortic wall may be variable according to the amount of methemoglobin formation. Intramural hemorrhage in the hyperacute phase can show isointense signal on the T1-weighted images and high signal intensity on T2-weighted images. For the following 1 to 2 days, intramural hematoma is visualized as areas of high signal intensity on T1- and T2-weighted images (Fig. 93-15).45 As with CT, MR images obtained before the administration of contrast material (T1-weighted especially) are critical for proper detection of intramural hematoma.
Angiography
Catheter angiography may fail to identify intramural hematoma and is rarely the first choice for diagnosis of intramural hematoma because no intimal disruption is usually present. However, when the intramural hematoma is large and extensive, a thickened aortic wall may be demonstrated on catheter angiography as an indirect sign of an intramural hematoma.46
Synopsis of Treatment Options
Medical
The therapeutic strategy for intramural hematoma is similar to that for aortic dissection. Recent advances in diagnostic modalities and appropriate treatment of intramural hematoma have led to improved prognosis and survival of patients. Nevertheless, the overall mortality still remains high at 20.7%.39
Acute intramural hematoma involving the descending aorta only has an in-hospital mortality risk of less than 1% to 5%, similar to that seen with descending or type B dissection, and thus medical treatment is the standard of choice.47 Evolution to localized dissection is not necessarily an indication for surgical or endovascular treatment. Acute intramural hematoma confined to the aortic arch remains a controversial subject. Evangelista and colleagues39 stated that aggressive medical treatment alone, with a target heart rate below 60 beats/min and blood pressure below 120/80 mm Hg and at least one additional initial imaging study to exclude frank aortic dissection or early aneurysmal expansion, appears to be a reasonable strategy for management of such patients.
Intramural hematoma is often well managed with medical therapy to lower blood pressure. Prognosis in older patients with intramural hematoma is acceptable with medical therapy, perhaps because severe atherosclerosis can limit the expansion of hemorrhage with adequate blood pressure control.48 Patients whose conditions can be stabilized with antihypertensive therapy may have a good long-term prognosis.
Surgical/Interventional
As in classic aortic dissection, the mortality rate is much higher for an intramural hematoma involving the ascending aorta than for an intramural hematoma involving the descending aorta. Regression may occur but is less common in intramural hematoma involving the ascending aorta, and surgical treatment has been favored. If there are no complications, such as persistent chest pain or periaortic bleeding, surgical intervention should take place within 24 to 72 hours.45
Rapid aortic dilation with signs of imminent aortic rupture and periaortic bleeding in a patient with persistent pain is an indication for endovascular treatment. Such treatment can be undertaken with good results when the ends of the stent are implanted in the healthy aortic wall and not on the hematoma.45
Wall configuration in acute intramural hematoma has been reported to change rapidly. Bleeding into the aortic media may be self-limited, but it is a dynamic process that may lead to classic communicating dissection, aortic rupture, rapid aneurysmal dilation of the aorta, or circumferential and longitudinal extension of the bleeding.49 Intramural hematoma may progress to classic aortic dissection in 28% to 47% of patients and it carries a risk of aortic rupture in 20% to 45% of cases.50 Fluid extravasation, such as pericardial and pleural effusions and mediastinal hemorrhage, is a frequent finding in patients with acute intramural hematoma. This may indicate impending aortic rupture and is considered to be an indication for emergent surgery.51
The size of the ascending aorta at the first examination may be important in predicting the progress of an intramural hematoma. In one study, patients with an aortic diameter of less than 5 cm experienced regression of the hematoma during medical therapy; whereas those with a larger diameter (>5 cm) had a tendency for progression to dissection or rupture.48 A maximum thickness of a hematoma greater than 11 mm on the initial CT scan may also be a significant factor for predicting development of aortic dissection and aortic aneurysm.42 Therefore, treatment strategies may be individualized according to the condition of the patient. Emergency surgery should be recommended in symptomatic patients, those with rapid progression during follow-up, and those with a large ascending aorta.
PENETRATING ATHEROSCLEROTIC ULCER
Definition
A penetrating atherosclerotic ulcer is a condition in which an aortic atherosclerotic plaque ruptures, followed by ulcer formation and penetration through the internal elastic lamina into the aortic media.1
Prevalence and Epidemiology
This lesion usually affects patients who have hypertension and diffuse atherosclerosis. Penetrating atherosclerotic ulcer is more often observed in the descending aorta, which is generally more involved by atherosclerosis.52 Involvement of the ascending aorta is rare.20
Etiology and Pathophysiology
The atherosclerotic ulcer is initially confined to the intima and is usually asymptomatic.53 The progression of the intimal erosion can develop into a deep atheromatous ulcer, which penetrates through the elastic lamina into the medial layer. If an atherosclerotic ulcer penetrates into the aortic media that is very rich with the vasa vasorum, the medial layer may be exposed to pulsatile arterial flow, which can initiate hemorrhage into the media without an intimal flap. However, this lesion is mostly localized because its longitudinal propagation is limited by atherosclerotic fibrosis and calcification of the aortic wall. Further extension of penetrating atherosclerotic ulcer to the aortic adventitia can cause progressive dilation of the aorta, which leads to a pseudoaneurysm or even aortic rupture.7
Manifestations of Disease
Imaging Indications and Algorithm
Imaging findings of penetrating atherosclerotic ulcers differ from those of acute aortic dissection. An intimal flap is absent, and a contrast-filled space is not visualized in the medial layer, although intramural hematomas are frequently associated with penetrating atherosclerotic ulcer. A penetrating atherosclerotic ulcer may be distinguished from a simple atherosclerotic plaque by the presence of a discrete ulcer crater or a focal contrast-filled outpouching (Fig. 93-16). However, ulcerated atherosclerotic plaque shows irregular margins of the intima without evidence of contrast material extending beyond the intimal layer (Fig. 93-17).
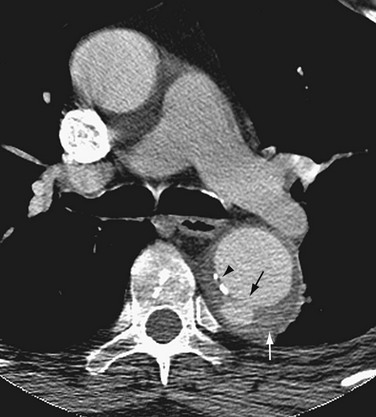
 FIGURE 93-16 CTA of penetrating atherosclerotic ulcer along the posterior wall of the descending aorta (black arrow). The white arrow shows an associated intramural hematoma. The arrowhead indicates intimal calcification. Compare with ulcerated plaque in Figure 93-17.
FIGURE 93-16 CTA of penetrating atherosclerotic ulcer along the posterior wall of the descending aorta (black arrow). The white arrow shows an associated intramural hematoma. The arrowhead indicates intimal calcification. Compare with ulcerated plaque in Figure 93-17.
(From from Kapustin AJ, Litt HI. Diagnostic imaging for aortic dissection. Semin Thorac Cardiovasc Surg 2005; 17:214-223.)
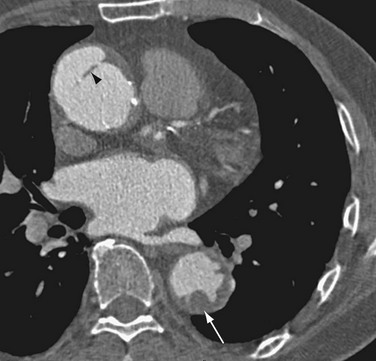
 FIGURE 93-17 Contrast-enhanced CT image shows irregular ulcerated atherosclerotic plaque in the descending thoracic aorta, without penetration beyond the intima (arrow). The ascending aorta shows aortic dissection, which has an intimal flap with spotty calcification along the luminal side of the aortic wall (arrowhead). Compare with penetrating atherosclerotic ulcer of the aorta in Figure 93-16.
FIGURE 93-17 Contrast-enhanced CT image shows irregular ulcerated atherosclerotic plaque in the descending thoracic aorta, without penetration beyond the intima (arrow). The ascending aorta shows aortic dissection, which has an intimal flap with spotty calcification along the luminal side of the aortic wall (arrowhead). Compare with penetrating atherosclerotic ulcer of the aorta in Figure 93-16.
Imaging Techniques and Findings
Ultrasonography
TEE is relatively insensitive for diagnosis of aortic ulcers. A penetrating atherosclerotic ulcer is more difficult to identify with TEE than with CT or MR imaging.54 However, TEE has the ability to classify the different types of aortic ulcers related to their pathogenesis. TEE can directly visualize the aortic lumen and wall and differentiates between ulcerated atherosclerotic plaques confined to the intima and penetrating atherosclerotic ulcers extending into the medial layer.12 Ulcerated atherosclerotic plaques may demonstrate a thickened intima with irregular margins, whereas a penetrating atherosclerotic ulcer shows a discrete ulcer crater with extension beyond the intima. If the penetrating atherosclerotic ulcer is associated with intramural hematoma, the aortic wall is thickened, and intimal calcifications, if present, are displaced inward. In some patients, TEE may fail to identify the aortic ulcer and may reveal only intramural hematoma from a tiny aortic ulcer mouth.55
Computed Tomography
CT findings of a penetrating atherosclerotic ulcer may include a focal ulcer, intramural hematoma, displacement of calcified intima, thickened and enhancing aortic wall, pleural effusion, mediastinal fluid, or even pseudoaneurysm.56
CT scans performed before the administration of contrast material readily demonstrate aortic atherosclerotic plaques with ulcer craters, which are manifested as low-attenuation material along the vessel wall, with intimal calcifications frequently scattered along the luminal side of the aorta. The intramural hematoma, if it is also present, may be visualized as an area of high attenuation on the CT images obtained before contrast enhancement. Intimal calcifications can be displaced inward by surrounding intramural hematoma.56
Contrast-enhanced CT depicts a penetrating atherosclerotic ulcer as a focal contrast collection projecting beyond the intimal border of the aortic wall without an intimal dissection flap or false lumen (Fig. 93-18). The contrast collection may extend longitudinally up to several centimeters. Penetrating ulcers are sometimes associated with aortic wall thickening and possible mural enhancement. Massive hemomediastinum may be associated with a complicated penetrating atherosclerotic ulcer, which requires immediate surgical treatment.20
Magnetic Resonance
Although CT is commonly used in the initial screening of penetrating atherosclerotic ulcers, MRI can also depict a penetrating atherosclerotic ulcer as a focal contrast-filled outpouching without an intimal flap or false lumen in the aortic wall (Fig. 93-19). As in intramural hematoma, the hemorrhage associated with penetrating atherosclerotic ulcers can be demonstrated as a variable signal intensity lesion on MRI. An intramural hematoma combined with penetrating atherosclerotic ulcer is usually visualized as localized areas of high signal intensity on the T1- and T2-weighted images.
Angiography
Catheter angiography is often not the initial examination for diagnosis of penetrating atherosclerotic ulcers. If the ulcer projects tangentially from the aortic lumen, catheter angiography can show a penetrating atherosclerotic ulcer as a localized contrast-filled outpouching of the aortic wall. However, the ability of catheter angiography to show penetrating atherosclerotic ulcers is limited with false-negative diagnoses unless the ulcer is profiled on the image projections.54 Angiography may not detect or may underestimate the presence of an intramural hematoma associated with a penetrating atherosclerotic ulcer.
Synopsis of Treatment Options
Medical
Symptomatic treatment of acute penetrating atherosclerotic ulcers should be similar to that of other acute aortic syndromes. Treatment of penetrating atherosclerotic ulcers generally depends on their evolutional patterns, such as persistent symptoms, progressive dilation of the aorta, or rebleeding of the aortic wall.12 Patients with penetrating atherosclerotic ulcers complicated by localized intramural hematoma generally undergo aggressive medical treatment in an intensive care unit.56 Medical treatment focuses on the control of hypertension.
Surgical/Interventional
Patients with hemodynamic instability or uncontrollable pain should be recommended for surgical treatment. Patients who have developed pseudoaneurysms or aortic rupture are treated with emergency surgery.54
Endovascular treatment has been favored in elderly patients with atherosclerosis because of surgical risk. Indications for surgical treatment include persistent or recurrent pain, expanding intramural hematoma or pseudoaneurysm, distal embolization, and hemodynamic instability.56 Surgical treatment of a penetrating atherosclerotic ulcer requires placement of an interposition graft at the site of the ulcer. The interposition graft is relatively extensive because it should cover the aortic wall involved by intramural hematoma, which may lead to higher morbidity such as paraplegia.54
RUPTURED AORTIC ANEURYSM
Etiology and Pathophysiology
Aortic aneurysm rupture usually occurs when the mechanical stress on the aortic wall exceeds the strength of the wall tissue.57 The risk of rupture is closely related to the size of the aneurysm. A previous study reported that the median diameter of the aorta at the diagnosis of rupture was 6.0 cm in the ascending aorta and 7.2 cm in the descending thoracic aorta.58 The expansion rate of aortic aneurysms may affect the risk of rupture in relation to the size of the aneurysms. The median expansion rate was much faster in patients with aneurysms of more than 4 cm in diameter (0.3 to 0.8 cm/yr) than in patients with aneurysms of less than 4 cm in diameter (0.2 cm/yr).59 The risk of rupture may also depend on the cause of the aneurysm; rate of rupture is relatively high in mycotic aneurysms and pseudoaneurysms. In practice, ruptured aortic aneurysm is more frequently observed in the aortic arch and descending aorta, where it is more common but less life-threatening than in the ascending aorta.20
Manifestations of Disease
Clinical Presentation
Clinical symptoms in patients with unstable aortic aneurysm are similar to those of other acute aortic syndromes. Although patients with unstable aortic aneurysm often complain of chest or back pain, they are usually accompanied by hypotension when the aneurysm has ruptured, and, therefore, ruptured aortic aneurysm may not be routinely included as a part of acute aortic syndrome.21
Imaging Techniques and Findings
Computed Tomography
CT is frequently used in the emergency department to evaluate suspected aortic disease and can accurately demonstrate the size and extent of the aneurysm or pseudoaneurysm, associated dissection of the aorta, presence and extent of mural thrombus, and other critical information about the aortic wall.60 CT also shows mediastinal hematoma and its relationship to the aorta, pleural effusion or associated lung collapse, and rarely pericardial effusion. In an emergent situation, CT can be performed without the intravenous administration of contrast material.
The findings of aortic rupture on CT images obtained before contrast enhancement include high-attenuation thickening of the aortic wall, adjacent mediastinal hematoma, hemothorax, and hemopericardium (Fig. 93-20). Even without signs of frank rupture, demonstration of crescentic high attenuation in the thickened wall of the aorta or within the mural thrombus on CT images obtained before contrast enhancement is indicative of early aortic rupture or impending aortic rupture.61,62 This sign represents acute hemorrhage into the preexisting mural thrombus or the aneurysm wall, which can be a potential site for rupture. Contrast-enhanced CT may demonstrate deformities of the lumen and irregular thickening of the aortic wall with or without active extravasation of contrast material into the periaortic spaces. The pattern of mural calcification can also be important, with a focal discontinuity in otherwise circumferential calcification depicted in patients with ruptured aortic aneurysm.62 Draping of the posterior aspect of the aorta over the adjacent vertebral body is another sign of aortic wall insufficiency in abdominal aortic aneurysms, even in the absence of retroperitoneal hemorrhage.63
Magnetic Resonance
The efficacy of MRI in the diagnosis of ruptured aortic aneurysm is controversial because of examination length and monitoring difficulties in potentially unstable patients. Irregular wall thickening of the aorta can be depicted by MRI, which may show variable signal intensity according to the stages of hemorrhage. Hemorrhage within the mural thrombus or thickened aortic wall is seen as focal areas of high signal intensity on T1- and T2-weighted spin-echo MR images.64 However, it is not clear that all areas of hemorrhage visualized on the MR images are related to the ruptured aneurysms or hyperattenuating crescent signs on CT images.
Synopsis of Treatment Options
Surgical/Interventional
Ruptured aortic aneurysm should be treated aggressively with immediate surgical repair. Although techniques have improved, surgery for ruptured aortic aneurysm is still associated with a high morbidity and mortality rate. However, a study reported that patients with ruptured aortic aneurysm had an early mortality rate of 21%.65 This lower mortality rate suggests that most surgical patients are hemodynamically stable and are, to a certain degree, self-selected. Patients who are hemodynamically unstable do not usually survive long enough to be transported to the operating room.
Postoperative complications include paraplegia, renal failure, and other cardiopulmonary events. The development of paraplegia is the most serious complication after repair of aneurysm rupture. Paraplegia is usually due to interruption of the intercostal blood supply to the spinal cord.7 The rate of paraplegia is much higher, with an incidence rate of 54%, in patients with aneurysm rupture than in patients without aneurysm rupture (10%). Renal failure depends on the preoperative condition of the patient; the incidence of renal impairment is reported to be between 3% and 27%.65,66 Visceral and renal perfusion protection should be important goals in the treatment of ruptured aortic aneurysms.
KEY POINTS
 Acute aortic syndromes include aortic dissection, intramural hematoma, penetrating atherosclerotic ulcer, and ruptured aortic aneurysm.
Acute aortic syndromes include aortic dissection, intramural hematoma, penetrating atherosclerotic ulcer, and ruptured aortic aneurysm.Kapustin AJ, Litt HI. Diagnostic imaging for aortic dissection. Semin Thorac Cardiovasc Surg. 2005;17:214-223.
Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003;181:309-316.
Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic aneurysm leak and rupture and traumatic aortic transection. AJR Am J Roentgenol. 2003;181:303-307.
Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part II: therapeutic management and follow-up. Circulation. 2003;108:772-778.
Roberts DA. Magnetic resonance imaging of thoracic aortic aneurysm and dissection. Semin Roentgenol. 2001;36:295-308.
1 Vilacosta I, Roman JA. Acute aortic syndrome. Heart. 2001;85:365-368.
2 Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003;181:309-316.
3 Zamorano JL, Perez de Isla L, Gonzalez R, et al. [Imaging diagnosis in acute aortic syndromes]. Rev Esp Cardiol. 2003;56:498-508.
4 Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271-1278.
5 Clouse WD, Hallett JWJr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79:176-180.
6 Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897-903.
7 Ahmad F, Cheshire N, Hamady M. Acute aortic syndrome: pathology and therapeutic strategies. Postgrad Med J. 2006;82:305-312.
8 Coady MA, Rizzo JA, Elefteriades JA. Pathologic variants of thoracic aortic dissections. Penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin. 1999;17:637-657.
9 Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. N Engl J Med. 1997;336:1876-1888.
10 Muna WF, Spray TL, Morrow AG, Roberts WC. Aortic dissection after aortic valve replacement in patients with valvular aortic stenosis. J Thorac Cardiovasc Surg. 1977;74:65-69.
11 Wooley CF, Sparks EH, Boudoulas H. Aortic pain. Prog Cardiovasc Dis. 1998;40:563-589.
12 Evangelista Masip A. [Natural history and therapeutic management of acute aortic syndrome]. Rev Esp Cardiol. 2004;57:667-679.
13 DeBakey ME, McCollum CH, Crawford ES, et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery. 1982;92:1118-1134.
14 Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237-247.
15 Kapustin AJ, Litt HI. Diagnostic imaging for aortic dissection. Semin Thorac Cardiovasc Surg. 2005;17:214-223.
16 Shiga T, Wajima Z, Apfel CC, et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350-1356.
17 Petasnick JP. Radiologic evaluation of aortic dissection. Radiology. 1991;180:297-305.
18 Blanchard DG, Kimura BJ, Dittrich HC, DeMaria AN. Transesophageal echocardiography of the aorta. JAMA. 1994;272:546-551.
19 Yamada E, Matsumura M, Kyo S, Omoto R. Usefulness of a prototype intravascular ultrasound imaging in evaluation of aortic dissection and comparison with angiographic study, transesophageal echocardiography, computed tomography, and magnetic resonance imaging. Am J Cardiol. 1995;75:161-165.
20 Romano L, Pinto A, Gagliardi N. Multidetector-row CT evaluation of nontraumatic acute thoracic aortic syndromes. Radiol Med (Torino). 2007;112:1-20.
21 Bhalla S, West OC. CT of nontraumatic thoracic aortic emergencies. Semin Ultrasound CT MR. 2005;26:281-304.
22 Manghat NE, Morgan-Hughes GJ, Roobottom CA. Multi-detector row computed tomography: imaging in acute aortic syndrome. Clin Radiol. 2005;60:1256-1267.
23 Roos JE, Willmann JK, Weishaupt D, et al. Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology. 2002;222:271-277.
24 Urban BA, Bluemke DA, Johnson KM, Fishman EK. Imaging of thoracic aortic disease. Cardiol Clin. 1999;17:659-682. viii
25 Roberts DA. Magnetic resonance imaging of thoracic aortic aneurysm and dissection. Semin Roentgenol. 2001;36:295-308.
26 Krinsky GA, Rofsky NM, DeCorato DR, et al. Thoracic aorta: comparison of gadolinium-enhanced three-dimensional MR angiography with conventional MR imaging. Radiology. 1997;202:183-193.
27 Liu Q, Lu JP, Wang F, et al. Three-dimensional contrast-enhanced MR angiography of aortic dissection: a pictorial essay. Radiographics. 2007;27:1311-1321.
28 Erbel R, Engberding R, Daniel W, et al. Echocardiography in diagnosis of aortic dissection. Lancet. 1989;1:457-461.
29 Cigarroa JE, Isselbacher EM, DeSanctis RW, Eagle KA. Diagnostic imaging in the evaluation of suspected aortic dissection. Old standards and new directions. N Engl J Med. 1993;328:35-43.
30 LePage MA, Quint LE, Sonnad SS, et al. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol. 2001;177:207-211.
31 Williams DM, Joshi A, Dake MD, et al. Aortic cobwebs: an anatomic marker identifying the false lumen in aortic dissection—imaging and pathologic correlation. Radiology. 1994;190:167-174.
32 Ince H, Nienaber CA. [Management of acute aortic syndromes]. Rev Esp Cardiol. 2007;60:526-541.
33 David TE, Feindel CM. An aortic valve–sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617-621.
34 Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part II: therapeutic management and follow-up. Circulation. 2003;108:772-778.
35 Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546-1552.
36 Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539-1545.
37 Bortone AS, Schena S, D’Agostino D, et al. Immediate versus delayed endovascular treatment of post-traumatic aortic pseudoaneurysms and type B dissections: retrospective analysis and premises to the upcoming European trial. Circulation. 2002;106:I234-I240.
38 Evangelista A, Dominguez R, Sebastia C, et al. Prognostic value of clinical and morphologic findings in short-term evolution of aortic intramural haematoma. Therapeutic implications. Eur Heart J. 2004;25:81-87.
39 Evangelista A, Mukherjee D, Mehta RH, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111:1063-1070.
40 Schlatmann TJ, Becker AE. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977;39:21-26.
41 Carerj S, Cerrito M, Oreto G. Aortic intramural haematoma leading to aortic dissection. Heart. 2004;90:254.
42 Song JM, Kang DH, Song JK, et al. Clinical significance of echo-free space detected by transesophageal echocardiography in patients with type B aortic intramural hematoma. Am J Cardiol. 2002;89:548-551.
43 Song JK. Diagnosis of aortic intramural haematoma. Heart. 2004;90:368-371.
44 Mohr-Kahaly S. Aortic intramural hematoma: from observation to therapeutic strategies. J Am Coll Cardiol. 2001;37:1611-1613.
45 Evangelista Masip A. [Progress in the acute aortic syndrome]. Rev Esp Cardiol. 2007;60:428-439.
46 Bansal RC, Chandrasekaran K, Ayala K, Smith DC. Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography. J Am Coll Cardiol. 1995;25:1393-1401.
47 O’Gara PT, DeSanctis RW. Acute aortic dissection and its variants. Toward a common diagnostic and therapeutic approach. Circulation. 1995;92:1376-1378.
48 Kang DH, Song JK, Song MG, et al. Clinical and echocardiographic outcomes of aortic intramural hemorrhage compared with acute aortic dissection. Am J Cardiol. 1998;81:202-206.
49 Ohmi M, Tabayashi K, Moizumi Y, et al. Extremely rapid regression of aortic intramural hematoma. J Thorac Cardiovasc Surg. 1999;118:968-969.
50 von Kodolitsch Y, Csosz SK, Koschyk DH, et al. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003;107:1158-1163.
51 Isselbacher EM, Cigarroa JE, Eagle KA. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation. 1994;90:2375-2378.
52 Lissin LW, Vagelos R. Acute aortic syndrome: a case presentation and review of the literature. Vasc Med. 2002;7:281-287.
53 Pauls S, Orend KH, Sunder-Plassmann L, et al. Endovascular repair of symptomatic penetrating atherosclerotic ulcer of the thoracic aorta. Eur J Vasc Endovasc Surg. 2007;34:66-73.
54 Levy JR, Heiken JP, Gutierrez FR. Imaging of penetrating atherosclerotic ulcers of the aorta. AJR Am J Roentgenol. 1999;173:151-154.
55 Movsowitz HD, David M, Movsowitz C, et al. Penetrating atherosclerotic aortic ulcers: the role of transesophageal echocardiography in diagnosis and clinical management. Am Heart J. 1993;126:745-747.
56 Kazerooni EA, Bree RL, Williams DM. Penetrating atherosclerotic ulcers of the descending thoracic aorta: evaluation with CT and distinction from aortic dissection. Radiology. 1992;183:759-765.
57 Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic aneurysm leak and rupture and traumatic aortic transection. AJR Am J Roentgenol. 2003;181:303-307.
58 Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476-491.
59 Guirguis EM, Barber GG. The natural history of abdominal aortic aneurysms. Am J Surg. 1991;162:481-483.
60 Ledbetter S, Stuk JL, Kaufman JA. Helical (spiral) CT in the evaluation of emergent thoracic aortic syndromes. Traumatic aortic rupture, aortic aneurysm, aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer. Radiol Clin North Am. 1999;37:575-589.
61 Mehard WB, Heiken JP, Sicard GA. High-attenuating crescent in abdominal aortic aneurysm wall at CT: a sign of acute or impending rupture. Radiology. 1994;192:359-362.
62 Siegel CL, Cohan RH, Korobkin M, et al. Abdominal aortic aneurysm morphology: CT features in patients with ruptured and nonruptured aneurysms. AJR Am J Roentgenol. 1994;163:1123-1129.
63 Schwartz SA, Taljanovic MS, Smyth S, et al. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR Am J Roentgenol. 2007;188:W57-W62.
64 Castrucci M, Mellone R, Vanzulli A, et al. Mural thrombi in abdominal aortic aneurysms: MR imaging characterization—useful before endovascular treatment? Radiology. 1995;197:135-139.
65 Lemaire SA, Rice DC, Schmittling ZC, Coselli JS. Emergency surgery for thoracoabdominal aortic aneurysms with acute presentation. J Vasc Surg. 2002;35:1171-1178.
66 Cambria RP, Davison JK, Zannetti S, et al. Thoracoabdominal aneurysm repair: perspectives over a decade with the clamp-and-sew technique. Ann Surg. 1997;226:294-303.

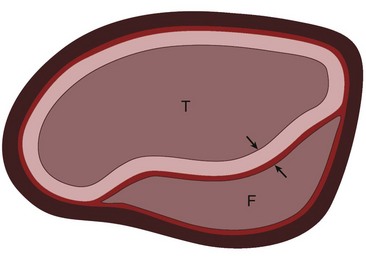
 FIGURE 93-1
FIGURE 93-1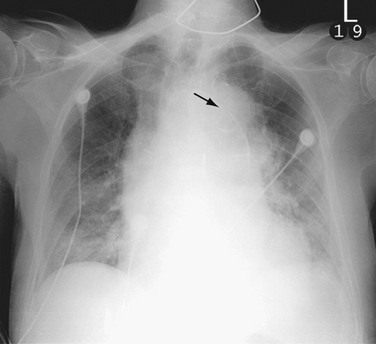
 FIGURE 93-3
FIGURE 93-3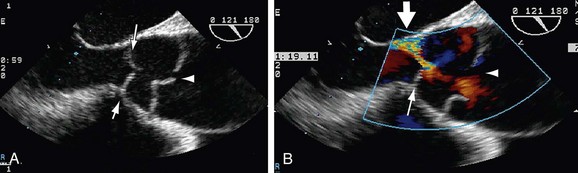
 FIGURE 93-4
FIGURE 93-4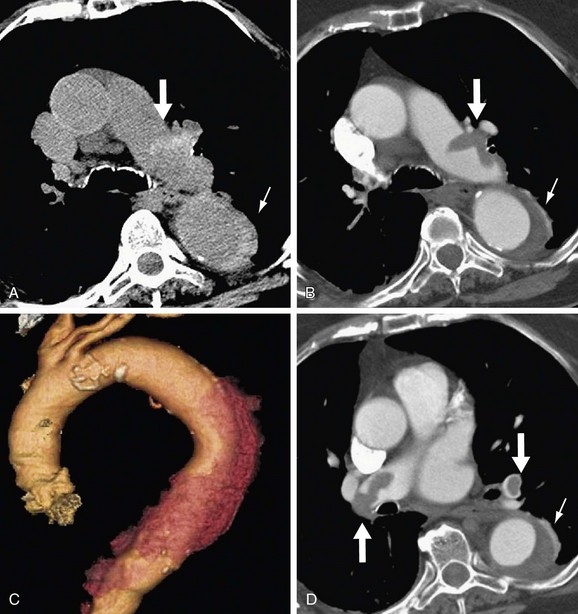
 FIGURE 93-5
FIGURE 93-5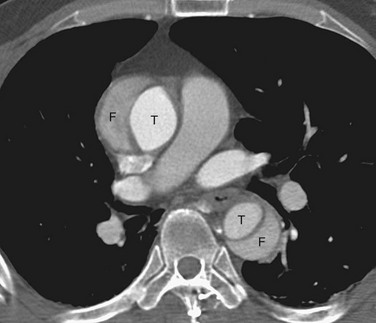
 FIGURE 93-6
FIGURE 93-6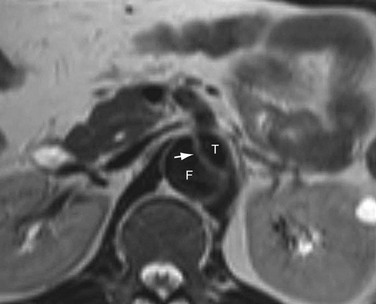
 FIGURE 93-8
FIGURE 93-8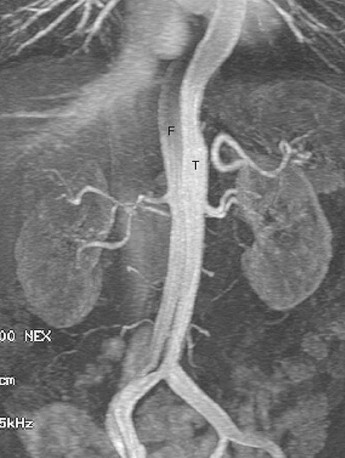
 FIGURE 93-9
FIGURE 93-9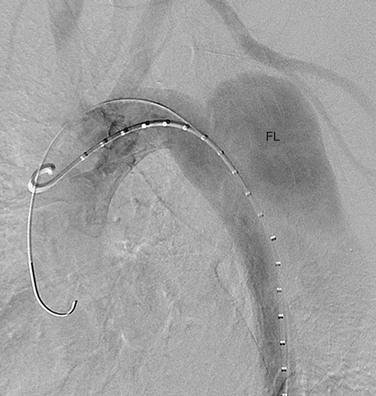
 FIGURE 93-10
FIGURE 93-10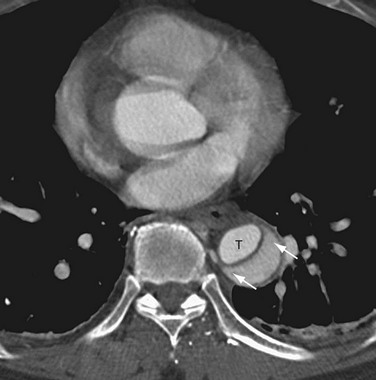
 FIGURE 93-11
FIGURE 93-11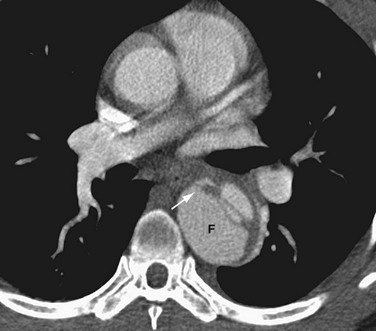
 FIGURE 93-12
FIGURE 93-12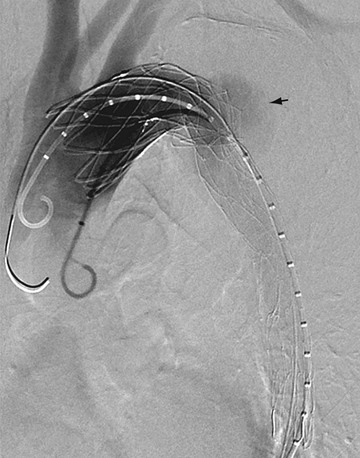
 FIGURE 93-13
FIGURE 93-13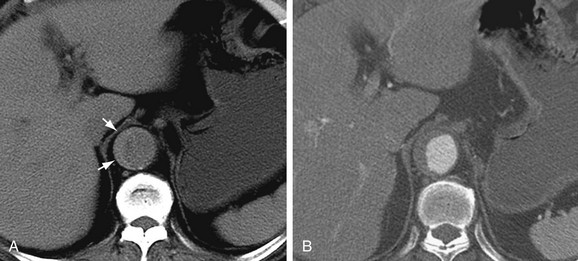
 FIGURE 93-14
FIGURE 93-14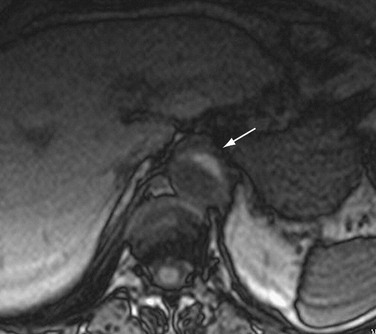
 FIGURE 93-15
FIGURE 93-15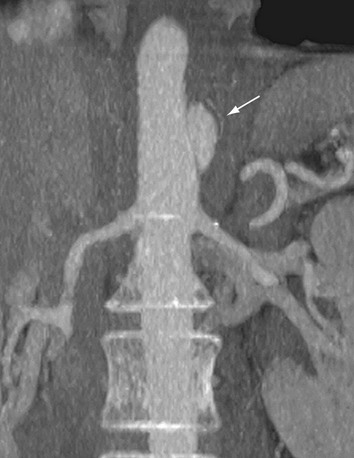
 FIGURE 93-18
FIGURE 93-18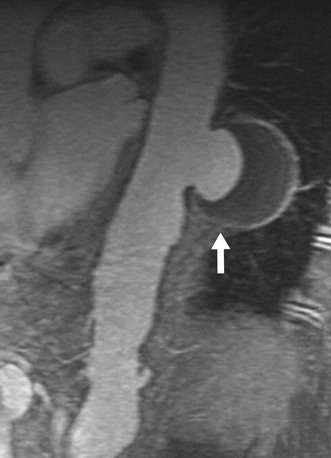
 FIGURE 93-19
FIGURE 93-19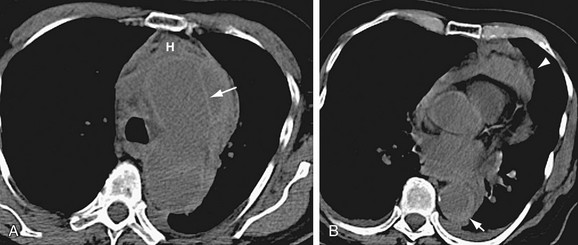
 FIGURE 93-20
FIGURE 93-20

