Acute Aortic Dissection
Introduction
Thoracic aortic dissection (TAD) occurs with communication between the thoracic aorta lumen and wall with a separation of the thoracic aortic wall layers. Thoracic aortic dissection is to be contrasted with thoracic aortic aneurysm (TAA), which is defined as dilation of the thoracic aorta to a diameter of ≥1.5 times normal. Thoracic aortic aneurysm is a risk factor for TAD. Thoracic aortic aneurysm, although an important morbidity that may be associated with acute catastrophic leakage and death, is in and of itself not the acute emergency that is signified by a TAD. The international registry of acute aortic dissection reported an overall mortality of 27.4% with this condition. Surgical mortality was 26% for proximal (type A dissection) versus 58% for medical management and for distal (type B dissection), with 10.7% for medical management and 31% for surgical management.1
History
In 1819, Laenec introduced “aortic dissection” into the medical literature when he described an intimal tear distal to the aortic valve associated with a longitudinal space in the aortic wall postmortem.2 An autopsy report in 1760 described aortic dissection and pericardial tamponade as the causes of death of King George II.3 In 1863, Peacock reported a summary of findings in 80 patients with aortic dissection and classified the disease into three stages: intimal tear, propagation of the dissection with the potential for rupture, and recanalization of the lumen.4 In 1896, Marfan described the aortic connective tissue abnormality now known to be associated with this entity.5 In 1955, DeBakey treated a descending thoracic aortic dissection with resection and reapproximation of dissected layers with graph interposition.6 In 1963, DeBakey and colleagues repaired an ascending aortic dissection complicated by aortic valvular insufficiency.7
Types of TAD
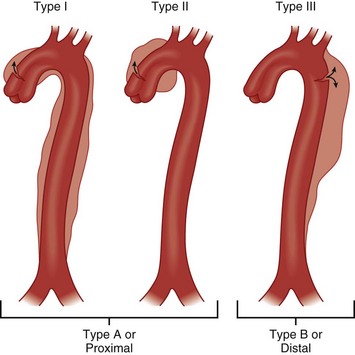
The thoracic aorta is divided anatomically into the ascending portion, the transverse portion, and the descending portion.8 The ascending aorta has two sections, the aortic root (valvular annulus and sinuses) and the tubular portion extending to the origin of the innominate artery. The transverse portion of the aorta is a short segment, and the brachiocephalic arteries come off this portion of the aorta. The descending thoracic aorta begins immediately distal to the left subclavian artery and extends to the diaphragm. The anatomic location of TAD has important clinical and prognostic implications. The most commonly used classification scheme for TAD is the Stanford System (Fig. 33.1), which has mostly replaced the first classification system, the DeBakey System (see Fig. 33.1). An advantage of the DeBakey System is that it does distinguish between dissections that are confined only to the ascending aorta (DeBakey type 2) and dissections that involve the entire aorta (DeBakey type 1). Using the Stanford System, which is more simplistic, any involvement of the ascending aorta classifies the dissection as a Stanford A, whereas lack of ascending aorta involvement classifies the dissection as a Stanford type B.
Risk Factors for Thoracic Aortic Dissection
Multiple risk factors for TAD have been identified.8 Men are at greater risk for developing TAD than women; approximately two thirds of patients with TAD are male.9 The sex distribution is consistent across different classifications of aortic dissection. Women with TAD are more likely than men to have a history of hypertension. Women are older at the time of diagnosis of TAD when compared to men. The average age at diagnosis of TAD is 65 years and is younger for type A TAD than for type B TAD.8 Specific risk factors for TAD are shown in Table 33.1. It is thought that the higher the blood pressure, the greater the probability of aortic dissection. In the presence of coarctation of the aorta, acute dissection typically occurs proximally rather than distal to the coarctation. The majority of patients with aortic dissection have hypertensive disease at the time of diagnosis.10 It is thought that blood pressure control decreases the occurrence of aortic dissection. However, hypertension is not thought to be the sole cause of dissection in a given patient. Many think that pregnancy is an independent risk factor for aortic dissection, although analysis of the available data and the conclusions from these data is hampered by the small number of patients affected and coexisting additional predisposing factors such as hypertension.11–13 In addition to blunt trauma, thoracic aortic dissection may be induced following establishing antegrade or retrograde arterial perfusion during cardiopulmonary bypass. Intra-aortic balloon counter-pulsation use may also trigger aortic dissection. There is increased risk of type A TAD after aortic valve replacement, especially with larger aortic diameters.14 Finally, diagnostic angiography and cardiac catheterization may be complicated by aortic dissection (see Table 33.1).14
Table 33.1
Specific Risk Factors for Thoracic Aortic Dissection
| Age and gender | Smoking | Trauma |
| Atherosclerosis | Aortitis | Pregnancy |
| Hypertension | Diabetes mellitus | Drug use* |
| Congenital cardiovascular defects† | Connective tissue syndromes‡ | Aortic dilatation |
*Cocaine, amphetamine, sildenafil.
†Bi-aortic value, aortic coarctation.
‡Marfan syndrome, Ehlers-Danlos syndrome, Turner syndrome, familial TAD.
Pathophysiology
The aortic wall is composed of three layers: the innermost intima, the media (smooth muscle and elastic connective tissue), and the outermost adventitia. Hemodynamic stresses to the aortic inner walls can result from risk factors such as prolonged hypertension or inherently weakened connective tissue walls as seen in some connective tissue syndromes or a bicuspid aortic valve that alters the flow pattern of blood ejected out of the aorta. A dissection may be initiated by a tear or ulceration of the medial layer facilitated by degeneration from normal aging or compounded by the risk factors listed in Table 33.1. A classic aortic dissection is classified by an intimal tear into the media of the aortic wall, resulting in separation of the medial layer and formation of a false channel, allowing blood to flow into this channel. An intramural hematoma (IMH) can also be the trigger for a dissection when an accumulation of blood separates the medial layers; in this circumstance, the inciting entrance tear is lacking. IMH is more common in the elderly hypertensive patient’s descending aorta. Finally, aortic ulcers can disrupt the aortic wall and result in aortic rupture or dissection. Patients with aortic ulcers are older than those with IMH. The false lumen of a TAD has the potential to extend both distally and proximally, potentially leading to obstruction of arterial origins from the aortic trunk, rupture back into the true vascular lumen (which can be lifesaving), extension into the pericardial sac with pericardial tamponade, or rupture into the pleural cavity with devastating hemorrhagic shock and death (Fig. 33.2A,B).
Rupture is the most common cause of death during the early acute phase of TAD. The most frequent rupture route leading to death is into the pericardium causing tamponade. Compromise of arch vessels may lead to neurologic symptoms and injury. Death may also occur with involvement of the aortic root, producing primary ostial compromise and acute myocardial infarction or severe aortic regurgitation. Rarely, fistulas and high-degree heart block may be produced. The thin wall of any residual pseudo-aneurysm following TAD tends to enlarge and over time is at high risk of rupture. Total thrombosis of the false lumen is rare, whereas distal reentry into the true lumen may help decompress the false lumen and increase the chance of survival. Postoperative false lumen patency is a predictor of late mortality.15
Diagnosis
Symptoms
The diagnosis of acute TAD is complicated by the infrequent occurrence of this clinical condition as well as the diagnostic difficulty and is particularly problematic because of the potential catastrophic outcome.16 Typical symptoms of TAD are excruciating, severe at onset, pain of a sharp and tearing nature. The location of the pain (anterior chest, neck, jaw, inner scapular, and lumbar/abdominal) is linked to the location of the dissection. Symptoms, other than pain, include visceral symptoms (vomiting, diaphoresis, and syncope).
Physical Exam
Although physical examination findings may be absent, if present, they are useful in directing the clinician’s attention to this diagnosis.16 Inequality of pulses in the upper extremity and a blood pressure differential of 20 to 30 mm Hg between the two extremities may be seen based on the location of the dissection. A new aortic regurgitation murmur occurs in a significant number of patients. Proximal dissection may also interfere with coronary artery blood flow, producing cardiogenic shock or rupture into the pericardium, producing pericardial tamponade. Pericardial tamponade would be supported by findings of jugular venous distention, muffled heart tones, tachycardia, and hypotension. Mass compression effects can produce findings such as superior vena cava syndrome, Horner syndrome, hoarseness, dyspnea, or dysphagia. Syncope is seen in 1 of 10 cases of TAD. Syncope likely results from acute cardiac dysfunction or vascular outflow obstruction of the carotid arteries. Vasovagal pain response may also be a potential etiology of syncope. When syncope is related to hypovolemic shock from rupture through the adventitia into the pleural space, the prognosis is grave.
Imaging
Chest Radiograph
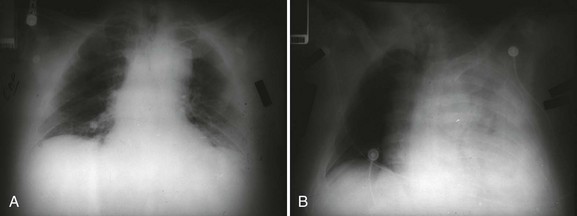
The great majority of patients with thoracic aortic dissection has abnormalities on the chest radiograph. A normal chest radiograph may therefore help in decreasing the likelihood of aortic dissection. A study by Klompas looked at 1337 chest radiographs in patients with thoracic aortic dissection and reported abnormalities in 90% of patients.17 The most common changes associated with dissection are abnormal aortic contour, widening of the mediastinum, pleural effusion, displacement of intimal calcification, abnormalities of the aortic knob, and displacement of the trachea or nasogastric tube to the right. In the absence of an abnormal aortic contour or mediastinal widening, a diagnosis of dissection is less likely.
Computed Tomography
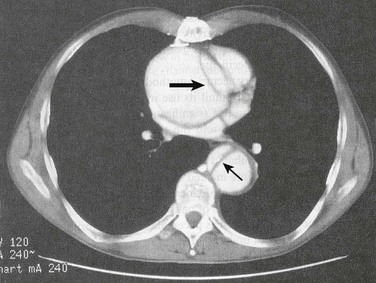
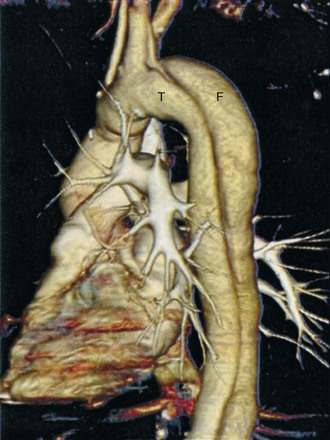
The most common diagnostic modality used for diagnosis of TAD is computed tomography (CT) (Fig. 33.3). A CT angiogram shows high sensitivity and specificity in diagnosis and exclusion of TAD. It is the optimal imaging modality for ruling out aortic dissection in patients with low clinical pretest probability. Limitations of this technique include use of ionizing radiation and contrast media, need for transfer to imaging station, and limited ability to access the aortic valve. Advantages are ready availability, turnaround time, delineation of entire aorta, and diagnosis of other disorders causing the patient’s symptoms. Advances in CT angiographic techniques—such as spiral multidetection scanner technology that allows volume-rendered, three-dimensional reconstruction of a CT angiogram—are likely superior to magnetic resonance images (see Fig. 33.4 as an example of the technology).
Echocardiography
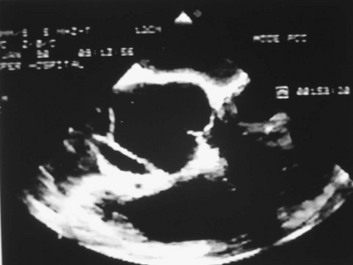
Transthoracic echocardiography (TTE) should be performed early in the evaluation of any patient in whom the diagnosis of ascending aortic dissection is entertained. If a pericardial effusion is present, signs of tamponade can be identified. TTE can help identify aortic dissection if multiple views are obtained—specifically, suprasternal, subcostal, and right parasternal views. The intimal flap may be identified within the lumen, with motion that is not synchronized with the surrounding structures. To minimize the chance of a false-positive result, the flap should be visualized in more than one view. The addition of color Doppler helps to identify flow artifacts (as opposed to an intimal flap) within the ascending aorta by demonstrating flow between the true and false channels at the site of an intimal tear and by showing a difference in the timing or direction of flow within the two lumens.18 Color Doppler permits detection and quantification of aortic regurgitation. TTE is especially useful for diagnosing dissections involving the ascending aorta but is much less sensitive for descending dissections. If a dissection is not visualized in the patient with chest pain, finding regional wall motion abnormalities may suggest the alternative diagnosis of coronary ischemia.
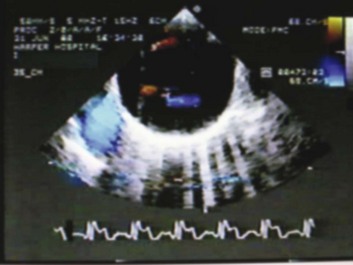
Transthoracic echocardiography (TEE) (Fig. 33.5) can be performed safely on most critically ill patients in a monitored setting.19,20 To avoid precipitating hypertension, tachycardia, or gagging (straining) in a patient with suspected dissection, conscious sedation should be administered. Nearly all of the thoracic aorta can be visualized, including most of the arch. The area from the distal ascending aorta to the midarch, however, is difficult to evaluate with TEE because of interposition of the airway between the esophagus and the aorta.21 The sensitivity of TEE for thoracic aortic dissection is close to 100%, but specificity is lower, owing primarily to reverberation artifacts that may be visualized within the ascending aorta, simulating an intimal flap.22 Color flow Doppler with TEE may demonstrate flow through or on either side of the suspected intimal flap (Fig. 33.6). TEE is helpful for evaluating involvement of the coronary ostia and aortic valve. As with TTE, regional and overall ventricular wall motion can be evaluated and the presence of pericardial effusion can be determined.
Aortography
Once the gold standard for diagnosis of TAD, aortography fell into disfavor as the diagnostic study of choice owing to its invasive nature and a relatively high false-negative rate.23 The sensitivity of aortography is lower than that of CT, and its specificity is no better. Reliance on iodinated contrast does not ensure direct visualization of a thrombosed lumen or intramural hematoma, or occasionally even confirm the presence of a second, false lumen.24 Aortography has had a resurgence with the growth of endovascular intervention in the management of dissections where it facilitates stenting across intimal tears, fenestration of intimal flaps, and opening occluded branch vessels.25,26
Magnetic Response Imaging (MRI)
The most reliable diagnostic finding with MRI is demonstration of two lumens separated by an intimal flap. Gadolinium-enhanced magnetic resonance angiography (MRA) allows the visualization of blood flow, which can be used to detect the presence and magnitude of aortic regurgitation or to demonstrate communication between the true and false lumens at the site of an intimal tear. An intimal flap can be identified in most cases, except when the false lumen is completely thrombosed. The diagnostic accuracy of MRI for aortic dissection approaches 100%.27 Problematic is (1) that it cannot be performed in patients with pacemakers or defibrillators, aneurysm clips, or ferrous metal implants, (2) claustrophobia, and (3) life support equipment (ventilators, monitors, intravenous infusion pumps) with associated logistical issues. Image acquisition, despite advances in the technology, is more time consuming than with CT. Because it entails no radiation exposure, MRI may be an appropriate initial study in the stable patient22 and for long-term follow-up evaluation.22,28
Summary
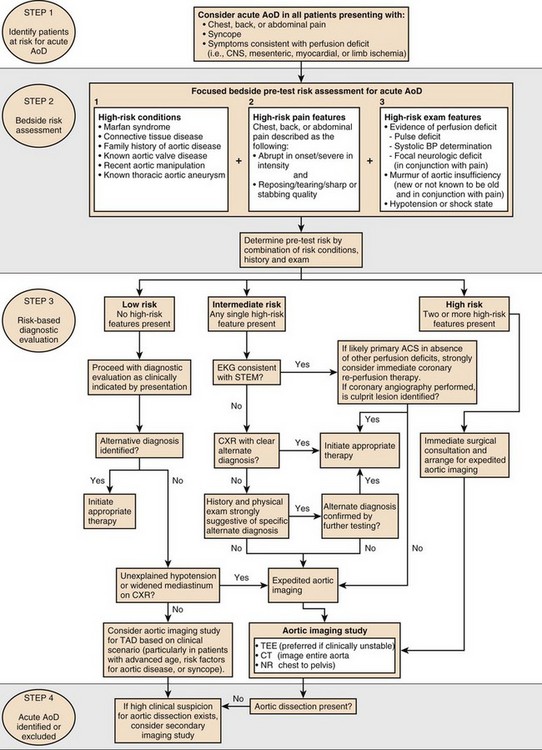
Table 33.1 shows a comparison of diagnostic imaging modalities for evaluation of thoracic aortic dissection. The 2010 American Heart Association (AHA) guidelines for diagnosis of dissection give a class I recommendation for using either urgent transesophageal echo, MRI, or CT angiogram to determine a definitive diagnosis in patients with high clinical suspicion.29 If the initial diagnostic test is negative in patients with high clinical suspicion, a second imaging modality should be completed. The diagnosis algorithm from the 2010 guidelines for the diagnosis and management of TAD is shown in Figure 33.7.
Perioperative Management
General Principles
Medical therapy is critical for survival with both type A and type B TAD.30–33 Immediate initiation of medical therapy is imperative, both before surgical intervention for type A TAD and as definitive therapy for patients with type B TAD that is uncomplicated. The primary objectives of therapy are rapidly normalizing arterial blood pressure in hypertensive patients and reducing the force of left ventricular contractility in all patients. Following admission to the intensive care unit (ICU), vital signs are monitored continually; blood pressure should be compared between upper extremities, and the same for carotid pulses. Arterial line insertion is indicated. A radial arterial line on the side of higher blood pressure or a femoral arterial line is placed. For type B dissection, a right radial arterial line is preferred. Intravenous drugs are indicated for medical therapy. In order to limit shear force, which is propagating the dissection, it is important to control the rapidity of the rise of the systole, which is done by avoiding tachycardia (goal heart rate is 60 to 75 beats per minute), depressing contractility, and decreasing blood pressure. Because blood pressure is maintained at low normal, a Foley catheter is recommended in order to judge adequate tissue perfusion. Pain is a marker of continued dissection of a TAD, and subsiding of pain can be assumed to be a marker of halting of dissection. Medical therapy is the treatment of choice for uncomplicated type B TAD. It may also be successfully used in patients who are poor surgical candidates or in select patients with uncomplicated and stable type A TAD.
The mortality of nonoperative management of type A dissection may be up to 1% to 2% per hour in the early phase after symptom onset.34 The goals of medical management should be stabilization, management of hypertension, and evaluation of neurologic and metabolic function while the operating room is being prepared for the patient. Most centers favor transport directly to the operating room after the surgical team has been mobilized to avoid any delay that would increase mortality. Exceptions to this rule are patients who may present with severe malperfusion that may preclude operative intervention (severe cerebral or visceral ischemia). These exceptions need to be thoroughly evaluated with CT scan and neurologic consultation if central nervous system issues are present. In the case of patients presenting with severe acidosis and elevated lactate levels, CT scan of the abdomen to evaluate for bowel infarction, which if present may make the patient inoperable, is indicated.
Specific Medications
Control of blood pressure and heart rate in the presence of TAD is essential.35 Intravenous beta-blockers are the first line of therapy for aortic dissection and are immediately initiated to avoid tachycardia, which may occur with some medications chosen to lower blood pressure.36 Beta-blockers, in addition to controlling heart rate, also depress contractility and lower blood pressure, both goals of therapy. Intravenous esmolol is the ideal drug as it is a quick onset and short-acting agent and can be titrated to effect. Intravenous diltiazem is an alternative to esmolol for decreasing contractility and avoiding tachycardia when there are contraindications to the use of beta-blockers, such as in the active asthmatic. This target blood pressure will then be anticipated to persist for 6 to 8 hours.
Table 34.2 reviews drugs used in the medical therapy of acute TAD.
Operative Approach
Ascending Aortic Dissections
Surgical intervention is indicated in all patients with proximal dissections, with the exception of patients with serious concomitant conditions that preclude surgery.37 Stroke is the most common contraindication to surgery because there is real concern that anticoagulation (cardiopulmonary bypass) as well as reperfusion can result in further neurologic deterioration by converting the ischemic stroke to a hemorrhagic stroke. Every attempt must be made to perform a careful neurologic assessment prior to undertaking surgical intervention. Additional evidence of severe malperfusion (bowel ischemia) is also a contraindication to surgery.
Preoperative evaluation is essential to assess for the presence of aortic regurgitation, pericardial effusion, the extension of the dissection into the major aortic branches, and the localization of entry and reentry sites, and the presence of thrombosis in the false lumen yields information that can be helpful in planning the surgical approach.38
The operative mortality rate for patients with aortic dissections ranges from 5% to 10% and may approach 70% in cases with complications. The independent predictors of operative mortality include the presence of cardiac tamponade, the site of the tear, the time to operation, the presence of renal/visceral ischemia, renal dysfunction, and the presence of pulmonary disease.39
If there is no primary tear site in the sinus of Valsalva, most surgeons favor reconstruction of the aortic root with glue aortoplasty, and valve resuspension.40 Indications for performing a full aortic root replacement with a valved conduit and a left and right coronary artery reimplantation (Bentall operation) include a tear through the sinus of Valsalva or an aortic valve that cannot be reconstructed. This can be performed with a mechanical valved conduit, pericardial conduit, or porcine heterograft.40–44 Valve-sparing aortic root replacement may be also performed in patients who have a long life expectancy. This complex procedure requires resection of all aorta distal to the annulus, creation of neosinuses, and coronary artery reimplantation with reconstitution of aortic lumen continuity.45
In addition, stent grafting of the distal thoracic aorta may be performed in an antegrade fashion while on a period of circulatory arrest (stented elephant trunk technique). This may stabilize the distal aorta, prevent future aneurysmal dilation, or facilitate a proximal endovascular landing zone for future endovascular therapy should that become necessary.46
Descending Aortic Dissection and Endovascular Therapy
Uncomplicated acute type B dissection is best managed medically. Approximately 15% of this type of dissection will develop aneurysmal degeneration and require surgical intervention within 5 years of the initial event. The indications for performing early surgery in patients with distal dissections are the rapid expansion of a dissecting aneurysm, rupture into the left chest, impending rupture, persistent and uncontrollable pain, or impairment of the blood flow to an organ or limb.37,47–49 Open surgical repair carries extremely high morbidity and mortality, and endovascular stent grafting is the currently favored approach for the acute treatment of malperfusion.50,51
The clinical success of endovascular stent placement for aortic dissection ranges from 76% to 100% with a reported 30-day mortality rate of up to 25%.51–56 Data on the long-term follow-up of these patients are scarce.
About 13% of patients with aortic dissections receive stent-graft treatment, and this proportion is steadily increasing. With more data available and more advancement in operator expertise, stent graft placement may, in the future, become the standard treatment for most cases of distal aortic dissection, because waiting for the complications to occur may not be prudent given that the operative mortality rate in these situations approaches 70%.54
Treatment of Aortic Intramural Hematoma and Atherosclerotic Aortic Ulcer
The treatment of patients with both aortic intramural hematomas and atherosclerotic aortic ulcers is similar to that for patients with classic aortic dissections and depends on the aortic site involved. Both aortic intramural hematoma and atherosclerotic aortic ulcer are more common in the descending aorta and therefore are treated with aggressive medical therapy. Medical therapy should consist of the optimal control of blood pressure (BP), a decrease in aortic pulse dP/dt, as well as close long-term follow-up. Surgery is preferred for the treatment of patients with intramural hematomas and atherosclerotic aortic ulcers in the ascending aorta and aortic arch, and for patients with progressive dilatation and aneurysm formation of the aorta, irrespective of the site of involvement.57–61 In a meta-analysis of 143 patients with aortic intramural hematomas, of whom 30 patients (21%) died, 20 deaths (67%) were due to aortic dissection or rupture.62
Interest has also grown in the use of endovascular treatment of type A aortic dissection with short segment stent grafting in high-risk patients.63 Use of short segment grafts to cover an ascending tear is possible if the dissection is limited to the aorta just above the sinotubular junction and there is no associated valvular compromise. Placement may be performed retrograde or antegrade through the ventricle.
Outcomes and Prognosis
The rationale for surgical treatment of acute type A TAD is universally recognized. Patients with acute ascending aortic dissection treated medically fare far worse than those with dissection involving the descending aorta. Little controversy exists over the treatment of choice for the acute type B variety. For most patients, unless life- or limb-threatening vascular compromise is present, medical therapy is considered superior to surgical treatment.64,65 If a complication such as rupture necessitating emergency surgery arises, however, the mortality rate is very high. Fortunately, this occurs infrequently. If operation is required for type B TAD within the first 2 weeks, mortality remains high.66 Analysis of all early postoperative complications shows a much lower stroke rate for descending aorta repairs compared to repair of ascending dissections, although the incidence of pulmonary complications and spinal cord injury is higher.
References
1. Hagan, P, Nienaber, C, Isselbacher, E, et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA. 2000; 283:897–903.
2. Laennec, RTH. De l’ascultations médiate, ou traité du diagnostic des maladies des poumons et du coeur, fondé principalemente sur ce nouveau moyen d’exploration. Paris: JA Brosson & JD Chaudé; 1819.
3. Nicholls, F. Observations concerning the body of His Late Majesty. Philos Trans London. 1762; 52:265.
4. Peacock, TB. Report of cases of dissecting aneurysm. Trans London Pathol Soc. 1863; 14:87.
5. Marfan, A. Un cas de deformation congenitale des quatre membres, plus prononcée aux extremités, caracterisée par l’allongement des os avec un certain degré d’amincissement. Soc Hop Paris Bull Mem. 1896; 13:220–226.
6. DeBakey, ME, Cooley, DA, Creech, O, Jr. Surgical considerations of dissecting aneurysm of the aorta. Ann Surg. 1955; 142:586.
7. Olsson, C, Eriksson, N, Ståhle, E, Thelin, S. Surgical and long-term mortality in 2634 consecutive patients operated on the proximal thoracic aorta. Eur J Cardiothorac Surg. 2007; 31:963–969.
8. LeMaire, SA, Russell, L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011; 8:103–113.
9. Grubb, KJ, Kron, IL. Sex and gender in thoracic aortic aneurysms and dissection. Semin Thorac Cardiovasc Surg. 2011; 23:124–125.
10. Meszaros, I, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000; 117:1271–1278.
11. Nienaber, CA, Fattori, R, Mehta, RH, et al. Gender-related differences in acute aortic dissection. Circulation. 2004; 109:3014–3021.
12. Januzzi, JL, Isselbacher, EM, Fattori, R, et al. Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004; 43:665–669.
13. Oskoui, R, Lindsay, JJr. Aortic dissection in women < 40 years of age and the unimportance of pregnancy. Am J Cardiol. 1994; 73:821–823.
14. Januzzi, JL, Sabatine, MS, Eagle, KA, et al. Iatrogenic aortic dissection. Am J Cardiol. 2002; 89:623–626.
15. Bernard, Y, Zimmermann, H, Chocron, S, Litzler, JF, et al. False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol. 2001; 87:1378–1382.
16. Upadhye, S, Schiff, K. Acute aortic dissection in the emergency department: Diagnostic challenges and evidence-based management. Emerg Med Clin North Am. 2012; 30:307–327.
17. Bushnell, J, Brown, J. Clinical assessment for acute thoracic aortic dissection. Ann Emerg Med. 2005; 46:90–92.
18. Iliceto, S, Nanda, NC, Rizzon, P, et al. Color Doppler evaluation of aortic dissection. Circulation. 1987; 75:748.
19. Adachi, H, Omoto, R, Kyo, S, et al. Emergency surgical intervention of acute aortic dissection with the rapid diagnosis by transesophageal echocardiography. Circulation. 1991; 84:III–114.
20. Pearson, AC, Castello, R, Labovitz, AJ, et al. Safety and utility of transesophageal echocardiography in the critically ill patient. Am Heart J. 1990; 119:1083.
21. Blanchard, DG, Kimura, BJ, Dittrich, HC, DeMaria, AN. Transesophageal echocardiography of the aorta. JAMA. 1994; 272:546.
22. Nienaber, CA, Von Kodolitsch, Y, Volkmar, N, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993; 328:1–9.
23. Cigarroa, JE, Isselbacher, EM, DeSanctis, RW, Eagle, KA. Diagnostic imaging in the evaluation of suspected aortic dissection: Old standards and new directions. N Engl J Med. 1993; 328:35.
24. Shuford, WH, Sybers, RG, Weens, HS. Problems in the aortographic diagnosis of dissecting aneurysm of the aorta. N Engl J Med. 1969; 280:225–231.
25. Prendergast, BD, Boon, NA, Buckenham, T. Aortic dissection: Advances in imaging and endoluminal repair. Cardiovasc Intervent Radiol. 2002; 25:95–97.
26. Hartnell, GG, Gates, J. Aortic fenestration: A why, when, how-to guide. Radiographics. 2005; 25:175–189.
27. Nienaber, CA, Spielmann, RP, Kodolitsch, Y, et al. Diagnosis of thoracic aortic dissection. Circulation. 1992; 85:434.
28. Kapustin, AJ, Litt, HI. Diagnostic imaging for aortic dissection. Semin Thorac Cardiovasc Surg. 2005; 17:214–223.
29. Hiratzka, LF, Bakris, GL, Beckman, JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010; 121:e266–e369.
30. Moon, MR. Approach to the treatment of aortic dissection. Surg Clin North Am. 2009; 89:869–893.
31. Fattori, R, Mineo, G, Di Eusanio, M. Acute type B aortic dissection: Current management strategies. Curr Opin Cardiol. 2011; 26:488–493.
32. Elefteriades, JA. Thoracic aortic aneurysm: Reading the enemy’s playbook. Curr Probl Cardiol. 2008; 33:203–277.
33. Feldman, M, Shah, M, Elefteriades, JA. Medical management of acute type A aortic dissection. Ann Thorac Cardiovasc Surg. 2009; 15:286–293.
34. Mészáros, I, Mórocz, J, Szlávi, J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000; 117:1271–1278.
35. Wittels, K. Aortic emergencies. Emerg Med Clin North Am. 2011; 29:789–800.
36. Elefteriades, JA. Does medical therapy for thoracic aortic aneurysms really work? Are beta-blockers truly indicated? PRO. Cardiol Clin. 2010; 28:255–260.
37. Borst, HG, Laas, J. Surgical treatment of thoracic aortic aneurysms. Adv Card Surg. 1993; 4:47–87.
38. Jamieson, WR, Munro, AI, Miyagishima, RT, et al. Aortic dissection: early diagnosis and surgical management are the keys to survival. Can J Surg. 1982; 25:145–149.
39. Miller, DC, Mitchell, RS, Oyer, PE, et al. Independent determinants of operative mortality for patients with aortic dissections. Circulation. 1984; 70:I153–I164.
40. Guilmet, D, Bachet, J, Goudot, B, et al. Aortic dissection: Anatomic types and surgical approaches. J Cardiovasc Surg (Torino). 1993; 34:23–32.
41. Guilmet, D, Bachet, J, Goudot, B, et al. Use of biological glue in acute aortic dissection: Preliminary clinical results with a new surgical technique. Thorac Cardiovasc Surg. 1979; 77:516–521.
42. Carpentier, A. Glue aortoplasty as an alternative to resection and grafting for the treatment of aortic dissection. Semin Thorac Cardiovasc Surg. 1991; 3:213–214.
43. Borst, HG, Haverich, A, Walterbusch, G, et al. Fibrin adhesive: An important hemostatic adjunct in cardiovascular operations. Thorac Cardiovasc Surg. 1982; 84:548–553.
44. Hata, M, Shiono, M, Orime, Y, et al. The efficacy and mid-term results with use of gelatin resorcin formalin (GRF) glue for aortic surgery. Ann Thorac Cardiovasc Surg. 1999; 5:321–325.
45. Kallenbach, K, Oelze, T, Salcher, R, et al. Evolving strategies for treatment of acute aortic dissection type A. Circulation. 2004; 110:II243–II249.
46. Uchida, N, Katayama, A, Tamura, K, et al. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothoracic Surg. 2011; 40:1066–1071.
47. Carrel, T, Pasic, M, Vogt, P. Retrograde ascending aortic dissection: A diagnostic and therapeutic challenge. Eur J Cardiothorac Surg. 1992; 7:146–152.
48. Elefteriades, JA, Hartleroad, J, Gusberg, RJ, et al. Long term experience with descending aortic dissection: The complication-specific approach. Ann Thorac Surg. 1992; 53:11–21.
49. Sasaki, S, Yasuda, K, Kunihara, T, et al. Surgical results of Stanford type B aortic dissection: Comparisons between partial and subtotal replacement of the dissected aorta. J Cardiovasc Surg (Torino). 2000; 41:227–232.
50. Duebener, LF, Lorenzen, P, Richardt, G, et al. Emergency endovascular stent-grafting for life-threatening acute type B aortic dissections (Commentary). Ann Thorac Surg. 2004; 78:1261–1266.
51. Slonim, SM, Miller, DC, Mitchell, RS, et al. Percutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissection. Thorac Cardiovasc Surg. 1999; 117:1118–1126.
52. Slonim, SM, Nyman, U, Semba, CP, et al. Aortic dissection: Percutaneous management of ischemic complications with endovascular stents and balloon fenestration. J Vasc Surg. 1996; 23:241–251.
53. Dake, MD, Kato, N, Mitchell, RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999; 340:1546–1552.
54. Nienaber, CA, Fattori, R, Lund, G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999; 340:1539–1545.
55. Stolf, NA, Pego Fernandes, PM, Souza, LR, et al. Self-expanding endovascular stent-graft implant for treatment of descending aortic diseases. J Card Surg. 1999; 14:9–15.
56. Williams, DM, Lee, DY, Hamilton, BH, et al. The dissected aorta: Percutaneous treatment of ischemic complications: Principles and results. J Vasc Interv Radiol. 1997; 8:605–625.
57. Robbins, RC, McManus, RP, Mitchell, RS, et al. Management of patients with intramural hematoma of the thoracic aorta. Circulation. 1993; 88:II1–II10.
58. Nienaber, CA, von Kodolitsch, Y, Petersen, B, et al. Intramural hemorrhage of the thoracic aorta: Diagnostic and therapeutic implications. Circulation. 1995; 92:1465–1472.
59. Muluk, SC, Kaufman, JA, Torchiana, DF, et al. Diagnosis and treatment of thoracic aortic intramural hematoma. J Vasc Surg. 1996; 24:1022–1029.
60. Murray, JG, Manisali, M, Flamm, SD, et al. Intramural hematoma of the thoracic aorta: MR image findings and their prognostic implications. Radiology. 1997; 204:349–355.
61. Harris, KM, Braverman, AC, Gutierrez, FR, et al. Trans-esophageal echocardiographic and clinical features of aortic intramural hematoma. Thorac Cardiovasc Surg. 1997; 114:619–626.
62. Fann, JI, Miller, DC. Endovascular treatment of descending thoracic aortic aneurysms and dissections. Surg Clin North Am. 1999; 79:551–574.
63. Dake, M, Kata, N, Mitchell, S, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999; 340:1546–1552.
64. Umana, JP, Lai, DT, Mitchell, RS, et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg. 2002; 194:896–910.
65. Roseborough, G, Burke, J, Sperry, J, et al. Twenty year experience with acute distal thoracic aortic dissections. J Vasc Surg. 2004; 40:235–246.
66. Gallo, A, Davies, RR, Coe, MP, et al. Indications, timing, and prognosis of operative repair of aortic dissections. Semin Thorac Cardivasc Surg. 2005; 17:224–235.