Chapter 182 Actinomyces
Clinical Manifestations
The 3 major forms of actinomycosis—cervicofacial, abdominal and pelvic, and pulmonary—arise by different routes but may progress to other forms of the disease. Actinomycosis in children suggests an underlying immunodeficiency, especially chronic granulomatous disease (Chapter 124).
Cervicofacial Actinomycosis
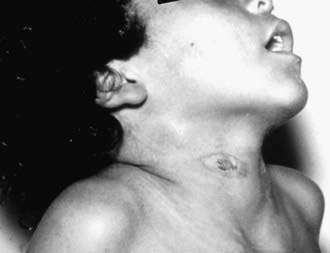
In the patient with cervicofacial actinomycosis, there is often a history of oral trauma, oral surgery, dental procedures, or caries facilitates entry of organisms into cervicofacial tissues. Cervicofacial actinomycosis usually manifests as a painless, slow-growing, hard mass and can produce cutaneous fistulas, a condition commonly known as lumpy jaw (Fig. 182-1). Less frequently, cervicofacial actinomycosis manifests clinically as an acute pyogenic infection with a tender, fluctuant mass with trismus, firm swelling, and fistulas with drainage containing the characteristic sulfur granules. Bone is not involved early in the disease, but periostitis, mandibular osteomyelitis, or perimandibular abscess may develop. Infection may spread by way of sinus tracts to the cranial bones, possibly giving rise to meningitis. The ability of Actinomyces to burrow through tissue planes and even bone is a key difference between actinomycosis and nocardiosis. The cervicofacial form of actinomycosis has the best prognosis and is usually cured with surgical debridement and excision combined with antibiotic therapy.
Bartlett AH, Rivera AL, Krishnamurthy R, et al. Thoracic actinomycosis in children: case report and review of the literature. Pediatr Infect Dis J. 2008;27:165-169.
Clarridge JEIII, Zhang Q. Genotypic diversity of clinical Actinomyces species: phenotype, source, and disease correlation among genospecies. J Clin Microbiol. 2002;40:3442-3448.
Feder HMJr. Actinomycosis manifesting as an acute painless lump of the jaw. Pediatrics. 1990;85:858-864.
Olah E, Berger C, Boltshauser E, et al. Cerebral actinomycosis before adolescence. Neuropediatrics. 2004;35:239-241.
Park JK, Lee HK, Ha HK, et al. Cervicofacial actinomycosis: CT and MR imaging findings in seven patients. Am J Neuroradiol. 2003;24:331-335.
Reddy I, Ferguson DAJr, Sarubbi FA. Endocarditis due to Actinomyces pyogenes. Clin Infect Dis. 1997;25:1476-1477.
Robison JL, Vaudry WL, Dobrovolsky W. Actinomycosis presenting as osteomyelitis in the pediatric population. Pediatr Infect Dis J. 2005;24:365-369.
Sabbe LJM, Van De Merwe D, Schouls L, et al. Clinical spectrum of infections due to the newly described Actinomyces species A. turicensis, A. radingae, and A. europaeus. J Clin Microbiol. 1999;37:8-13.
Sharma M, Briski LE, Khatib R. Hepatic actinomycosis: an overview of salient features and outcome of therapy. Scand J Infect Dis. 2002;34:386-391.
Smego RAJr, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255-1261.
Tyrrell J, Noone P, Prichard JS. Thoracic actinomycosis complicated by Actinobacillus actinomycetemcomitans: case report and review of literature. Respir Med. 1992;86:341-343.