Actinobacillus, Aggregatibacter, Kingella, Cardiobacterium, Capnocytophaga, and Similar Organisms
1. Describe the general characteristics of the bacteria included in this chapter.
2. Describe the normal habitat and the routes of transmission for the organisms included in this chapter.
3. Identify the major clinical diseases associated with Actinobacillus, Aggregatibacter, Kingella, Cardiobacterium, and Capnocytophaga spp.
4. Explain the incubation conditions for the bacteria discussed in this chapter including oxygenation, time, and temperature.
6. List the media used to cultivate the organisms discussed in this chapter.
7. Discuss the unique colonial presentation of the various genera of the clinically significant species.
Epidemiology, Pathogenesis, and Spectrum of Disease, and Antimicrobial Therapy
The organisms listed in Table 31-1 are part of the normal flora of the nasopharynx or oral cavity of humans and other animals and are parasitic. Species associated with animals are specifically indicated in the table at the beginning of the chapter. As such, they generally are of low virulence and, except for those species associated with periodontal infections, usually only cause infections in humans after introduction into sterile sites following trauma such as bites, droplet transmission from human to human, sharing paraphernalia, or manipulations in the oral cavity. Cardiobacterium spp. are not only associated with the human oropharynx and oral cavity, but they may also be identified in the gastrointestinal and urogenital tract. The natural habitat for Dysgonomonas is unknown. Rare isolates have been identified in the feces of immunocompromised patients.
TABLE 31-1
| Organism | Habitat (Reservoir) | Mode of Transmission |
| Aggregatibacter actinomycetemcomitans | Normal flora of human oral cavity | Endogenous; enters deeper tissues by minor trauma to mouth, such as during dental procedures |
| Actinobacillus spp. | Normal oral flora of animals such as cows, sheep, and pigs; not part of human flora | Rarely associated with human infection; transmitted by bite wounds or contamination of preexisting wounds during exposure to animals |
| Kingella spp. | Normal flora of human upper respiratory and genitourinary tracts | Infections probably caused by patient’s endogenous strains |
| Cardiobacterium hominis and Cardiobacterium valvarum | Normal flora of human upper respiratory tract | Infections probably caused by patient’s endogenous strains |
| Capnocytophaga gingivalis, Capnocytophaga ochracea, Capnocytophaga sputigena, and other species | Subgingival surfaces and other areas of human oral cavity | Infections probably caused by patient’s endogenous strains |
| Capnocytophaga canimorsus and Capnocytophaga cynodegmi | Oral flora of dogs | Dog bite or wound (scratch), long exposure to dogs Capnocytophaga cynodegmi |
| Dysgonomonas capnocytophagoides and other species | Uncertain; possibly part of human gastrointestinal flora | Uncertain; possibly endogenous |
The types of infections caused by these bacteria vary from periodontitis to endocarditis (Table 31-2). Actinobacillus spp. cause granulomatous disease in animals and have been associated with soft tissue infection in humans following animal bites. Additionally, A. equuli and A. suis have been isolated from the human respiratory tract. Additional species have been isolated from patients that have developed meningitis following trauma or surgery. Actinobacillus spp. may harbor a pore-forming protein toxin known as an RTX toxin that is cytotoxic and hemolytic. A. actinomycetemcomitans is often associated with periodontitis. Virulence factors include the RTX leukotoxin, cytotoxic distending toxin, and the EmaA adhesin. Three of these organisms, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, and Kingella spp., are the A, C, and K, respectively, of the HACEK group of organisms that cause slowly progressive (i.e., subacute) bacterial endocarditis, soft tissue infections, and other infections. Capnocytophaga are associated with septicemia and endogenous infections in immunocompromised patients. Infections with C. canimorsus and C. cynodegmi following a dog or cat bat can result in serious illness including disseminated intravascular coagulation, renal failure, shock, and hemolytic-uremic syndrome. Kingella spp. can also be involved in other serious infections involving children, especially osteoarthritic infections. The pathogenic mechanisms are unknown, and disease associated with Dysgonomonas spp. is quite variable and includes diarrhea, bacteremia, blood, and wound infections.
TABLE 31-2
Pathogenesis and Spectrum of Diseases
| Organism | Virulence Factors | Spectrum of Diseases and Infections |
| Aggregatibacter spp. | Unknown; probably of low virulence; an opportunistic pathogen | A. actinomycetemcomitans has been associated with destructive periodontitis that may cause bone loss or endocarditis; endocarditis, often following dental manipulations; soft tissue and human bite infections, often mixed with anaerobic bacteria and Actinomyces spp.; A. aphrophilus is an uncommon cause of endocarditis and is the H member of the HACEK group of bacteria associated with slowly progressive (subacute) bacterial endocarditis |
| Actinobacillus spp. | Unknown for human disease; probably of low virulence | Rarely cause infection in humans but may be found in animal bite wounds, such as meningitis or bacteremia; association with other infections, such as meningitis or bacteremia, is extremely rare and involves compromised patients |
| Kingella spp. | Unknown; probably of low virulence; opportunistic pathogens | Endocarditis and infections in various other sites, especially in immunocompromised patients; K. kingae associated with blood, bone, and joint infections of young children; periodontitis and wound infections |
| Cardiobacterium hominis | Unknown; probably of low virulence | Infections in humans are rare; most commonly associated with endocarditis, especially in persons with anatomic heart defects |
| Capnocytophaga gingivalis, Capnocytophaga ochracea, and Capnocytophaga sputigena | Unknown; produce wide variety of enzymes that may mediate tissue destruction | Most commonly associated with periodontitis and other types of periodontal disease; less commonly associated with bacteremia in immunocompromised patients |
| Capnocytophaga canimorsus and Capnocytophaga cynodegmi | Unknown | Range from mild, local infection at bite site to bacteremia culminating in shock and disseminated intravascular coagulation; most severe in splenectomized or otherwise debilitated (e.g., alcoholism) patients but can occur in healthy people; miscellaneous other infections such as pneumonia, endocarditis, and meningitis may also occur |
| Dysgonomonas capnocytophagoides and other species | Unknown; probably of low virulence | Role in disease is uncertain; may be associated with diarrheal disease in immunocompromised patients; rarely isolated from other clinical specimens, such as urine, blood, and wounds |
Infections are frequently treated using β-lactam antibiotics, occasionally in combination with an aminoglycoside (Table 31-3). β-lactamase production has been described in Kingella spp., but the impact of this resistance mechanism on the clinical efficacy of beta-lactams is uncertain. When in vitro susceptibility testing is required, Clinical and Laboratory Standards Institute (CLSI) document M45 does provide guidelines for testing A. actinomycetemcomitans, Cardiobacterium spp., and Kingella spp.
TABLE 31-3
Antimicrobial Therapy and Susceptibility Testing
| Organism | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods* |
| Aggregatibacter actinomycetemcomitans | No definitive guidelines; for periodontitis, debridement of affected area; potential agents include ceftriaxone, ampicillin, amoxicillin-clavulanic acid, fluoroquinolone, or trimethoprim-sulfamethoxazole; for endocarditis, penicillin, ampicillin, or a cephalosporin (perhaps with an aminoglycoside) may be used | Some strains appear resistant to penicillin and ampicillin, but clinical relevance of resistance is unclear | See CLSI document M45 |
| Actinobacillus spp. | No guidelines (susceptible to extended-spectrum cephalosporins and fluoroquinolones) | Unknown (same as Aggregatibacter) | Not available |
| Kingella denitrificans, Kingella kingae | A beta-lactam with or without an aminoglycoside; other active agents include erythromycin, trimethoprim/ sulfamethoxazole, and ciprofloxacin | Some strains produce beta-lactamase that mediates resistance to penicillin, ampicillin, ticarcillin, and cefazolin | See CLSI document M45 |
| Cardiobacterium hominis | For endocarditis, penicillin with or without an aminoglycoside; usually susceptible to other β-lactams, chloramphenicol, and tetracycline | Unknown (same as Aggregatibacter) | See CLSI document M45 |
| Capnocytophaga gingivalis, Capnocytophaga ochracea, Capnocytophaga sputigena | No definitive guidelines; generally susceptible to clindamycin, erythromycin, tetracyclines, chloramphenicol, imipenem, and other beta-lactams | β-lactamase–mediated resistance to penicillin | Not available |
| Capnocytophaga canimorsus, Capnocytophaga cynodegmi | Penicillin is drug of choice; also susceptible to penicillin derivatives, imipenem, and third-generation cephalosporins | Unknown | Not available |
| Dysgonomonas capnocytophagoides | No guidelines; potentially effective agents include chloramphenicol, trimethoprim/sulfamethoxazole, tetracycline, and clindamycin | Often resistance to β-lactams and ciprofloxacin | Not available |
*Validated testing methods include those standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and those commercial methods approved by the Food and Drug Administration (FDA).
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Cultivation
Media of Choice
Colonial Appearance
Table 31-4 describes the colonial appearance and other distinguishing characteristics (e.g., hemolysis and pigment) of each genus on 5% sheep blood agar. Most species will not grow on MacConkey agar; exceptions are noted in Table 31-4.
TABLE 31-4
Colonial Appearance and Characteristics on 5% Sheep Blood Agar
| Organism | Appearance |
| Aggregatibacter actinomycetemcomitans | Pinpoint colonies after 24 hours; rough, sticky, adherent colonies surrounded by a slight greenish tinge after 48 hours; characteristic finding is presence of a four- to six-pointed star-like configuration in the center of a mature colony growing on a clear medium (e.g., brain-heart infusion agar) resembling crossed cigars, which can be visualized by examining the colony under low power (100×) of a standard light microscope |
| Aggregatibacter aphrophilus | Round; convex with opaque zone near center on chocolate agar |
| Aggregatibacter segnis | Convex, grayish white, smooth or granular at 48 hours on chocolate agar |
| Actinobacillus equuli* | Small colonies at 24 hours that are sticky, adherent, smooth or rough, and nonhemolytic |
| A. lignieresii* | Resembles A. equuli |
| A. suis* | Beta-hemolytic but otherwise resembles A. equuli and A. lignieresii |
| A. ureae | Resembles the pasteurellae (see Chapter 32) |
| Cardiobacterium hominis | After 48 hours, colonies are small, slightly alpha-hemolytic, smooth, round, glistening and opaque; pitting may be produced |
| Capnocytophaga spp. | After 48 to 74 hours, colonies are small- to medium-size, opaque, shiny; nonhemolytic; pale beige or yellowish color may not be apparent unless growth is scraped from the surface with a cotton swab; gliding motility may be observed as outgrowths from the colonies or as a haze on the surface of the agar, similar to swarming of Proteus |
| Dysgonomonas capnocytophagoides | Pinpoint colonies after 24 hours; small, wet, gray-white colonies at 48 to 72 hours; usually nonhemolytic, although some strains may produce a small zone of beta-hemolysis; characteristic odor alternately described as fruity strawberry-like odor or bitter |
| Kingella denitrificans | Small, nonhemolytic; frequently pits agar; can grow on Neisseria gonorrhoeae selective agar (e.g., Thayer-Martin agar) |
| K. kingae | Small, with a small zone of beta-hemolysis; may pit agar |
Approach to Identification
Table 31-5 outlines some conventional biochemical tests that are useful for differentiating among Actinobacillus, Aggregatibacter, Cardiobacterium, and Kingella; these are four of the five HACEK bacteria that cause subacute bacterial endocarditis. A. aphrophilus does not require either X or V factors for growth. However, it is catalase negative and ferments lactose or sucrose. A. actinomycetemcomitans yields the opposite reactions in these tests.
TABLE 31-5
Biochemical and Physiologic Characteristics of Actinobacillus spp. and Related Organisms
| Organism | Oxidase | Fermentation of:† | |||||||
| Catalase | Nitrate Reduction | Indole | Urea | Esculin Hydrolysis | Xylose | Lactose | Trehalose | ||
| Aggregatibacter actinomycetemcomitans | 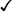 |
+ | + | − | − | − | v | − | − |
| A. equuli | 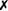 |
v | + | − | (+)* | − | + | + | (+) |
| A. lignieresii | 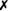 |
v | + | − | (+)* | − | + or (+) | v | − |
| A. suis | 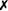 |
v | + | − | (+)* | + | + | + or (+) | + |
| A. ureae | 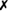 |
v | + | − | (+)* | − | − | − | − |
| Cardiobacterium hominis | 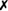 |
− | − | + | − | − | − | − | ND |
| Aggregatibacter aphrophilus | 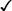 |
− | + | − | − | − | − | (+) | (+) |
| Kingella denitrificans | 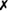 |
− | (+)‡ | − | − | − | − | − | ND |
| K. kingae | 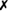 |
− | − | − | − | − | − | − | ND |
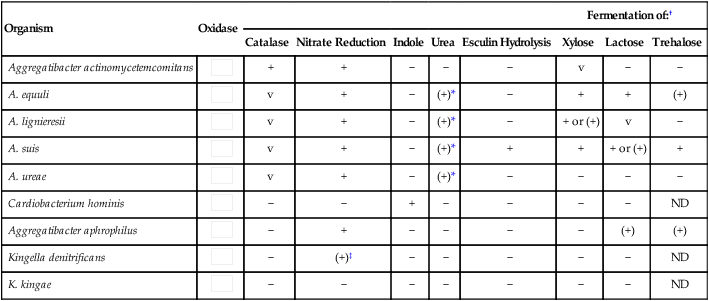
ND, No data; v, variable; +, >90% of strains positive; (+), >90% of strains positive but reaction may be delayed (i.e., 2 to 7 days); −, >90% of strains negative.
*May require a drop of rabbit serum on the slant or a heavy inoculum.
†May require the addition of 1 to 2 drops rabbit serum per 3 mL of fermentation broth to stimulate growth.
‡Nitrate is usually reduced to gas.
Data compiled from Weyant RS, Moss CW, Weaver RE, et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1996, Williams & Wilkins.
Table 31-6 shows key conventional biochemicals that can be used to differentiate Capnocytophaga spp., Dysgonomonas capnocytophagoides, and aerotolerant Leptotrichia buccalis.
TABLE 31-6
| Organism | Oxidase | Catalase | Esculin Hydrolysis | Indole | Nitrate Reduction | Xylose Fermentation |
| Capnocytophaga spp. (CDC group DF-1)* | − | − | (v) | − | v | − |
| C. canimorsus (CDC group DF-2)† | (+) | (+) | v | − | − | −‡ |
| C. cynodegmi (CDC group DF-2-like)† | (+) | (+) | + or (+) | − | − | − |
| Leptotrichia buccalis* | − | − | v | − | − | −‡ |
| Dysgonomonas capnocytophagoides* | − | − | (+) | (v) | − | + or (+)‡ |
| CDC group DF-3-like | − | v | v | (+) | − | −‡ |
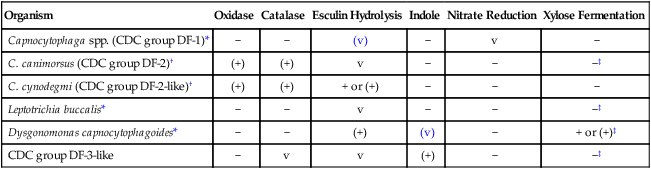
+, >90% of strains positive; (+), >90% of strains positive, but reaction may be delayed (i.e., 2 to 7 days); −, >90% of strains negative; v, variable; (v), positive reactions may be delayed.
*Lactic acid is the major fermentation end product of glucose fermentation for Leptotrichia buccalis, and succinic acid and propionic is the major fermentation end product of glucose fermentation for Capnocytophaga spp. (CDC group DF-1) and Dysgonomonas capnocytophagoides.
†C. canimorsus does not ferment the sugars inulin, sucrose, or raffinose; C. cynodegmi will usually ferment one or all of these sugars.
‡May require the addition of 1 to 2 drops of rabbit serum per 3 mL of fermentation broth to stimulate growth.
Data compiled from Jensen KT, Schonheyder H, Thomsen VF: In-vitro activity of β-lactam and other antimicrobial agents against Kingella kingae, J Antimicrob Chemother 33:635, 1994; and Weyant RS, Moss CW, Weaver RE, et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1996, Williams & Wilkins.
Comments Regarding Specific Organisms
Actinobacillus spp. are facultative anaerobic, nonmotile, gram-negative rods. The genus Actinobacillus is similar to Aggregatibacter and Pasteurella (see Chapter 30), which must also be considered when a fastidious gram-negative rod requiring rabbit serum is isolated. A. actinomycetemcomitans, the most frequently isolated of the aggregatibacters, can be distinguished from A. aphrophilus by its positive test for catalase and negative test for lactose fermentation.
Kingella spp. are catalase negative, which helps to separate them from Neisseria spp. (see Chapter 40), with which they are sometimes confused. K. denitrificans may be mistaken for Neisseria gonorrhoeae when isolated from modified Thayer-Martin agar. Nitrate reduction is a key test in differentiating K. denitrificans from N. gonorrhoeae, which is nitrate negative.
The species in the former CDC group DF-1—that is, C. ochracea, C. sputigena, and C. gingivalis—are catalase and oxidase negative; however, members of CDC group DF-1 cannot be separated by conventional biochemical tests. C. canimorsus and C. cynodegmi are catalase and oxidase positive; these species are also difficult to differentiate from each other. However, for most clinical purposes, a presumptive identification to genus—that is, Capnocytophaga—is sufficiently informative and precludes the need to identify an isolate to the species level. Presumptive identification of an organism as Capnocytophaga spp. can be made when a yellow-pigmented, thin, gram-negative rod with tapered ends that exhibits gliding motility (see Table 31-4) and does not grow in ambient air is isolated.







