Chapter 19 Actigraph Assessment of Periodic Leg Movements and Restless Legs Syndrome
As noted in Chapter 17 on periodic leg movements in sleep (PLMS) and waking (PLMW) the interpretation of the significance of these movements has changed since they were first reported by Symonds.1 An examination of the electromyelograph (EMG) activation (lasting 0.5 to 10 seconds) seen with these movements has dispelled the initial concept that they were a form of myoclonus, which has a much briefer muscle activation. However, the supposed myoclonic nature of these movements led to the use of clonazepam as a treatment.2 The second clinical interpretation of PLMS focused on the conspicuous arousals seen with these movements; it was postulated that they caused significant sleep disruption with consequent daytime sleepiness.3 Evaluations of those patients with PLMS who are without any other significant sleep or neurologic disorder, however, have failed to find any relation between periodic limb movements (PLM) and either insomnia or daytime sleepiness.4–8 One of the early studies even showed an unexpected relation between increased PLMS and decreased inadvertent napping in the daytime.9 This dilemma of no apparent relation between these events conspicuously disturbing sleep and sleep-related complaints, however, appears to reflect the multiple causes of PLMS that were not well evaluated in prior studies. An examination of the multiple conditions associated with PLMS showed that many of these involve dopaminergic abnormalities, such as rapid eye movement (REM) behavior disorder,10 narcolepsy,11 Parkinson’s disease,12 and restless legs syndrome (RLS).13 Thus, PLMS can be considered a motor expression of the dopaminergic abnormalities associated with these disorders.4 In this view, the frequency and perhaps intensity of the PLMS would reflect the severity of this aspect of the underlying disease process. The effects of the severity of the underlying disease may produce significant sleep disruption for multiple reasons with PLMS only a minor component of this process. Thus, in most of these diseases, there is no established relation between subjective report of poor sleep and the frequency of PLMS. The one exception is RLS where at least on the first night of recording one study reported a significant correlation between subjective report of PLMS/hr and sleep complaint6 and two independent studies have reported a correlation between subjective report of disease severity and the rate of PLMS.14,15 Because there has been no reported “first-night” effect with increased rate of PLMS.11 this relation with RLS would be expected to continue for all nights recorded, but the extreme variability of PLMS per hour in RLS patients makes it likely that some single nights might not show this relationship (e.g., no relation to sleep compliant was found on the second PSG night in one study6). This emphasizes the importance of having multiple nights of recording for RLS patients. Overall, there appears to be a relation between disease severity and PLMS for RLS not reported in the other disorders with increased rates of PLMS.
PLM can, therefore, provide an objective measure of disease severity that is particularly important for RLS. Because PLM usually involve an actual movement of the leg, it becomes possible to use piezoelectric accelerometers in battery-operated ambulatory monitors to record these movements over several nights. The technology to do this has been developed and is presented here in the full context of PLM measurement, particularly for RLS. In addition, the leg activity meters provide a gross assessment of day and night activity, and the relative ratio of activity in these different time periods may also prove useful for RLS evaluation. First, we consider the PLM measurement issues. These determine to a large extent the requirements for leg activity monitoring. Then, we consider the application of these monitors for evaluating RLS and the potential for future developments using this technology.
Problems With Periodic Leg Movement Measurement on a Polysomnogram
The series of leg movements making up a PLM are usually measured by the electrical activity of the anterior tibialis EMG. Electrodes placed on the skin at the midline of this muscle provide the basic measurement of this physiologic event. Each leg is recorded separately. The new World Association of Sleep Medicine (WASM) standards for measuring and scoring these movements16 provide the basic standardization for the physiologic recordings and scoring and officially recognize leg activity monitoring as an alternative method for measuring leg movements in sleep. The motivation for even considering activity monitoring to evaluate PLM stems largely from the following five significant problems that occur when these movements are limited to the EMG measurements in a sleep laboratory polysomnogram (PSG).
Second, the night-to-night variability is very large in many cases; in particular, this is true of PLM associated with RLS, a disease process that itself appears to have marked daily variation in severity.11,17 This variability increases sample sizes required for studies involving only 1 or 2 nights of PSG measurement across many subjects, but it becomes particularly significant when addressing the disease state of a given individual. Multiple nights, probably 3 to 5, are need to find an estimate of the range and variation in PLM for a given individual.18
Third, the EMG usually involves only the anterior tibialis leg muscle. As noted in the chapter on periodic limb movements (Chapter 17), PLM events involve activation of several other muscles producing somewhat mixed and complicated leg movements (e.g., as reported by Provini and colleagues19). EMG of only one muscle, albeit probably the most significant one, fails to capture the full duration and the magnitude of the actual movement event. Thus, the standard PSG procedure probably somewhat underreports the actual movement event.
Leg Activity Monitoring
Advantages and Limitations
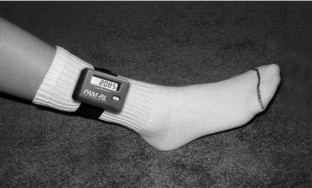
Remarkably, four of the five major PLM measurement problems for the PSG can be largely resolved by using ambulatory leg activity monitoring. A well-calibrated leg activity monitor provides quantitative assessment of the magnitude of the acceleration or the force in the leg movement, permits recording over 5 nights to assess nightly variations, and measures the full range of the motion produced by any complicated interaction of the muscle activation, not just that from activity of the anterior tibialis. It may, however, miss small movements that involve co-contraction of opposing muscles or movement of only the great toe. Finally, leg activity monitors are easy to use, the patients can put them on themselves and take them off, and they can be sent and returned in the mail. (See Fig. 19-1 for an example of one such leg monitor.) They are also relatively inexpensive.
The primary limitation of the leg activity monitoring is the failure to separate PLM by sleep-wake state. These monitors detect all PLM in all stages of sleep and waking; they are recording the sum of PLMW and PLMS. Because for most situations the PLMW are relatively small and the wake time during the night is also small, this presents only a minor error for estimates of the PLMS. Patients with disorders, however, may be awake or even up out of bed a lot during the night; this is particularly true for more severe RLS patients for whom sleep efficiency is often less than 50%.14 Fortunately, the PLMW are as important as the PLMS, at least for diagnosing RLS,20 so they can be combined into one measure. The only major issue is not counting time or movement events that occur when the patient is out of bed or sitting up on the bed. This can be easily done using a position monitor in the leg activity monitor.
Minimum Technical Requirements for Periodic Leg Movement
Although it may be possible to estimate PLM events from statistical analyses of general activity during sleep, such an effort fails to provide the basic measurement of leg movement events. At a minimum, the activity monitor needs to be designed to measure all the standard aspects of leg movement events used for the definition of PLM16 and those added measures of amplitude, durations, and period that are now features of more complete analyses of PLM. The parameters used also need to be flexible enough to measure young children as well as large adults. The following are the minimum parameters needed to meet these standards and handle the measurement problems noted above.
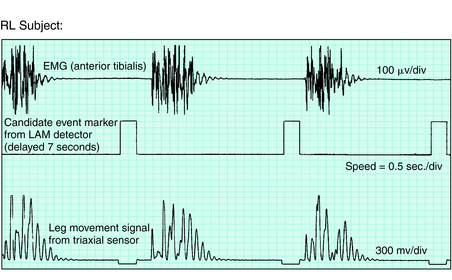
Figure 19-1 presents a photograph of one monitor (PAM-RL; IM Systems, Baltimore, MD) that meets these criteria. Figure 19-2 shows the remarkable parallel between the anterior tibialis EMG and the activity monitor signal from this meter during simultaneous recording from a PLM of an RLS patient. Note the close match between the EMG and activity data for both duration and morphology of the events. This particular meter is an example of one that provides position recording and adjustable threshold settings. Figure 19-3 shows the activity with change in leg position from lying down to sitting or standing. The data summary from this monitor is provided separately for each of these two body positions.
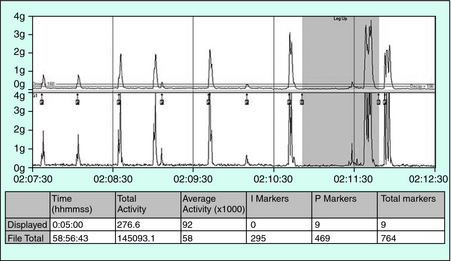
FIGURE 19-3. Leg activity during lying down and then sitting or standing as indicated by the darker backgrounds displayed.
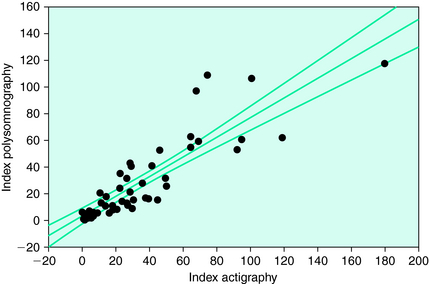
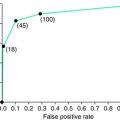
This particular monitor has been validated compared with PLM on a PSG for both RLS and insomnia patients by the meter manufacturer21 and for RLS, insomnia, and SDB patients by a respected research group with no association with the manufacturer of the meter.22 The first study found overall close agreement between the meter and the PSG data (r = 0.89 with no significant variation from the identity line). The latter study similarly shows an excellent correlation between the PAM-RL device and PSG (r = 0.87) but notes that the PAM-RL slightly overestimates the PLMS (Fig. 19-4). The researchers reported this was more of a problem for SDB and insomnia patients. This overestimation tendency of an activity meter is consistent with the fact that it counts both PLMW and PLMS and that it also evaluates movement magnitudes that are more general than might be observed on the EMG from only the anterior tibialis. The latter may particularly occur for SDB and restless insomnia producing atypical types of movement arousals.
Assessment for Restless Legs Syndrome
PLM are particularly important for RLS because they represent the motor sign of the disorder. Although this sign is somewhat nonspecific, it is fairly sensitive and therefore supports the diagnosis.23 Both the PLM during waking (PLMW) while lying in bed at night and the PLM during sleep (PLMS) support the RLS diagnosis. One study comparing RLS patients with control subjects reported diagnostic sensitivity and specificity for PLMS of 78% and 76% and for PLMW of 87% and 80%, respectively.20 The activity meter provides the sum of these two variables and presumably would have a diagnostic specificity and sensitivity that fall between these values. The genetic linkage found for the RLS phenotype on chromosome 12q24 in French Canadians is associated with a phenotype defined as a higher PLM rate. In an Icelandic population, the same association with the 12q locus has been found by the PAM-RL activity meter (David Rye, personal communication). Another locus within the BTBD9 gene (chromosome 6p), studied in Icelandic and American populations, is associated with increased PLM in subjects with RLS, again measured over multiple nights using the PAM-RL. This use of the activity meter for genetic studies to possibly better define the phenotype deserves more consideration. This may particularly be the case if, as has been proposed, the PLM provide a measure of an underlying dopaminergic abnormality.4
The PLMS correlate reasonably well (r = 0.45) with the subjective ratings of RLS severity using either the Johns Hopkins RLS severity scale25 or the International Restless Legs Syndrome Study Group’s severity scale (IRLS).15 This degree of correlation seems more than reasonable given the differences between objective and subjective measures and the fact that the subjective assessments are generally obtained several hours after the patient had the symptoms and during a time when the patient likely has few, if any, RLS symptoms. Thus, the PLM index provides a sensitive measure of RLS severity and has been found to be very sensitive to treatment effects with at most a minimal placebo effect in clinical trials.26 The activity measure of PLM should, therefore, provide a sensitive measure of RLS severity and an excellent method for a simple objective evaluation of treatment benefits. These have been used successfully to confirm with objective measures the subjective report of treatment benefit for RLS from intravenous iron27 and to confirm the subjective report of better treatment response for carbidopa/levodopa than for the opioid propoxyphene.28
Motor Activity at Night
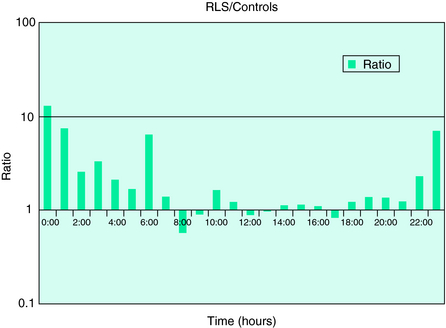
Because RLS patients experience both restlessness and PLM during the night, it is also possible to assess their sleep disruption by the degree of motor activity during the night. In most individuals, the hours in bed during the night are the most quiescent and the least movement can be recorded. In one study, motor activity at night—recorded either in absolute terms or as a percentage of mean daytime activity—was significantly higher in a set of treated RLS patients (with some residual symptoms) than in normal control subjects (Fig. 19-5). Future studies will be needed to determine sensitivity and specificity of this measure and the degree to which it is independent of the PLMS rate or intensity. Nocturnal motor activity is likely to be elevated in a number of sleep disorders besides RLS. Therefore, increased activity at night may be a better indicator of the severity of RLS—or its response to treatment—than a good diagnostic measure.
Future Considerations for Activity Monitoring in Restless Legs Syndrome
1. Symonds CP. Nocturnal myoclonus. J Neurol Neurosurg Psychiatry. 1953:166-171.
2. Oshtory MA, Vijayan N. Clonazepam treatment of insomnia due to sleep myoclonus. Arch Neurol. 1980;37:119-120.
3. Dhanuka AK, Singh G. Periodic limb movement disorder: A clinical and polysomnographic study. Neurol India. 2001;49:366-370.
4. Montplaisir J, Michaud M, Denesle R, Gosselin A. Periodic leg movements are not more prevalent in insomnia or hypersomnia but are specifically associated with sleep disorders involving a dopaminergic impairment. Sleep Med. 2000;1:163-167.
5. Mendelson W. Periodic Leg Movements: Lack of Association With Subjective Sleep Quality. Washington, DC: APSS, 1996;214.
6. Hornyak M, Riemann D, Voderholzer U. Do periodic leg movements influence patients’ perception of sleep quality? Sleep Med. 2004;5:597-600.
7. Nicolas A, Lesperance P, Montplaisir J. Is excessive daytime sleepiness with periodic leg movements during sleep a specific diagnostic category? Eur Neurol. 1998;40:22-26.
8. Mosko SS, Dickel MJ, Paul T, et al. Sleep apnea and sleep-related periodic leg movements in community resident seniors. J Am Geriatr Soc. 1988;36:502-508.
9. Ancoli-Israel S, Kripke DF, Klauber MR, et al. Periodic limb movements in sleep in community dwelling elderly. Sleep. 1991;14:496-500.
10. Winkelman J. High rates of periodic leg movements of sleep in REM-sleep behavior disorder raise more questions about the relationship between sleep-related movement disorders. Sleep Med 2003;4:261-262.
11. Hornyak M, Kopasz M, Feige B, et al. Variability of periodic leg movements in various sleep disorders: Implications for clinical and pathophysiologic studies. Sleep. 2005;28:331-335.
12. Wetter TC, Collado-Seidel V, Pollmacher T, et al. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep. 2000;23:361-367.
13. Lugaresi E, Cirignotta F, Coccagna G, et al. Nocturnal myoclonus and restless legs syndrome. In: Fahn S, Marsden CD, Van Woert MH, editors. Myoclonus. New York: Raven Press; 1986:295-307.
14. Allen RP, Earley CJ. Validation of the Johns Hopkins Restless Legs Severity Scale. Sleep Med. 2001;2:239-242.
15. Garcia-Borreguero D, Larrosa O, de la Llave Y, et al. Correlation between rating scales and sleep laboratory measurements in restless legs syndrome. Sleep Med. 2004;5:561-565.
16. Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006;7:175-183.
17. Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6:259-267.
18. Allen RP. Is one night enough for measurement of periodic limb movements in sleep of restless legs patients? Sleep. 2005;28:331-335.
19. Provini F, Vetrugno R, Meletti S, et al. Motor pattern of periodic limb movements during sleep. Neurology. 2001;57:300-304.
20. Michaud M, Paquet J, Lavigne G, et al. Sleep laboratory diagnosis of restless legs syndrome. Eur Neurol. 2002;48:108-113.
21. Gorny S, Allen R, Krausman D, et al. Evaluation of the PAM-RL system for the detection of periodic leg movements during sleep in the lab and home environments. Sleep. 1998;21(suppl):183.
22. Sforza E, Johannes M, Claudio B. The PAM-RL ambulatory device for detection of periodic leg movements: A validation study. Sleep Med. 2005;6:407-413.
23. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the Restless Legs Syndrome Diagnosis and Epidemiology Workshop at the National Institutes of Health. Sleep Med. 2003;4:101-119.
24. Desautels A, Turecki G, Montplaisir J, et al. Identification of a major susceptibility locus for restless legs syndrome on chromosome 12q. Am J Hum Genet. 2001;69:1266-1270.
25. Allen R, Earley C. Validation of a diagnostic questionnaire for the restless legs syndrome (RLS). Neurology. 2001;58(8 suppl 3):A4.
26. Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907-914.
27. Earley CJ, Heckler D, Horská A, et al. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231-235.
28. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
29. Michaud M, Poirier G, Lavigne G, et al. Restless legs syndrome: Scoring criteria for leg movements recorded during the suggested immobilization test. Sleep Med. 2001;2:317-321.
30. Montplaisir J, Boucher S, Nicolas A, et al. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless legs syndrome. Mov Disord. 1998;13:324-329.