CHAPTER 45 Acquired Immune Deficiency Syndrome
Acquired immunodeficiency syndrome (AIDS) is caused by infection with human immunodeficiency virus (HIV) and is defined by the Centers for Disease Control and Prevention as occurring in any HIV-infected individual with a helper T-cell (CD4+) count less than 200/µL blood.1 Before adoption of this definition, HIV-positive individuals with either opportunistic infections or rare malignancies associated with immunocompromise were said to have developed an AIDS-defining illness. This terminology persists, and AIDS-defining illnesses are often found coincident with CD4+ T-cell counts that meet the criteria for the definition of AIDS.
HIV is a retrovirus that infects cells by docking and binding at two essential sites on a target cell: the CD4 receptor and one of two chemokine receptors (CCRs), CCR5 or CXCR4.2 Although the primary cell of focus in HIV infection is the T lymphocyte, it is important to know that other cell types, including resident cells of the brain, are also susceptible. Once an individual is infected, CD4+ T lymphocytes are gradually depleted until cell-mediated immunity finally deteriorates and AIDS develops. The latency between HIV infection and the development of AIDS may be many years. The symptoms and signs of HIV infection are protean and may go unrecognized as being related to HIV. This, along with the stigma associated with HIV infection, leads to frequent delays in diagnosis and treatment, thereby further spreading the virus and compromising the health of infected individuals.3
Effective HIV treatment, in the form of highly active antiretroviral therapy (HAART), now exists, and as a result in some communities AIDS has become a chronic illness.4,5 Accordingly, AIDS patients may require neurosurgical intervention for any number of conditions affecting the population at large. The risk of exposure to HIV by surgeons and allied health care workers during diagnostic procedures is real but small.6 In the early days of the AIDS epidemic, when the mechanisms of disease transmission were unknown, many justified reluctance to perform invasive procedures because of this risk. The poor prognosis associated with the condition was used to bolster this claim. Today, decisions regarding the need for and timing of neurosurgical procedures are dictated by an understanding of AIDS itself. Biopsy or resection of intracranial lesions that might be performed in otherwise healthy individuals is often delayed in an AIDS patient pending response to trials of medical therapy for conditions commonly encountered. However, cases must be approached individually and be based on an understanding of risk-benefit ratios in this population.
Human Immunodeficiency Virus Infection of the Nervous System
Both the central nervous system (CNS) and the peripheral nervous system can be directly affected by HIV. Although controversy still exists regarding the extent to which CNS cells (excluding microglia) can harbor HIV, immunologic responses to HIV-infected cells in the CNS probably account for many of the neurological complications associated with HIV/AIDS. These complications include many of the more elusive disorders causing difficulties in diagnosis and treatment, as outlined in Table 45-1.
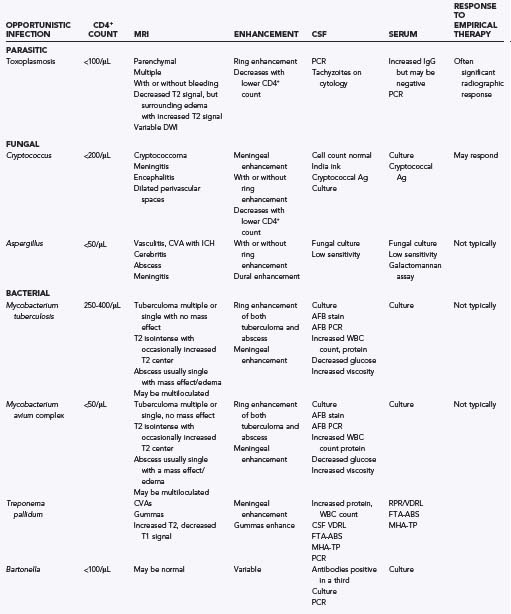
TABLE 45-1 Common Neurological Conditions Associated with HIV Infection (Independent of Subsequent Opportunistic Infection, AIDS-Associated Malignancies, and Treatment-Related Toxicities)*
Acute Retroviral Syndrome
Acute HIV infection results in an acute retroviral syndrome in the majority of those exposed.7 Although these symptoms are often systemic in nature, aseptic meningitis occurs in almost 25% of infected individuals.8 Other neurological manifestations of acute HIV infection include facial nerve palsies, radiculopathy, and acute demyelinating polyneuropathy.9 These symptoms are usually manifested within 2 to 6 weeks of infection and may be the primary reason that an infected individual seeks medical attention after HIV infection.
The neurological signs and symptoms of acute retroviral syndrome are probably distinct from the infection of CNS tissue itself. Nevertheless, HIV infection of the CNS is an early event that is thought to occur through trafficking of infected monocytes/macrophages and CD4+ T lymphocytes into the brain. Microglia, or resident CNS macrophages, are early reservoirs of HIV infection. Furthermore, astrocytes can also be infected by HIV.10 Astrocyte infection is CD4+ lymphocyte independent and results in nonproductive virus. However, the presence of HIV within them may account for many of the chronic effects of CNS infection by HIV, especially in the pediatric population.11
Human Immunodeficiency Virus–Associated Encephalopathy
HIV infection of the brain is associated with a spectrum of clinical findings often referred to as either HIV-associated encephalopathy (HAE) or HIV-associated dementia.12 The disorder is manifested differently in children and adults.13 HAE in adult patients is characterized by a gradual decline in cognitive functioning, often occurring long after the initial infection. Motor slowing is usually evident, sometimes coincident with but often after the onset of cognitive decline. Both are progressive, and behavioral changes may also become apparent. Neuroimaging reveals diffuse atrophy. Nonenhancing periventricular white matter changes may also be seen, and the electroencephalogram usually shows diffuse slowing. Cerebrospinal fluid (CSF) evaluation often reveals a mild lymphocytic pleocytosis and protein elevation. Focal lesions on imaging or more extreme findings on CSF evaluation should prompt evaluation for other or coexisting illnesses.
HAE in children is similar to that in adults in many ways, but because the infection occurs during brain development, some differences may be seen. Specifically, evidence for more widespread infection of astrocytes and even neurons may be found.14,15 Developmental delay may be the first sign of HIV infection and can be manifested as either motor or language delay. Behavioral consequences may also be seen. Frequently, these manifestations exist together. Again, comorbid conditions associated with AIDS may be present and should be ruled out before a diagnosis of HAE is made.
Myelopathy
The spinal cord is commonly involved in HIV infection. The characteristic lesion in adults consists of vacuolar changes, predominantly in the lateral and posterior columns of the thoracic cord, although any level may be affected.16 Even though present in up to 50% of autopsy cases, clinical manifestations of myelopathy are much less frequently described, either because they are less pronounced than other neurological symptoms (i.e., those of dementia and neuropathy) or because they are attributed to some other cause.17 Common symptoms include spasticity, weakness, sensory ataxia, urinary incontinence, and erectile dysfunction. The disorder is usually a late manifestation of AIDS. Although also common in children, the nature of the myelopathic changes differs from that commonly seen in adults.18
The cause of vacuolar myelopathy remains poorly understood, but HIV-infected macrophages are often seen. There is no compelling evidence for overt HIV infection of spinal cord tissue itself. Two hypotheses, not mutually exclusive, that might explain the pathologic features include (1) toxicity associated with proinflammatory cytokine production by infected macrophages and (2) impaired intraspinal methylation essential for myelination and neurotransmitter metabolism.19 Pathologic examination reveals a symmetrical axon-sparing process initially, which in advanced cases may disrupt axons and appear more symmetrical.
The diagnosis is usually clinical, but findings on magnetic resonance imaging (MRI) include atrophic changes of the thoracic cord with occasional increased signal on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences.20 CSF is often normal. Occasionally, a mild lymphocytic pleocytosis and protein elevation are observed.17 Somatosensory evoked potentials are frequently abnormal even before clinical symptoms develop.20
HIV-associated vacuolar myelopathy tends to be insidious in onset. There is no effective treatment. Therefore, in any patient with a rapidly progressive myelopathic disorder, especially one associated with focal back pain or marked CSF abnormalities, alternative diagnoses should be sought because treatments may be available.17 Other possibilities include disorders caused by other retroviruses (human T-cell lymphotropic virus types I and II), cytomegalovirus (CMV), herpes simplex virus type 2, and herpes zoster. Less common causes include those associated with malignancies, especially lymphoma and myeloma. Ischemic myelopathies are rarely reported.
Human Immunodeficiency Virus–Associated Stroke
HIV infection is associated with an increased risk for stroke.21 However, attribution of HIV itself as being causative of stroke is problematic. HIV infection has been associated with the presence of both antiphospholipid antibodies and protein S deficiency in HIV-positive stroke patients.22,23 HIV has also been associated with vasculitis.24 Although rare in children, stroke should be considered in those with HIV and an acute onset of neurological symptoms because cerebrovascular disease is well documented.25 In addition, dyslipidemia, a common side effect of HAART therapy, carries an increased risk for stroke. To date, however, no compelling evidence for a strong association between HAART and an increased incidence of stroke has been documented.21 In conclusion, stroke must be considered in the differential diagnosis of any HIV-positive individual with an abrupt onset of neurological symptoms.
Human Immunodeficiency Virus–Associated Neuropathy
Peripheral neuropathy is often encountered in HIV/AIDS patients and is considered the most common neurological manifestation of the disease.26 Acute HIV infection may result in acute inflammatory polyradiculopathy either in isolation or as part of the acute retroviral syndrome described earlier.9 A painful distal symmetrical polyneuropathy is present in many AIDS patients at diagnosis. It is thought to be due to the direct effects of HIV on peripheral nerves or the neurotoxic effects of proinflammatory cytokines that result from viral infection.27 Related metabolic and nutritional deficiencies may also contribute to distal symmetrical polyneuropathy. Patients complain of numbness and burning of the feet, which are exquisitely sensitive to touch. The hands may be involved as well, but this is usually a late complication. Weakness is rare. Distal reflexes are diminished relative to proximal reflexes in a symmetrical fashion. Electrical studies demonstrate reduced nerve conduction velocities or amplitudes, or both, along with evidence of denervation and reinnervation distally.28 Rarely is evaluation of the CSF or nerve/muscle biopsies indicated. Later in the course of the disease, opportunistic infections may cause radiculopathies and mononeuritis multiplex. Finally, many of the agents included in HAART are known neurotoxins (see later).
Acute inflammatory polyradiculopathy is treated by immunomodulation, most often either plasmapheresis or intravenous immune globulin. Treatment of distal symmetrical polyneuropathy includes identifying and correcting any comorbid nutritional or infectious factors as well as the use of many medications, including analgesics of all classes, anticonvulsants, anesthetic agents, and select antidepressants. An “analgesic ladder” has been constructed by the World Health Organization to aid in the treatment of symptoms from distal symmetrical polyneuropathy, but many reports suggest that pain is inadequately treated in these patients.29
Human Immunodeficiency Virus–Associated Myopathy
Symmetrical and proximal myopathy is a common finding in those with HIV/AIDS.30 Its etiology is unknown but it is found in those without underlying explanations, such as opportunistic infection or myopathy-causing therapies, including HAART. Presumed causes include HIV itself, as well as associated inflammatory cytokines. The process affects the hip flexors and neck muscles disproportionately. Serum creatine kinase is frequently elevated, and electrical studies show classic myopathic changes.31 Muscle biopsies are characterized by degeneration of myofibers. Inflammation may or may not be present. Management includes identifying and treating any underlying causes (including HAART) and administration of steroidal and nonsteroidal anti-inflammatory agents and intravenous immune globulin.
Infection
Infections of many kinds are common in those with HIV/AIDS, and the nervous system is frequently involved. Impaired cell-mediated immunity predisposes HIV-infected individuals to opportunistic infections rarely encountered in the population at large. Opportunistic infections encountered in the CNS of persons with HIV/AIDS are outlined in Table 45-2 and are the focus of this section. As noted earlier, the clinical manifestations and radiographic features of various opportunistic infections can be quite similar, and they can also mimic other AIDS-associated conditions (i.e., malignancies and nonopportunistic infections such as Treponema pallidum or Bartonella, which are common in patients with HIV/AIDS). Furthermore, several processes may coexist and thus complicate diagnosis and treatment. As a result, histologic confirmation of a suspected infectious process may be warranted. Serologic studies, cell culture, and molecular techniques such as polymerase chain reaction (PCR) are also commonly used.
Toxoplasmosis
T. gondii, a ubiquitous parasite, is the most common. Human exposure occurs through contact with oocytes shed by its definitive host, the cat, and the risk for AIDS-related CNS toxoplasmosis is related to the prevalence of Toxoplasma exposure in a particular population or area.31 After infection, persistent and asymptomatic cysts form in both the brain and muscle. Reactivation occurs as a consequence of impaired cellular immunity and often causes an encephalitis without significant meningeal involvement. Multiple lesions can develop subacutely in the brain and cause location-dependent symptoms.
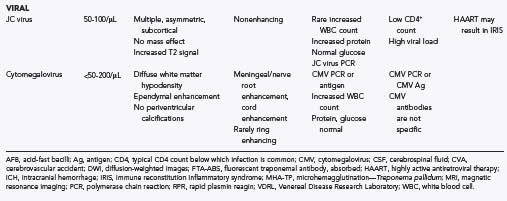
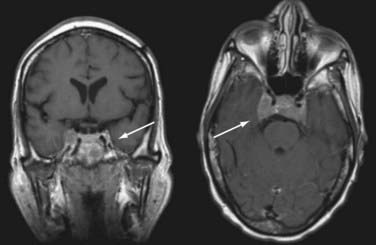
In typical cases of toxoplasmosis, both computed tomography (CT) and MRI reveal varying numbers of nodular or ring-enhancing lesions with surrounding edema (Fig. 45-1). The degree of enhancement varies and may be less pronounced in those with advanced AIDS and weaker immune responses.32 The lesions are most common at the gray-white interface and in the basal ganglia and thalamus.33 Although signal characteristics vary, the lesions are often hyperintense on T2-weighted sequences. The center of the lesion may be hypointense on diffusion-weighted imaging, with the surrounding edema being hyperintense.34
The differential diagnosis of ring-enhancing lesions includes lymphoma and other infections, especially tuberculosis and cryptococcosis. Although the lesions in lymphoma can be nodular and ring enhancing, a homogeneous pattern of enhancement is more common. Small parenchymal hemorrhages are common in toxoplasmosis and infrequent in lymphoma.35 Diffusion-weighted imaging may also help differentiate between toxoplasmosis and lymphoma in that the latter is often characterized by a uniformly restricted pattern.36 Periventricular and callosal involvement is more common in lymphoma as well.
Serum and CSF studies may help in determining the etiology of CNS lesions, but many caveats exist. Antibody titers for Toxoplasma are not very helpful in immunocompromised individuals.37–39 Serum or CSF PCR, in contrast, is associated with good specificity and reasonable sensitivity.40 CSF cytology can occasionally demonstrate the Toxoplasma organisms.41
Because of the high incidence of toxoplasmic encephalitis in HIV-infected patients and the difficulty in establishing a diagnosis by noninvasive means, empirical treatment is often initiated in patients with characteristic enhancing mass lesions. Toxoplasmosis often responds rapidly to therapy with pyrimethamine and sulfadiazine. Radiologic improvement is usually apparent within 2 to 4 weeks, but in rare cases, it may take up to 6 months to see a response.42
In cases of failed diagnosis or failed response to empirical treatment of presumed toxoplasmosis, the neurosurgeon may be called to perform a biopsy. Biopsy specimens of toxoplasmotic tissue are often necrotic, and when histopathologic evaluation fails to reveal organisms, other means of establishing the diagnosis are necessary,42,43 including isolation of active T. gondii from cultured biopsy specimens. Toxoplasma infection can also be established by identification of Toxoplasma-specific DNA by PCR performed on either fresh or formalin-fixed, paraffin-embedded tissue.42,44
Toxoplasmosis is usually responsive to treatment.45 Unfortunately, treatment does not affect the dormant bradyzoite form of the infection, and there is a high potential for reactivation. In the event of suspected recurrence or progression because of drug resistance, repeat biopsy may be warranted.
Fungal Infections
C. neoformans infection, like toxoplasmosis, is endemic. The organism is found in bird excrement and infects humans via inhalation, although overt pulmonary infection and pneumonia are rare.46,47 In the setting of HIV infection, both meningoencephalitis and isolated encephalitis may occur, usually in those with CD4+ T-cell counts less than 200 cells/µL. The clinical manifestations probably represent reactivation of latent infection.47 The symptoms and signs of cryptococcal meningitis develop over a period of weeks to months. Because of an impaired immune response to infection, the findings may be more subtle and insidious in onset in HIV-infected patients. Cranial neuropathies are common because of the predilection for meningeal involvement at the base of the brain. Hydrocephalus is also common, and strokes sometimes occur. Direct involvement of the brain parenchyma, when present, may be either focal or diffuse, and symptoms and signs vary according to the area or areas affected.48
Radiologic findings in cryptococcosis are relatively nonspecific. Occasionally, ring-enhancing lesions occur and can be difficult to differentiate from toxoplasmosis. Less commonly, cryptococcosis causes pseudocysts in the CSF spaces, which are seen as dilated perivascular spaces.42 Sputum culture is unreliable because of fungi colonizing the upper respiratory tract. Blood cultures are often positive for cryptococcosis in HIV-positive patients with CNS involvement.49 Positive serum cryptococcal antigen by latex agglutination may also help support the diagnosis.47,50 CSF cell counts are frequently unremarkable in HIV/AIDS patients infected with Cryptococcus.51,52 Therefore, India ink stains, cryptococcal antigen assay, and fungal culture are useful in making the diagnosis. These studies have high specificity, but their sensitivity is variable. CSF culture is positive in 56% to 99% of all patients with cryptococcal meningitis, India ink staining in 75% to 98%, and latex agglutination in 80% to 98%.53,54
On histopathologic review, cryptococcomas reveal chronic granulomatous changes with only a few organisms. Occasionally, these lesions are surrounded by more profound inflammation. In cryptococcosis, thickening and opacification of the meninges is commonly seen. India ink and mucicarmine stains of the meninges may reveal budding yeast. If biopsy is performed on the brain parenchyma, pseudocysts may be found. These gelatinous dilations of the perivascular spaces are described as “soap bubbles.” They are most common in the basal ganglia but can be found elsewhere in the parenchyma as well.42
HIV-associated CNS cryptococcal infection, if untreated, is fatal. Treatment often consists of fluconazole or amphotericin B (or one of its lipid derivatives). The majority of patients respond to treatment, but as with toxoplasmosis, the relapse rate is high.51,54–56 The mortality associated with cryptococcal meningoencephalitis remains 10% to 30%.57,58 Because hydrocephalus is a common problem, the neurosurgeon may often be consulted to place a ventricular shunt.
Aspergillus is another fungal infection commonly encountered in HIV/AIDS patients. It is a septate hyaline mold found in plants and soil that causes severe sinopulmonary infections in immunocompromised hosts. From there it can spread either hematogenously or by direct invasion from the sinuses to the CNS.59 Most patients with CNS aspergillosis will also have evidence of ongoing or previous sinopulmonary infection. Aspergillus can cause diffuse cerebritis, focal abscesses, or meningitis. It can also cause a vascular invasion and result in stroke.60 Biopsy may be required to establish a definitive diagnosis in these severely immunocompromised patients.
Mycobacterial Infections
The prevalence of Mycobacterium tuberculosis is high in the developing world and increasing in the United States and other developed nations. HIV/AIDS patients are particularly prone to reactivation of tuberculosis and to extrapulmonary infection.61 In cases of CNS involvement, patients often have a history of pulmonary tuberculosis.62 The atypical mycobacteria M. avium and M. intracellulare are endemic in the environment, found in water, soil, and animal hosts. In profoundly immunocompromised AIDS patients (CD4+ T-cell counts <50 cells/µL), they cause a systemic syndrome classified as Mycobacterium avium complex (MAC). CNS involvement, when present, mimics tuberculosis and is difficult to distinguish from it.63
Meningitis is the most common manifestation of tubercular infection of the CNS in both immunocompetent and immunocompromised individuals. In immunocompromised patients, typical meningeal signs can be absent.64,65 Tubercular meningitis, like fungal meningitis, may be associated with stroke.66 Parenchymal brain lesions are less common, but two types are seen: fibrotic tuberculomas and tubercular abscesses containing actively dividing mycobacteria.62
The radiologic features of tubercular meningitis are indistinguishable from those associated with fungal infections. Leptomeningeal thickening may be noted, particularly in the area at the base of the skull. MRI may be helpful in distinguishing tuberculomas from tubercular abscesses. Tuberculomas may be single or multiple. Like abscesses, they more commonly appear in the supratentorial space and can often be found at the gray-white interface. On T2-weighted MRI sequences they are isointense, frequently with a low-signal center.42 On contrast-enhanced studies they are at times described as “target lesions.” There may be ring enhancement with a small area of enhancement or calcification in the center of the lesion.33 A mass effect is not usually present.66 Although tubercular abscesses are also most commonly found in the supratentorial space, they have a number of unique imaging characteristics. These abscesses are often solitary. They are generally larger and can appear multiloculated on imaging studies.33 They usually enhance, cause a mass effect, and are surrounded by significant edema. Magnetic resonance spectroscopy (MRS) can be used to help differentiate these lesions from neoplasms. Tubercular abscesses will have elevated lipid and lactate peaks without a significant increase in cell membrane markers such as choline.67
Purified protein derivative skin tests for M. tuberculosis are unreliable in patients with HIV. CSF cultures are positive in approximately a third to two thirds of patients with tubercular meningitis, and it may be weeks before they turn positive.64,68 Acid-fast bacillus staining is often negative. Acid-fast bacillus PCR has higher sensitivity, but a negative PCR result does not rule out M. tuberculosis in the CNS. CSF typically demonstrates an elevated white blood cell count with a lymphocytic predominance. This is less pronounced in the setting of HIV.69 CSF protein and CSF viscosity are also elevated. CSF glucose is typically decreased.
Tubercular meningitis only rarely requires a biopsy for diagnosis, but it may cause obstructive hydrocephalus requiring placement of an extraventricular drain or shunt by the neurosurgeon. M. tuberculosis brain abscesses, however, frequently require surgical drainage. These multiloculated lesions typically have pus with abundant acid-fast bacilli that can be easily cultured. Vascular granulation tissue may be present at the periphery of the lesions. Histologic evaluation of parenchymal lesions in patients with MAC demonstrates poorly formed granulomas with the greatest concentration of organisms located perivascularly. Necrosis and parenchymal infarctions can also be found.70,71
CNS tuberculosis in the setting of HIV is treated with multidrug regimens. Treatment duration varies but can extend to a year or more. The role of steroids in treating tubercular meningitis in the setting of HIV/AIDS is unknown.72 The standard of care for the parenchymal tubercular CNS lesions in AIDS patients is even less firmly defined. Steroids may be added to the treatment regimen to decrease inflammation and the mass effect. In addition to establishing a diagnosis, surgical decompression for a significant mass effect or increase in intracranial pressure may be necessary.73 Treatment of CNS MAC in HIV patients involves HAART in addition to a multidrug regimen that includes ethambutol and macrolide antibiotics. Mortality, however, is higher, with a large proportion of patients dying of this disease.74,75
Viral Infections
Progressive Multifocal Leukoencephalopathy
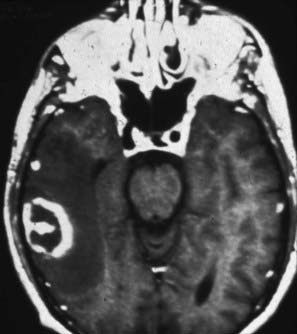
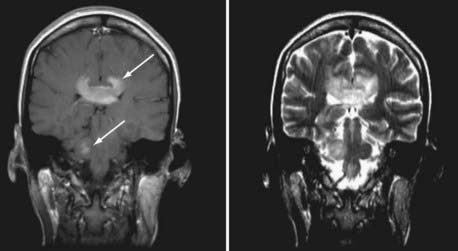
JC virus is a ubiquitous DNA polyomavirus that infects most individuals before adulthood. It remains latent in B lymphocytes, monocytes-macrophages, hematopoietic stem cells (CD34+), and renal epithelial cells. Reactivated in the setting of immune suppression, progressive multifocal leukoencephalopathy (PML) is usually diagnosed in HIV-positive patients when CD4+ T-cell counts fall to less than 100 cells/µL. Once reactivated, JC virus can spread via B lymphocytes to the CNS, where it replicates in oligodendrocytes and leads to their death. Demyelination follows. Subacute neurological decline with focal symptoms develops, depending on the location of the lesions. Seizures may occur. The disease progresses and is often fatal. In the pre-HAART era, evidence of PML was found in up to 5% of HIV-positive patients at autopsy,76 but the incidence has decreased significantly during the HAART era.77 MRI reveals areas of increased signal in the white matter on T2/FLAIR sequences, with corresponding decreased signal intensity on T1-weighted images (Fig. 45-2) This is most pronounced posteriorly but can be found anywhere in the brain, including the posterior fossa. The lesions are often multifocal and have an asymmetric distribution. Because of the blunted immune response, enhancement, edema, and a mass effect are frequently absent. Enhancement after contrast administration, although rare, is associated with improved survival.78 There is less atrophy than is typically associated with HAE.42 Unlike some opportunistic infections, which may improve radiographically with treatment, PML rarely does. Correlative imaging studies such as MRS are seldom helpful.
Anti–JC virus antibodies are present in up to 75% of healthy adults in developed countries.79 JC virus is detected in serum by PCR in 2% to 3% of the population, and its detection is not pathognomonic for JC disease.80 PML is often associated with a decreased CD4+ T-cell count and elevated viral load.77 CSF rarely demonstrates pleocytosis. Protein is elevated in approximately half of the patients, but there is usually no decrease in glucose.77 The lack of inflammatory findings in the CSF correlates with the lack of meningeal signs in the patient. CSF PCR for JC virus is highly specific, but the sensitivities reported have been quite variable (57% to 90%).81,82 For unclear reasons, the sensitivity of CSF JC virus PCR appears to be decreasing during the HAART era.83
With the low sensitivity of CSF studies and the lack of radiographic response to empirical therapy, brain biopsy plays an important role in this setting. Approximately half of the patients who undergo biopsy because of negative CSF PCR have a diagnosis of PML on biopsy. Histologic examination reveals demyelination accompanied by “bizarre”-looking astrocytes with pleomorphic nuclei, as well as enlarged oligodendrocyte nuclei containing virions on electron microscopy. The spherical and filamentous virion particles are sometimes described as “spaghetti and meatballs.” There is only scant evidence of perivascular inflammation.42 Immunohistochemistry can be performed to evaluate for the presence of the JC virus capsid protein VP-1 in oligodendrocyte nuclei. JC virus PCR can also be performed on the biopsy tissue.
No specific treatment of PML is available. There have been some reports of the viral DNA inhibitor cidofovir being used.84,85 The mainstay of treatment has been immune reconstitution via HAART. In the HAART era, survival has increased to a median of greater than 1 year. This is particularly likely if the CD4+ count is greater than 50 at the time of initial PML diagnosis.77 One of the potential side effects of treatment with HAART is an exacerbated immune response in the CNS. This immune reconstitution syndrome is discussed in greater depth later in this chapter.
Cytomegalovirus
CMV can cause myriad neurological syndromes, including encephalitis, myelitis, polyradiculopathy, and polyneuropathy. Encephalitis often involves the periventricular tissue and the brainstem, tends to occur concomitantly with meningitis, and may be associated with stroke. The myelitis is subacute in onset and can resemble similar syndromes found in patients with autoimmune disorders or other infectious conditions. It has been hypothesized that both direct viral infection of the spinal cord and a misdirected immune attack play roles in the development of this syndrome.86 The polyradiculopathy associated with CMV most commonly affects the lumbar spinal nerve roots. Patients have bilateral lower extremity weakness and bowel and bladder symptoms. Sensory symptoms include numbness and paresthesias. Neurological examination reveals a decrease in reflexes consistent with a lower motor neuron syndrome.87 Finally, patients may also have a subacute syndrome of sensory and lower motor symptoms secondary to a multifocal polyneuropathy.
MRI in patients with encephalitis may demonstrate focal findings, particularly increased signal on T2/FLAIR images, with rare enhancement in the ependymal region, the periventricular white matter, and elsewhere in the brain or brainstem. The periventricular calcifications of congenital CMV infection are not seen in HIV.33 If there is a meningeal component, leptomeningeal enhancement may be noted. Myelopathy exhibits similar findings in the spinal cord. Patients with radiculopathy may demonstrate enhancement of the nerve roots. A high percentage of the population has previously been exposed to CMV and is seropositive, so antibody titers are not helpful. CSF may reveal increased protein and glucose levels, as well as white blood cells. CMV grows very slowly in culture, which has largely been superseded by CMV antigenemia and PCR-based assays. Both have high sensitivity and specificity in HIV-positive individuals with encephalitis.88 However, CMV PCR positivity is not sufficient by itself for diagnosis because CSF CMV PCR may also be positive in individuals with systemic CMV infection in the absence of neurological syndrome.89 Histologic evaluation, when performed, reveals eosinophilic intranuclear inclusions with surrounding halos. Specific treatment of CMV consists of ganciclovir or foscarnet, or both. HAART may also help reconstitute the immune system. Unfortunately, the mortality rate is high, with death often occurring within weeks.
Treponema pallidum and Bartonella
Infection by the bacterium T. pallidum, the cause of syphilis, is common in HIV/AIDS patients and should be considered in the differential diagnosis of those with neurological symptoms. The manifestations of the disease differ in HIV-infected individuals as a consequence of their immunocompromised condition. The tertiary stage of syphilis is associated with diffuse systemic involvement, including the CNS. Syphilitic meningitis90 or meningovasculitis,33 the symptoms of which resemble many of the other opportunistic infections of the CNS discussed earlier, commonly develop in patients with HIV. A third CNS syphilitic syndrome that appears more frequently in HIV-infected patients is subacute polyradiculopathy.91 The classic manifestations of tertiary syphilis, such as general paresis of the insane, tabes dorsalis, and cerebral gummas, are less common in the HIV/AIDS setting.92
The gram-negative bacteria Bartonella henselae and Bartonella quintana are the causative agents of cat-scratch disease and bacillary angiomatosis, respectively. Usually self-limited in immunocompetent individuals, systemic disease with florid meningoencephalitis is much more common in AIDS patients. The diagnosis should be suspected in those with typical skin lesion or lymphadenopathy.93–95
Acquired Immune Deficiency Syndrome–Related Malignancies
Malignancies associated with HIV/AIDS include Kaposi’s sarcoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma (NHL), squamous cell carcinoma, plasmacytoma, and leiomyosarcoma.96,97 When encountered in the AIDS population, these tumors are frequently associated with viral infections, which are presumed to play a role in their pathogenesis. Direct involvement of the CNS by these tumors is rare, with one exception: primary central nervous system lymphoma (PCNSL). It should be remembered that other primary and metastatic CNS tumors also occur at high frequency in HIV-positive patients, especially with the advent of HAART and the prolongation of life associated with its use.98 In the following section we review AIDS-related PCNSL, followed by a discussion of other AIDS-related malignancies.
Primary Central Nervous System Lymphoma
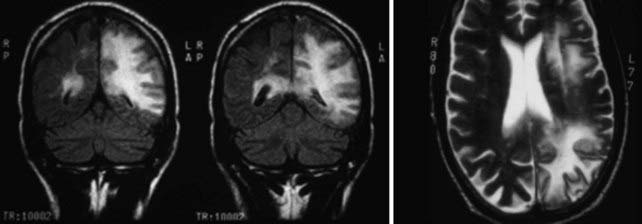
PCNSL is a rare NHL that accounts for less than 5% of primary CNS tumors.99 Before the emergence of HIV/AIDS, PCNSL was most often seen either in the setting of known immunocompromised status or with advanced age. The emergence of the AIDS epidemic was mirrored by a rise in the incidence of PCNSL, and it soon became an AIDS-defining illness. Unlike PCNSL in the population at large, the AIDS-associated variant is relatively resistant to treatment and usually follows an aggressive course. Fortunately, with the advent of HAART, it has become less common and may be more amenable to treatment. In approximately 2% of HIV-infected individuals in the developed world, PCNSL will be diagnosed in their lifetime, but autopsy series suggest that the incidence may be as high as 10%.100
PCNSL usually demonstrates homogeneous contrast-enhanced lesions with either a hypodense appearance (on CT) or low signal intensity (on T1-weighted MRI) (Fig. 45-3). The lesion or lesions are frequently concentrated in the periventricular regions. The use of metabolic imaging, especially single-photon emission spectroscopy after thallium administration, may show avid uptake of the radiotracer in areas of contrast enhancement.101 Other metabolic imaging techniques, including positron emission tomography and MRS, have been less widely used, and their sensitivity and specificity remain unclear.
A strong association exists between AIDS-associated PCNSL and infection with Epstein-Barr virus (EBV).101 The potential mechanisms responsible for EBV transformation of lymphoid cells in this setting include coinfection with both EBV genotypes, the presence of particular deletions in EBV-associated membrane proteins, and aberrant regulation of EBV-associated gene promoters and enhancers.102,103 For this reason, attempts to diagnose suspected PCNSL from CSF by PCR, even in the absence of suspicious cells, are ongoing.104,105 Because EBV infection is common, the presence of EBV-specific DNA in CSF alone should not be considered diagnostic of PCNSL.
The neurosurgeon will often be called on to biopsy suspected PCNSL because the course of the disease is aggressive and treatment delays can result in significant morbidity. The diagnosis itself (usually by stereotactic biopsy) has high specificity in most cases.106,107 Exceptions occur, especially when patients have received corticosteroids before biopsy because these drugs are acutely lympholytic and may transiently obscure the diagnosis. As with all PCNSL, there is no role for surgical intervention beyond diagnostic biopsy in AIDS patients.
Although PCNSL is fairly common, similar lesions caused by infectious agents in individuals with HIV/AIDS are more common. Worldwide, toxoplasmosis accounts for the majority of these lesions (see earlier). In some regions of the developing world, cerebral tuberculosis is also commonly encountered. Positive CSF cultures and stains for either of these conditions do not exclude the coexistence of PCNSL. Nevertheless, empirical treatment of these conditions often precedes attempts at biopsy in many cases. Despite its high specificity, biopsy in HIV/AIDS patients carries an increased risk for morbidity and mortality over that in the general population.108 Risks for infection, bleeding, and death are increased. Thus, many advocate the use of empirical antimicrobial treatment for a defined period in conjunction with close clinical observation for signs of either improvement or deterioration. The majority of patients with cerebral toxoplasmosis show signs of clinical improvement within 3 days of treatment, and improvement on neuroimaging follows within 7 to 10 days. In the case of suspected tuberculosis, improvement occurs less rapidly. Therefore, if this approach is adopted, careful clinical assessment must be maintained, and biopsy should be performed in any person with early deterioration after the initiation of empirical treatment of presumed infectious disease.
Treatment of AIDS-related PCNSL is difficult because immune suppression plays a central role. In the pre-HAART era, survival after diagnosis rarely exceeded 3 months, even with treatment, although palliation of symptoms was often achieved.109 Whole-brain radiotherapy (WBRT) has played an important role in the treatment of AIDS-related PCNSL. Before the use of HAART, chemotherapy—a mainstay of treatment in immunocompetent patients—was associated with high morbidity and mortality. Survival after WBRT alone in PCNSL patients is approximately 15 months110,111 and decreases to 2 to 5 months in AIDS patients.112 The addition of HAART to this regimen results in improved survival over the use of WBRT alone.113 Protease inhibitors, an important component of HAART, may enhance the effects of radiation.114,115
Methotrexate-based chemotherapy regimens, the standard of care in immunocompetent patients, are being used with greater frequency in those with AIDS-related PCNSL. HAART has rendered more AIDS patients suitable for chemotherapy and has led to cautious optimism in improving survival.115 Furthermore, treatment strategies that also target EBV are under way and may improve survival.116 Despite these advances, AIDS-related PCNSL remains difficult to treat, and improved survival outcomes remain an elusive goal for many.
Other Acquired Immune Deficiency Syndrome–Related Malignancies
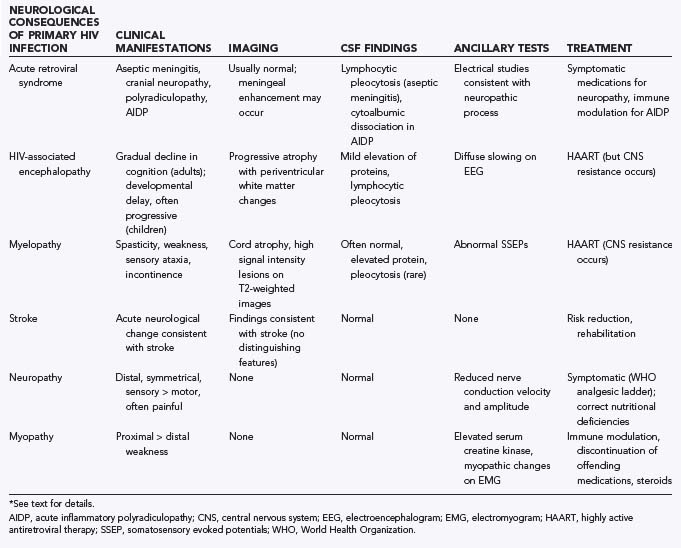
Both Kaposi’s sarcoma117 and leiomyosarcoma118–120 are known to develop in the brains of HIV-infected individuals, but they are rare. Systemic NHL can involve the leptomeninges and, in some series, has been observed in the majority of cases.120,121 The use of HAART has dramatically decreased the incidence of meningeal involvement from systemic NHL.121 As shown in Figure 45-4, meningeal involvement can be extensive and cause significant neurological morbidity. Finally, lung cancer is also increased in patients with HIV and is a frequent cause of NS metastasis.98
Human Immunodeficiency Virus Treatment–Related Neurotoxicities
The value of HAART in the treatment of HIV/AIDS is unquestionable. As with all therapies, however, side effects can be dose limiting. The predominant neurological consequence of HAART therapy is peripheral neuropathy. The nucleoside analogues, a critical component of HAART therapy, are associated with a dose-dependent distal sensory polyneuropathy.122,123 More recently, protease inhibitors have also been implicated.124 The incidence of distal sensory polyneuropathy has decreased as awareness of this effect has led to lower doses in contemporary HAART regimens. As noted earlier, the causes of peripheral neuropathy in HIV-positive persons may be multifactorial and include HIV infection itself, opportunistic infection, and nutritional deficiencies.
IRIS has been described in the CNS in the setting of many opportunistic infections, including PML, CMV, M. tuberculosis, MAC, and Cryptococcus.125–127 The risk for development of this syndrome appears to be higher when initiating HAART. It also appears more likely when multiple infections are present.125,128,129
The neurological symptoms of IRIS, like those of the opportunistic infections and HIV-associated malignancies, are protean and largely dependent on location. Generalized symptoms can occur (e.g., impaired concentration and somnolence) and might also be the result of proinflammatory cytokines and other irritants on nervous system tissue. The onset of symptoms typically occurs within 2 months of initiating HAART.125
Imaging studies often reveal contrast enhancement. When opportunistic infection does not cause significant enhancement, such as in PML, imaging can be of significant value in differentiating infection from IRIS.130 Unfortunately, many opportunistic infections are also associated with radiographic enhancement, sometimes obscuring the difference between treatment effect and progressive disease. The neurosurgeon may be called on to perform a biopsy to help determine the cause of the clinical deterioration. Histologic evaluation in patients with IRIS generally reveals diffuse cytotoxic T-cell infiltrates.130 Residual histologic characteristics of the opportunistic infection might also be present.
Management of IRIS is problematic. The overarching goal of HAART—control and prevention of AIDS-associated illnesses—must be balanced against the short- and long-term consequences of bystander immune effects. Seldom should HAART be discontinued in the setting of IRIS. Some authors have suggested using steroids to dampen the immune response, particularly if significant cerebral edema or a mass effect is present.130
Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs. 2007;21:293.
Cherry CL, McArthur JC, Hoy JF, et al. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol. 2003;26:195.
Collazos J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs. 2003;17:869.
Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19:446.
Di Rocco A. Diseases of the spinal cord in human immunodeficiency virus infection. Semin Neurol. 1999;19:151.
Dorsey SG, Morton PG. HIV peripheral neuropathy: pathophysiology and clinical implications. AACN Clin Issues. 2006;17:30.
Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009;199:77.
Hamlyn E, Easterbrook P. Occupational exposure to HIV and the use of post-exposure prophylaxis. Occup Med (Lond). 2007;57:329.
Kastrup O, Wanke I, Maschke M. Neuroimaging of infections of the central nervous system. Semin Neurol. 2008;28:511.
Marra CM. Bacterial and fungal brain infections in AIDS. Semin Neurol. 1999;19:177.
Mitsuyasu RT. Non–AIDS-defining malignancies in HIV. Top HIV Med. 2008;16:117.
Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol. 2006;61:393.
Ortiz G, Koch S, Romano JG, et al. Mechanisms of ischemic stroke in HIV-infected patients. Neurol. 2007;68:1257.
Shelburne SAIII, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67.
Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17:1787.
Smith AB, Smirniotopoulos JG, Rushing EJ. From the archives of the AFIP: central nervous system infections associated with human immunodeficiency virus infection: radiologic-pathologic correlation. Radiographics. 2008;28:2033.
Travis J, Varma A, duPlessis D, et al. Immune reconstitution associated with progressive multifocal leukoencephalopathy in human immunodeficiency virus: a case discussion and review of the literature. Neurologist. 2008;14:321.
Van Rie A, Harrington PR, Dow A, et al. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11:1.
Wadhwa A, Kaur R, Bhalla P. Profile of central nervous system disease in HIV/AIDS patients with special reference to cryptococcal infections. Neurologist. 2008;14:247.
Whiteman M, Espinoza L, Post MJ, et al. Central nervous system tuberculosis in HIV-infected patients: clinical and radiographic findings. AJNR Am J Neuroradiol. 1995;16:1319.
1 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1.
2 Coakley E, Petropoulos CJ, Whitcomb JM. Assessing chemokine co-receptor usage in HIV. Curr Opin Infect Dis. 2005;18:9.
3 Herek GM, Capitanio JP, Widaman KF. HIV-related stigma and knowledge in the United States: prevalence and trends, 1991-1999. Am J Public Health. 2002;92:371.
4 Palella FJJr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853.
5 Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557.
6 Hamlyn E, Easterbrook P. Occupational exposure to HIV and the use of post-exposure prophylaxis. Occup Med (Lond). 2007;57:329.
7 Soogoor M, Daar ES. Primary human immunodeficiency virus type 1 infection. Curr HIV/AIDS Rep. 2005;2:55.
8 Michaels J, Sharer LR, Epstein LG. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1:71.
9 Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19:446.
10 Fischer-Smith T, Bell C, Croul S, et al. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318.
11 Van Rie A, Harrington PR, Dow A, et al. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11:1.
12 McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus–associated dementia. Semin Neurol. 1999;19:129.
13 Mintz M. Clinical comparison of adult and pediatric neuroAIDS. Adv Neuroimmunol. 1994;4:207.
14 Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32:253.
15 Canto-Nogues C, Sanchez-Ramon S, Alvarez S, et al. HIV-1 infection of neurons might account for progressive HIV-1–associated encephalopathy in children. J Mol Neurosci. 2005;27:79.
16 Petito CK, Navia BA, Cho ES, et al. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;312:874.
17 Di Rocco A. Diseases of the spinal cord in human immunodeficiency virus infection. Semin Neurol. 1999;19:151.
18 Dickson DW, Belman AL, Kim TS, et al. Spinal cord pathology in pediatric acquired immunodeficiency syndrome. Neurology. 1989;39:227.
19 Surtees R, Hyland K, Smith I. Central-nervous-system methyl-group metabolism in children with neurological complications of HIV infection. Lancet. 1990;335:619.
20 Sartoretti-Schefer S, Blattler T, Wichmann W. Spinal MRI in vacuolar myelopathy, and correlation with histopathological findings. Neuroradiology. 1997;39:865.
21 Pierelli F, Garrubba C, Tilia G, et al. Multimodal evoked potentials in HIV-1–seropositive patients: relationship between the immune impairment and the neurophysiological function. Acta Neurol Scand. 1996;93:266.
22 Ortiz G, Koch S, Romano JG, et al. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257.
23 Hoffmann M, Berger JR, Nath A, et al. Cerebrovascular disease in young, HIV-infected, black Africans in the KwaZulu Natal province of South Africa. J Neurovirol. 2000;6:229.
24 Qureshi AI, Janssen RS, Karon JM, et al. Human immunodeficiency virus infection and stroke in young patients. Arch Neurol. 1997;54:1150.
25 Chetty R. Vasculitides associated with HIV infection. J Clin Pathol. 2001;54:275.
26 Park YD, Belman AL, Kim TS, et al. Stroke in pediatric acquired immunodeficiency syndrome. Ann Neurol. 1990;28:303.
27 So YT, Holtzman DM, Abrams DI, et al. Peripheral neuropathy associated with acquired immunodeficiency syndrome. Prevalence and clinical features from a population-based survey. Arch Neurol. 1988;45:945.
28 Manji H. Neuropathy in HIV infection. Curr Opin Neurol. 2000;13:589.
29 Dorsey SG, Morton PG. HIV peripheral neuropathy: pathophysiology and clinical implications. AACN Clin Issues. 2006;17:30.
30 Verma S, Estanislao L, Mintz L, et al. Controlling neuropathic pain in HIV. Curr HIV/AIDS Rep. 2004;1:136.
31 Luft BJ, Hafner R, Korzun AH, et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med. 1993;329:995.
32 Kastrup O, Wanke I, Maschke M. Neuroimaging of infections of the central nervous system. Semin Neurol. 2008;28:511.
33 Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol. 2006;61:393.
34 Chong-Han CH, Cortez SC, Tung GA. Diffusion-weighted MRI of cerebral toxoplasma abscess. AJR Am J Roentgenol. 2003;181:1711.
35 Trenkwalder P, Trenkwalder C, Feiden W, et al. Toxoplasmosis with early intracerebral hemorrhage in a patient with the acquired immunodeficiency syndrome. Neurology. 1992;42:436.
36 Schroeder PC, Post MJ, Oschatz E, et al. Analysis of the utility of diffusion-weighted MRI and apparent diffusion coefficient values in distinguishing central nervous system toxoplasmosis from lymphoma. Neuroradiology. 2006;48:715.
37 Zufferey J, Sugar A, Rudaz P, et al. Prevalence of latent toxoplasmosis and serological diagnosis of active infection in HIV-positive patients. Eur J Clin Microbiol Infect Dis. 1993;12:591.
38 Machala L, Maly M, Hrda S, et al. Antibody response of HIV-infected patients to latent, cerebral and recently acquired toxoplasmosis. Eur J Clin Microbiol Infect Dis. 2008.
39 Meira CS, Costa-Silva TA, Vidal JE, et al. Use of the serum reactivity against Toxoplasma gondii excreted-secreted antigens in cerebral toxoplasmosis diagnosis in human immunodeficiency virus–infected patients. J Med Microbiol. 2008;57:845.
40 Colombo FA, Vidal JE, Penalva de Oliveira AC, et al. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples. J Clin Microbiol. 2005;43:5044.
41 Palm C, Tumani H, Pietzcker T, et al. Diagnosis of cerebral toxoplasmosis by detection of Toxoplasma gondii tachyzoites in cerebrospinal fluid. J Neurol. 2008;255:939.
42 Smith AB, Smirniotopoulos JG, Rushing EJ. From the archives of the AFIP: central nervous system infections associated with human immunodeficiency virus infection: radiologic-pathologic correlation. Radiographics. 2008;28:2033.
43 Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002;185(Suppl 1):S73.
44 Held TK, Kruger D, Switala AR, et al. Diagnosis of toxoplasmosis in bone marrow transplant recipients: comparison of PCR-based results and immunohistochemistry. Bone Marrow Transplant. 2000;25:1257.
45 Collazos J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs. 2003;17:869.
46 Sorrell TC, Ellis DH. Ecology of Cryptococcus neoformans. Rev Iberoam Micol. 1997;14:42.
47 Wadhwa A, Kaur R, Bhalla P. Profile of central nervous system disease in HIV/AIDS patients with special reference to cryptococcal infections. Neurologist. 2008;14:247.
48 Arayawichanont A, Prayoonwiwat N, Churojana A, et al. Successful medical treatment of multiple cryptococcomas: report of a case and literature review. J Med Assoc Thai. 1999;82:991.
49 Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794.
50 Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65.
51 Zuger A, Louie E, Holzman RS, et al. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234.
52 Kovacs JA, Kovacs AA, Polis M, et al. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103:533.
53 Saha DC, Xess I, Jain N. Evaluation of conventional & serological methods for rapid diagnosis of cryptococcosis. Indian J Med Res. 2008;127:483.
54 McCarthy KM, Morgan J, Wannemuehler KA, et al. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. AIDS. 2006;20:2199.
55 Grant IH, Armstrong D. Fungal infections in AIDS. Cryptococcosis. Infect Dis Clin North Am. 1988;2:457.
56 Bicanic T, Harrison T, Niepieklo A, et al. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069.
57 Robinson PA, Bauer M, Leal MA, et al. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28:82.
58 Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008;47:123.
59 Sundaram C, Umabala P, Laxmi V, et al. Pathology of fungal infections of the central nervous system: 17 years’ experience from southern India. Histopathology. 2006;49:396.
60 Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs. 2007;21:293.
61 Shafer RW, Kim DS, Weiss JP, et al. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine (Baltimore). 1991;70:384.
62 Rock RB, Olin M, Baker CA, et al. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21:243.
63 Little AA, Gebarski SS, Blaivas M. Nontuberculous mycobacterial infection of a metastatic brain neoplasm in an immunocompromised patient. Arch Neurol. 2006;63:763.
64 Sanchez-Portocarrero J, Perez-Cecilia E, Jimenez-Escrig A, et al. Tuberculous meningitis. Clinical characteristics and comparison with cryptococcal meningitis in patients with human immunodeficiency virus infection. Arch Neurol. 1996;53:671.
65 Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326:668.
66 Whiteman M, Espinoza L, Post MJ, et al. Central nervous system tuberculosis in HIV-infected patients: clinical and radiographic findings. AJNR Am J Neuroradiol. 1995;16:1319.
67 Gupta RK, Vatsal DK, Husain N, et al. Differentiation of tuberculous from pyogenic brain abscesses with in vivo proton MR spectroscopy and magnetization transfer MR imaging. AJNR Am J Neuroradiol. 2001;22:1503.
68 Girgis NI, Sultan Y, Farid Z, et al. Tuberculosis meningitis, Abbassia Fever Hospital-Naval Medical Research Unit No. 3-Cairo, Egypt, from 1976 to 1996. Am J Trop Med Hyg. 1998;58:28.
69 Laguna F, Adrados M, Ortega A, et al. Tuberculous meningitis with acellular cerebrospinal fluid in AIDS patients. AIDS. 1992;6:1165.
70 Marra CM. Bacterial and fungal brain infections in AIDS. Semin Neurol. 1999;19:177.
71 Bottieau E, Noe A, Florence E, et al. Multiple tuberculous brain abscesses in an HIV-infected patient successfully treated with HAART and antituberculous treatment. Infection. 2003;31:118.
72 Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741.
73 Hall WA, Truwit CL. The surgical management of infections involving the cerebrum. Neurosurgery. 2008;62(Suppl 2):519.
74 Jacob CN, Henein SS, Heurich AE, et al. Nontuberculous mycobacterial infection of the central nervous system in patients with AIDS. South Med J. 1993;86:638.
75 Flor A, Capdevila JA, Martin N, et al. Nontuberculous mycobacterial meningitis: report of two cases and review. Clin Infect Dis. 1996;23:1266.
76 Petito CK. Neuropathology of human immunodeficiency virus: questions and answers. Hum Pathol. 1996;27:623.
77 Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009;199:77.
78 Berger JR, Levy RM, Flomenhoft D, et al. Predictive factors for prolonged survival in acquired immunodeficiency syndrome–associated progressive multifocal leukoencephalopathy. Ann Neurol. 1998;44:341.
79 Stolt A, Sasnauskas K, Koskela P, et al. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84:1499.
80 Pelosini M, Focosi D, Rita F, et al. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of literature. Ann Hematol. 2008;87:405.
81 Fong IW, Toma E. The natural history of progressive multifocal leukoencephalopathy in patients with AIDS. Canadian PML Study Group. Clin Infect Dis. 1995;20:1305.
82 Vago L, Cinque P, Sala E, et al. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:139.
83 Marzocchetti A, Di Giambenedetto S, Cingolani A, et al. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43:4175.
84 De Luca A, Giancola ML, Ammassari A, et al. Cidofovir added to HAART improves virological and clinical outcome in AIDS-associated progressive multifocal leukoencephalopathy. AIDS. 2000;14:F117.
85 Gasnault J, Kousignian P, Kahraman M, et al. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J Neurovirol. 2001;7:375.
86 Rigamonti A, Usai S, Ciusani E, et al. Atypical transverse myelitis due to cytomegalovirus in an immunocompetent patient. Neurol Sci. 2005;26:351.
87 Anders HJ, Goebel FD. Cytomegalovirus polyradiculopathy in patients with AIDS. Clin Infect Dis. 1998;27:345.
88 Bestetti A, Pierotti C, Terreni M, et al. Comparison of three nucleic acid amplification assays of cerebrospinal fluid for diagnosis of cytomegalovirus encephalitis. J Clin Microbiol. 2001;39:1148.
89 Quereda C, Corral I, Laguna F, et al. Diagnostic utility of a multiplex herpesvirus PCR assay performed with cerebrospinal fluid from human immunodeficiency virus–infected patients with neurological disorders. J Clin Microbiol. 2000;38:3061.
90 Ramsey RG, Geremia GK. CNS complications of AIDS: CT and MR findings. AJR Am J Roentgenol. 1988;151:449.
91 Ferrari S, Vento S, Monaco S, et al. Human immunodeficiency virus–associated peripheral neuropathies. Mayo Clin Proc. 2006;81:213.
92 Johns DR, Tierney M, Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987;316:1569.
93 Karem KL, Paddock CD, Regnery RL. Bartonella henselae, B. quintana, and B. bacilliformis: historical pathogens of emerging significance. Microbes Infect. 2000;2:1193.
94 Schwartzman WA, Patnaik M, Angulo FJ, et al. Bartonella (Rochalimaea) antibodies, dementia, and cat ownership among men infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:954.
95 Margolis B, Kuzu I, Herrmann M, et al. Rapid polymerase chain reaction–based confirmation of cat scratch disease and Bartonella henselae infection. Arch Pathol Lab Med. 2003;127:706.
96 Gates AE, Kaplan LD. AIDS malignancies in the era of highly active antiretroviral therapy. Oncology (Williston Park). 2002;16:657.
97 Gates AE, Kaplan LD. AIDS malignancies in the era of highly active antiretroviral therapy. Oncology (Williston Park). 2002;16:441.
98 Mitsuyasu RT. Non–AIDS-defining malignancies in HIV. Top HIV Med. 2008;16:117.
99 Paulus W, Jellinger K, Morgello S, et al. Malignant lymphomas. In: Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumors of the Nervous System. Lyon (France): IARC Press; 2000:108-203.
100 Loureiro C, Gill PS, Meyer PR, et al. Autopsy findings in AIDS-related lymphoma. Cancer. 1988;62:735.
101 Ruiz A, Ganz WI, Post MJ, et al. Use of thallium-201 brain SPECT to differentiate cerebral lymphoma from Toxoplasma encephalitis in AIDS patients. AJNR Am J Neuroradiol. 1994;15:1885.
102 Correa RM, Fellner MD, Durand K, et al. Epstein Barr virus genotypes and LMP-1 variants in HIV-infected patients. J Med Virol. 2007;79:401.
103 Martini M, Capello D, Serraino D, et al. Characterization of variants in the promoter of EBV gene BZLF1 in normal donors, HIV-positive patients and in AIDS-related lymphomas. J Infect. 2007;54:298.
104 Corcoran C, Rebe K, van der Plas H, et al. The predictive value of cerebrospinal fluid Epstein-Barr viral load as a marker of primary central nervous system lymphoma in HIV-infected persons. J Clin Virol. 2008;42:433.
105 Ivers LC, Kim AY, Sax PE. Predictive value of polymerase chain reaction of cerebrospinal fluid for detection of Epstein-Barr virus to establish the diagnosis of HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2004;38:1629.
106 Levy RM, Russell E, Yungbluth M, et al. The efficacy of image-guided stereotactic brain biopsy in neurologically symptomatic acquired immunodeficiency syndrome patients. Neurosurgery. 1992;30:186.
107 Skolasky RL, Dal Pan GJ, Olivi A, et al. HIV-associated primary CNS lymphoma: morbidity and utility of brain biopsy. J Neurol Sci. 1999;163:32.
108 Luzzati R, Ferrari S, Nicolato A, et al. Stereotactic brain biopsy in human immunodeficiency virus–infected patients. Arch Intern Med. 1996;156:565.
109 Donahue BR, Sullivan JW, Cooper JS. Additional experience with empiric radiotherapy for presumed human immunodeficiency virus–associated primary central nervous system lymphoma. Cancer. 1995;76:328.
110 Laperriere NJ, Cerezo L, Milosevic MF, et al. Primary lymphoma of brain: results of management of a modern cohort with radiation therapy. Radiother Oncol. 1997;43:247.
111 Shenkier TN, Voss N, Chhanabhai M, et al. The treatment of primary central nervous system lymphoma in 122 immunocompetent patients: a population-based study of successively treated cohorts from the British Colombia Cancer Agency. Cancer. 2005;103:1008.
112 Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105:496.
113 Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17:1787.
114 Pajonk F, McBride WH. Survival of AIDS patients with primary central nervous system lymphoma may be improved by the radiosensitizing effects of highly active antiretroviral therapy. AIDS. 2002;16:1195.
115 Aboulafia DM, Ratner L, Miles SA, et al. Antiviral and immunomodulatory treatment for AIDS-related primary central nervous system lymphoma: AIDS Malignancies Consortium pilot study 019. Clin Lymphoma Myeloma. 2006;6:399.
116 Bossolasco S, Falk KI, Ponzoni M, et al. Ganciclovir is associated with low or undetectable Epstein-Barr virus DNA load in cerebrospinal fluid of patients with HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2006;42:e21.
117 Omeis I, Siems AL, Harrington W, et al. Spinal Kaposi sarcoma presenting without cutaneous manifestations. Case report. J Neurosurg Spine. 2007;7:558.
118 Ritter AM, Amaker BH, Graham RS, et al. Central nervous system leiomyosarcoma in patients with acquired immunodeficiency syndrome. Report of two cases. J Neurosurg. 2000;92:688.
119 Citow JS, Kranzler L. Multicentric intracranial smooth-muscle tumor in a woman with human immunodeficiency virus. Case report. J Neurosurg. 2000;93:701.
120 Lowenthal DA, Straus DJ, Campbell SW, et al. AIDS-related lymphoid neoplasia. The Memorial Hospital experience. Cancer. 1988;61:2325.
121 Navarro JT, Vall-Llovera F, Mate JL, et al. Decrease in the frequency of meningeal involvement in AIDS-related systemic lymphoma in patients receiving HAART. Haematologica. 2008;93:149.
122 Villelabeitia-Jaureguizar K, Rivas-Gonzalez P, Ibarra-Luzar JI, et al. [Clinical and subclinical neuropathy in patients with human immunodeficiency virus receiving antiretroviral therapy.]. Rev Neurol. 2006;42:513.
123 Cherry CL, McArthur JC, Hoy JF, et al. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol. 2003;26:195.
124 Pettersen JA, Jones G, Worthington C, et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor–mediated neurotoxicity. Ann Neurol. 2006;59:816.
125 Shelburne SAIII, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67.
126 Travis J, Varma A, duPlessis D, et al. Immune reconstitution associated with progressive multifocal leukoencephalopathy in human immunodeficiency virus: a case discussion and review of the literature. Neurologist. 2008;14:321.
127 Kishida S, Ajisawa A. Probable cerebral Mycobacterium avium complex–related immune reconstitution inflammatory syndrome in an HIV-infected patient. Intern Med. 2008;47:1349.
128 Robertson J, Meier M, Wall J, et al. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis. 2006;42:1639.
129 Stoll M, Schmidt RE. Immune restoration inflammatory syndromes: apparently paradoxical clinical events after the initiation of HAART. Curr HIV/AIDS Rep. 2004;1:122.
130 Riedel DJ, Pardo CA, McArthur J, et al. Therapy insight: CNS manifestations of HIV-associated immune reconstitution inflammatory syndrome. Nat Clin Pract Neurol. 2006;2:557.