Acquired Abnormalities
Duodenal Obstruction
Superior Mesenteric Artery Syndrome
Overview: First described at autopsy by von Rokitansky in 1861,1 the term superior mesenteric artery (SMA) syndrome refers to obstruction of the third portion of the duodenum at its crossing between the aorta and the SMA. It also is known by other terms, including cast syndrome and Wilkie syndrome after David Percival Wilkie, who termed it “chronic duodenal ileus” in his description of 75 patients in 1927.2 The overall prevalence is cited to be between 0.013% and 0.3% of patients undergoing upper gastrointestinal (UGI) examinations.3–6
Etiology: The normal angle between the aorta and the SMA is between 25 and 60 degrees, and the normal aorto-SMA distance is 10 to 28 mm,3 parameters that have been found to be significantly correlated to adult body mass index, particularly in females.7 The etiology of SMA syndrome has been ascribed to an abnormally low angle between the aorta and the SMA, bringing them in close apposition at the crossing of the third portion of the duodenum. The abnormally low angle and decreased distance between the aorta and the SMA is thought to occur because of an abnormally low origin of the SMA or an abnormally high position of the duodenojejunal junction at the ligament of Treitz, so that the third portion of the duodenum is located nearer to the origin of the SMA.1,7,8
Although asthenic individuals, particularly after acute weight loss, are most susceptible to the development of SMA syndrome, in a series by Biank et al., it was found that this presentation occurred in only approximately 50% of their patients. In their study population, the body mass index ranged between the 3rd and 97th percentile for age, with a mean at the 39th percentile. Premorbid conditions included Nissen fundoplication, cerebral palsy, traumatic brain injury, and posterior spinal fusion.6 Other persons predisposed to SMA syndrome include burn victims and patients with lumbar hyperlordosis or who are in spinal traction or a spinal cast.9 According to one report the syndrome occurred in several members of one family, raising the question of genetic predisposition.10
Clinical Presentation: The clinical syndrome occurs more commonly in females between the ages of 10 and 39 years, although it has been described rarely in neonates.11–13 The most common clinical presentation includes abdominal pain, vomiting, nausea, early satiety, and anorexia6,14; acute presentation may follow one of the aforementioned premorbid conditions.
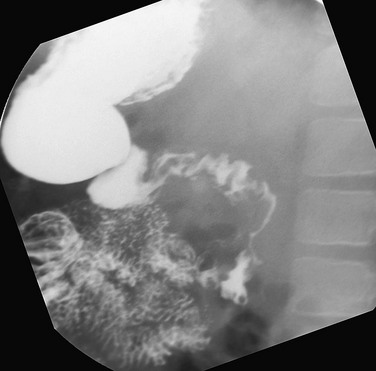
Imaging: Plain radiographs may show gastric dilatation, but the stomach may be decompressed by vomiting or by an enteric tube. Contrast UGI study shows dilatation and partial obstruction of the third portion of the duodenum, terminating in a straight line at the expected location of the crossing of the SMA, with active but poorly effective peristalsis across the point of obstruction (Fig. 104-1, A). A useful maneuver to confirm that the obstruction is a consequence of SMA syndrome is to place the patient in a left side down decubitus position, which tends to relieve SMA obstruction.
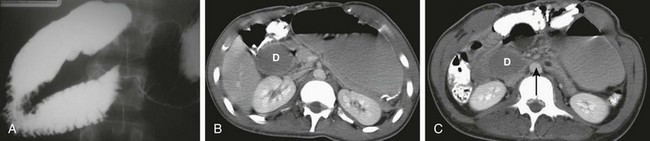
Figure 104-1 Superior mesenteric artery syndrome.
A, A 15-year-old girl with rapid weight loss. An anteroposterior spot image from an upper gastrointestinal examination demonstrates complete obstruction at the third portion of the duodenum. B and C, Two images from a computed tomography scan in a 22-year-old who had sustained a gunshot wound, resulting in paraplegia. The stomach and duodenum (D) are dilated. The arrow points to the compression of the duodenum at the crossing of the superior mesenteric artery, with resolution of dilatation of the duodenal lumen beyond the point of compression.
Ultrasound has been used to measure both the angle and the distance between the aorta and the SMA but may have difficulty outlining their relationship to the duodenum unless the latter is fluid-filled. Computed tomography (CT) (Fig. 104-1, B and C) and magnetic resonance imaging (MRI) are able to assess the dilatation of the duodenum, as well as its relationship to the SMA.
Treatment: Nonoperative treatment centers on weight gain with high-calorie enteral nutrition either by mouth or transpyloric tube feedings beyond the point of obstruction, or with hyperalimentation; such nonoperative intervention is successful in most patients.6 Medical treatment is most likely to be successful when the syndrome is of acute onset.15
Operative treatment is reserved for patients in whom medical management fails and typically consists of a duodenojejunostomy.16 Some authors advocate obtaining a biopsy specimen of the duodenum and jejunum before undertaking surgery to exclude infection, an infiltrative or neoplastic process, or intestinal pseudoobstruction as a cause of the duodenal dilatation.1
Duodenal Hematoma
Overview: A duodenal hematoma is an infrequent injury in pediatric patients, occurring in fewer than 3% of children with abdominal injuries, and usually is associated with blunt abdominal trauma, both accidental and nonaccidental. The hematoma often extends beyond the ligament of Treitz into the proximal jejunum as a duodenojejunal hematoma.17 In a series of 33 children with a blunt duodenal injury, nonaccidental trauma was the most common cause of duodenal injury (24%), followed by motor vehicle crashes, handlebar injures, sports injuries, and other forms of direct trauma.18
Etiology: The retroperitoneal duodenum contains a vascular submucosal and subserosal plexus and lies relatively fixed against the rigid spine, thus facilitating injury after blunt abdominal trauma. Bleeding is contained within the submucosa and subserosa and encroaches into the lumen of the duodenum. In children, this injury typically occurs after directed blunt trauma such as a direct punch or kick to the epigastrium, or as a result of handlebar injuries. Motor vehicle injuries such as those involving sudden deceleration forces also can produce a shearing injury and result in an intramural hematoma.17,19 Duodenal hematomas also have been reported in patients with coagulation abnormalities after a relatively minor trauma, after undergoing an endoscopic duodenal biopsy,19–23 or when pancreatitis develops, with the latter postulated to be a result of disruption of the intramural vasculature by pancreatic enzymes.24,25
Clinical Presentation: The clinical presentation includes abdominal pain and vomiting, which typically is bilious. When a duodenal hematoma occurs after blunt trauma, pancreatitis related to an associated pancreatic injury may develop. A luminal obstruction sufficient to produce symptoms may not occur immediately after injury, and the child may not present until several days after the traumatic event. When a duodenal hematoma occurs after significant trauma such as a motor-vehicle accident, the CT examination may show the diagnosis before symptom onset. One should always remember that this injury, in the appropriate clinical setting, should raise the concern for inflicted trauma.17,20,26
Imaging: Plain radiographic films may be the initial modality in a child presenting with epigastric abdominal pain, tenderness, and vomiting. Depending on the degree of obstruction and the severity of vomiting, the radiographs may show distension of the stomach and duodenum, with paucity of distal bowel contents (Fig. 104-2, A).
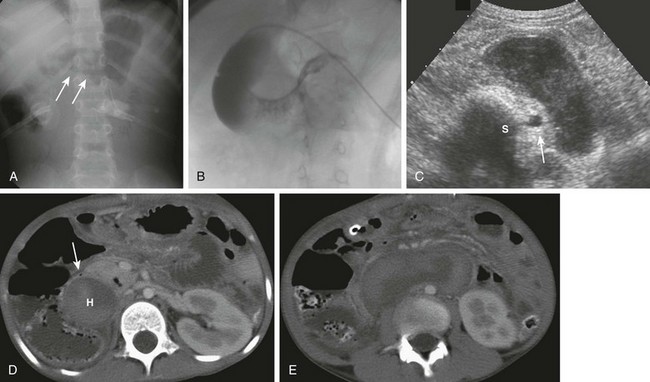
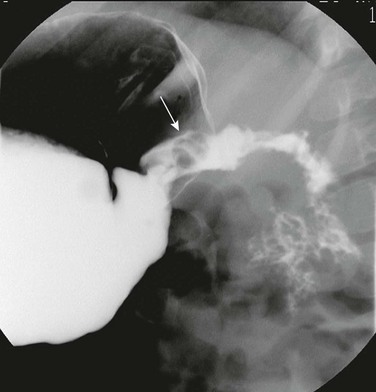
Figure 104-2 Duodenal hematoma.
A, An 8-year-old with agenesis of the corpus callosum who had undergone an upper endoscopy with a duodenal biopsy and subsequently developed persistent bilious vomiting. The abdominal radiograph shows a polypoid density (arrows) encroaching upon the gas along the inferior border of the antrum. Gaseous distension of the duodenum is seen with very little gas distally. B, An upper gastrointestinal examination from a 10-year-old boy with cerebral palsy and mental retardation who presented with dehydration and inability to tolerate feedings. Introduction of contrast material into the duodenum during an unsuccessful attempt at gastrojejunal tube placement shows an obstructing duodenal hematoma as a filling defect upon the third and distal second portions of the duodenum. C, An abdominal ultrasound on the same patient shown in part A demonstrates the heterogeneous duodenal hematoma, following the course of the third portion of the duodenum. S, Spine. Arrow points to the aorta. D, A computed tomography (CT) image at the level of the junction of the second and third portions of the duodenum in the same patient in parts A and C shows the large heterogeneous hematoma (H) obliterating the duodenal lumen. The arrow points to the duodenal wall and gas bubble in the residual obliterated lumen. E, A more distal CT section through the third portion of the duodenum (an analogous section to ultrasound in part C) shows again the heterogeneous hematoma, which is obliterating the lumen of the third portion of the duodenum.
Findings of a UGI examination depend on the ability of some of the contrast material to traverse the obstructed portion of the duodenum. If no or very little contrast material moves through, the luminal border of the distended duodenum may show a lobulated contour. As contrast material passes through the obstruction, the lobulated contour of the filling defect will outline the column of contrast across the duodenum (Fig. 104-2, B). Smaller hematomas may demonstrate more subtle findings, such as some thickening of the duodenal folds.17
Ultrasound (Fig. 104-2, C) typically shows a distended first portion of the duodenum, with a filling defect encroaching upon and obliterating its lumen.27,28 The echogenicity of the hematoma is variable, depending on the status of the clot; the clue to the diagnosis is the fact that the mass follows the course of the duodenum. On Doppler interrogation, the lesion has no flow.
CT demonstrates similar findings, with dilatation of the duodenum and a nonenhancing mass that follows the course of the duodenum, obliterating its lumen (Fig. 104-2, D and E).
MRI often is not performed in children with duodenal hematomas. If it is performed, it again will show a mass following the course of the duodenum, obliterating its lumen, with signal characteristics consistent with an evolving hematoma that may appear in concentric layers of differing signal intensity.29
Treatment: Patients with duodenal perforation need emergent surgery, but the treatment for a duodenal hematoma generally consists of decompression of the stomach and duodenum via a nasogastric tube and maintenance of hydration and nutrition with total parenteral nutrition until the hematoma resolves. In one series of 27 children with a duodenal injury who were treated conservatively, the mean time to resolution of symptoms ranged between 12 and 16 days.18,30 If complete obstruction is present without improvement, it may be necessary to evacuate the hematoma surgically.18
Duodenal Intussusception
Overview: Duodenal intussusception may be seen in both antegrade and retrograde directions, typically around a gastrojejunal (GJ) tube.
Etiology: Antegrade duodenojejunal intussusception is a relatively common complication of the placement of postpyloric GJ tubes, because the tube acts as a lead point for the intussusception. Children with GJ tubes have a 16% to nearly 50% rate of antegrade intussusception.31,32 It is believed that intussusceptions are more likely in boys, in younger patients, in patients with larger bore tubes and tubes with a distal pigtail that may act as a lead point, and when prokinetic agents are administered.32,33 Intussusception also can occur around a weighted nasojejunal tube.34
Retrograde intussusception, on the other hand, is much less common and is believed to occur as a tube with an inflated terminal balloon, which has migrated distally as a result of peristalsis, is pulled back toward the stomach.35,36
Clinical Presentation: The presentation typically is the onset of vomiting, which is bilious in approximately half the cases31 and may be accompanied by abdominal pain/irritability. However, sometimes these intussusceptions are asymptomatic and are discovered at the time of a routine tube check,37 and therefore they may be transient. Hematemesis or bloody gastric aspirates typically are described in patients with retrograde intussusceptions.35,36
Although it bypasses the duodenum, retrograde intussusception also occurs after gastric surgery, such as a gastrojejunostomy. The intussuscepted jejunum may be gangrenous; obstruction and hematemesis are typical in acute presentations.38
Imaging: Abdominal radiographs and ultrasound are helpful in making the diagnosis (e-Fig. 104-3). Radiographs may reveal gastric outlet obstruction, and in cases of retrograde intussusception, gas within the stomach will demonstrate the distal mass protruding into the gastric antrum. Ultrasound demonstrates the classic appearance of bowel within bowel at the gastric outlet31,35,39 and may show a balloon as a lead point. Particularly in retrograde intussusception, upper GI examination will demonstrate the classic “coiled spring” appearance as the contrast material passes into the intussusception; the amount of contrast material may be small and the findings relatively subtle, and thus a high index of suspicion is helpful in arriving at the correct diagnosis.39 On a CT image, the intussusception will be seen extending along the course of the duodenum, and it is seen particularly well when it is outlined by air or contrast medium.40
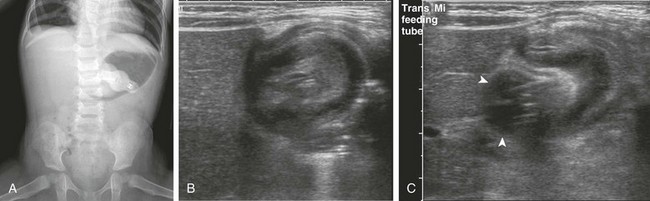
e-Figure 104-3 Retrograde duodenogastric intussusception in a 3-year-old boy with a gastrostomy tube who presented with vomiting.
A, An anteroposterior view of the abdomen demonstrates a gastrostomy tube, a dilated air-filled stomach with a masslike lesion at the antrum, and a paucity of distal bowel gas consistent with gastric outlet obstruction. B, Right upper quadrant ultrasound scan demonstrates an intussusception. C, An ultrasound image immediately adjacent to that in part B confirms that the gastrostomy tube balloon (arrowheads) is intimately related to the intussusception.
Treatment: Introduction of air or saline solution through the tube has been successful in reducing intussusception,31 although antegrade intussusceptions can be treated by replacement of the GJ tube over a wire.33 This complication can be prevented by changing to a smaller bore GJ tube, using a catheter without a pigtail, or shortening the length of GJ tube.41 If the intussusception occurs around a nasojejunal tube, the tube should be removed.37
Retrograde intussusception is usually managed by deflation of the balloon,35 although in some cases of supervening bowel ischemia, surgical resection may be necessary.42
Duodenal Inflammatory Conditions
Duodenal Ulcer Disease
Overview: Ulcer disease can involve the duodenum and, as in the stomach, it is associated with Helicobacter pylori infection, a pathogen that is estimated to colonize or infect approximately half the world’s population.43 The prevalence of ulcer disease is lower in children than in adults, but duodenal ulcers are more common than gastric ulcers in children after the neonatal period.
Etiology: Duodenal ulcerations result when the inherent defense mechanisms are overwhelmed, including epithelial cell renewal and regeneration, duodenal bicarbonate production, preservation of mucosal blood flow, and production of prostaglandins.44 The understanding of peptic and duodenal ulcer disease in both adults and children has changed dramatically since the elucidation of the role of H. pylori infection (then known as Campylobacter pyloridis) in its development by Warren and Marshall in 1984,45,46 for which they were awarded the Nobel Prize in 2005. Because H. pylori is trophic for gastric epithelium, duodenal ulcers related to H. pylori are believed to occur at sites of gastric metaplasia47 and thus are related to increased acid secretion and decreased bicarbonate output by the duodenum, resulting in lower luminal pH and precipitating bile acids, which otherwise would exert an inhibitory effect on the growth of H. pylori. Acid hypersecretion additionally promotes the activation of pepsinogen to pepsin, facilitating mucosal ulceration.47
A higher rate of H. pylori infection has been reported in children with duodenal ulcers (62%) than in those with peptic ulcers (20%), although other sources do not always confirm this relationship.48 Transmission of H. pylori is mainly by the oral-oral or fecal-oral route and requires contact with intestinal secretions; light contact, such as kissing, is not believed to be effective.49,50 Colonization with this pathogen has greater prevalence in locations with suboptimal sanitary infrastructure.50 Childhood is the primary period of colonization by H. pylori, with cases often clustering within families as a result of intrafamilial transmission.50 In Western nations the prevalence of H. pylori colonization is decreasing and has been reported in as few as 27% of children from Western European centers, although it is thought that some underreporting may be occurring because the specimens are being cultured from the stomach rather than the duodenum.
Clinical Presentation: Children with duodenal ulcer disease may present with epigastric tenderness, pain that wakes the child from sleep at night, hematemesis, melena, anorexia, poor weight gain, and vomiting.48,51 Other manifestations include failure to thrive with growth disturbance and poor weight gain, iron deficiency anemia, and idiopathic thrombocytopenic purpura.50
Imaging: Fluoroscopy and upper GI examinations largely have been supplanted in the diagnosis of duodenal ulcer disease by upper endoscopy, which has become the gold standard for diagnosis, in addition to allowing biopsy and culture of detected abnormalities. When upper GI examination is performed, double contrast is more sensitive in the detection of ulcer disease, although this procedure is much more difficult to perform in younger patients and impossible to perform in infants.
Despite the shift to endoscopy, duodenal ulcers should be detected during upper GI examination if they are present. Duodenitis, which can be a result of ulcer disease, is manifested by thickening of the duodenal folds, and this finding has a sensitivity of 45% in detecting duodenal inflammation (e-Fig. 104-4).51 Free peritoneal air may be seen if perforation has occurred (Fig. 104-5, A). Barium is the usual contrast agent, but contrast material that has a high iodine concentration and low osmolality and is water soluble should be used if perforation is a concern. The imaging appearance of duodenal ulcers in children is similar to that in adults. The most common finding in an acute ulcer is contrast within an ulcer crater (Fig. 104-5, B). Radiating folds that represent surrounding mucosal edema also may be seen. When the ulcer is on the nondependent wall of the duodenum, air will fill the ulcer outlined by barium. Chronic duodenal ulcers may lead to a scarred and deformed duodenum, but this appearance is less common in children than in adults. So-called “giant duodenal ulcers” are rare and occur more commonly in association with NSAIDs, in patients with end-stage renal disease, and in patients with Crohn disease.47
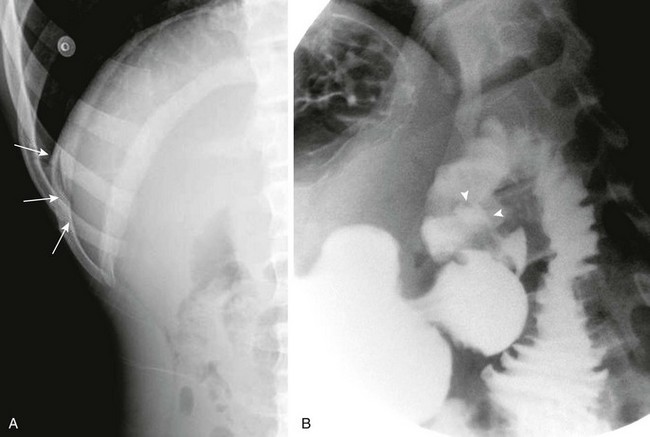
Figure 104-5 A duodenal ulcer.
A, A left lateral decubitus radiograph in a boy with a 1-week history of abdominal pain demonstrates pneumoperitoneum lateral to the liver (arrows). B, A spot image of the duodenal bulb from an upper gastrointestinal (UGI) examination with water-soluble contrast material for the same patient shown in part A reveals a collection of contrast in the ulcer crater (arrowheads). (B, Courtesy Dr. Tamar Ben-Ami, Chicago, IL.)
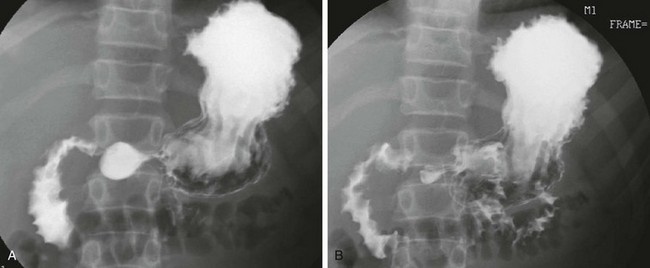
e-Figure 104-4 Nonspecific duodenitis in a 14-year-old boy with abdominal pain.
Filled (A) and collapsed (B) views of the duodenal sweep from an upper gastrointestinal examination demonstrate fold thickening. Findings at biopsy revealed nonspecific inflammation. Cultures were negative, and no specific diagnosis was made.
Ultrasound typically is not performed in patients with known duodenal ulcer disease. Thickening of the bowel wall has been described, as well as surrounding fluid and gas in cases of perforation.52
On CT, thickening of the duodenal wall may be detected, along with contrast material within an ulcer crater.53 In cases of perforation, periduodenal fluid and retroperitoneal and intraperitoneal free air may be identified24 (Fig. 104-6).
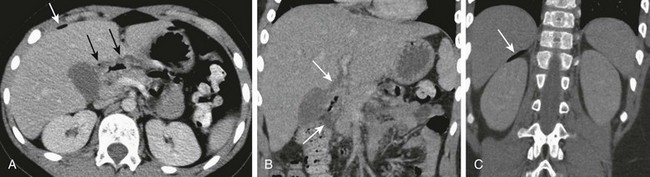
Figure 104-6 A perforated duodenal ulcer.
A, A computed tomography scan in a 9-year-old boy with a 2-day history of abdominal pain, fever, and decreased activity shows thickening of the duodenum, with a small amount of periduodenal fluid and air (black arrows), and free air anterior to the liver (white arrow). B, A coronal reformatted image again shows thickening of the duodenum and periduodenal fluid (arrows). C, A more posterior coronal reformatted image shows additional free air (arrow) superior to the right kidney.
Treatment: Although the initial reports by Marshall and Warren indicated that the pyloric Campylobacter organisms were sensitive to multiple antibiotics,54 not all current strains demonstrate similar wide susceptibility. Eradication of H. pylori in symptomatic patients requires triple therapy with a proton pump inhibitor and two antibiotics for a 2-week treatment course.50
Zollinger-Ellison Syndrome
Overview: The Zollinger-Ellison syndrome refers to autonomous oversecretion of the hormone gastrin by a gastrinoma, resulting in unusually severe and refractory ulcer disease, typically accompanied by diarrhea. The condition is rare, with an incidence quoted as one to three new cases per million population.55 Gastrinomas and the Zollinger-Ellison syndrome can occur sporadically or, in approximately 15% to 38% of cases, as part of the multiple endocrine neoplasia type 1 (MEN-1) syndrome55–57; conversely, the Zollinger-Ellison syndrome occurs in approximately 21% to 70% of patients with MEN-1.
The Zollinger-Ellison syndrome is typically diagnosed in the fifth decade, with approximately 90% of cases presenting between the ages of 20 and 60 years.57 Approximately 8.6% occur in children, about four times more commonly in boys, and typically no earlier than the second decade, with the youngest case reported in a 5-year-old girl.56,58,59 The syndrome tends to present at a younger age when it is part of the MEN-1 syndrome.
Most gastrinomas are located in the wall of the duodenum or in the head of the pancreas, but they also can be located in more distant regions, including the heart, ovary, kidneys, and mesentery. Tumors can be both small and multiple, presenting a challenge to diagnosis.60–62 However, most gastrinomas occur in the so-called gastrinoma triangle, which is located to the right of the SMA and bounded superiorly by the confluence of the cystic and common bile ducts, the junction of the second and third portions of the duodenum inferiorly, and medially by the junction of the head and body of the pancreas.60,63
Etiology: The etiology of the syndrome is the autonomous neoplastic overproduction of gastrin, resulting in acid hypersecretion and extensive ulcer disease. The cause of sporadic gastrin-secreting tumors is not known; however, MEN-1 is the result of an autosomal-dominant mutation in the 10 exon tumor suppressor gene MEN-1, encoding the 610 amino acid protein MENIN. Parathyroid hyperplasia, pancreatic endocrine tumors (of which gastrinoma is the most common), pituitary adenomas, and adrenal adenomas occur in this syndrome. Patients also are prone to the development of carcinoid tumors, gastric, thymic, and bronchial lesions, lipomas, and skin lesions, including melanomas.57
Clinical Presentation: Patients may present with a triad of abdominal pain, weight loss, and diarrhea in conjunction with ulcer disease, which typically is negative for H. pylori. This constellation of symptoms is highly suggestive of Zollinger-Ellison syndrome. Patients also may present with hematemesis, tarry stools, and anemia. Patients with gastrinoma and Zollinger-Ellison syndrome should undergo further investigation for MEN-1.57,58 Some patients with MEN-1 may present initially with evidence of hyperparathyroidism, such as urolithiasis; manifestation of gastrinoma may follow, sometimes up to 25 years later.64 Approximately 60% of gastrinomas are malignant, and 50% have metastasized at diagnosis.60,65
The primary ulcer site is the duodenum, and ulcers can occur in the distal duodenum as well as the jejunum, sites that should raise concern for gastrinoma. In a pediatric series of patients with Zollinger-Ellison syndrome, the primary ulcer site was the duodenum in 72% and the jejunum in 28%.58
Successful initial treatment with proton pump inhibitors may delay the diagnosis by assuaging the symptoms.55,57 The diagnosis is made by demonstration of hypersecretion of gastrin, determined by measurement of fasting gastrin levels and acid secretion. However, in patients who are treated with proton pump inhibitors, fasting serum gastrin levels may be sufficiently elevated to overlap with those seen in patients with Zollinger-Ellison syndrome57; in such cases, proton pump inhibitors should be withdrawn for approximately 1 week before repeating the test.55 In addition to fasting gastrin levels, provocative tests also are used, utilizing secretin, calcium, and meal stimulation.57
Imaging: The classic finding on upper GI examination is the presence of ulcers in the duodenum, particularly the distal duodenum, or in the jejunum, as well as thickening of duodenal and gastric folds.57 Ulcer disease is now more often evaluated endoscopically; up to 94% of patients with Zollinger-Ellison syndrome are reported to have thickened gastric folds.55
Ultrasonography has not been found to be sensitive in detecting gastrinomas either inside or outside the liver and pancreas, showing a sensitivity of approximately 19%.65 With CT, identification of hypervascular pancreatic gastrinomas is optimized with the rapid bolus technique, with thin collimation and reformatted images. With 10-mm collimation, CT has a sensitivity of 38%,65 although much better performance could reasonably be expected with today’s rapid scanners and thin collimation. With MRI, detection is optimized by a high field strength magnet, water-sensitive sequences, and administration of contrast material; MR sensitivity is quoted as 45%.65 On the other hand, somatostatin receptor scintigraphy using [111In-DTPA-DPhe1] octreotide was found to be 70% sensitive in the detection of the tumors.57,65 In a later publication of 151 patients with gastrinomas,61 diagnostic sensitivity of imaging was similar: 24% for ultrasound, 39% for CT, 46% for MRI, and 79% for somatostatin-receptor scintigraphy. In approximately one third of patients, all imaging studies are negative.61 Imamura has described a venous sampling technique that is helpful in detecting more than 90% of gastrinomas that are <5 mm. The technique involves venous sampling after selective arterial injection of secretin or calcium to elicit gastrin release from the gastrinoma, termed the selective arterial secretagogue injection test (SASI).62,66
Treatment: Control of hyperacidity is one of the key elements in survival.57,67 At one time, control of hyperacidity was achieved with gastrectomy; today, acid suppression is achieved medically with proton pump inhibitors. Thus the management of patients is geared toward treatment of the gastrinoma itself and other manifestations of the MEN-1 syndrome if it is present. Although most gastrinomas demonstrate slow growth, 25% show more rapid growth, and approximately 60% to 90% demonstrate malignant behavior.61 In a study of 151 patients, more than half of the patients with sporadic gastrinomas were free of disease after surgery, and most of these patients were able to achieve a long-term cure; this approach is preferred in patients with sporadic gastrinoma.61 In patients with MEN-1 and gastrinoma, some authors advocate a pancreas-preserving total duodenectomy to achieve a cure in some of the patients.62
Inflammatory Bowel Disease
Overview: Crohn disease most commonly involves the distal small bowel and colon and will be discussed in Chapters 105 and 107.
Although involvement of the duodenum with Crohn disease is nearly universally accompanied by ileal or ileocolic disease,68,69 there is a wide difference in the reported percentage of patients with Crohn disease who have duodenal involvement, with increasing prevalence in more recent reports.70 This finding is likely related to the more frequent use of endoscopy for the diagnosis, along with the fact that a large percentage of patients with endoscopic and histologic disease do not have clinical symptoms separable from those of the more distal involvement.69,71,72 The duodenum is the most frequently affected segment in both pediatric and adult patients with upper tract Crohn disease.72
Etiology: The etiology of Crohn disease remains unknown, although a genetic predisposition is underscored by familial occurrence and concordance of the disease in monozygotic twins (44% to 58%).73 Other factors that affect the development of the disease include immune dysfunction, gut flora, and environmental factors such as active or passive cigarette smoking, appendectomy, diet, perinatal infections, and measles infection.73
Clinical Presentation: Patients with duodenal involvement tend to present at a younger age than do patients with distal involvement,74 typically with nonspecific abdominal pain and general malaise and weight loss.75
Complications related to duodenal involvement include involvement of the ampullary region of the duodenum, leading to biliary and pancreatic obstruction.76 Pancreatitis may occur, although it is attributed to other causes such as medications used to treat the primary disease.77 Fistula formation also occurs, although it is believed that fistulas between the duodenum and small bowel or colon usually originate in the latter, rather than in the duodenum.69
Imaging: Just as the diagnosis of Crohn disease has shifted from radiography to endoscopy, the imaging modalities have shifted from radiography to cross-sectional imaging, including ultrasound with color or power Doppler, CT, and MRI. However, if upper GI examination is requested in a patient with abdominal pain and weight loss, the diagnostic findings should be appreciated.
The typical imaging findings on upper GI examination in patients with Crohn disease are similar to those found in the distal bowel. Double-contrast studies are more sensitive than single-contrast examinations in identifying the aphthous lesions that characterize the early stages of the disease. These lesions typically will be found in the antrum, pylorus, and duodenum. Duodenal fold thickening, luminal narrowing, wall thickening, and fistula and sinus tract formation are also findings seen at upper GI examination (Fig. 104-7).
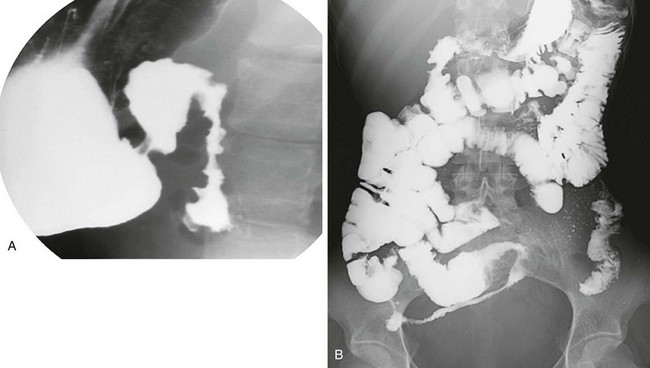
Figure 104-7 Crohn disease.
A, A right anterior oblique upper gastrointestinal spot image demonstrates duodenal mucosal fold thickening. B, A small bowel follow-through again identifies the duodenal changes, as well as typical involvement of the terminal ileum with luminal narrowing and spiculation. (Courtesy Lakshmana Das Narla, Richmond, VA.)
Ultrasound is able to detect bowel wall thickening, and Doppler is able to detect the hyperemia characteristic of active Crohn disease, similar to terminal ileal disease.78–81
CT is helpful in identifying involved bowel segments and surrounding pathology, such as abscess and fistula formation, and is most helpful in patients who are acutely ill, patients with bowel obstruction, and other patients who are unable to tolerate MRI because of procedure length and other requirements such as sedation in younger patients. Duodenal findings include fold thickening, increased enhancement, and stricture formation.24,53,82
MR enterography is becoming the ideal modality in the routine imaging evaluation of patients with Crohn disease. The findings in the duodenum are similar to those in other bowel segments.83,84
Treatment: Therapy for duodenal Crohn disease typically includes exclusion of H. pylori. Treatment then consists of proton pump inhibition to inhibit gastric secretion and promote ulcer healing, as well as corticosteroids. Azathioprine and 6-mercaptopurine are used to maintain remission. Strictures may be amenable to endoscopic balloon dilatation. Patients who do not respond to medical management, or in whom a massive gastrointestinal hemorrhage develops, may need surgical intervention.69
Cystic Fibrosis
Overview: Cystic fibrosis has multiple manifestations in the gastrointestinal tract; those affecting the small bowel and colon are covered in Chapters 103 and 105. Duodenal involvement includes duodenal fold thickening, ulceration, and stricture formation.85
Etiology: Duodenal and gastric ulceration has been reported in as many as 10% of autopsy reports of pediatric patients with cystic fibrosis.86 The etiology of these duodenal abnormalities is thought to be related to impaired secretion of bicarbonate by the duodenum and pancreas, which curtails the ability to buffer gastric acid.87 Thickening of the duodenal folds is believed to be related to hyperacidity, abnormal mucus production, and hypertrophy of Brunner glands.86 A hyperacid duodenal environment also leads to precipitation of bile salts and decreased fat absorption.
Inflammatory changes in the gut of patients with cystic fibrosis have been found through identification of T-cell activation products88 and output of inflammatory proteins.89 The etiology of inflammatory changes in the duodenal mucosa may include factors such as increased antigenic load as a result of pancreatic insufficiency, defective digestion of luminal contents,88 and altered intestinal flora.90
Clinical Presentation: Patients usually present with abdominal pain; hematemesis may occur if ulcer disease is present.86 Patients also may manifest symptoms of severe gastroesophageal reflux, which is thought to be partly related to gastrointestinal dysmotility and the need for frequent postural drainage.91
Imaging: The findings on upper GI examination typically demonstrate thick and coarse duodenal folds (e-Fig. 104-8), particularly along the first and second portions; ulceration86; or frank stricture formation.85,87 Distortion and kinking of the duodenal contours at times simulates a mass lesion.92
Duodenal Neoplasms
Duodenal neoplasms are uncommon. The frequency of benign tumors of the small bowel at autopsy has been reported as being 0.16%, and of these, approximately 18% are located in the duodenum. Fewer than 2% of malignant gastrointestinal tumors arise in the small bowel.95,96 Neoplasms that are characteristic to the duodenum include lesions related to Brunner glands, adenomas associated with polyposis syndromes, and duodenal carcinoid tumors. Brunner gland hyperplasia has been described as being of three types: diffuse through the duodenum; circumscribed and confined to its first portion; and Brunner gland adenoma, which refers to lesions larger than 1 cm.95 Brunner gland hyperplasia and duodenal polyps may be seen in approximately 15.2% of uremic patients of all ages undergoing upper GI examination, compared with approximately 0.3% of nonuremic patients.95
Duodenal polyps are reported in approximately 0.4% of children undergoing upper endoscopy.97 Most duodenal polyps not associated with Brunner glands are associated with polyposis syndromes. Familial adenomatous polyposis (FAP) is the most commonly associated systemic abnormality in patients with duodenal adenomatous polyps; conversely, adenomatous duodenal polyps occur in approximately 90% of patients with FAP.96,97 In an endoscopic study of 24 pediatric patients with FAP, periampullary adenomas were found in 41%.98 Children with Peutz-Jeghers syndrome develop hamartomatous polyps in the duodenum,97 although adenomatous changes develop in 3% to 6% of hamartomas.96 Brunner gland hypertrophy develops in patients with juvenile polyposis,97 but cancers of the duodenum also have been reported in this population.96
Duodenal carcinoids are associated with the Zollinger-Ellison syndrome, particularly in association with MEN-1 (G-cell carcinoids, of which approximately one third manifest as Zollinger-Ellison syndrome) and with neurofibromatosis type 1 (D-cell carcinoids that produce somatostatin). Approximately two thirds of duodenal carcinoids are G-cell gastrin tumors, whereas somatostatin-producing D-cell tumors represent approximately one fifth of duodenal carcinoids, nearly exclusively located in the periampullary region.99
Etiology: The hyperplasia of Brunner glands that occurs in uremic patients is thought to be related to elevated gastrin levels and hyperacidity that occur in patients with end-stage renal insufficiency.95,100,101 Brunner gland adenomas are associated with chronic renal failure, chronic pancreatitis, peptic ulcer disease, and infection with H. pylori.102
Clinical Manifestations: Patients with Brunner gland hyperplasia typically are asymptomatic. When symptoms occur, patients tend to present with bleeding, obstruction, pain, vomiting, and diarrhea.95 Large lesions such as Brunner gland adenoma can present with gastric outlet obstruction, biliary obstruction, and pancreatitis when affecting the periampullary region.102
Duodenal carcinoids may manifest as Zollinger-Ellison syndrome. However, D-cell somatostatin-producing tumors arising in the duodenum are usually too small to produce clinically apparent symptoms related to hormone overproduction; such symptoms are present in less than 10% of cases and typically include abdominal pain, diarrhea, steatorrhea, and weight loss. Unlike carcinoids occurring in other parts of the small bowel, those that occur in the duodenum are not associated with serotonin overproduction and thus do not produce symptoms of the carcinoid syndrome.99
Imaging: On upper GI examination, a filling defect within the duodenal bulb may be demonstrated (e-Fig. 104-9). On ultrasound and CT, large or prolapsing lesions may present a confusing picture and be mistaken for a pancreatic mass, which can have significant surgical implications if the true nature and location of the lesion are not documented preoperatively. A giant Brunner gland adenoma may demonstrate gastric outlet obstruction on abdominal radiographs.102 Multiple polyps, such as are seen in patients with FAP or Peutz-Jeghers syndromes, appear as multiple filling defects, at times complicated by intussusception (Fig. 104-10). Duodenal carcinoids have been described as intraluminal or mural filling defects, sometimes with ulcerations, and occasionally producing obstruction by intussusception; duodenal folds may be thickened in a small number of patients. On CT, duodenal carcinoids have been described as lesions with marked contrast enhancement in the arterial phase, decreased enhancement in later phases, and possible invasion into the adjacent pancreas. On MRI they have been described as having low signal on T1 weighting, with a heterogeneously high signal on water-sensitive sequences, and positive gadolinium enhancement.99
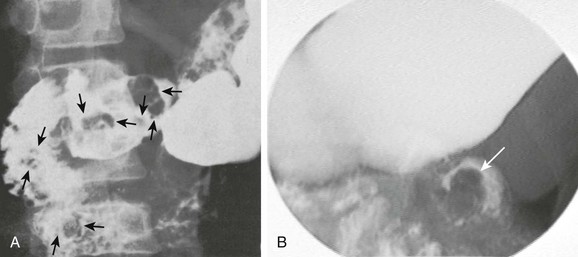
Figure 104-10 Peutz-Jeghers syndrome with duodenal polyps.
A, Multiple polyps in the duodenum (arrows) in a 12-year-old boy with Peutz-Jeghers syndrome. He also had polyps in the stomach, colon, and the remainder of the small intestine. B, A 9-year-old boy with Peutz-Jeghers syndrome presenting with symptoms of intermittent obstruction. An upper gastrointestinal spot image of the proximal jejunum demonstrates a filling defect (arrow) due to duodenojejunal intussusception. A hamartomatous duodenal polyp was found to be the lead point at endoscopy. (Courtesy R. Paul Guillerman, Houston, TX.)
Treatment: Brunner gland hyperplasia does not require specific treatment if the patient is asymptomatic. Conservative treatment consists of control of gastric acidity with proton pump antagonists and H2 blockers. Brunner gland adenomas may be amenable to endoscopic removal, although larger or more diffuse lesions may require more extensive surgical polypectomy or segmental or more extensive duodenal resection.102 Patients with FAP undergo periodic endoscopic screening, even pediatric patients starting at the time of initial screening colonoscopy. In children, upper gastrointestinal symptoms are present in only a small number of patients and are not predictive of the presence of duodenal adenomas. Management of complicating periampullary adenocarcinoma includes pancreaticoduodenectomy. Endoscopic ampullectomy may be helpful in some patients with early lesions.98
Agrons, GA, et al. Gastrointestinal manifestations of cystic fibrosis: radiologic-pathologic correlation. Radiographics. 1996;16(4):871–893.
Attard, TM, Abraham, SC, Cuffari, C. The clinical spectrum of duodenal polyps in pediatrics. J Pediatr Gastroenterol Nutr. 2003;36(1):116–119.
Ellison, EC, Johnson, JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46(1):13–106.
Gaines, BA, et al. Duodenal injuries in children: beware of child abuse. J Pediatr Surg. 2004;39(4):600–602.
Hughes, UM, et al. Further report of small-bowel intussusceptions related to gastrojejunostomy tubes. Pediatr Radiol. 2000;30(9):614–617.
Kalach, N, et al. Frequency and risk factors of gastric and duodenal ulcers or erosions in children: a prospective 1-month European multicenter study. Eur J Gastroenterol Hepatol. 2010;22(10):1174–1181.
Levy, AD, et al. Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology. 2005;237(3):967–972.
Long, FR, et al. Duodenitis in children: correlation of radiologic findings with endoscopic and pathologic findings. Radiology. 1998;206(1):103–108.
Mashako, MN, et al. Crohn’s disease lesions in the upper gastrointestinal tract: correlation between clinical, radiological, endoscopic, and histological features in adolescents and children. J Pediatr Gastroenterol Nutr. 1989;8(4):442–446.
Merrett, ND, et al. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg. 2009;13(2):287–292.
References
1. Raissi, B, Taylor, BM, Taves, DH. Recurrent superior mesenteric artery (Wilkie’s) syndrome: a case report. Can J Surg. 1996;39(5):410–416.
2. Wilkie, D. Chronic duodenal ileus. Am J Med Sci. 1927;173(5):643.
3. Neri, S, et al. Ultrasound imaging in diagnosis of superior mesenteric artery syndrome. J Intern Med. 2005;257(4):346–351.
4. Anderson, JR, Earnshaw, PM, Fraser, GM. Extrinsic compression of the third part of the duodenum. Clin Radiol. 1982;33(1):75–81.
5. Goin, LS, Wilk, SP. Intermittent arteriomesenteric occlusion of the duodenum. Radiology. 1956;67(5):729–737.
6. Biank, V, Werlin, S. Superior mesenteric artery syndrome in children: a 20-year experience. J Pediatr Gastroenterol Nutr. 2006;42(5):522–525.
7. Ozkurt, H, et al. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat. 2007;29(7):595–599.
8. Strong, E. Mechanics of arteriomesenteric duodenal obstruction and direct surgical attack upon etiology. Ann Surg. 1958;148(5):725–730.
9. Jain, R. Superior mesenteric artery syndrome. Curr Treat Options Gastroenterol. 2007;10(1):24–27.
10. Ortiz, C, et al. Familial superior mesenteric artery syndrome. Pediatr Radiol. 1990;20(8):588–589.
11. Mosalli, R, et al. Superior mesenteric artery syndrome: a rare cause of complete intestinal obstruction in neonates. J Pediatr Surg. 2011;46(12):e29–e31.
12. Okugawa, Y, et al. Superior mesenteric artery syndrome in an infant: case report and literature review. J Pediatr Surg. 2007;42(10):E5–E8.
13. Caspi, B, et al. Prenatal manifestation of superior mesenteric artery syndrome. Prenat Diagn. 2003;23(11):932–934.
14. Marchant, EA, Alvear, DT, Fagelman, KM. True clinical entity of vascular compression of the duodenum in adolescence. Surg Gynecol Obstet. 1989;168(5):381–386.
15. Makam, R, et al. Laparoscopic management of superior mesentric artery syndrome: a case report and review of literature. J Minim Access Surg. 2008;4(3):80–82.
16. Richardson, WS, Surowiec, WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377–378.
17. Kleinman, PK, Brill, PW, Winchester, P. Resolving duodenal-jejunal hematoma in abused children. Radiology. 1986;160(3):747–750.
18. Clendenon, JN, et al. Management of duodenal injuries in children. J Pediatr Surg. 2004;39(6):964–968.
19. Deambrosis, K, et al. Delayed duodenal hematoma and pancreatitis from a seatbelt injury. West J Emerg Med. 2011;12(1):128–130.
20. Gaines, BA, et al. Duodenal injuries in children: beware of child abuse. J Pediatr Surg. 2004;39(4):600–602.
21. Dunkin, D, Benkov, KJ, Rosenberg, HK. Duodenal and rectal hematomas complicating endoscopic biopsy: use of sonography in pediatrics. J Ultrasound Med. 2009;28(11):1575–1580.
22. Iuchtman, M, et al. Post-traumatic intramural duodenal hematoma in children. Isr Med Assoc J. 2006;8(2):95–97.
23. Szajewska, H, et al. Intramural duodenal hematoma: an unusual complication of duodenal biopsy sampling. J Pediatr Gastroenterol Nutr. 1993;16(3):331–333.
24. Jayaraman, MV, et al. CT of the duodenum: an overlooked segment gets its due. Radiographics. 2001;21:S147–S160. [Spec No].
25. Ma, JK, et al. Pancreatic-induced intramural duodenal haematoma. Asian J Surg. 2008;31(2):83–86.
26. Hilmes, MA, et al. CT identification of abdominal injuries in abused pre-school-age children. Pediatr Radiol. 2011;41(5):643–651.
27. Hernanz-Schulman, M, Genieser, NB, Ambrosino, M. Sonographic diagnosis of intramural duodenal hematoma. J Ultrasound Med. 1989;8(5):273–276.
28. Megremis, S, et al. Sonographic diagnosis and monitoring of an obstructing duodenal hematoma after blunt trauma: correlation with computed tomographic and surgical findings. J Ultrasound Med. 2004;23(12):1679–1683.
29. Hahn, PF, et al. Duodenal hematoma: the ring sign in MR imaging. Radiology. 1986;159(2):379–382.
30. Shilyansky, J, et al. Diagnosis and management of duodenal injuries in children. J Pediatr Surg. 1997;32(6):880–886.
31. Hughes, UM, et al. Further report of small-bowel intussusceptions related to gastrojejunostomy tubes. Pediatr Radiol. 2000;30(9):614–617.
32. Friedman, JN, et al. Complications associated with image-guided gastrostomy and gastrojejunostomy tubes in children. Pediatrics. 2004;114(2):458–461.
33. Connolly, BL. Gastrointestinal interventions—emphasis on children. Tech Vasc Interv Radiol. 2003;6(4):182–191.
34. Hughes, U, Connolly, B. Small-bowel intussusceptions occurring around nasojejunal enteral tubes—three cases occurring in children. Pediatr Radiol. 2001;31(6):456–457.
35. Fisher, D, Hadas-Halpern, I. Jejunoduodenogastric intussusception—a rare complication of gastrostomy tube migration. Pediatr Radiol. 2001;31(6):455.
36. Gasparri, MG, et al. Retrograde jejunogastric intussusception. South Med J. 2000;93(5):499–500.
37. Navarro, O, Daneman, A. Intussusception. Part 3: Diagnosis and management of those with an identifiable or predisposing cause and those that reduce spontaneously. Pediatr Radiol. 2004;34(4):305–312. [quiz 369].
38. Prasad, S, Ramachandra, L, Deepak, S. Gangrenous jejunogastric intussusception. Internet J Surg. 19(1), 2009.
39. Connolly, BL, et al. Recognition of intussusception around gastrojejunostomy tubes in children. AJR Am J Roentgenol. 1998;170(2):467–470.
40. Weber, A, Nadel, S. CT appearance of retrograde jejunoduodenogastric intussusception: a rare complication of gastrostomy tubes. AJR Am J Roentgenol. 1991;156(5):957–959.
41. Soscia, J, Friedman, JN. A guide to the management of common gastrostomy and gastrojejunostomy tube problems. Paediatr Child Health. 2011;16(5):281–287.
42. Osuntokun, B, et al. Duodenogastric intussusception: a rare cause of gastric outlet obstruction. J Pediatr Gastroenterol Nutr. 2004;39(3):299–301.
43. Pacheco, AR, et al. Involvement of the Helicobacter pylori plasticity region and cag pathogenicity island genes in the development of gastroduodenal diseases. Eur J Clin Microbiol Infect Dis. 2008;27(11):1053–1059.
44. Vakil, N. Peptic ulcer disease. In: Feldman M, Friedman L, Brandt L, eds. Sleisenger and Fordtran’s gastrointestinal and liver disease. Philadelphia: Saunders, 2010.
45. Marshall, BJ, et al. Attempt to fulfill Koch’s postulates for pyloric Campylobacter. Med J Aust. 1985;142(8):436–439.
46. Marshall, BJ, Warren, JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315.
47. Soll, A, Graham, D. Peptic ulcer disease. In: Yamada T, ed. Textbook of gastroenterology. Hoboken, NJ: Wiley-Blackwell, 2009.
48. Kalach, N, et al. Frequency and risk factors of gastric and duodenal ulcers or erosions in children: a prospective 1-month European multicenter study. Eur J Gastroenterol Hepatol. 2010;22(10):1174–1181.
49. Boyanova, L, et al. Helicobacter pylori and Helicobacter heilmannii in untreated Bulgarian children over a period of 10 years. J Med Microbiol. 2007;56(pt 8):1081–1085.
50. Gold, B. Helicobacter pylori infection. In Liacouras CA, Piccoli D, eds.: Pediatric gastroenterology: requisites, 1st ed, Philadelphia: Mosby, 2008.
51. Long, FR, et al. Duodenitis in children: correlation of radiologic findings with endoscopic and pathologic findings. Radiology. 1998;206(1):103–108.
52. Minardos, I, et al. Peptic ulcer perforation: sonographic imaging of active fluid leakage. J Clin Ultrasound. 2006;34(1):38–41.
53. Zissin, R, et al. Pictorial review. CT of duodenal pathology. Br J Radiol. 2002;75(889):78–84.
54. Marshall, BJ, et al. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985;142(8):439–444.
55. Gibril, F, Jensen, RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6(6):454–463.
56. Grosfeld, JL, et al. Pancreatic tumors in childhood: analysis of 13 cases. J Pediatr Surg. 1990;25(10):1057–1062.
57. Ellison, EC, Johnson, JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46(1):13–106.
58. Rosenlund, ML. The Zollinger-Ellison syndrome in children: a review. Am J Med Sci. 1967;254(6):884–892.
59. Abe, K, Niikawa, N, Sasaki, H. Zollinger-Ellison syndrome with Marden-Walker syndrome. Association of two rare diseases in a 5-year-old girl. Am J Dis Child. 1979;133(7):735–738.
60. Eire, PF, et al. Uncommon case of gastrinoma in a child. Eur J Pediatr Surg. 1996;6(3):173–174.
61. Norton, JA, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341(9):635–644.
62. Imamura, M, Komoto, I, Ota, S. Changing treatment strategy for gastrinoma in patients with Zollinger-Ellison syndrome. World J Surg. 2006;30(1):1–11.
63. Passaro, E, Jr., et al. The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Arch Surg. 1998;133(1):13–16. [discussion 17].
64. Gibril, F, et al. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83(1):43–83.
65. Gibril, F, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas. A prospective study. Ann Intern Med. 1996;125(1):26–34.
66. Imamura, M, et al. Usefulness of selective arterial secretin injection test for localization of gastrinoma in the Zollinger-Ellison syndrome. Ann Surg. 1987;205(3):230–239.
67. Wilson, SD. Zollinger-Ellison syndrome in children: a 25-year follow-up. Surgery. 1991;110(4):696–702. [discussion 702-703].
68. Wagtmans, MJ, et al. Crohn’s disease of the upper gastrointestinal tract. Neth J Med. 1997;50(2):S2–S7.
69. Kefalas, CH. Gastroduodenal Crohn’s disease. Proc (Bayl Univ Med Cent). 2003;16(2):147–151.
70. van Hogezand, RA, et al. Proximal Crohn’s disease: review of the clinicopathologic features and therapy. Inflamm Bowel Dis. 2001;7(4):328–337.
71. Lenaerts, C, et al. High incidence of upper gastrointestinal tract involvement in children with Crohn disease. Pediatrics. 1989;83(5):777–781.
72. Mashako, MN, et al. Crohn’s disease lesions in the upper gastrointestinal tract: correlation between clinical, radiological, endoscopic, and histological features in adolescents and children. J Pediatr Gastroenterol Nutr. 1989;8(4):442–446.
73. Beattie, RM, et al. Inflammatory bowel disease. Arch Dis Child. 2006;91(5):426–432.
74. Wagtmans, MJ, et al. Clinical aspects of Crohn’s disease of the upper gastrointestinal tract: a comparison with distal Crohn’s disease. Am J Gastroenterol. 1997;92(9):1467–1471.
75. Lukovich, P, et al. [Crohn’s disease of the duodenum. Clinical signs, diagnosis, conservative and surgical treatment]. Orv Hetil. 2008;149(11):505–508.
76. Yung, K, et al. Ampullary stenosis with biliary obstruction in duodenal Crohn’s disease: a case report and review of the literature. Dig Dis Sci. 2005;50(6):1118–1121.
77. Pitchumoni, CS, Rubin, A, Das, K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44(4):246–253.
78. Parente, F, et al. Bowel ultrasound in assessment of Crohn’s disease and detection of related small bowel strictures: a prospective comparative study versus x ray and intraoperative findings. Gut. 2002;50(4):490–495.
79. Thomson, M, et al. Graded compression and power Doppler ultrasonography versus endoscopy to assess paediatric Crohn disease activity pre- and posttreatment. J Pediatr Gastroenterol Nutr. 2012;54(3):404–408.
80. Alison, M, et al. Ultrasonography of Crohn disease in children. Pediatr Radiol. 2007;37(11):1071–1082.
81. Spalinger, et al. Doppler US in patients with Crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217(3):787–791.
82. Dillman, JR, et al. CT enterography of pediatric Crohn disease. Pediatr Radiol. 2010;40(1):97–105.
83. Tolan, DJ, et al. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010;30(2):367–384.
84. Toma, P, et al. CT and MRI of paediatric Crohn disease. Pediatr Radiol. 2007;37(11):1083–1092.
85. Phelan, MS, et al. Radiographic abnormalities of the duodenum in cystic fibrosis. Clin Radiol. 1983;34(5):573–577.
86. Agrons, GA, et al. Gastrointestinal manifestations of cystic fibrosis: radiologic-pathologic correlation. Radiographics. 1996;16(4):871–893.
87. Robertson, MB, Choe, KA, Joseph, PM. Review of the abdominal manifestations of cystic fibrosis in the adult patient. Radiographics. 2006;26(3):679–690.
88. Raia, V, et al. Evidence of chronic inflammation in morphologically normal small intestine of cystic fibrosis patients. Pediatr Res. 2000;47(3):344–350.
89. Smyth, RL, et al. Intestinal inflammation in cystic fibrosis. Arch Dis Child. 2000;82(5):394–399.
90. Bruzzese, E, et al. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther. 2004;20(7):813–819.
91. Levine, J. Gastrointestinal manifestations of systemic diseases. In: Yamada T, ed. Textbook of gastroenterology. Hoboken, NJ: Wiley Online Library, 2009.
92. Taussig, LM, Saldino, RM, Di Sant’Agnese, PA. Radiographic abnormalities of the duodenum and small bowel in cystic fibrosis of the pancreas (mucoviscidosis). Radiology. 1973;106(2):369–376.
93. van der Doef, HP, et al. Gastric acid inhibition for fat malabsorption or gastroesophageal reflux disease in cystic fibrosis: longitudinal effect on bacterial colonization and pulmonary function. J Pediatr. 2009;155(5):629–633.
94. De Matos, V, Mascarenhas, M. Cystic fibrosis. In Liacouras C, Piccoli D, eds.: Pediatric gastroenterology: requisites, 1st ed, Philadelphia: Mosby, 2007.
95. Paimela, H, et al. Multiple duodenal polyps in uraemia: a little known clinical entity. Gut. 1984;25(3):259–263.
96. Bresalier, R, Anadasabapathy, S. Tumors of the small intestine. In: Yamada T, ed. Textbook of gastroenterology. Hoboken, NJ: Wiley Online Library, 2009.
97. Attard, TM, Abraham, SC, Cuffari, C. The clinical spectrum of duodenal polyps in pediatrics. J Pediatr Gastroenterol Nutr. 2003;36(1):116–119.
98. Attard, TM, et al. Multicenter experience with upper gastrointestinal polyps in pediatric patients with familial adenomatous polyposis. Am J Gastroenterol. 2004;99(4):681–686.
99. Levy, AD, et al. Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology. 2005;237(3):967–972.
100. Dekker, W. Clinical relevance of gastric and duodenal polyps. Scand J Gastroenterol Suppl. 1990;178:7–12.
101. Tori, A, Gupta, SK. Clinical quiz. Uremia. J Pediatr Gastroenterol Nutr. 2005;40(5):609–610.
102. Gupta, V, Gupta, P, Jain, A. Giant Brunner’s gland adenoma of the duodenal bulb presenting with ampullary and duodenal obstruction mimicking pancreatic malignancy. JOP. 2011;12(4):413–419.