Chapter 28 Acne Cosmeceutical Myths
The purpose of this chapter is to dispel some of the commonly held myths regarding acne cosmeceuticals. These myths may be held by dermatologists and patients alike. They are perpetuated by the popular press and exuberant marketing efforts that present ideas or concepts that seem to make common sense but cannot be verified by the scientific method. This chapter presents the material by first stating the acne cosmeceutical myth and subsequently exploring where the truth may lie. The acne myths discussed were collected from the authors and editors of this text by canvassing dermatologists in their respective practices and dermatology training programs. It is hoped that this material serves to further cosmeceutical science by providing concise analyses to frequent acne misconceptions.
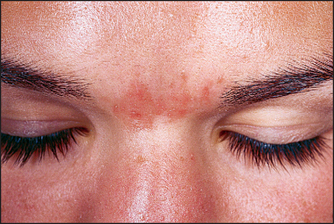
COSMECEUTICALS DO NOT PRODUCE ACNE IF LABELED NONCOMEDOGENIC AND NONACNEGENIC
Similar to hypoallergenic, noncomedogenic and nonacnegenic are marketing claims carrying no implied regulation (Fig. 28.1). They were also developed to create a new consumer image for cosmetic lines designed to minimize acne. In order to make the claim noncomedogenic, rabbit ear or human comedogenicity testing should be undertaken. Both the animal and the human model are based on the presence of new comedone formation after the exposure of skin to the finished cosmetic. Human testing is considered to be more accurate, but the results are highly dependent on the skill of the contract testing laboratory. Acnegenic claims are based on human use testing and the evaluation of volunteer subjects following product use for an increase in the presence of acne. Many manufacturers, however, make noncomedogenic and nonacnegenic claims based on the safety profiles of the individual ingredients in the formulation. This is inaccurate. Noncomedogenic and nonacnegenic claims should be made based on clinical testing of the finished formulation. The dermatologist should still consider all products labeled noncomedogenic or nonacnegenic as problematic.
MINERAL OIL IS COMEDOGENIC
Mineral oil is one of the most common ingredients in skin care products and colored cosmetics (Fig. 28.2). It is a lightweight inexpensive oil that is odorless and tasteless. One of the common concerns regarding the use of mineral oil is its presence on several lists of comedogenic substances. These comedogenic lists were developed many years ago, yet remain frequently quoted in the dermatologic literature. There are several important points to consider. First, there are different grades of mineral oil. There is industrial grade mineral oil, which is used as a machine lubricant, that is not of the purity required for skin application. Cosmetic grade mineral oil is the purest form without contaminants. Industrial grade mineral oil may be comedogenic, but cosmetic grade mineral oil is not. Quality manufacturers only purchase quality products from quality suppliers who guarantee the quality of the materials they provide. I believe that cosmetic grade mineral oil is noncomedogenic and I have never found it to be comedogenic in any of the testing I have performed for the skin care industry.
SUNSCREENS PRODUCE ACNE
Most of the sunscreens on the market today are based primarily on UVB-absorbing ingredients, such as octyl methoxycinnamate, oxybenzone, homosalate, etc. Many also have UVA-absorbing ingredients, such as avobenzone, titanium dioxide, or zinc oxide, as secondary sunscreens. All of the UVB sunscreens and avobenzone function by transforming ultraviolet radiation to heat energy through a process known as resonance delocalization. This heat energy is appreciated by many patients who will state that they do not like wearing sunscreens, since the gels or lotions make them feel hot. In some patients, I believe that the increased sweating induced by the sunscreens accompanied by the warm sunny weather cause increased activity by the eccrine glands. This may cause miliaria rubra that may be magnified by the occlusive nature of the water-resistant, rubproof product. Thus, I believe that much of the problem with sunscreen-induced breakout is the formation of papules or pustules around the eccrine duct ostia without the sebaceous gland involvement that characterizes true acne.
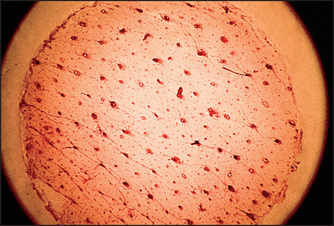
GLYCOLIC ACID APPLICATION CAN REDUCE PORE SIZE
Salicylic acid is an oil-soluble chemical exfoliant that can remove debris from the pore, creating the appearance of skin smoothness, but it too cannot measurably reduce pore size. It is important to distinguish between real pore size reduction and an improved cosmetic appearance. Figure 28.3 demonstrates the appearance of pores containing keratotic debris. Removal of this debris with a salicylic acid peel can improve the appearance of the pores and can shrink the size of dilated pores due to debris presence, but the physical size of the pore cannot be altered.
TRETINOIN TOPICALLY AIDS IN THE TREATMENT OF ACNE SCARRING
Whether tretinoin aids in the treatment of acne scarring is rather controversial, even among dermatologists. Tretinoin normalizes follicular keratinization, which improves comedonal, pustular, and superficial papular acne. This effect may smooth out the skin around acne scars simply by treating the acne. Tretinoin also increases collagen production after extended use, which may improve acne scarring. The degree to which tretinoin improves pitted acne scarring has never been quantified and more research is needed in this area. However, it is unlikely that this research will be forthcoming from the pharmaceutical industry, since tretinoin is now generic and there is currently no indication for acne scarring, only acne treatment.
A COMPLEX SKIN CARE REGIMEN OF MULTIPLE CLEANSERS, MOISTURIZERS, AND ANCILLARY SKIN CARE PRODUCTS IS NECESSARY FOR CLEAR SKIN
There are many different approaches to skin care. There is the no nonsense bar of soap and water twice daily approach and the 20 step skin care routine approach. Which is better? I am not sure I know the answer. In Japan, skin care is a complex ritual of multiple cleansers, toners, and moisturizers. The Japanese also feel that they have the most sensitive skin of all races and the incidence of atopic dermatitis is dramatically rising in their country. Is this due to the use of extensive skin care products? I also do not know the answer to this question. However, there is no doubt that the more the skin is manipulated, the more opportunity there is for problems to arise. Perhaps the old adage of everything in moderation is the best advice, even when it comes to skin care (Fig. 28.4).