Acetaminophen
Perspective
Acetaminophen is one of the most commonly used antipyretic and analgesic agents throughout the world. Acetaminophen is found as an isolated product or in combination medications for the treatment of cold symptoms, pain, and headache. In 2010, an intravenous (IV) formulation was approved in the United States (an IV formulation was available in Europe and Australia previously) for the treatment of pain1–3 and as an antipyretic.4 Given the widespread use and availability of acetaminophen, toxicity is a concern in all intentional ingestions as well as with repeated supratherapeutic dosing and drug abuse. Acetaminophen toxicity is one of the leading causes of hospital admission, antidote use, and fatalities from oral poisonings in the United States.5
Principles of Disease
Acetaminophen is absorbed rapidly, with peak plasma concentrations generally occurring within 1 hour and complete absorption within 4 hours. Once it is absorbed, acetaminophen inhibits prostaglandin E2 (PGE2) synthesis, leading to antipyresis and analgesia. Inhibition of PGE2 synthesis is by either direct cyclooxygenase-2 inhibition or inhibition of membrane-associated prostaglandin synthase.6,7
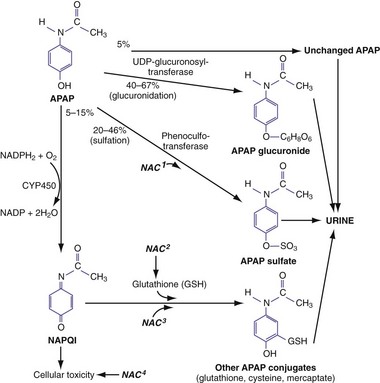
In therapeutic doses, acetaminophen is primarily metabolized by conjugation with glucuronide (40-67%) and sulfate (20-46%) into nontoxic metabolites that are excreted in the urine8 (Fig. 148-1). A small percentage (<5%) is oxidized by cytochrome P450 2E1 (CYP2E1) (and to a lesser extent 1A4 and 3A4) to a highly cytotoxic metabolic intermediary, N-acetyl-p-benzoquinone imine (NAPQI).9,10 In therapeutic doses, NAPQI is short-lived, combining rapidly with glutathione and other thiol-containing compounds to form nontoxic metabolites that are excreted in the urine. With typical therapeutic acetaminophen dosing, glutathione stores and the ability to regenerate glutathione easily keep up with NAPQI production.
After large ingestions or repeated supratherapeutic ingestions, the amount of NAPQI produced begins to outstrip glutathione stores and the liver’s ability to regenerate glutathione, leading to unbound NAPQI. The highly reactive electrophile NAPQI covalently binds to cell proteins in the liver, which initiates a cascade of events that lead to hepatic cell death. Renal injury may also occur with or without liver injury11 and may be mediated by renal CYP enzymes or activation of prostaglandin synthase.
Acetaminophen-induced liver damage initially occurs in hepatic zone III (centrilobular) because oxidative metabolism is concentrated in this area. With severe toxicity, necrosis of the entire liver parenchyma may occur. The clinical effects of severe acetaminophen toxicity are the result of severe fulminant liver failure rather than a direct acetaminophen effect. These effects include multiorgan failure, systemic inflammatory response syndrome, hypotension, cerebral edema, and death.12
The principal therapy for acetaminophen toxicity is N-acetylcysteine (NAC), which is effective by two separate mechanisms. Soon after overdose, NAC serves as a glutathione precursor and a sulfur-containing glutathione substitute (see Fig. 148-1), binding to and thereby detoxifying NAPQI and avoiding subsequent hepatotoxicity. In addition, NAC may decrease NAPQI formation by enhancing acetaminophen conjugation with sulfate to nontoxic metabolites.
Even after acetaminophen hepatotoxicity is evident, NAC acts as a free radical scavenger and an antioxidant and alters hepatic microcirculation and oxygen delivery.13 In patients with acetaminophen-induced hepatic failure, IV administration of NAC decreases the rates of cerebral edema, hypotension, and death even when no acetaminophen remains.12
Clinical Features
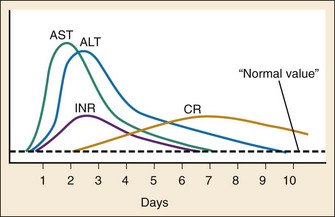
Early after acute acetaminophen ingestion, patients may be asymptomatic or have mild nonspecific symptoms (e.g., nausea, vomiting, anorexia, malaise, diaphoresis) (Table 148-1). Liver injury becomes evident after a period of 8 to 36 hours as an elevation in aspartate transaminase (AST).14 Once liver injury has begun, patients may have right upper quadrant pain or tenderness, vomiting, and jaundice. AST concentrations continue to rise rapidly14 and usually peak in 2 to 4 days, corresponding to maximal liver injury. Alanine transaminase (ALT), prothrombin time, and bilirubin typically begin to rise and peak shortly after AST values. With severe toxicity, AST, ALT, and the prothrombin time may all be elevated within 24 hours (Fig. 148-2).14 With maximal liver injury, patients have signs and symptoms consistent with fulminant liver failure, including metabolic acidosis, coagulopathy, and hepatic encephalopathy. Death may occur from hemorrhage, adult respiratory distress syndrome, sepsis, multiorgan failure, or cerebral edema. The risk of renal injury increases with the severity of hepatic injury; renal injury occurs in less than 2% of patients without hepatotoxicity and in 25% of patients with severe hepatotoxicity.
Diagnostic Strategies
Risk Assessment with Acute Acetaminophen Ingestion
The initial diagnostic strategy of an acute ingestion is well established. The first step is to determine the patient’s risk of acute acetaminophen exposure. Patients who report an acute intentional ingestion of acetaminophen should have laboratory risk stratification regardless of the reported amount ingested. It is likely that significantly more than 150 mg/kg must be acutely ingested before significant liver toxicity occurs; however, history alone may not be reliable. A serum acetaminophen concentration should be considered in all intentional overdoses because approximately 1.4 to 8.4% of patients with intentional ingestions who deny acetaminophen ingestion actually have a detectable but usually subtoxic concentration.15–17
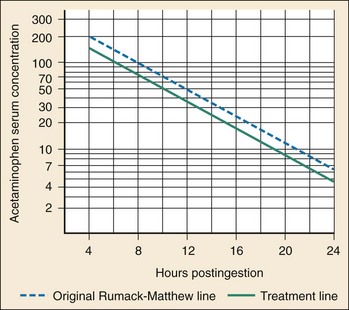
Once a patient is determined to be at risk and a time of ingestion has been established or estimated, the next step is to determine a serum acetaminophen concentration 4 hours after ingestion or as soon as possible after 4 hours. The serum acetaminophen concentration and the time of ingestion determine the need for antidotal therapy by plotting of the serum acetaminophen concentration against the time since ingestion on the treatment nomogram (Fig. 148-3), an adaptation of the Rumack-Matthew nomogram. If the serum acetaminophen concentration is on or above the treatment line (that starts at 150 µg/mL (990 µmol/L) at 4 hours and decreases to 4.7 µg/mL (31 µmol/L) at 24 hours), treatment with NAC should be initiated. If the serum acetaminophen concentration is below the treatment line and the most severe possible scenario has been taken for the time of ingestion, the patient requires no antidote.18 The treatment nomogram is a highly sensitive approach and may be used for all acute ingestions. Alternative approaches in patients with alcoholism,19 in patients with coingestions of antimuscarinic agents,20,21 in patients with unknown ingestion times,22 and after the administration of IV formulations23 have been suggested, but these remain controversial and unvalidated. Strict adherence to the approach described here is recommended.
Measurement of serum acetaminophen concentration before 4 hours is typically not necessary. It is likely that a serum acetaminophen concentration less than 10 µg/mL (66 µmol/L) between 1 and 4 hours after ingestion excludes significant ingestion of acetaminophen; however, there are few data on which to base this conclusion. Absorption of acetaminophen may not be complete before 4 hours, and any serum acetaminophen concentration greater than 10 µg/mL (66 µmol/L) is difficult to interpret. Finally, serum acetaminophen concentrations measured before 4 hours cannot be plotted on the treatment nomogram. Fortunately, there is little need to treat patients before 6 to 8 hours after ingestion; patients treated with NAC up to 6 hours after ingestion, even after very large doses, have no increased risk of hepatotoxicity regardless of their serum acetaminophen concentration.24 For most overdose patients, the risk of hepatotoxicity does not significantly increase unless NAC is delayed for 8 hours or longer after ingestion.24 This is generally enough time for a serum acetaminophen concentration to be determined at 4 hours and the laboratory evaluation to be completed. For patients at risk whose serum acetaminophen concentration cannot be obtained before 8 hours after ingestion, a loading dose of NAC should be considered.
There are few data for development of a strategy of risk assessment after a large IV acetaminophen overdose. Several pharmacokinetic factors suggest that some alteration of the typical nomogram-based assessment may be necessary. After infusion, IV acetaminophen generates serum concentrations that are significantly higher than an equivalent oral dose. In addition, the nomogram that is used for acute ingestions takes into account an absorptive period that is not necessary for an IV infusion. These and other factors suggest that an alternative approach may be necessary, but experience is limited. We adhere to a conservative approach, which suggests that patients be treated with NAC if they either (1) are treated with more than 60 mg/kg IV acetaminophen in one dose or (2) they have a serum acetaminophen concentration that is above 50 µg/mL (330 µmol/L) at 4 hours after the infusion stops.25
Risk Assessment with Chronic Ingestion
The risk of hepatotoxicity from chronic ingestion of acetaminophen is increased with both an increasing total dose of acetaminophen and a longer duration in which it has been ingested in supratherapeutic quantities. With this in mind, laboratory testing for serum acetaminophen concentration and AST should be initiated in any patient who fits the criteria in Table 148-2.26
Table 148-2
Indications to Initiate Testing for Serum Acetaminophen Concentration and AST in Chronic Acetaminophen Ingestions26
| Age ≥6 years | Ingestion of >10 g/day or >200 mg/kg/day (whichever is smaller) during a 24-hr period |
| or | Ingestion of >6 g/day or >150 mg/kg/day (whichever is smaller) during a 48-hr period or longer |
| or | Symptomatic (e.g., RUQ pain or tenderness, jaundice, vomiting) |
| Children <6 years | Ingestion of >200 mg/kg/day during a 24-hr period |
| or | Ingestion of >150 mg/kg/day during a 48-hr period |
| or | Ingestion of >100 mg/kg/day during a 72-hr or longer period |
| or | Symptomatic (e.g., RUQ pain or tenderness, jaundice, vomiting) |
Ingestion of therapeutic amounts of acetaminophen appears to be safe.27,28 However, rare reports of transaminitis and liver injury during therapeutic dosing suggest that some patients may be at increased risk for liver injury, possibly because of genetic variation or specific risk factors.29 Patients who chronically ingest isoniazid or ethanol may have increased CYP2E1 activity and theoretically be at higher risk for chronic acetaminophen toxicity. Similarly, patients who have prolonged fasting (e.g., malnourished, AIDS, severe prolonged vomiting) and children with febrile illnesses30–32 have reason to be at higher risk. All of these risk factors are controversial and require additional study. Given that we are unable to accurately predict the rare patient at high risk, patients who have symptoms consistent with liver injury (e.g., right upper quadrant pain or tenderness, jaundice) after taking acetaminophen merit risk determination regardless of the amount that they reportedly ingest.
Once serum acetaminophen concentration and AST are determined, further risk assessment is necessary. Conceptually, patients with chronic ingestions may benefit from antidotal therapy if they have evidence of liver injury or if they have evidence of acetaminophen excess that may lead to liver injury. With this in mind, patients with chronic supratherapeutic acetaminophen exposure with significant elevations of AST (e.g., >50 IU/L) should be treated with NAC regardless of their serum acetaminophen concentration.33,34 A higher cutoff for AST (e.g., twice normal, or >120 IU/L) has been suggested but is unstudied.35,36 In patients with an AST that is not elevated (e.g., <50 IU/L), NAC should be initiated if the serum acetaminophen concentration is higher than expected. After a typical therapeutic dose of acetaminophen, serum acetaminophen concentration peaks below 30 µg/mL (199 µmol/L) and is less than 10 µg/mL (66 µmol/L) at 4 hours.
Risk Assessment in Pregnant Women
Fetal acetaminophen toxicity is rare, but adverse outcomes have been reported in all stages of pregnancy.37 Acetaminophen crosses the placenta and may be present in concentrations in the fetus that are as high as or higher than those in the mother.37 In the early gestational period, acetaminophen toxicity can be associated with fetal death.37 CYP enzymes appear in the fetus during the second trimester, and activity increases with gestational age, which may put the maturing fetus (e.g., third trimester) or newborn at risk of toxicity.
Management
Limiting Gastrointestinal Absorption
Activated charcoal effectively binds acetaminophen in vitro38 and limited studies have suggested some efficacy,39,40 but there is no evidence that administration of activated charcoal to patients translates into improved clinical outcomes.
N-Acetylcysteine
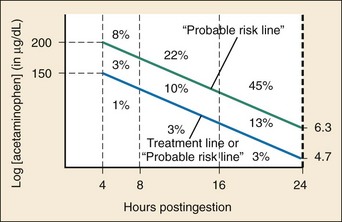
When it is indicated, NAC should be administered as early as possible. Delay of NAC administration for more than 8 hours after ingestion increases the risk of hepatotoxicity (Fig. 148-4).
NAC can be administered by the oral (PO) or IV route, with advantages and disadvantages for each. All formulations of NAC (PO and IV) are effective when they are started within 8 hours of ingestion.24,41–44 The main role of early NAC administration is to prevent hepatotoxicity by detoxifying NAPQI and decreasing NAPQI production. The risk of liver injury (i.e., AST > 1000 IU/L) in this group with treatment with NAC is less than 4%, and the mortality rate approaches zero41,44 (see Fig. 148-4).
Both PO and IV NAC are equally effective in treating patients who present 8 to 24 hours after ingestion, although the overall rate of liver injury (i.e., AST > 1000 IU/L) in this group is significantly higher (approximately 30%).41,42,45
Once liver failure (e.g., coagulopathy, encephalopathy) is evident, however, the IV route is the only route that has been systematically studied.12 IV NAC decreases the risk of hypotension, cerebral edema, and death in patients with acetaminophen-related hepatic failure.12 Oral NAC should be used only if IV NAC is not available.
The main differences between IV and PO NAC are in their side effect profiles (Table 148-3). Approximately 2 to 6% of patients treated with IV NAC have anaphylactoid reactions,43,46–48 although rates of up to 30% have been reported in prospective trials.49,50 The majority of these symptoms are mild and consist of transient skin rashes and flushing. More severe reactions have been reported in less than 1% of patients and include angioedema, bronchospasm, hypotension, and at least one death.47–51 Symptoms typically occur within 30 minutes of the start of the loading infusion. These anaphylactoid reactions are dose, rate, and concentration dependent.46,47
Table 148-3
Side Effect Profile for N-Acetylcysteine Formulations
| NAC FORMULATION | COMMON SIDE EFFECTS | SEVERE SIDE EFFECTS |
| PO NAC | Vomiting (23%)52 | Very rare |
| IV NAC | Mild anaphylactoid reactions (e.g., rash, flushing, pruritus, vomiting), 2-18%46–49,53 | Severe anaphylactoid reactions (e.g., hypotension), <1%43,46–49 |
Anaphylactoid reactions are much less frequent with PO NAC. Skin rash, serious systemic reactions, and anaphylactic reactions are rarely reported with the PO formulation. However, approximately 23% of patients receiving PO NAC vomit, delaying timely antidotal delivery.52 PO NAC is extremely unpalatable, largely because of a “rotten egg” odor and taste. Palatability may be improved by administering NAC diluted with either soda or juice and serving it in a covered container through a straw. Any dose that is vomited within 1 hour of administration should be repeated. Antiemetics (e.g., ondansetron, metoclopramide) are advisable before PO NAC dosing, but there are few data about effectiveness of this approach.
Anaphylactoid reactions to IV NAC are typically mild (e.g., flushing) and occur during the initial 15- to 60-minute infusion. Mild reactions can be managed with antihistamines (e.g., IV diphenhydramine) without stopping the infusion. Serious reactions can be managed by slowing or pausing the infusion, giving a fluid bolus, and administering diphenhydramine or glucocorticoids intravenously if necessary.53 Epinephrine is rarely required. Although these reactions require close observation and treatment as necessary, they do not preclude subsequent doses.53
N-Acetylcysteine in Pregnancy
Treatment of the mother with NAC is safe and effective,54 and NAC effectively crosses the placenta.55 Administration of IV NAC to the mother has the theoretic advantage of increased NAC delivery to the fetus compared with PO NAC. IV administration circumvents first-pass metabolism, presumably exposing the fetal circulation to higher maternal serum concentrations. On the basis of large published studies, we recommend continuation of therapy for 72 hours.37
Disposition
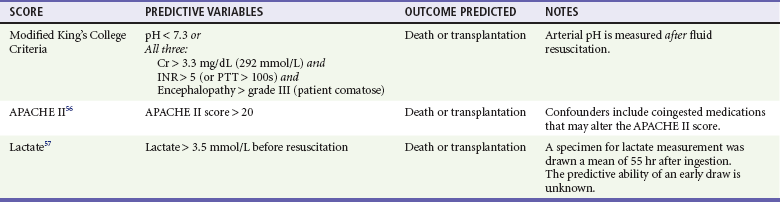
If a patient presents with established hepatotoxicity, transfer to a higher level center that specializes in the care of patients with liver failure may be advisable, as is the case for any other patient presenting with liver failure. Clinical predictors of severe hepatic failure are listed in Table 148-4.56,57
References
1. Sinatra, RS, et al. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822.
2. Wininger, SJ, et al. A randomized, double-blind, placebo-controlled, multicenter, repeat-dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clin Ther. 2010;32:2348.
3. Grissa, MH, et al. Paracetamol vs piroxicam to relieve pain in renal colic. Results of a randomized controlled trial. Am J Emerg Med. 2011;29:203.
4. Walson, PD, Jones, J, Chesney, R, Rodarte, A. Antipyretic efficacy and tolerability of a single intravenous dose of the acetaminophen prodrug propacetamol in children: A randomized, double-blind, placebo-controlled trial. Clin Ther. 2006;28:762.
5. Bronstein, AC, et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila). 2010;48:979.
6. Aronoff, D, Neilson, E. Antipyretics: Mechanism of action and clinical use in fever suppression. Am J Med. 2001;111:304.
7. Graham, GG, Kieran, FS. Mechanism of action of paracetamol. Am J Ther. 2005;12:46.
8. Schenker, S, Speeg, K, Perez, A, Finch, J. The effects of food restriction in man on hepatic metabolism of acetaminophen. Clin Nutr. 2001;20:145.
9. Manyike, PT, Kharasch, ED, Kalhorn, TF, Slattery, JT. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther. 2000;67:275.
10. Hazai, E, Vereczkey, L, Monostory, K. Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Comm. 2002;291:1089.
11. Boutis, K, Shannon, M. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. J Toxicol Clin Toxicol. 2001;39:441.
12. Keays, R, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: A prospective controlled trial. Br Med J. 1991;303:1026.
13. Jones, AL. Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: A critical review. J Toxicol Clin Toxicol. 1998;36:277.
14. Green, TJ, et al. When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol (Phila). 2010;48:787–792.
15. Lucanie, R, Chiang, WK, Reilly, R. Utility of acetaminophen screening in unsuspected suicidal ingestions. Vet Hum Toxicol. 2002;44:171.
16. Ashbourne, JF, Olson, KR, Khayam-Bashi, H. Value of rapid screening for acetaminophen in all patients with intentional drug overdose. Ann Emerg Med. 1989;18:1035.
17. Hartington, K, Hartley, J, Clancy, M. Measuring plasma paracetamol concentrations in all patients with drug overdoses: Development of a clinical decision rule and clinicians willingness to use it. Emerg Med J. 2002;19:408.
18. Hendrickson, RG. Acetaminophen. In Goldfrank L, et al, eds.: Goldfrank’s Toxicologic Emergencies, 9th ed, New York: McGraw-Hill, 2010.
19. Ali, FM, Boyer, EW, Bird, SB. Estimated risk of hepatotoxicity after an acute acetaminophen overdose in alcoholics. Alcohol. 2008;42:213.
20. Hendrickson, RG, McKeown, NJ, West, PL, Burke, CR. Bactrian (“double hump”) acetaminophen pharmacokinetics: A case series and review of the literature. J Med Toxicol. 2010;6:337–344.
21. Klein-Schwartz, W, Doyon, S. Intravenous acetylcysteine for the treatment of acetaminophen overdose. Expert Opin Pharmacother. 2011;12:119.
22. Sivilotti, ML, et al. Multiplying the serum aminotransferase by the acetaminophen concentration to predict toxicity following overdose. Clin Toxicol (Phila). 2010;48:793.
23. Beringer, RM, Thompson, JP, Parry, S, Stoddart, PA. Intravenous paracetamol overdose: Two case reports and a change to national treatment guidelines. Arch Dis Child. 2011;96:307.
24. Sivilotti, ML, et al. A risk quantification instrument for acute acetaminophen overdose patients treated with N-acetylcysteine. Ann Emerg Med. 2005;46:263.
25. Gray, T, Hoffman, RS, Bateman, DN. Intravenous paracetamol—an international perspective of toxicity. Clin Toxicol (Phila). 2011;49:150.
26. Dart, RC, et al. Acetaminophen poisoning: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2006;44:1.
27. Dart, RC, Bailey, E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. 2007;27:1219.
28. Lavonas, EJ, Reynolds, KM, Dart, RC. Therapeutic acetaminophen is not associated with liver injury in children: A systematic review. Pediatrics. 2010;126:e1430.
29. Watkins, PB, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: A randomized controlled trial. JAMA. 2006;296:87.
30. American Academy of Pediatrics, Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108:1020.
31. Caravati, EM. Unintentional acetaminophen ingestion in children and the potential for hepatotoxicity. J Toxicol Clin Toxicol. 2000;38:291.
32. Mohler, CR, et al. Prospective evaluation of mild to moderate pediatric acetaminophen exposures. Ann Emerg Med. 2000;35:239.
33. Daly, FF, O’Malley, GF, Heard, K, Bogdan, GM, Dart, RC. Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion. Ann Emerg Med. 2004;44:393.
34. Wolf, SJ, Heard, K, Sloan, EP, Jagoda, AS. Clinical policy: Critical issues in the management of patients presenting to the emergency department with acetaminophen overdose. Ann Emerg Med. 2007;50:292.
35. Kozer, E, Greenberg, R, Zimmerman, DR, Berkovitch, M. Repeated supratherapeutic doses of paracetamol in children—a literature review and suggested clinical approach. Acta Paediatr. 2006;95:1165.
36. Alhelail, MA, Hoppe, JA, Rhyee, SH, Heard, KJ. Clinical course of repeated supratherapeutic ingestion of acetaminophen. Clin Toxicol (Phila). 2011;49:108.
37. Riggs, BS, et al. Acute acetaminophen overdose during pregnancy. Obstet Gynecol. 1989;74:247.
38. Offerman, SR. The clinical management of acetaminophen poisoning in a community hospital system: Factors associated with hospital length of stay. J Med Toxicol. 2011;7:4.
39. Buckley, NA, Whyte, IM, O’Connell, DL, Dawson, AH. Oral or intravenous N-acetylcysteine: Which is the treatment of choice for acetaminophen (paracetamol) poisoning? J Toxicol Clin Toxicol. 1999;37:759.
40. Spiller, HA, Winter, ML, Klein-Schwartz, W, Bangh, SA. Efficacy of activated charcoal administered more than four hours after acetaminophen overdose. J Emerg Med. 2006;30:1.
41. Buckley, NA, Whyte, IM, O’Connell, DL, Dawson, AH. Oral or intravenous N-acetylcysteine: Which is the treatment of choice for acetaminophen (paracetamol) poisoning? J Toxicol Clin Toxicol. 1999;37:759.
42. Prescott, L. Oral or intravenous N-acetylcysteine for acetaminophen poisoning? Ann Emerg Med. 2005;45:409.
43. Whyte, IM, Francis, B, Dawson, AH. Safety and efficacy of intravenous N-acetylcysteine for acetaminophen overdose: Analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin. 2007;23:2359.
44. Rumack, BH. Acetaminophen hepatotoxicity: The first 35 years. J Toxicol Clin Toxicol. 2002;40:3.
45. Kanter, MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm. 2006;63:1821.
46. Horowitz, BZ, Hendrickson, RG, Pisarro-Osilla, C. Not so fast!. Ann Emerg Med. 2006;47:122.
47. Kao, LW, et al. What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning? Ann Emerg Med. 2003;42:741.
48. Yarema, MC, et al. Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2009;54:606.
49. Kerr, F, Dawson, A, Whyte, IM. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005;45:402.
50. Sandilands, EA, Bateman, DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila). 2009;47:81.
51. Appelboam, AV, Dargan, PI, Knighton, J. Fatal anaphylactoid reaction to N-acetylcysteine: Caution in patients with asthma. Emerg Med J. 2002;19:594.
52. Bebarta, VS, et al. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol (Phila). 2010;48:424–430.
53. Bailey, B, McGuigan, MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Ann Emerg Med. 1998;31:710.
54. McElhatton, PR, Sullivan, FM, Volans, GN. Paracetamol overdose in pregnancy analysis of the outcomes of 300 cases referred to the Teratology Information Service. Reprod Toxicol. 1997;11:85.
55. Horowitz, RS, et al. Placental transfer of N-acetylcysteine following human maternal acetaminophen toxicity. J Toxicol Clin Toxicol. 1997;35:447.
56. Larson, AM, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1364.
57. Bernal, W, Donaldson, N, Wyncoll, D, Wendon, J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: A cohort study. Lancet. 2002;359:558.