Abnormalities of the Male Genital Tract
Overview
Ultrasound, including Doppler imaging in all its forms, is the main diagnostic imaging tool for evaluating the scrotum. Computed tomography (CT) is used predominantly to evaluate metastatic spread of testicular or other intrascrotal tumors. Magnetic resonance imaging (MRI) has been used in the search for undescended testes that remain in an intraabdominal position. MRI, like CT, can be used to analyze metastatic spread of testicular tumor. Its uses in intraabdominal, intrapelvic, and intrascrotal imaging are evolving.1
Ultrasound Technique
Scrotal ultrasound is performed with use of a high-frequency transducer. The superficial position of testes in the normally thin-walled scrotum allows excellent imaging with a transducer of 7.5 MHz or higher. Longitudinal, transverse, and coronal views are taken of each hemiscrotum. Transverse views, with the addition of a convex array transducer (e-Fig. 126-1), allow the best side-by-side comparison of both testes and their adnexa, especially when checking for differences in size, echogenicity, and vascularity (e-Fig. 126-2).1–6
e-Figure 126-1 Normal testes.
The testes of a teenager are seen as homogeneously echogenic on ultrasound in the transverse plane. This image was obtained using a convex linear array probe that allowed imaging of most of both testes. These transducers can show both testes in their entirety in most cases and therefore are an improvement over linear array transducers (which are limited by the size of their footprint) for side-by-side comparisons of testes.
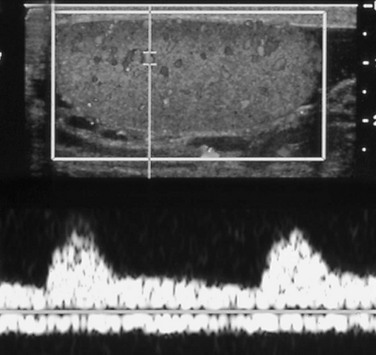
e-Figure 126-2 Normal Doppler flow in a testis.
The upper half of the image shows the normal testis, with color filling vessels with moving blood. Insonation of a point in the upper third of the testis provides a spectral pattern (in the lower half of the image) that shows a classic arterial signal with its systolic and diastolic components above the baseline and a relatively unchanging and continuous venous signal below it.
Normal Findings
The testes should be ovoid, nearly symmetric in size (Box 126-1), and homogeneously echogenic. A highly echogenic linear focus (seen posteriorly and superiorly) represents the mediastinum testis (Fig. 126-3), which is the inward extension of the tightly adherent covering of the testis, the tunica albuginea. Fibrous septa extending from the mediastinum testes divide the testes into more than 250 lobules. The spermatic cord, draining veins, lymphatics, nerves, vas deferens, and a single testicular artery run within the mediastinum testis.6–9
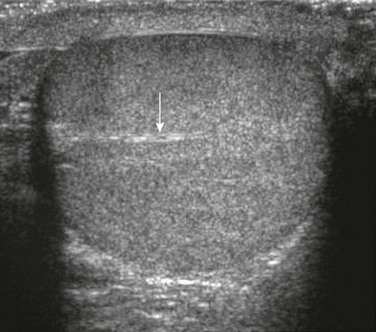
Figure 126-3 Mediastinum testis.
A transverse sonogram shows a linear echogenicity (arrow) within a normal testis that is the mediastinum testis.
The head of the epididymis (e-Fig. 126-4) sits atop the superior pole of each testis. The head is continuous with the epididymal body and tail, which travel inferiorly along the posterolateral margin of the testis. The echogenicity of the epididymis is normally homogeneous. It may be of equal or of slightly greater or lesser echogenicity than that of the testes.6–9
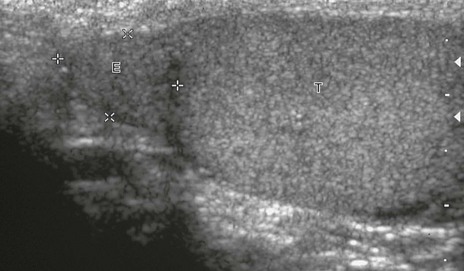
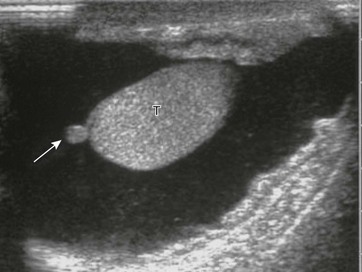
e-Figure 126-4 Normal epididymis.
In this longitudinal sonogram, cursors mark off the head of the epididymis (E), which sits atop the superior testis (T). The normal epididymal body and tail, which travel along the side of the testis, are more difficult to separate from normal testis.
The scrotal wall should be between 3 and 6 mm thick. Beneath the scrotal wall are the two layers of the tunica vaginalis (Fig. 126-5), the outer (parietal) and the inner (visceral) layers, which are the residua of the processus vaginalis (i.e., peritoneum that descended with the testis from the abdomen). The visceral layer covers the testis on its anterior border and is attached to the tunica albuginea. Between the tunica’s two layers is a potential space that normally may contain as much as 1 to 2 mL of fluid. It is here that the fluid of a hydrocele may accumulate.6–9
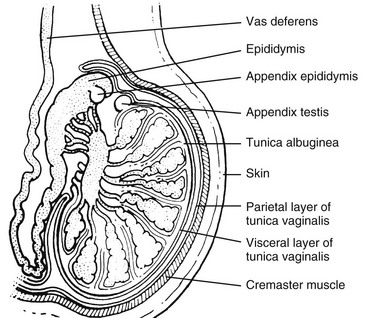
Figure 126-5 A schematic drawing of the scrotum and its contents as seen in the longitudinal plane through a single testis.
Note the tunica albuginea surrounding the lobules of the testes. The tunica albuginea, in turn, is surrounded by the visceral layer of the tunica vaginalis and the more superficial parietal layer of the tunica vaginalis. (From Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill; 2001. Reproduced with permission of McGraw-Hill Companies.)
Three small, persistent, vestigial remnants of the mesonephric and müllerian duct systems occasionally may be seen, usually only when a normal testis is surrounded by hydrocele fluid. These remnants are the appendix testis (e-Fig. 126-6) (a remnant of the müllerian duct), which is attached to the upper pole of the testis (and the most common one to potentially undergo torsion); the appendix epididymis (a remnant of the mesonephron), which is attached to the head of the epididymis; and the vas aberrans (a remnant of the mesonephron), which is attached to the epididymis at the junction of its body and tail.6–9
Cryptorchidism
Overview: By 32 weeks’ gestational age, the testes have descended into the scrotum via the inguinal canal in 93% of all male fetuses. By 6 weeks of age, only 4% of term infants have a nonpalpable testis. Of these infants, 20% have true cryptorchidism (undescended testes). Cryptorchidism is more common on the right (70%) and is bilateral in 10% to 33% of cases. Beyond the age of 1 year, the prevalence of true cryptorchidism is approximately 1%. The etiology of cryptorchidism is not clear; it may be hormonal or mechanical (e.g., lack of proper fixation of the testis or an abnormal gubernaculum), or a combination of the two.1
Imaging: Ultrasound is the initial procedure for localization of a testis that is not palpable within the scrotum (e-Fig. 126-7). The undescended testes may be smaller and hypoplastic, although usually it is of equal echogenicity compared with the normal testis. MRI with fat-suppression techniques generally is more effective than CT for intraabdominal testes. Normal testes have a homogeneous high signal on T2-weighted images.1,6,10
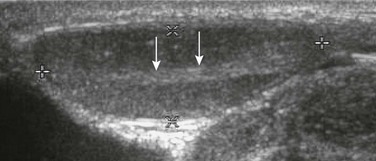
e-Figure 126-7 Analysis of groin for undescended testes.
Sonogram of inguinal region. Cursors mark off the borders of a normal undescended testis found in the right groin of a child. Arrows point to the mediastinum of the testis. Color Doppler imaging denoted normal flow to the testis, which appeared homogeneous and normal. Its superficial position required a high-frequency transducer (10 MHz).
Treatment: Surgical treatment for undescended testes is orchiopexy. It is performed, especially in cases of intraabdominal testes, because of the increased risk for the development of testicular neoplasia (a 10 to 40 times greater risk, with a seminoma being the most common neoplasm).11 Spontaneous descent during the first year of life may occur from an endogenous surge of luteinizing hormone, and thus surgical correction typically is delayed until 18 to 24 months.1,6,12 After orchiopexy, 53% of the testes are reported to be abnormal by either position, volume, structure, or perfusion.1,13–16
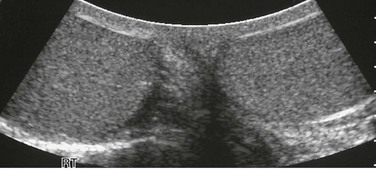
Hydrocele
Overview: A hydrocele, which is the most common scrotal mass in a child (Fig. 126-8), results from fluid accumulated within the layers of the tunica vaginalis. Several types of hydroceles are identified (Fig. 126-9). The processus vaginalis is closed in 50% to 75% of persons by the time they are born and in most of the remainder of children by the end of the first year of life. Residual fluid from testicular descent is responsible for the noncommunicating hydroceles reported in at least 15% of male fetuses beyond 28 weeks of life. If the processus fails to close, a communicating hydrocele can develop.
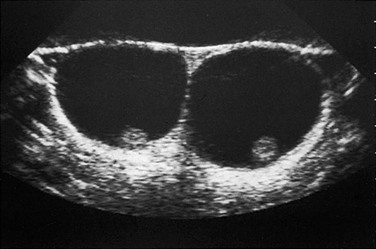
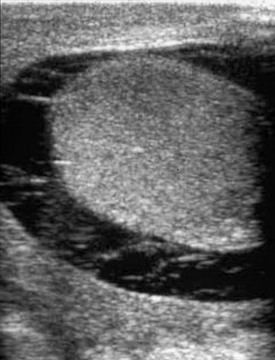
Figure 126-8 Hydroceles.
On transverse plane ultrasound, two normal testes are seen as small echogenic structures within a very enlarged scrotum of a young child with large hydroceles. Typically, high-frequency transducers (e.g., 7.5 MHz and greater) are used for improved near-field resolution of superficial structures such as the testes. In cases such as this one with large hydroceles, a lower-frequency transducer may be needed to better penetrate the greater distance between the anterior and posterior scrotal walls to image the testes.
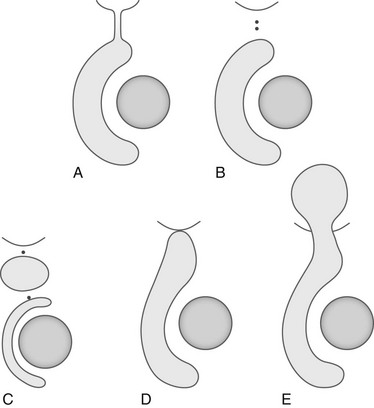
Figure 126-9 Hydrocele types.
A, Congenital or intermittent hydrocele. B, Scrotal hydrocele. C, Hydrocele of the cord. D, Inguinoscrotal hydrocele. E, Abdominoscrotal hydrocele.
In a hydrocele of the cord (a funicular hydrocele), the processus vaginalis is obliterated in its proximal and distal end and the hydrocele is contained in the patent space between these two points. In an inguinoscrotal hydrocele, the processus vaginalis is obliterated only at the internal inguinal ring and the hydrocele extends cephalad from the scrotum into the inguinal canal. In an abdominoscrotal hydrocele (e-Fig. 126-10), closure of the funicular process at the internal inguinal ring also occurs and forms a dumbbell-shaped cystic mass that protrudes into the extraperitoneal space above the inguinal area.1,17
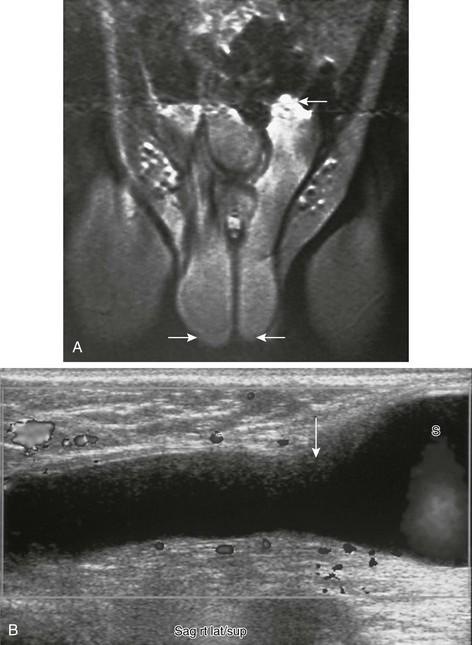
e-Figure 126-10 Abdominoscrotal hydrocele.
A, Tubular structures (arrows) are seen extending from each side of the scrotum superiorly into the abdominal cavity on a coronal T1-weighted magnetic resonance image. This child had bilateral abdominoscrotal hydroceles; the superior extent on the right is not well visualized on this image. He presented with an enlarged scrotum and palpable inguinal area masses, the size of which changed with scrotal compression. B, A longitudinal sonogram in another patient shows a right-sided tubular, fluid-filled structure extended from the scrotum (S) to above the inguinal area (arrow). (A from Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill; 2001. Reproduced with permission of McGraw-Hill Companies.)
In most cases, the etiology of a hydrocele found in the child or adolescent is idiopathic. Acquired hydroceles occur after scrotal trauma or as a complication of epididymitis-orchitis, testicular torsion, or intrascrotal neoplasm (reactive hydrocele). Increasing size of a hydrocele without an intrascrotal cause suggests a patent processus vaginalis and an associated inguinal hernia.1
Imaging: Scrotal ultrasound shows the cystic nature of the hydrocele as it appears to surround the normal homogeneously echogenic testes. Septations and debris may be present, particularly if the hydrocele is infected (i.e., a pyocele) or hemorrhagic (i.e., a hematocele) (e-Fig. 126-11). Echogenic debris (e.g., cholesterol crystals) often is seen in the fluid of chronic hydroceles (Fig. 126-12), along with occasional calcifications.1
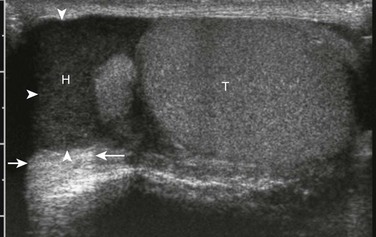
Figure 126-12 Chronic hydrocele (arrowheads).
Ultrasound in the longitudinal plane. Echogenic debris-filled fluid is seen in a chronic hydrocele (H) in the scrotum of a teenager. Excellent through transmission of sound (arrows) helps indicate that the echogenic material is complicated fluid rather than solid material. T, testicle.
Testicular Torsion
Overview: Torsion may occur at any age but is seen most often in adolescent boys between 11 and 18 years of age, perhaps because of the increase in testicular growth and weight during this time. The normal testis is strongly attached to the epididymis, which in turn is applied to the posterior scrotal wall. If these attachments fail to develop properly (the “clapper-in-a-bell” phenomenon), the testis, suspended within the tunica vaginalis, may rotate and the spermatic cord undergoes torsion. A person with acute torsion experiences sudden acute scrotal pain, accompanied by nausea and vomiting. An important finding on physical examination is a change in testicular axis from the normal vertical positioning of the testis in the scrotum to a more horizontal positioning. Within hours of torsion, a reddened scrotum develops, with or without enlargement.1
Imaging: Ultrasonography is the only diagnostic tool necessary to make the diagnosis of torsion and suggest surgical exploration. The testis is normal-sized to enlarged, with normal to decreased echogenicity (Fig. 126-13). Enlargement and hypoechogenicity are thought to be due to venous congestion. The echogenicity pattern is usually homogeneous. Epididymal enlargement may be an early finding in some cases of torsion. At least 10% of cases of torsion have associated reactive hydroceles. Ultrasound performed more than 48 hours after symptomatology (e-Fig. 126-14) may show a heterogeneous or hyperechoic testis from hemorrhage or hemorrhagic necrosis.1
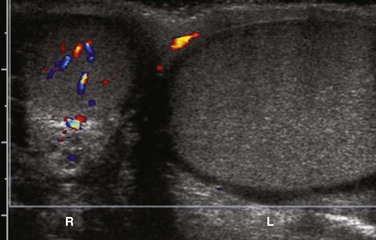
Figure 126-13 Testicular torsion and axis change.
A color Doppler ultrasound in the transverse plane shows color flow in the right testicle. The right testicle is oval to circular, because it has been evaluated in the transverse plane. The left testicle is elongated as if it is in longitudinal plane. This axis change is the ultrasound equivalent of the worrisome clinical finding suggestive of torsion when accompanied by a history of sudden pain. Lack of Color Doppler flow in the left testicle confirms a left testicular torsion.
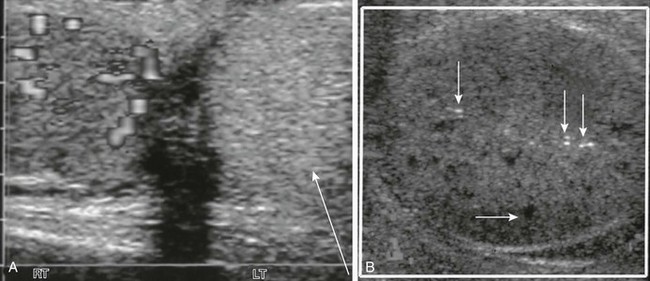
e-Figure 126-14 A longitudinal Doppler sonogram of a patient with a delayed diagnosis of testicular torsion shows an enlarged testis with heterogeneous echogenicity and no color flow.
According to this teenager’s history, the testis had twisted 4 days before he sought medical care. A, Transverse view of the testes shows a hyperechoic left testicle (arrow) with absent Doppler-able flow. A normal right testicle is present. B, Echoless areas (the horizontal arrow points to one) are consistent with destroyed areas of testicular parenchyma. A few bright echoes (vertical arrows) were consistent with areas of hemorrhage.
State-of-the-art color or power Doppler imaging methods have made ultrasound the gold standard for the imaging and preoperative diagnosis of testicular torsion. Venous flow is lost before arterial flow ceases. Color flow comparison to the contralateral testis is helpful and necessary, particularly when torsion is incomplete, in which case the diagnosis may be suggested not by absence of arterial flow but by blood flow asymmetry. The spermatic cord always should be seen, and it should be straight. When torsion is present, a spiral twist or Whirlpool sign is seen. Flow may be normal or increased in a torsed testis that spontaneously detorses.20–23
Treatment: Torsion must be corrected for testicular viability. Surgery within 24 hours leads to 60% to 70% testicular salvage, but after 24 hours salvage is only 20%. Because abnormal testicular fixation is considered a bilateral phenomenon, preventive orchiopexy often is performed on the contralateral nontwisted testis.16
Testicular Torsion in the Fetus and Newborn
Overview: Testicular torsion that occurs in the newborn period usually presents as a painless, firm scrotal mass often associated with bluish-red scrotal discoloration. The affected testis is generally nonviable. If the torsion occurred in intrauterine life, one is imaging the neonatal equivalent of delayed torsion.1
Imaging: Antenatal torsion may be diagnosed on fetal ultrasound examination. Antenatal torsion must be considered if the fetal testis or testes are heterogeneous in echogenicity or of asymmetric size (Fig. 126-15). Diagnosis in the newborn is made by similar imaging findings.24–27
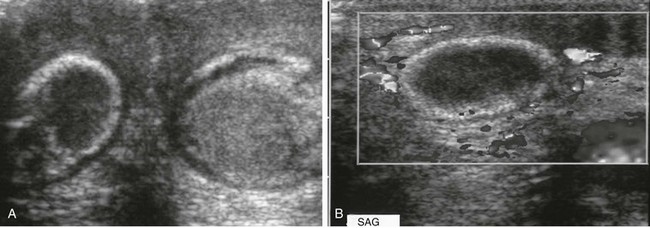
Figure 126-15 Bilateral antenatal testicular torsion.
A, A transverse sonogram shows two testes with torsion of different ages in a newborn with scrotal swelling. The testes are seen deep to a more superficial thickened scrotal wall. The right testis is hypoechoic with a somewhat hyperechoic periphery, suggesting an old torsion. The grayer left testis does not appear particularly abnormal on this image, although its periphery is more echogenic than the remainder of its parenchyma. Color Doppler imaging showed no flow in either testis. Upon surgery, both testes proved to have undergone torsion. The torsion of the left testis probably occurred later in fetal life. Tiny hydroceles are present bilaterally. Because the scrotum of this newborn was small, it could be seen completely by the high-frequency (14-MHz) linear array probe despite its small footpad. B, A longitudinal sonogram of the torsed right testis. No color flow is seen within the hypoechoic parenchyma or the surrounding hyperechoic periphery of this neonate’s testis, which underwent torsion during fetal life.
Torsion of Testicular and Epididymal Appendages
Overview: Testicular and epididymal appendage torsion occurs most typically in the 6- to 12-year-old age group. Patients present with localized pain, a pea-sized mass in the area of the upper pole of the testis, and at times, a pinpoint discoloration (the “blue dot” sign) seen through the overlying scrotal skin. The clinical picture may be indistinguishable from that of testicular torsion, albeit in a somewhat younger age group.
Imaging: Ultrasound demonstrates an enlarged torsed appendage as a small circular mass (Fig. 126-16) of variable echogenicity near the testis or epididymis of the affected side. Reactive epididymal swelling and hyperemia often is present. Usually the testicular echogenicity and vascular flow are normal.24,28
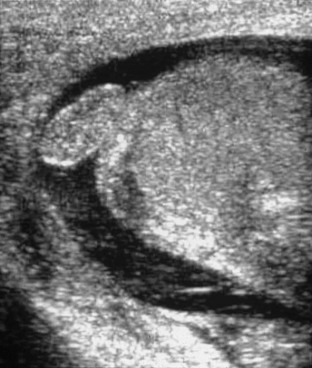
Figure 126-16 A torsed appendix testis.
A longitudinal sonogram of the superior portion of the scrotum shows a prominent oval structure superior to the testis of a child who presented with sudden testicular pain. The oval structure is of equal echogenicity to the testis and is an enlarged torsed testicular appendage. Color flow of the testis was normal, proving that no testicular torsion was present. Some debris is present in a small surrounding hydrocele.
Treatment: Twisted appendages generally are a self-limited disorder and respond best to nonsteroidal antiinflammatory medications and comfort measures such as limited activity and a warm compress. As the appendage infarcts and necroses, the pain resolves. Surgical intervention is indicated when the symptoms are prolonged and spontaneous resolution does not occur.29
Epididymitis
Overview: Epididymitis is the most common cause of an acute painful scrotum in the postpubescent male. The cause is usually bacterial, although in pediatric and some adolescent patients, epididymitis may be viral (e.g., as a result of mumps). Patients with epididymitis often are febrile, and at least half report dysuria. Pyuria is common, and nausea, vomiting, and leukocytosis may be present. An enlarged epididymis may be palpated, and scrotal edema and tenderness often are present.1
Complications of epididymitis include direct spread of the infection to the testis (i.e., epididymo-orchitis), which can be seen in as many as 20% of cases. Although it is an uncommon complication, an abscess may develop within the scrotum or testes and require drainage. In unusual cases, acute epididymitis may impede testicular blood flow, resulting in focal or diffuse infarction of the testis or epididymis even in the absence of torsion.1
Imaging: Ultrasound shows enlargement of the epididymis (e-Fig. 126-17) and commonly a reactive hydrocele or pyocele. Overlying scrotal wall thickening may be present. The echogenicity of the enlarged epididymis may be normal, slightly decreased, or slightly increased. The affected area of epididymis, although enlarged, usually maintains its normal shape. The diagnosis is supported by color Doppler imaging showing increased flow (Fig. 126-18). With epididymo-orchitis, a hypoechoic area of involvement is seen within the testis directly adjacent to the area of involved epididymis (e-Fig. 126-19). Color Doppler imaging usually shows increased flow to the involved portion of adjacent testis and the epididymis. In the prepubescent male, when epididymitis is the result of a congenital anomaly, a voiding cystourethrogram may be performed to detect abnormalities such as reflux of contrast agent into a vas deferens.30–32
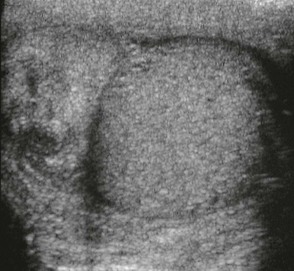
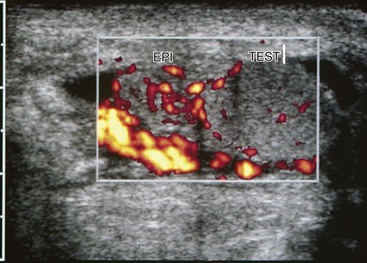
e-Figure 126-17 Epididymitis.
A longitudinal sonogram shows that the left epididymis of this teenager is very large compared with the normal testis.
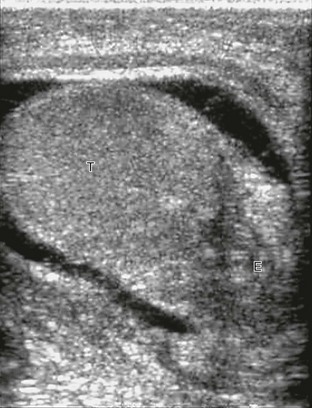
e-Figure 126-19 Epididymo-orchitis.
A longitudinal sonogram of the left testis shows that the scrotum is somewhat thickened, and a hydrocele is present. The echopenic portion of the epididymis (E) is seen inferiorly and is difficult to separate from the testis (T). The testis is hypoechoic where it is in contact with the inflamed epididymis. The findings are consistent with extension of the infection into the testis.
Henoch-Schönlein Purpura
Overview and Imaging: Henoch-Schönlein purpura is a generalized vasculitis of unknown cause characterized clinically by a purpuric rash, abdominal pain, joint manifestations, and often hematuria. Involvement of the epididymis and testis may result in scrotal swelling and tenderness. Most often, however, the scrotum alone is involved by severe purpura and swelling. Imaging may be indicated to exclude testicular torsion. Imaging normal testes with normal flow despite marked edema of the scrotal wall helps suggest the diagnosis and can prevent the need to explore the scrotum surgically.1,34,35
Testicular and Paratesticular Neoplasms
Overview: Testicular neoplasms are uncommon in infants and children, representing only 1% to 1.5% of all childhood malignancies; when they are present, they tend to occur in very young children, with a peak incidence at 2 years of age and with 60% of the cases occurring in children younger than 2 years. Such tumors usually present as a painless, nontender, and firm scrotal mass often of weeks’ to months’ duration. Scrotal pain and tenderness may occur if associated torsion of the testis occurs. Testicular tumors occasionally are bilateral.1 Primary testicular tumors are classified according to their tissue of origin (Box 126-2).
Imaging: The predominant imaging tool used to search for and evaluate tumors of the scrotum and its contents is ultrasound. Most testicular neoplasms tend to be hypoechoic (e-Fig. 126-20). Contained anechoic areas within tumors represent either cystic components or more often are evidence of focal necrosis as the tumor outstrips its blood supply. Acute hemorrhage appears echogenic, and echogenic foci with posterior shadowing may be seen with intratumoral calcifications. Reactive hydroceles are associated in at least 15% to 20% of cases. Color Doppler and power Doppler ultrasound demonstrate increased vascularity in the majority of malignant tumors and may help define the mass itself. Abdominal ultrasound, CT, or MRI often is performed for detection of pelvic or retroperitoneal lymph node enlargement and in the search for solid organ metastases. Chest radiographs and CT are used to search for pulmonary metastases.1,6
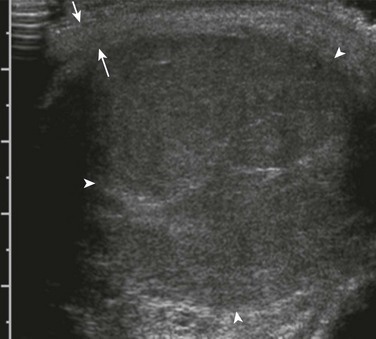
e-Figure 126-20 A testicular seminoma.
An ultrasound image in the longitudinal plane shows a large mass (arrowheads) surrounded by a periphery of normal testicular parenchyma (arrows). It is hypoechoic compared with the normal testicle, as is typical of most testicular neoplasms. The mass was found to be the cause of an enlarging scrotum in a 17-year-old.
Overview and Imaging: Most childhood testicular tumors (65% to 75%) are germ cell in origin. Yolk sac tumor is by far the most common malignant germ cell tumor (80% to 90%) in children. Three fourths of yolk sac tumors are diagnosed by 24 months of age. Hemorrhage, particularly after clot dissolution, frequently causes echo-free areas within this typically hypoechoic, somewhat well-circumscribed mass. An elevated serum alpha-fetoprotein level is a tumor marker in approximately 90% of affected patients.1,6
Testicular teratomas represent 10% to 15% of childhood germ cell tumors, with 65% of cases diagnosed before 2 years of age. They are composed of elements derived from all three germ cell layers and may contain cartilage, bone, and epidermal elements such as keratin, fibrous tissue, smooth muscle, and fat. Teratomas appear on ultrasound as complex masses with cystic and solid components (Fig. 126-21). Bony and dental elements are echogenic with posterior shadowing on ultrasound, whereas the adipose component appears echogenic but without shadowing. Prepubertal teratomas are said to almost always follow a benign course even if they contain islands of malignant germ cells (15%). Postpubertal teratomas, in contrast, are potentially malignant because of their propensity to develop components of other germ cell tumors, resulting in teratocarcinomas. The serum human chorionic gonadotropin level in these cases usually is elevated.1,6,36
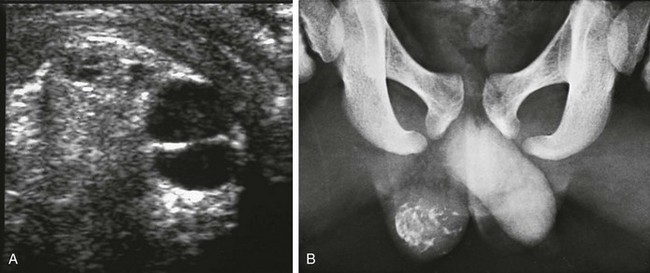
Figure 126-21 A testicular teratoma.
A, A transverse sonogram of the right testicle shows a complex testicular mass with debris-filled cystic foci and surrounding hyperechogenicity. B, A coned radiograph of the lower pelvis identifies a calcified right testicular lesion. (Courtesy Dr. Leslie E. Grissom.)
Gonadal Stromal Tumors
Overview and Imaging: Non–germ cell testicular tumor types represent only 25% to 30% of pediatric testicular tumors. Leydig cell tumors and Sertoli–granulosa cell tumors are very uncommon but represent the most common gonadal stromal tumors of childhood. They almost always are benign adenomas. Almost half (45%) of Leydig cell tumors are diagnosed between the ages of 2 and 9 years, with a peak incidence at 4 years of age. Leydig cell tumors typically are painless but may be associated with premature virilization (Fig. 126-22) if androgen is produced or with gynecomastia if estrogen is produced. Sertoli cell tumors represent 20% of the non–germ cell tumors. Half are diagnosed in the first year of life. Gynecomastia may be caused by estrogen production. Tumors containing a mixture of non–germ cell histologic patterns may occur.1,6,37,38
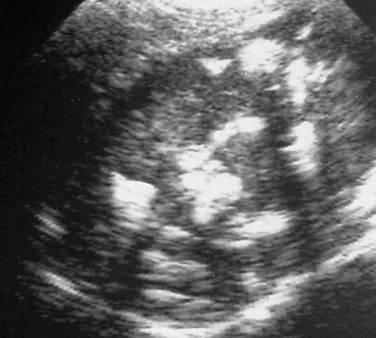
Figure 126-22 A Leydig cell tumor.
A longitudinal sonogram in a patient with precocious puberty (i.e., early virilization) shows multiple calcifications (bright echogenicities) scattered in the testicular parenchyma. Shadowing is present beyond one or two of the calcifications. (Courtesy Dr. Kenneth Glassberg.)
Epidermoid Cyst
Overview and Imaging: The epidermoid cyst, also known as a keratocyst, is a benign tumor of germ cell origin representing less than 1% of all testis tumors (Fig. 126-23). It consists of a simple squamous cell–lined echopenic area located within the testicular parenchyma, usually just below the tunica albuginea. The wall is made up of fibrous tissue, and its lumen contains cheesy keratinized material or amorphous debris. Patients usually present with a painless 0.5- to 4-cm nodule that often is picked up incidentally on a routine physical examination. Four basic ultrasound appearances are seen, including a halo with central increased echogenicity, a sharply defined mass with peripheral calcification, and a solid mass with an echogenic rim. The classic appearance is an onion-ring pattern with alternating hyperechoic and hypoechoic rings that typically is avascular.1,6,39–41
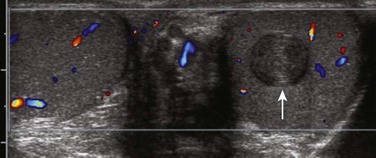
Figure 126-23 A testicular epidermoid.
A color Doppler ultrasound in the transverse plane shows that this teenager has a normal right testicle. The left testicle contains a mass (arrow) with onion skin layering. This image is typical for a testicular epidermoid. No color flow was seen within the mass, underlining the fact that these masses typically are avascular.
Secondary Testicular Neoplasms
Overview and Imaging: Testicular metastases are rare in children, representing far less than 1% of testicular tumors. Leukemia and lymphoma are the most common causes. Enlarged testes caused by leukemic or lymphomatous infiltration are uncommon but may be the primary manifestation of these diseases or the first sign of relapse after bone marrow remission. The lesions often are bilateral. At least 8% of patients with acute lymphocytic leukemia have testicular involvement at some point during the course of their disease. These enlarged testes have focal (Fig. 126-24) or diffuse areas of decreased echogenicity. Areas of hemorrhage appear echogenic, and lymphatic obstruction may lead to a hydrocele. Color Doppler imaging often shows significant increases in flow, often in an asymmetric pattern related to the position of the infiltrating masses within the testis.1
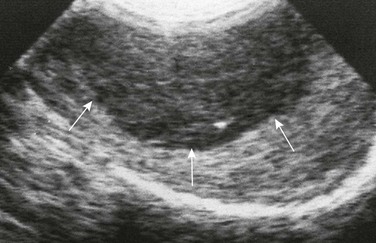
Figure 126-24 A patient with leukemia.
A longitudinal sonogram of an enlarged right testis of a teenager with leukemia who was thought to be in remission shows a hypoechoic mass (arrows). The echopenic area proved to be due to leukemic involvement of the testis. (From Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill; 2001. Reproduced with permission of McGraw-Hill Companies.)
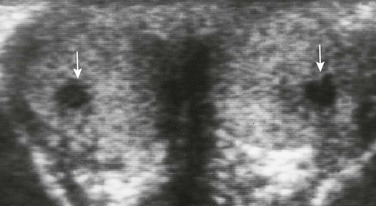
Overview and Imaging: Adrenal rests are simulators of intratesticular tumors. At times, aberrant cells from the adrenal cortex may travel with gonadal tissue and be incorporated into the testis during fetal life. With high levels of adrenocorticotropic hormone and associated cortical cell stimulation (as in persons with congenital adrenal hyperplasia and Cushing syndrome), these rests may enlarge (Fig. 126-25). Patients present with a testicular mass or enlargement. On ultrasound, several hypoechogenic or hyperechogenic masses generally are seen, usually in both testes. A typical color Doppler ultrasound pattern has been described in which multiple peripheral vessels radiate in a spokelike fashion from the centers of individual rests.1,41,42
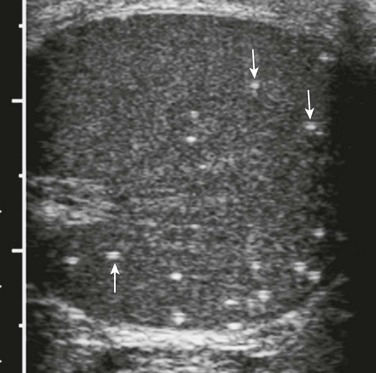
Testicular Microlithiasis
Overview and Imaging: Testicular microlithiasis is characterized by calcifications within the lumina of seminiferous tubules. It is denoted on ultrasound by individual, tiny (2 to 3 mm), hyperechoic foci without shadowing (Fig. 126-26) within the testicular parenchyma. Some clinicians reserve the diagnosis for cases in which more than five microliths are noted on a single ultrasound image (classic testicular microlithiasis). Testicular microlithiasis is reported in 2.4% of asymptomatic 0- to 19-year-old boys and also has been associated with cryptorchidism, infertility, male intersex, Klinefelter syndrome, and pulmonary alveolar microlithiasis. It usually is an incidental finding; however, there is a debated association between the presence of testicular microlithiasis and the presence or development of testicular neoplasm (reportedly 13 to 21.6 times greater risk), particularly germ cell neoplasia. Because of the association of microlithiasis and tumors, patients with testicular microlithiasis alone are followed up closely both clinically and by ultrasound (with follow-up by 6 months to 1 year) for possible tumor development.1,43–48
Intrascrotal Extratesticular Masses
Paratesticular Rhabdomyosarcoma
Overview and Imaging: Rhabdomyosarcoma is the most common malignant paratesticular mass in children, representing 10% of all intrascrotal tumors of childhood. It originates from the supporting stroma of the spermatic cord, testicular appendages, and paratesticular tunics and often is located superior to the testis. Two incidence peaks are seen for intrascrotal rhabdomyosarcoma, one at 2 to 4 years of age and the other between 15 and 17 years. Rhabdomyosarcomas present as a painless mass, grow rapidly, and frequently are large at presentation. They spread early to regional and retroperitoneal lymph nodes. Venous invasion and distant metastases, especially to the lungs, are not uncommon. On ultrasound examination, the mass is predominantly echogenic with focal anechoic areas caused by necrosis. As with all extratesticular masses, if the testes are invaded, differentiating the lesion from a mass of testicular origin may be difficult.1
Varicoceles
Overview: A common mass found within the scrotum of teenagers is a varicocele, which is composed of dilated veins of the pampiniform plexus. Varicoceles are graded according to their size (Box 126-3). When they are unilateral, 99% of varicoceles are left sided, where the spermatic vein enters the renal vein at a right angle. This anatomic situation is linked to a far greater incidence of venous incompetence compared with the right side, where the right spermatic vein enters the inferior vena cava directly at an oblique angle. The occurrence of bilateral varicoceles is common. However, finding a solitary right-sided varicocele is extremely uncommon, and the examiner must rule out an intraabdominal neoplasm or another mass as a cause.1,6
Imaging: On ultrasound examination, varicoceles are tortuous, tubular, echo-free structures that typically are situated superior, lateral, and/or posterior to the testis (Fig. 126-27). The diagnosis is confirmed by having the patient perform a Valsalva maneuver, supine or standing, and noting on color Doppler imaging that the tubular vessels, which may not show flow before the maneuver, will fill with color (e-Fig. 126-28).
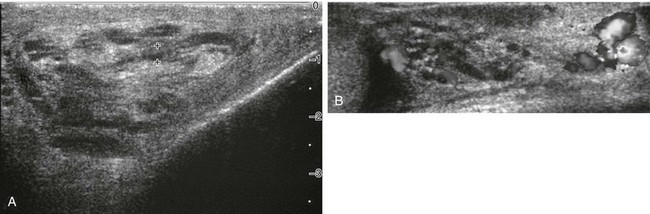
Figure 126-27 A varicocele.
A, A transverse sonogram shows tubular echoless structures lateral to a teenager’s left testis. One (cursors) is noted to be 2.7 mm wide. B, A color Doppler image during straining by the patient. Color fills several of the tubular structures. A venous spectral pattern was obtained, which proved that they were varicoceles. The normal testis is seen to the reader’s left.
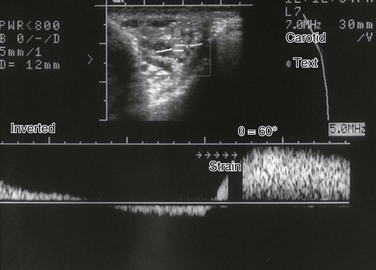
e-Figure 126-28 A varicocele.
An image of a triplex Doppler shows tubular structures in the scrotum. The lower half of the image shows the spectral pattern over time. Arrows point out when the patient strained. The strain resulted in significant venous-type flow increase, proving that the structures were due to a varicocele.
Treatment: Surgical or interventional varicocele ligation is recommended in cases in which ipsilateral testicular volume loss of 2 mL or greater occurs. Histologic analysis of such testes has shown various degrees of tubal hypoplasia, decreased spermatogenesis, focal fibrosis, or arrest of germ cell maturation.1,6,49–54
Ahmed, HU, Arya, M, Muneer, A, et al. Testicular and paratesticular tumours in the prepubertal population. Lancet Oncol. 2010;11(5):476–483.
Akbar, SA, Sayyed, TA, Jafri, SZ, et al. Multimodality imaging of paratesticular neoplasms and their rare mimics. Radiographics. 2003;23(6):1461–1476.
Coley, BD. Sonography of pediatric scrotal swelling. Semin Ultrasound CT MR. 2007;28(4):297–306.
Hörmann, M, Balassy, C, Philipp, MO, et al. Imaging of the scrotum in children. Eur Radiol. 2004;14(6):974–983.
Karmazyn, B, Steinberg, R, Livne, P, et al. Duplex sonographic findings in children with torsion of the testicular appendages: overlap with epididymitis and epididymoorchitis. J Pediatr Surg. 2006;41(3):500–504.
Marulaiah, M, Gilhotra, A, Moore, L, et al. Testicular and paratesticular pathology in children: a 12-year histopathological review. World J Surg. 2010;34(5):969–974.
Munden, MM, Trautwein, LM. Scrotal pathology in pediatrics with sonographic imaging. Curr Probl Diagn Radiol. 2000;29(6):185–205.
References
1. Cohen, HL. Abnormalities of the male genital tract. In Slovis T, ed.: Caffey’s pediatric diagnostic imaging, 11th ed, Philadelphia: Mosby (Elsevier), 2008.
2. Prader, A. Testicular size: assessment and clinical importance. Triangle. 1966;7:240–243.
3. Kuijper, E, Van Kooten, J, Verbeke, J, et al. Ultrasonographically measured testicular volumes in 0- to 6-year-old boys. Human Reproduction. 2008;23:792–796.
4. Cassorla, FG, Golden, SM, Johnsonbaugh, RD, et al. Testicular volume during early infancy. J Pediatrics. 1981;99:742–743.
5. Daniel, WA, Jr., Feinstein, RA, Howard-Peebles, P, et al. Testicular volumes of adolescents. J Pediatrics. 1982;101:1010–1012.
6. Cohen, HL, Haller, J. Scrotal ultrasound in the pediatric and adolescent patient. Radiol Rep. 1990;2:276–290.
7. Dogra, VS, Gottlieb, RH, Oka, M, et al. Sonography of the scrotum. Radiology. 2003;227:18–36.
8. Cohen, HL. Hematocle. In: Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill, 2001.
9. Akin, EA, Khati, NJ, Hill, MC. Ultrasound of the scrotum. Ultrasound Q. 2004;20:181–200.
10. Troughton, AH, Waring, J, Longstaff, A, et al. Role of magnetic resonance imaging in the investigation of undescended testes. Clin Radiol. 1990;41:178–181.
11. Woodward, P. Case 70: seminoma in an undescended testis. Radiology. 2004;231:388–392.
12. Tekgül, S, Riedmiller, H, Gerharz, E, et al. Cryptorchidism. In: Guidelines on paediatric urology. Arnhem, The Netherlands: European Association of Urology, European Society for Paediatric Urology; 2009.
13. Giwercman, A, Grindsted, J, Hansen, B, et al. Testicular cancer risk in boys with maldescended testis: a cohort study. J Urol. 1987;138:1214–1216.
14. Riebel, T, Herrmann, C, Wit, J, et al. Ultrasonographic late results after surgically treated cryptorchidism. Pediatr Radiol. 2000;30:151–155.
15. Pettersson, A, Richiardi, L, Nordenskjold, A, et al. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med. 2007;356(18):1835–1841.
16. Hadziselimovic, F. Treatment of cryptorchidism with GnRH. Urol Clin North Am. 1982;9:413–420.
17. Singh, A, Kao, S, D’Alessandro, M, et al. Case 164; funicular type of spermatic hydrocele. Radiology. 2010;257:890–892.
18. Lau, ST, Lee, YH, Caty, M. Current management of hernias and hydroceles. Semin Pediatr Surg. 2007;16(1):50–57.
19. Cimador, M, Castagnetti, M, De Grazia, E. Management of hydrocele in adolescent patients. Nat Rev Urol. 2010;7(7):379–385.
20. Paltiel, H. Acute left testicular torsion in an adolescent. In: Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill, 2001.
21. Jensen, MC, Lee, K, Halls, J, et al. Color Doppler sonography in testicular torsion. J Clin Ultrasound. 1990;18:446–448.
22. Karmazyn, B, Steinberg, R, Kornreich, L, et al. Clinical and sonographic criteria of acute scrotum in children: a retrospective study of 172 boys. Pediatr Radiol. 2005;35:302–310.
23. Baud, C, Veyrac, C, Couture, A, et al. Spiral twist of spermatic cord: a reliable sign of testicular torsion. Pediatr Radiol. 1998;28:950–954.
24. Vijayaraghavan, SB. Sonographic differential diagnosis of acute scrotum. J Ultrasound Med. 2006;25:563–574.
25. Zinn, H, Cohen, HL. Fetal and neonatal testicular torsion. In: Cohen HL, Sivit CJ, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill, 2001.
26. Zerin, JM, DiPietro, MA. Testicular infarction in a newborn: ultrasound findings. Pediatr Radiol. 1990;20:329–330.
27. Brown, SM, Casillas, V, Montalvo, B, et al. Intrauterine spermatic cord torsion in the newborn with sonographic and pathologic correlation. Radiology. 1990;177:755.
28. Cohen, HL, Shapiro, M, Haller, J, et al. Torsion of the testicular appendage. J Ultrasound Med. 1992;11:81–84.
29. Gatti, JM, Patrick Murphy, J. Current management of the acute scrotum. Semin Pediatr Surg. 2007;16(1):58–63.
30. Ralls, PW. Acute epididymo-orchitis. In: Herzberg BS, Hill MC, Cohen HL, eds. Ultrasound (third series). Reston, VA: American College of Radiology, 2005.
31. Trambert, MA, Mattrey, R, Levine, D, et al. Subacute scrotal pain: evaluation of torsion versus epididymitis with MR imaging. Radiology. 1990;175:53–56.
32. Middleton, WD, Siegel, BA, Melson, GL, et al. Acute scrotal disorders: prospective comparison of color Doppler US and testicular scintigraphy. Radiology. 1990;177:177–181.
33. Santillanes, G, Gausche-Hill, M, Lewis, RJ. Are antibiotics necessary for pediatric epididymitis? Pediatr Emerg Care. 2011;27(3):174–178.
34. Laor, T, Atala, A, Teele, RL. Scrotal ultrasonography in Henoch-Schönlein purpura. Pediatr Radiol. 1992;22(7):505–506.
35. Hara, Y, Tajiri, T, Matsuura, K, et al. Acute scrotum caused by Henoch-Schönlein purpura. Int J Urol. 2004;11(7):578–580.
36. Connor, S, Guest, P. Conversion of multiple solid testicular teratoma metastases to fatty and cystic liver masses following chemotherapy: CT evidence of “maturation,”. Br J Radiol. 1999;72:1114–1116.
37. Kirsch, A, Bastian, W, Cohen, HL, et al. Precocious puberty in a child with unilateral Leydig cell tumor of the testis following orchiopexy. J Urol. 1993;150:1483–1485.
38. Cohen, HL, Torrisi, J. Leydig cell tumor of the testicle. In: Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill, 2001.
39. Ulbright, T, Srigley, J. Dermoid cyst of the testis: a study of five postpubertal cases, including a pilomatrixoma-like variant, with evidence supporting its separate classification from mature testicular teratoma. Am J Surg Pathol. 2001;25:788–793.
40. Arellano, CM, Kozakewich, HP, Diamond, D, et al. Testicular epidermoid cysts in children: sonographic characteristics with pathological correlation. Pediatr Radiol. 2011;41(6):683–689. [quiz 796-797].
41. Neumann, DP, Abrams, GS, Hight, DW. Testicular epidermoid cysts in prepubertal children: case report and review of the world literature. J Pediatr Surg. 1997;32(12):1786–1789.
42. Avila, N, Premkumar, A, Shawker, T, et al. Testicular adrenal rest tissue in congenital adrenal hyperplasia: findings at gray-scale and color Doppler US. Radiology. 1996;198:99–104.
43. Vanzulli, A, DelMaschio, A, Paesano, P, et al. Testicular masses in association with adrenogenital syndrome: US findings. Radiology. 1992;183:425–429.
44. Cast, J, Nelson, W, Early, A, et al. Testicular microlithiasis: prevalence and tumor risk in a population referred for scrotal sonography. AJR Am J Roentgenol. 2000;175:1703–1706.
45. Skyrme, R, Fenn, N, Jones, A, et al. Testicular microlithiasis in a UK population: its incidence, association and follow-up. BJU Int. 2000;86:482–485.
46. Bach, A, Hann, L, Shi, W, et al. Is there an increased incidence of contralateral testicular cancer in patients with intratesticular microlithiasis? AJR Am J Roentgenol. 2003;180:497–500.
47. Hamper, UM. Testicular microlithiasis with germ cell tumor. In: Herzberg BS, Hill MC, Cohen HL, eds. Ultrasound (third series). Reston, AV: American College of Radiology, 2005.
48. Goede, J, Hack, W, Vander Voort-Doedens, LM, et al. Prevalance of testicular microlithiasis in asymptomatic males 0 to 19 years old. J Urol. 2009;182(4):1516–1520.
49. Kass, E, Chandra, R, Belman, A. Testicular histology in the adolescent with a varicocele. Pediatrics. 1987;79:996–998.
50. Kessler, A, Meirsdorf, S, Graif, M, et al. Intratesticular varicocele: gray scale and color Doppler sonographic appearance. J Ultrasound Med. 2005;24:1711–1716.
51. Ozcan, H, Aytac, S, Yagci, C, et al. Color Doppler ultrasonographic findings in intratesticular varicocele. J Clin Ultrasound. 1997;25:325–327.
52. Cohen, HL, Zinn, DL. Varicocele. In: Cohen HL, Sivit C, eds. Fetal and pediatric ultrasound: a casebook approach. New York: McGraw-Hill, 2001.
53. Castagnetti, M, Cimador, M, Catalano, P, et al. Evolving management of adolescent varicocele. J Pediatr Urol. 2008;4(2):107–112.
54. Preston, MA, Carnat, T, Flood, T, et al. Conservative management of adolescent varicoceles: a retrospective review. Urology. 2008;72(1):77–80.