Abdominal Trauma
Overview
Trauma in children accounts for more than 500,000 hospital admissions and 20,000 deaths per year. After cranial trauma, the abdomen is the second most common site of injury, and approximately 80% of abdominal injuries are due to blunt force trauma. The most common reported mechanism is motor vehicle crashes, followed by automobile–pedestrian injuries. Other common causes of injury include bicycle trauma and falls from a height. In young children, injuries also may result from intentional or nonaccidental trauma.1
Clinical Presentation
Clinical variables that have been associated with a high risk of injury include gross hematuria, abdominal tenderness, seat belt ecchymoses, and a low trauma score. Seat belt ecchymoses across the lower abdomen or flank represent an important high-risk marker for injury.2,3 Such ecchymoses are associated with a complex of injury to the lumbar spine, bowel, and bladder that accounts for most injuries to belted motor vehicle passengers.
Imaging
The rapid and accurate evaluation of injured children with CT has resulted in improved triage, has contributed to reduced morbidity and mortality, and along with improvements in supportive care, has played a critical role in the success of nonoperative management of solid organ injuries. CT findings have been shown to change the initial management plan in nearly half of children assessed after blunt abdominal trauma.4–6
Sonography in the Assessment of Abdominal Trauma
Sonography remains widely used in the screening of injured children and adults and has been shown to have high sensitivity and specificity in the detection of hemoperitoneum; however, its utility is limited in comparison with CT. Solely identifying fluid does not necessarily reveal the cause or the site of injury, and the presence of hemoperitoneum in a hemodynamically stable child typically does not affect clinical management decisions. Furthermore, sonography provides no diagnostic information regarding injury to the bony pelvis or lumbar spine, it cannot be used in the diagnosis of hollow viscus injury, and it has been shown to miss approximately one fourth to one third of solid organ injuries.7 Thus if one relies on identification of peritoneal fluid as a marker for hepatic and splenic injury, one will miss a significant number of injuries. Nevertheless, sonography has a potential role in diagnosing hemodynamically unstable patients because it can be performed rapidly at the bedside before the patient is taken to the operating room. In this role, it serves as a fast, noninvasive replacement for diagnostic peritoneal lavage. Recent studies suggest that contrast-enhanced sonography may have improved accuracy in delineating solid organ injuries.8
Computed Tomography Findings
The liver is the most frequently injured viscus after blunt trauma in children, in whom this organ is poorly protected from injury by overlying ribs because the immature chest wall is easily deformed by external forces. A hepatic laceration appears as a nonenhancing region of varying configuration (Fig. 110-1) that may be linear or branching. Lacerations may be associated with a parenchymal or a subcapsular hematoma.
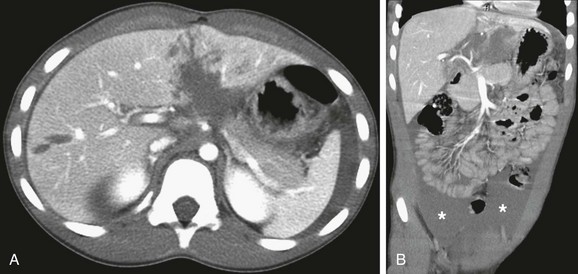
Figure 110-1 Hepatic laceration and hemoperitoneum.
Contrast-enhanced axial computed tomography scan through the upper abdomen (A) reveals a complex hepatic laceration involving segment four and a simple laceration of segment 5. A coronal reformat image (B) shows a large associated hemoperitoneum in the pelvis (asterisk).
The liver is surrounded by a thin capsule that in turn is covered by a peritoneal reflection of thin connective tissue. The presence of hemoperitoneum associated with hepatic injury principally relates to violation of the liver capsule at the site of injury. In several large series, hepatic injury was associated with hemoperitoneum in approximately two thirds of cases. Associated hemoperitoneum may be seen throughout the greater peritoneal cavity. Often the largest fluid pockets are located in the pelvis. Hepatic injury may not be associated with intraperitoneal hemorrhage if the injury does not extend to the surface of the liver, if the hepatic capsule is not disrupted, or if the injury extends to the liver surface in the bare area of the liver, which is devoid of peritoneal reflection (Fig. 110-2). Injury that extends to the bare area may lead to associated retroperitoneal hemorrhage, with blood often surrounding the right adrenal gland or extending into the anterior pararenal space.
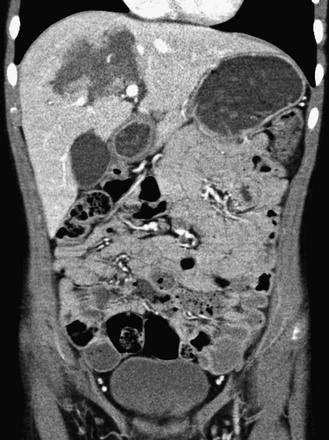
Figure 110-2 Hepatic laceration without hemoperitoneum.
Coronal reformat of a contrast-enhanced computed tomography scan reveals a laceration of segment 8. No associated hemoperitoneum is present.
Circumferential zones of periportal low attenuation may be seen in the liver after trauma. The presence of these low attenuation zones does not indicate hepatic injury. They most likely represent distended periportal lymphatics as a result of intravascular third-space fluid losses that occur after fluid resuscitation.9,10
Treatment: A number of grading scales have been proposed to quantify the severity of hepatic injury. These scales emphasize the anatomic extent of the injury, including capsular integrity, extent of subcapsular collection, extent of parenchymal disruption, and involvement of the vascular pedicle. The most widely used grading scale was developed by the American Association for the Surgery of Trauma. It was devised initially to reflect surgical findings but often is used to report severity of organ injury upon CT scanning. In children, these scales are not predictive of the need for operative management because in the vast majority of hepatic injuries, bleeding typically stops spontaneously and the injuries can be managed successfully without surgery regardless of the severity.11 This response likely is a result of the relatively smaller size of blood vessels and the enhanced vasoconstrictive response in children relative to adults. Between 1% and 3% of children with hepatic injury require surgical or endovascular hemostasis. However, injury grading scales often are used in the decision algorithm of patient management regarding intensity and length of hospitalization and activity restriction.
Splenic Injury
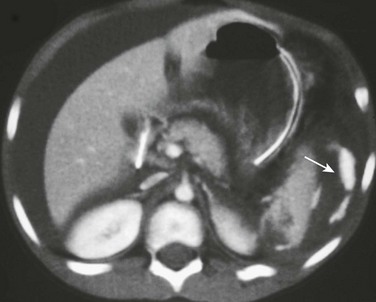
Splenic injury also is common after blunt trauma and frequently is associated with other organ injuries (Fig. 110-3). Because the spleen is much smaller than the liver, complex injury results in shattering or fragmentation of the organ (Fig 110-4). Associated intraparenchymal or subcapsular hematoma may be present. As with hepatic injury, associated intraperitoneal hemorrhage is not always present, especially when the splenic capsule remains intact. Absence of hemoperitoneum is observed in approximately 25% of splenic injuries. After injury involving the splenic hilum, blood also can track along the splenorenal ligament into the anterior pararenal space surrounding the pancreas.
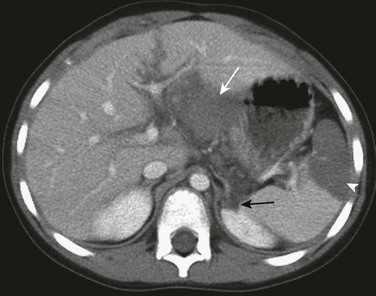
Figure 110-3 Hepatic, splenic, and renal injury.
A contrast-enhanced axial computed tomography scan through the upper abdomen reveals a laceration to segment 3 of the left hepatic lobe (white arrow), a splenic hematoma (arrowhead), and a small laceration to the left kidney (black arrow).
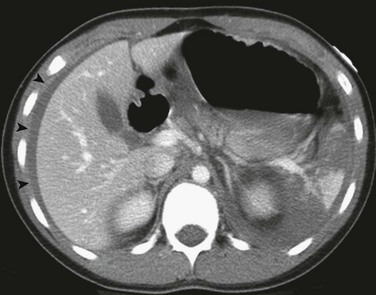
Figure 110-4 A shattered spleen.
A contrast-enhanced axial computed tomography scan through the upper abdomen shows a shattered spleen with only a small amount of central contrast enhancement. Note right perihepatic hemoperitoneum (arrowheads).
Treatment: Various injury grading scales have been described to objectively quantify injury to the spleen. As is true for hepatic injury, these scales are not predictive of surgical treatment in children because bleeding typically stops spontaneously and nonoperative management is successful in most splenic injuries.11,12 Grade of injury often is used for nonoperative clinical decision making, similar to the use of grading in hepatic injury.12–14
Pancreatic Injury
The best indicator of pancreatic injury at CT is unexplained peripancreatic fluid (i.e., fluid in the anterior pararenal space or lesser sac) (Fig. 110-5). This finding may be seen more often than the actual laceration. When fluid collects in the anterior pararenal space, it also may dissect between the pancreas and the splenic vein. However, pancreatic injury is only one cause of fluid in the anterior pararenal space.1 Other causes include third-space intravascular fluid loss, blood that extends from injury to the spleen or to the bare area of the liver, blood or bowel contents from a duodenal injury, and blood or urine that exudes from a renal injury after disruption of the renal fascia.
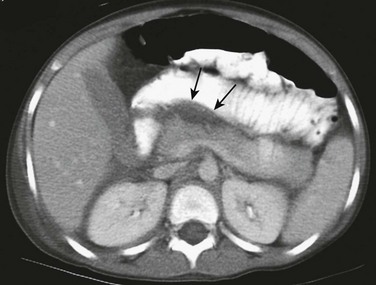
Figure 110-5 Pancreatic injury with associated peripancreatic fluid.
A contrast-enhanced axial computed tomography scan through the upper abdomen shows fluid in the anterior pararenal space surrounding the pancreas (arrows).
Pancreatic injury may be complicated by peripancreatic fluid collections, which may evolve into pancreatic pseudocysts. Approximately half of focal fluid collections that develop after pancreatic injury spontaneously resolve, and half evolve into pseudocysts that may require percutaneous or surgical drainage. The most common location for pseudocyst formation is the intrapancreatic or peripancreatic anterior pararenal space, or lesser sac (Fig. 110-6). However, pseudocysts may develop anywhere in the abdomen or pelvis.
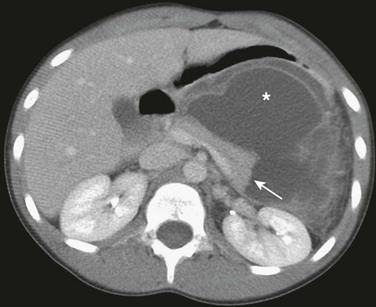
Figure 110-6 A pancreatic pseudocyst.
A contrast-enhanced axial computed tomography scan through the upper abdomen shows a laceration of the pancreatic tail (arrow) with a large, thick-walled focal fluid collection (asterisk) representing a pancreatic pseudocyst in the anterior pararenal space.
MR cholangiopancreatography can be very useful in subsequent imaging evaluation of pancreatic injury, especially in patients with transection of the pancreatic duct (Fig. 110-7).
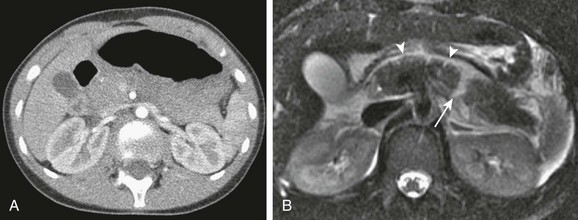
Figure 110-7 Pancreatic injury missed on an initial computed tomography (CT) scan.
A contrast-enhanced CT through the upper abdomen (A) shows a heterogeneous ill-defined pancreas but no focal laceration. An axial T2-weighted fast spin echo magnetic resonance image with fat saturation obtained the same day (B) shows peripancreatic fluid (arrowheads) and a laceration of the pancreatic body (arrow).
Treatment: Currently, divergent opinions have been expressed regarding the management of pancreatic injury. Some practitioners have shown that nonoperative management of most pancreatic injury is successful, even when the pancreatic duct is involved. Others believe that distal pancreatectomy for transections occurring to the left of the spine is the treatment of choice because it is definitive and is accompanied by an acceptable level of morbidity.15–17
Peritoneal Fluid and Hemorrhage
Occasionally, CT may reveal active hemorrhage in children who appear hemodynamically stable. The amount of hemoperitoneum noted on CT is not a measure of ongoing hemorrhage; rather, it reflects the cumulative amount of bleeding that occurred between the time of injury and the time the CT scan was obtained. The only sign of active hemorrhage on CT is the presence of focal or high attenuation areas (>90 HU) (Fig. 110-8). This finding also has been referred to in the literature as a contrast blush.13 The rate of active bleeding required for detection on CT is unclear. CT is useful in identifying active bleeding but may have difficulty in localizing the site of the hemorrhage. The torn blood vessel may be difficult to see because of diminished contrast enhancement caused by vasoconstriction and loss of blood containing contrast material. Occasionally, this finding may be observed only on delayed scanning.
Bowel and Mesenteric Injury
Bowel and mesenteric injuries are uncommon after blunt trauma, occurring in 6% to 16% of injured children. The most common mechanisms of injury associated with bowel and mesenteric injury are motor vehicle crashes, handlebar injuries, nonaccidental trauma, and falls. Intestinal injury is the result of direct force on the gastrointestinal tract and mesentery leading to a crush injury, rapid deceleration producing a shearing force between fixed and mobile portions of bowel or mesenteric attachments, and a sharp increase in intraluminal pressure resulting in rupture of the gut.20 Bowel rupture most commonly occurs in the mid to distal small intestine. The presence of seat belt ecchymosis and an acute hyperflexion (Chance) fracture of the lumbar spine are the only physical findings found to have a strong and significant association with bowel and mesenteric injury.3 Nonaccidental injury always must be considered in a child with a history of minor blunt trauma who has a bowel perforation.21,21a
Clinical signs and symptoms may be absent, minimal, or delayed, and CT plays an important role in early and accurate diagnosis. Delayed diagnosis can result in bowel ischemia, peritonitis, and, in rare cases, death as a result of sepsis. The main challenge for the radiologist is to distinguish between injuries that do and do not require surgical intervention. CT findings that are specific to bowel injury include the presence of extraluminal gas, extravasation of oral contrast material, and bowel discontinuity (Fig. 110-9).22,23 The latter two findings are very uncommon in pediatric practice. Extraluminal gas, however, is present in 20% to 30% of children with bowel injury and is a highly specific finding, with, unfortunately, low sensitivity. In a supine patient, extraluminal gas tends to accumulate at the convexity of the abdominal wall and in the porta hepatis (Fig. 110-10). Blunt abdominal injury also can lead to intravasation of gas into the mesenteric and portal venous system, presumably through mucosal disruption, or because of ischemic changes resulting from mesenteric tears.24 Injury to the retroperitoneal duodenum often results in localized bubbles of gas immediately adjacent to a thickened and distorted duodenum and pancreas. Review of the examination at a wide window setting is helpful in the detection of small amounts of extraluminal gas. Several other CT findings are common in injured patients but are less specific for significant bowel injury; these findings include focal bowel wall thickening and mesenteric fluid or stranding.
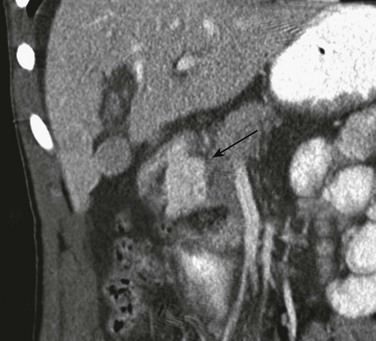
Figure 110-9 Duodenal perforation with extravasation of oral contrast material.
Coronal reformat of a contrast-enhanced computed tomography scan shows extravasation of oral contrast in the retroperitoneum (arrow) medial to the duodenal sweep.
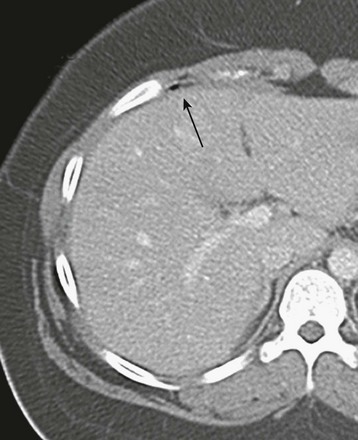
Figure 110-10 Ileal perforation and extraluminal gas.
A contrast-enhanced computed tomography image through the liver shows a small collection of extraluminal gas anterior to the right hepatic lobe (arrow).
Intramural hematoma results from hemorrhage into the bowel wall after a partial-thickness tear has occurred; these injuries usually can be managed nonoperatively. The most common location is the duodenum. The CT appearance is that of focal bowel wall thickening that often is eccentric (Fig. 110-11). Large duodenal hematomas may appear dumbbell shaped. Because the injury is intramural, no extraluminal air or extravasated contrast material should be present. Large hematomas can result in obstruction of the bowel proximal to the injury.
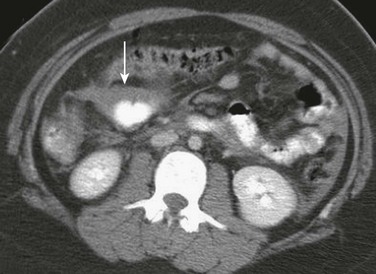
Figure 110-11 Mesenteric tear and ischemic bowel.
A contrast-enhanced axial computed tomography image through the mid abdomen shows a focal area of eccentric bowel wall thickening (arrow) due to a mesenteric tear. Ischemic bowel was confirmed at surgery.
CT signs that are highly specific for significant mesenteric injury are related to vascular injury and include mesenteric vascular beading, abrupt termination of mesenteric vessels, and mesenteric vascular extravasation (Fig. 110-12 and Box 110-1).22 These findings are rare in children, and their sensitivity and specificity are not known. Abnormalities that are less specific for the need for surgical intervention in mesenteric injury are mesenteric stranding and hematoma; these findings may represent a range of injuries from minor mesenteric bruising to underlying vascular disruption.
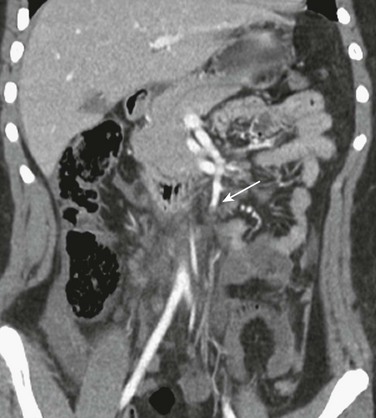
Figure 110-12 Superior mesenteric artery occlusion.
Coronal reformat of a contrast-enhanced computed tomography scan shows abrupt termination of the superior mesenteric artery (arrow). The patient had subsequent mesenteric ischemia and bowel perforation.
The most frequent CT finding associated with bowel rupture and mesenteric injury is “unexplained” peritoneal fluid (i.e., moderate to large amounts of fluid in the absence of solid viscus injury or bony pelvic fracture) (Fig. 110-13). Although nonspecific, unexplained peritoneal fluid is an important marker of potentially serious bowel or mesenteric injury. Approximately half the children with a moderate to large quantity of peritoneal fluid as the only finding on CT after blunt trauma have intestinal injury.25 Follow-up CT imaging in patients with initially equivocal findings and persistent abdominal symptoms has been reported to improve the detectability of intestinal injuries.23
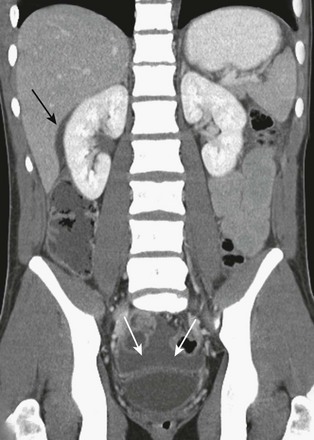
Figure 110-13 Bowel rupture with a moderate amount of “unexplained” peritoneal fluid.
Coronal reformat of a contrast-enhanced computed tomography (CT) image shows a moderate amount of peritoneal fluid in the cul-de-sac (white arrows), and in the subhepatic space (black arrow). No other abnormalities were noted on CT. A jejunal rupture was identified at surgery.
Multidetector scanners have improved the accuracy of CT for the diagnosis of bowel and mesenteric injuries. However, the reported sensitivity values (80%-95%) and specificity values (48%-84%) vary widely among studies.22,23
Hypoperfusion Complex
A characteristic complex of findings on CT associated with partially compensated hypovolemic shock in severely injured children has been characterized as the “hypoperfusion complex.” Most of these children have required extensive resuscitation for arterial hypotension on admission.26
CT findings in all children with the hypoperfusion complex include diffuse intestinal dilation with fluid. Abnormally intense contrast enhancement of bowel wall, mesentery, kidneys, aorta, and inferior vena cava is present, as well as diminished caliber of the aorta and inferior vena cava (Fig. 110-14). Variable findings include periportal low attenuation zones, intense adrenal, pancreatic and mesenteric enhancement, decreased pancreatic and splenic enhancement, peritoneal and retroperitoneal fluid, and bowel wall thickening.26,27
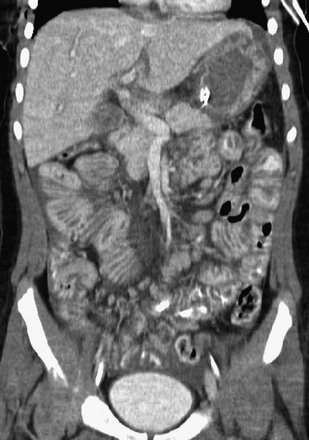
Figure 110-14 Hypoperfusion complex.
Coronal reformat of a contrast-enhanced computed tomography image shows diffuse intestinal thickening, dilation with fluid, intense contrast enhancement of the bowel wall, low periportal attenuation, and ascites indicative of partially compensated shock.
The hypoperfusion complex is a marker for a tenuous hemodynamic state and a predictor of a poor outcome. The mortality rate in children with this constellation of findings on CT approaches 80%.26
Brofman, N, Atri, M, Epid, D, et al. Evaluation of bowel and mesenteric trauma with multi-detector CT. Radiographics. 2006;26:1119–1131.
Lynn, KN, Werder, GM, Callaghan, RM, et al. Pediatric blunt splenic trauma: a comprehensive review. Pediatr Radiol. 2009;39:904–916.
Mattix, KD, Tataria, M, Holes, J, et al. Pediatric pancreatic trauma: predictors of nonoperative management failure and associated outcomes. J Pediatr Surg. 2007;42:340–344.
Sokolove, PE, Kupperman, N, Holmes, JF. Association between the “seat belt sign” and intra-abdominal injury in children with blunt torso trauma. Acad Emerg Med. 2005;12:808–813.
Van der Vlies, CH, Saltzherr, TP, Wilde, JCH, et al. The failure rate of nonoperative management in children with splenic or liver injury with contrast blush on computed tomography: a systematic review. J Pediatr Surg. 2010;45:1044–1051.
References
1. Bixby, SD, Callahan, MJ, Taylor, GA. Imaging in pediatric blunt abdominal trauma. Semin Roentgenol. 2008;43(1):72–82.
2. Sharma, OP, Oswanski, MF, Kaminsky, BP, et al. Clinical implications of the seat belt sign in blunt trauma. Am Surg. 2009;75(9):822–827.
3. Sivit, CJ, Taylor, GA, Newman, KD, et al. Safety-belt injuries in children with lap-belt ecchymosis: CT findings in 61 patients. AJR Am J Roentgenol. 1991;157(1):111–114.
4. Bond, SJ, Eichelberger, MR, Gotschall, CS, et al. Nonoperative management of blunt hepatic and splenic injury in children. Ann Surg. 1996;223(3):286–289.
5. Ruess, L, Sivit, CJ, Eichelberger, MR, et al. Blunt abdominal trauma in children: impact of CT on operative and nonoperative management. AJR Am J Roentgenol. 1997;169(4):1011–1014.
6. Neish, AS, Taylor, GA, Lund, DP, et al. Effect of CT information on the diagnosis and management of acute abdominal injury in children. Radiology. 1998;206(2):327–331.
7. Coley, BD, Mutabagani, KH, Martin, LC, et al. Focused abdominal sonography for trauma (FAST) in children with blunt abdominal trauma. J Trauma. 2000;48(5):902–906.
8. McGahan, JP, Horton, S, Gersovich, EO, et al. Appearance of solid organ injury with contrast-enhanced sonography in blunt abdominal trauma: preliminary experience. AJR Am J Roentgenol. 2006;187(3):658–666.
9. Patrick, LE, Ball, TI, Atkinson, GO, et al. Pediatric blunt abdominal trauma: periportal tracking at CT. Radiology. 1992;183(3):689–691.
10. Sivit, CJ, Taylor, GA, Eichelberger, MR, et al. Significance of periportal low-attenuation zones following blunt trauma in children. Pediatr Radiol. 1993;23(5):388–390.
11. Ruess, L, Sivit, CJ, Eichelberger, MR, et al. Blunt hepatic and splenic trauma in children: correlation of a CT injury severity scale with clinical outcome. Pediatr Radiol. 1995;25(5):321–325.
12. Lynn, KN, Werder, GM, Callaghan, RM, et al. Pediatric blunt splenic trauma: a comprehensive review. Pediatr Radiol. 2009;39(9):904–916. [quiz 1029-1030].
13. Lutz, N, Mahboubi, S, Nance, ML, et al. The significance of contrast blush on computed tomography in children with splenic injuries. J Pediatr Surg. 2004;39(3):491–494.
14. Cloutier, DR, Baird, TB, Gormley, P, et al. Pediatric splenic injuries with a contrast blush: successful nonoperative management without angiography and embolization. J Pediatr Surg. 2004;39(6):969–971.
15. Canty, TG, Sr., Weinman, D. Management of major pancreatic duct injuries in children. J Trauma. 2001;50(6):1001–1007.
16. Mattix, KD, Tataria, M, Holmes, J, et al. Pediatric pancreatic trauma: predictors of nonoperative management failure and associated outcomes. J Pediatr Surg. 2007;42(2):340–344.
17. Shilyansky, J, Sena, LM, Kreller, M, et al. Nonoperative management of pancreatic injuries in children. J Pediatr Surg. 1998;33(2):343–349.
18. Nwomeh, BC, Nadler, EP, Meza, MP, et al. Contrast extravasation predicts the need for operative intervention in children with blunt splenic trauma. J Trauma. 2004;56(3):537–541.
19. Sivit, CJ, Peclet, MH, Taylor, GA. Life-threatening intraperitoneal bleeding: demonstration with CT. Radiology. 1989;171(2):430.
20. Abbas, SM, Upadhyay, V. Hollow viscus injury in children: Starship Hospital experience. World J Emerg Surg. 2007;2:14.
21. Sivit, CJ, Taylor, GA, Eichelberger, MR. Visceral injury in battered children: a changing perspective. Radiology. 1989;173(3):659–661.
21a. Hilmes, MA, Hernanz-Schulman, M, Greeley, CS, et al. CT identification of abdominal injuries in abused pre-school-age children. Pediatr Radiol. 2011;41:643–651.
22. Atri, M, Hanson, JM, Grinblat, L, et al. Surgically important bowel and/or mesenteric injury in blunt trauma: accuracy of multidetector CT for evaluation. Radiology. 2008;249(2):524–533.
23. Brofman, N, Atri, M, Hanson, JM, et al. Evaluation of bowel and mesenteric blunt trauma with multidetector CT. Radiographics. 2006;26(4):1119–1131.
24. Gurland, B, Dolgin, SE, Shlasko, E, et al. Pneumatosis intestinalis and portal vein gas after blunt abdominal trauma. J Pediatr Surg. 1998;33(8):1309–1311.
25. Christiano, JG, Tummers, M, Kennedy, A. Clinical significance of isolated intraperitoneal fluid on computed tomography in pediatric blunt abdominal trauma. J Pediatr Surg. 2009;44(6):1242–1248.
26. Sivit, CJ, Taylor, GA, Bulas, DI, et al. Posttraumatic shock in children: CT findings associated with hemodynamic instability. Radiology. 1992;182(3):723–726.
27. Sivit, CJ, Eichelberger, MR, Taylor, GA. CT in children with rupture of the bowel caused by blunt trauma: diagnostic efficacy and comparison with hypoperfusion complex. AJR Am J Roentgenol. 1994;163(5):1195–1198.