CHAPTER 61 ABDOMINAL COMPARTMENT SYNDROME, DAMAGE CONTROL, AND THE POST-TRAUMATIC OPEN ABDOMEN
Major contributors to improved survival in the field of trauma and surgical critical care over the past decade include the early recognition and preventive strategies in the management of the abdominal compartment syndrome and the systematic, staged surgical approach to the trauma patient in extremis called damage control. However, in our efforts to cure one disease, we have created another: the post-traumatic open abdomen, defined as a large postoperative ventral hernia with the abdominal viscera covered by a temporary dressing or closure.
ABDOMINAL COMPARTMENT SYNDROME
The effects of increased intra-abdominal pressure were first described in 1863 by Marey and Burt, who reported the relationship between intrathoracic pressure and elevated intra-abdominal pressure. However, it was not until the 1980s that Kron and Richards coined the term “abdominal compartment syndrome” (ACS).1,2 They reported a separate series of patients that developed a tense, distended abdomen with elevated pulmonary artery pressures and increased intraabdominal pressures postoperatively despite normal mean arterial blood pressure and cardiac performance. All of these patients improved with re-exploration and abdominal decompression.
Intra-abdominal hypertension and the abdominal compartment syndrome are not synonymous. ACS is a late manifestation of uncontrolled intra-abdominal hypertension produced by ongoing ischemia and splanchnic hypoperfusion and the resuscitative measures to counteract the hemorrhagic shock state (Figure 1).

Figure 1 A cycle of ischemia producing intra-abdominal hypertension and the abdominal compartment syndrome.
(Reproduced with permission from Michael Rotondo, MD.)
Table 1 lists the risk factors associated with ACS. Mortality from the fulminant abdominal compartment syndrome has been reported to be as high as 67%.
Table 1 Etiology of Abdominal Compartment Syndrome: Who Is at Risk?
In addition to the accumulation of blood within the perineal cavity, other factors may contribute to occupying space within the abdominal cavity. This can occur by any shock-induced visceral ischemia and reperfusion edema including major injuries outside the abdominal cavity. Several recent reports have described this “secondary abdominal compartment syndrome,” which occurs most frequently with major pelvic and long bone fractures, and hemorrhagic chest injuries. However, secondary ACS can occur in any setting associated with hemorrhagic shock.3–5
Balough and associates reviewed patients with major torso trauma and found that both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiorgan failure. Fourteen percent of these patients develop abdominal compartment syndrome, all of which require aggressive resuscitation using crystalloid, blood, and blood products early in their initial management in the emergency department. Therefore, the current emphasis in critical care management of the severely injured patients focuses on identification of predictive factors for the development of ACS and the recognition of intra-abdominal hypertension and treatment before full development of the syndrome.7–12
Intra-abdominal hypertension affects multiple organ systems in a graded fashion. The deleterious consequences appear gradually, and the adverse effects of elevated intra-abdominal pressure occur at lower levels than previously thought and manifest before the development of the fulminant syndrome (Table 2).
Table 2 Effects of Intra-Abdominal Hypertension on Organ Systems
| Head | ↑ Intracranial pressure |
| ↓ Cerebral perfusion pressure | |
| Heart | ↓ CO |
| ↓ Venous return | |
| ↑ Pulmonary artery occlusion pressure and central venous pressure | |
| ↑ Systemic vascular resistance | |
| Lungs | ↑ Peak inspiratory pressure |
| ↑ Pulmonary artery wedge pressure | |
| ↓ Dynamic compliance (Cdyn) | |
| ↑ Arterial oxygen pressure (PaO2) | |
| ↑ Arterial carbon dioxide pressure (PaCO2) | |
| ↑ Intrapulmonary shunt (Qsp/Qt) | |
| ↑ Fraction of dead space to total expired tidal volume (VD/VT) | |
| Liver | ↓ Portal flow |
| ↓ Mitochondria | |
| ↑ Lactate | |
| Kidney | ↓ Urine output |
| ↓ Renal flow | |
| ↓ Glomerular filtration rate (GFR) | |
| Intestines | ↓ Celiac flow |
| ↓ Superior mesenteric arterial (SMA) flow | |
| ↓ Mucosal flow | |
| Abdomen wall | ↓ Compliance |
| ↓ Rectus flow |
The classic picture of ACS includes a patient with a tense, distended abdomen and ventilatory insufficiency including hypoxia and hypercarbia, as well as increased peak inspiratory pressures. Progressive oliguria occurs despite adequate mean arterial pressure and cardiac output. This is followed by decreased cardiac performance and subsequent cardiovascular collapse unless treatment is instituted immediately (Table 3).
Table 3 Abdominal Compartment Syndrome: Classic Clinical Picture
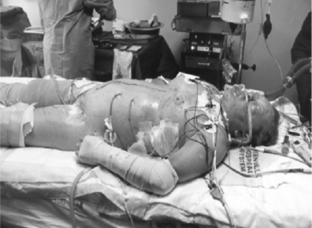
Figure 2 illustrates a high-risk patient for ACS: tense abdomen, respiratory failure, and progressive oliguria in a multitrauma patient requiring aggressive resuscitation for hemorrhagic shock.
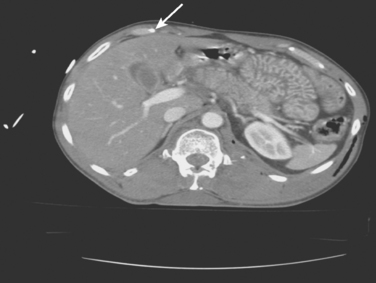
Common computed tomography findings in this patient population include extraperitoneal hematoma and/or extravasation, intra- and retro-peritoneal edema, and “shock bowel” defined as an intense mucosal enhancement producing a prominent, feather-like appearance to the small intestines (Figure 3).
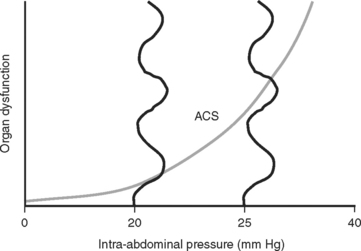
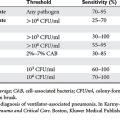
The consensus at the World Congress on Abdominal Compartment Syndrome defines this disease entity as persistent bladder pressures over 20 mm of mercury with the new onset of organ failure. Figure 4 demonstrates the progression of organ dysfunction as intra-abdominal pressure increases over this level. Once defined, ACS mandates immediate decompressive celiotomy (Figure 5).
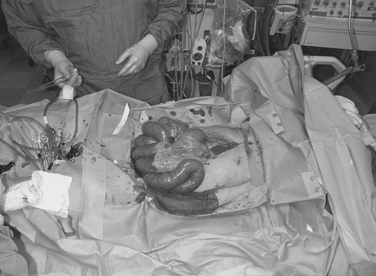
Figure 5 Bedside decompressive celiotomy for abdominal compartment syndrome (note massive small bowel edema).
This often rapidly reverses all the adverse affects of increased intra-abdominal pressure and dramatically improves oxygenation and pulmonary compliance, returning peak inspiratory pressures toward normal and promptly reversing the oliguria with a brisk diuresis of resuscitation fluids. Before decompression, all attempts to correct acid–base and electrolyte disturbances including potassium, magnesium, and calcium may avoid cardiac dysrhythmias after decompression. A respiratory therapist should also be immediately available to readjust ventilatory settings to prevent additional pulmonary barotrauma.
DAMAGE CONTROL
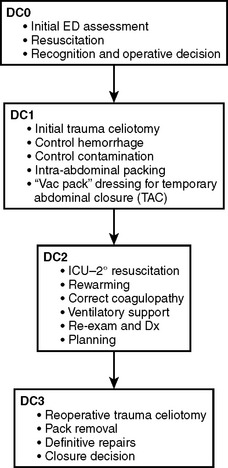
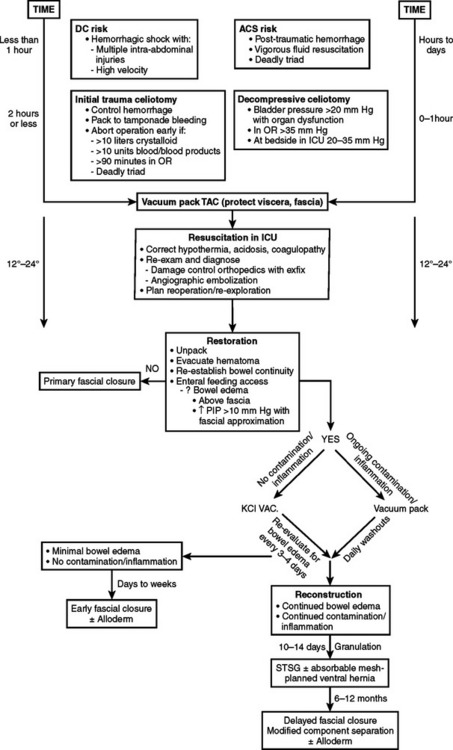
An abbreviated celiotomy and intra-abdominal packing for hemorrhage control in the abdomen was first described by Stone13 in 1983. However, the term “damage control” was first coined by Rotondo and Schwab at the University of Pennsylvania in 1993. The current management scheme has been revised to include four stages (Figure 6).14–16
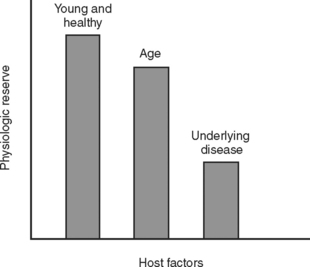
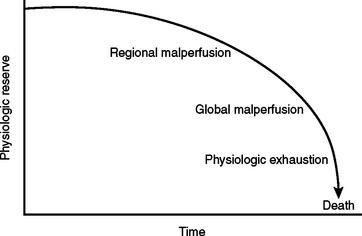
During the initial trauma celiotomy (DC1), it is important to determine each patient’s physiologic reserve in order to make appropriate operative decisions. Physiologic reserve is defined as an individual’s unique ability to tolerate injury. It is a function of several host factors including age, gender, pre-existing disease, genetics, and immunocompetence (Figure 7). As physiologic reserve becomes depleted during hemorrhagic shock, regional and then global malperfusion occur, leading to physiologic exhaustion and subsequent death (Figure 8).11
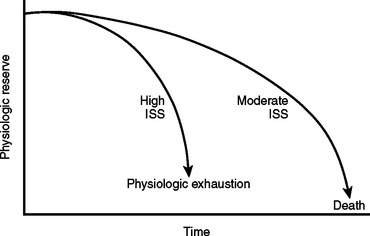
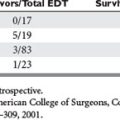
Mortality associated with damage control procedures ranges between 25%–60%. Each patient responds and reacts differently to stress and injury, and each individual has a limited amount of compensatory reserve until physiologic exhaustion is reached. Additionally, the extent of injury severity determines the slope leading to physiologic exhaustion (Figure 9).
During the initial trauma celiotomy, the surgeon must recognize the need for immediate control of major hemorrhage and contamination with maximum replacement of coagulation factors including platelets, fresh frozen plasma, and cryoprecipitate, and in certain circumstances, factor VIIa for microvascular bleeding. Intraoperative monitoring of temperature, arterial blood gases, and volume of resuscitative fluids are important in determining whether a patient is descending down the physiologic curve toward physiologic exhaustion. In addition, for patients suffering hollow viscus injuries, contamination is controlled with linear staples to transect the bowel ends and leave them in discontinuity until a later stage of damage control management.
Table 4 lists clinical parameters that should prompt the surgical team to initiate damage control maneuvers, abort the operation, and return to the ICU for resuscitation and restoration of reserve. Asensio17,18 recommends instituting damage control early, well before reaching the upper limits of physiologic exhaustion, and describes statistically validated criteria. Using these statistically validated intraoperative predictors of instituting damage control, patients with post-traumatic open abdomen incurred less hypothermia and fewer postoperative complications including intra-abdominal abscess and fistula formation. Patients with early damage control were also subjectively noted to have less bowel edema and were able to undergo definitive abdominal wall closure during their initial hospital stay.
Table 4 Clinical Guidelines to Abort Initial Trauma Celiotomy and Initiate Damage Control Maneuvers
| Hypothermia | <35° C |
| Acidosis | pH <7.2 |
| Base deficit (BD) ≥–8 | |
| Lactate ≥4 | |
| Coagulopathy | Activated partial thromboplastic time (aPTT) >60 |
| International normalized ratio (NR) >1.6 | |
| Ongoing resuscitation | Persistent shock systolic blood pressure <90 |
| >10 liters crystalloid | |
| >10 units packed red blood cells | |
| Operative time | >60–90 minutes with abdominal cavity opened |
Temporary Abdominal Closure
A TAC is defined as any technique that contains the abdominal viscera during the acute phase of care. Indications for creating a TAC are listed in Table 5.
Historically, a wide variety of techniques for TAC have been used to contain the abdominal viscera during the acute phase of care. However, the “vacuum pack” technique using the method described by Barker et al.19 best fulfills the first two principles in the management of the post-traumatic open abdomen: protecting the bowel and preserving the fascia.
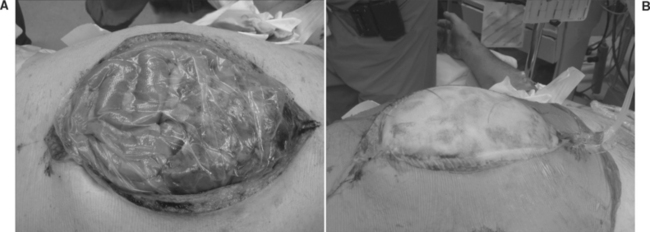
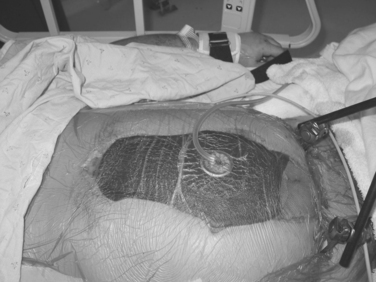
The bowel and abdominal viscera are protected by placing a perforated, nonadherent material such as a Steri-Drape (isolation bag 50 ′ 50 cm or 1010 large bowel bag 45 ′ 60 cm, 3M Health Care, St. Paul, MN) over the intestines. This is followed by subfascial placement of a moist surgical towel with two large sump drains (Jackson-Pratt, Baxter Health Care Corp., Deerfield, IL) that are brought out through the superior portion of the wound. The entire abdominal defect is then covered with a large adhesive drape (Ioban, 3M Health Care, St. Paul, MN). The sump drains are attached to wall suction to create continuous negative pressure. This technique also counteracts the lateral retraction of the fascial edges and preserves the rectus musculofascial complex for subsequent closure (Figure 10).
Advantages of the Vacuum Pack are listed in Table 6. Using this technique, the period before definitive fascia closure can be safely extended for up to a week after the initial celiotomy.20
Table 6 Advantages of Vacuum Pack Technique
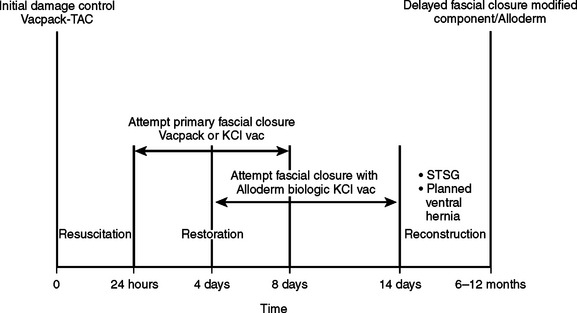
Three potential stages (Figure 11) follow the placement of the vacuum pack for the post-traumatic open abdomen:
Resuscitation
The goals of the secondary resuscitation in the ICU after damage control or decompressive celiotomy for ACS are repayment of the oxygen debt sustained, restoration of adequate perfusion to meet metabolic needs, and reversal of hemorrhagic shock. This includes correction of the coagulopathy, clearing of the arterial lactic acidosis, correcting electrolyte disturbances, and rewarming the patient. A checklist of essential items necessary to perform this secondary resuscitation is listed in Table 7.
Table 7 Secondary Intensive Care Unit Resuscitation Checklist
| Rewarming/Correct Hypothermia | |
Additional resuscitative measures during ICU management also include the use of arteriography and embolization, especially for severe pelvic arterial hemorrhage and extensive hepatic vascular injuries. Complex lower extremity and pelvic fractures can also be temporarily realigned with the use of external fixators, deferring complex orthopedic reconstructive work until later stages in the patients’ hospital course when the inflammatory response has dissipated.
Restoration
The complication rate associated with the post-traumatic open abdomen is between 25%–50%. The most common complications are listed in Table 8.
Table 8 Complications Associated with Post-Traumatic Open Abdomen
| Abdominal | Extra-Abdominal |
|---|---|
| Wound infection | Ventilator-associated pneumonia |
| Wound dehiscence | Aspiration pneumonitis |
| Abdominal wall fasciitis/necrosis | Bloodstream infection |
| Intra-abdominal abscess/sepsis | Urinary tract infection |
| Enteroatmospheric fistula | Deep venous thrombosis/pulmonary embolism |
| Pressure ulcers (occiput, scapulae, sacrum, heels) | |
| Multiple organ dysfunction syndrome/multiple organ system failure |
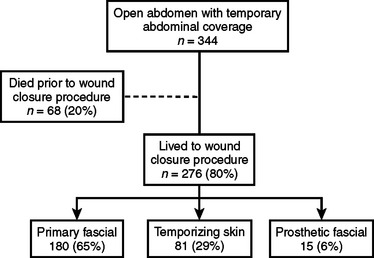
Figure 12 describes a recent study on complications after 344 post-traumatic open abdomen patients with TAC. The authors, Miller and colleagues,21 reported a 65% success rate on early primary fascial closure. The remaining patients underwent delayed closures by either applying a split thickness skin graft (temporizing skin) to a granulating abdominal wound or using synthetic prosthetic mesh material (prosthetic fascial).
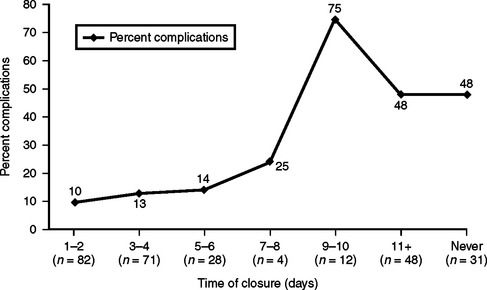
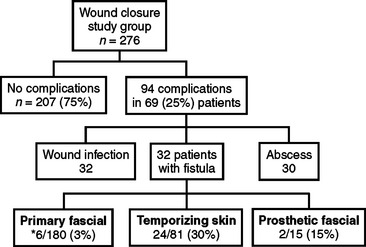
Figure 13 compares wound complications versus days to fascial closure. All three circumstances can potentially result in fistula formation, an expensive, labor-intensive, and morbid complication associated with the post-traumatic open abdomen. This occurred in 32 of 276 (8.6%) patients in this study (Figure 14).
In reviewing the literature, the incidence of fistula formation in this patient population is between 2%–25%. The etiology of fistulae formation is multifactorial and includes the presence of abdominal infection, bowel ischemia and/or obstruction, exposure of the bowel to atmospheric air for prolonged periods of time (causing desiccation), and the use of packs, dressings, or prosthetic materials that adhere to the serosa of the intestines.22,23
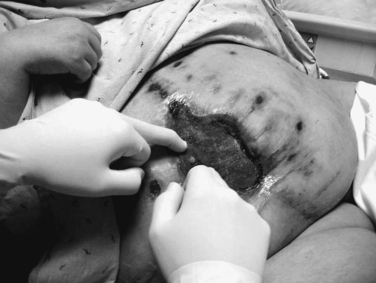
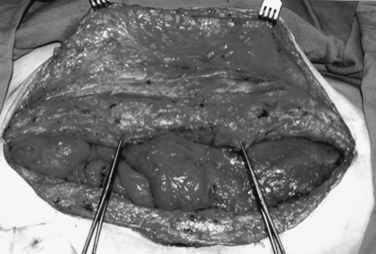
An enteroatmospheric fistula is defined as a postoperative complication with the open abdomen in which an enterotomy in the GI tract leaks succus or frank stool into the open wound. The absence of overlying soft tissue precludes closure (Figure 15).
Protecting the exposed abdominal viscera from injury and desiccation using the vacuum pack, limiting access to the wound to one or two senior surgeons, and early tension-free closure of the fascia are all good preventive measures. However, once a fistula forms, the adjacent viscera must be protected, and the effluent from the fistula controlled. Intubation of the fistula in the middle of the fixed visceral block is not recommended, and in this circumstance, repair of the fistula should not be performed until the patient is infection-free and in a stable physiologic state and has replenished protein and calorie stores. This usually occurs several months later. At that time the fistula can be resected in conjunction with delayed abdominal wall reconstruction.
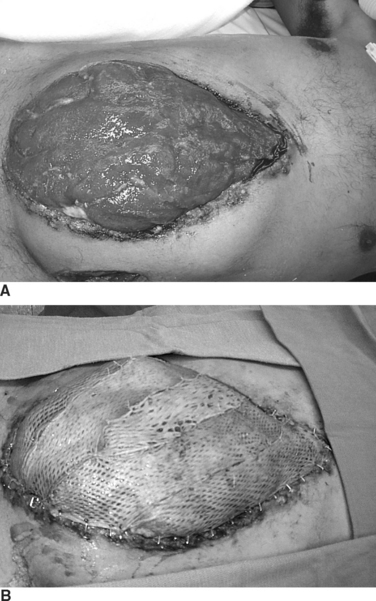
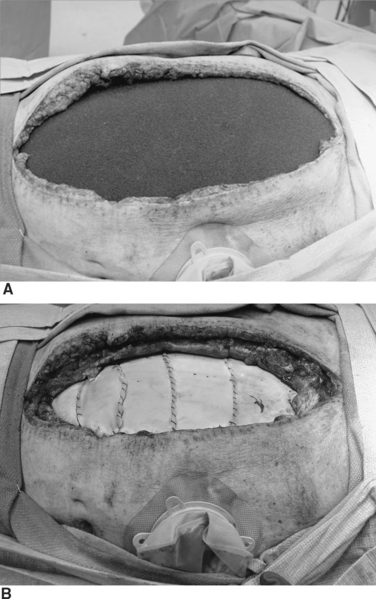
Because all post-traumatic open abdomens are either colonized or chronically infected, it is now realized that use of synthetic prosthetic material is contraindicated. If the abdomen cannot be closed by the end of the first week, granulation tissue starts to develop, and a “frozen abdomen” is evident by the end of the second week. This creates a hostile environment for early fascial closure and thus necessitates a planned ventral hernia with a delayed reconstruction. A split-thickness skin graft (STSG) is applied over the granulation bed and the patient then requires a 6–12 month recovery period for protein and calorie stores to be replenished before successful abdominal wall reconstruction can be performed (Figure 16). This decision, although life-saving, is very costly and is associated with a loss of productive lifestyle and an inability to return to the workforce.
New solution
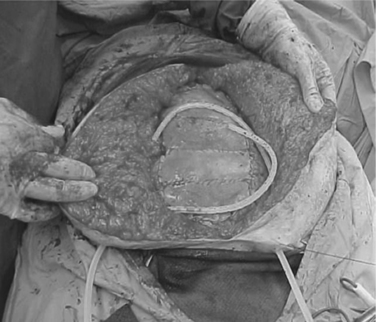
Similar to the vacuum pack, the KCI VAC technique places a perforated, nonadherent plastic drape beneath the anterior abdominal wall to protect the bowel. However, the surgical towel in the middle layer is replaced with a polyurethane sponge cut to size to fit the facial defect. An 18 French suction tube is inserted into the sponge, which is then covered with an adherent occlusive drape, and constant application of negative pressure is obtained with wall suction (Figure 17).
This technique also protects the bowel and preserves the fascia and recaptures loss of abdominal domain. Additional advantages are listed in Table 9.
The KCI VAC allows for successful primary fascial closure or closure with the use of Alloderm in most post-traumatic open abdomen patients, even up to 3 weeks after initial damage control procedures or decompressive celiotomy.
Alloderm is a biologic material derived from partial thickness skin of tissue donors treated for removal of all cellular components so that only the native connective tissue matrix remains. Unlike synthetic prosthetic materials, which the body recognizes as foreign, causing encapsulation, Alloderm supports cellular growth and integration with rapid revascularization and transition to the patient’s own tissue. This material serves as a bio-scaffold for the native autologous tissue (Figure 18).
Alloderm is also tolerant of contamination and can be used for closure in situations where synthetic material is contraindicated (Figure 19). It is best to underlay the Alloderm 4–5 cm lateral to the fascial edges using interrupted 0-prolene sutures and place this biologic material under moderate stretch to prevent future laxity of the abdominal wall.
Even though long-term results regarding recurrence rates and the development of abdominal wall laxity are not yet available, this method of fascial approximation certainly has significant advantages over the planned ventral hernia method of management with its inherent high risk of wound complications as mentioned previously.24–30 The combination of using the KCI VAC and placement of Alloderm fulfills the final essential principle with post-traumatic open abdomen: expedite early fascial closure.
Nutritional support for post-traumatic open abdomen
Although enteral feedings are the preferred route, it is not always possible in severely injured trauma patients with an open abdomen resulting from a prolonged inflammatory response with ongoing bowel wall edema, abdominal distention, high gastric output, and persistent adynamic ileus. In this circumstance, early enteral nutrition should be supplemented with parental nutrition to reach acceptable protein and calorie goals. In addition, enteral nutrition can continue safely during other extra-abdominal operations and procedures.31,32
Tsuei and colleagues33 studied nasoenteric feeding in patients with an open abdomen and found it a safe effective method of nutritional support capable of meeting the nutritional goals in most patients. However, there have been no clinical studies to determine the exact percentage of caloric goals required to achieve maintenance of gut integrity and prevent bacterial translocation during the open abdomen. In animal models, over 50% of goal calories were needed to effectively reduce translocation and maintain adequate gut permeability.
Planned Ventral Hernia and Delayed Abdominal Wall Reconstruction
Once the split thickness skin graft can be elevated from the underlying intestines (pinch sign–Figure 20), the inflammatory response has resolved and the underlying granulation tissue dissipated. This usually requires 6–12 months of recovery. Only then can reconstruction be performed safely.
The split thickness skin graft is removed and the fascia is mobilized from the surrounding tissue. The skin and subcutaneous tissue are then raised as lateral flaps circumferentially (Figure 21). Options for bridging the fascial gap at this point include the component separation technique and/or closure with Alloderm.
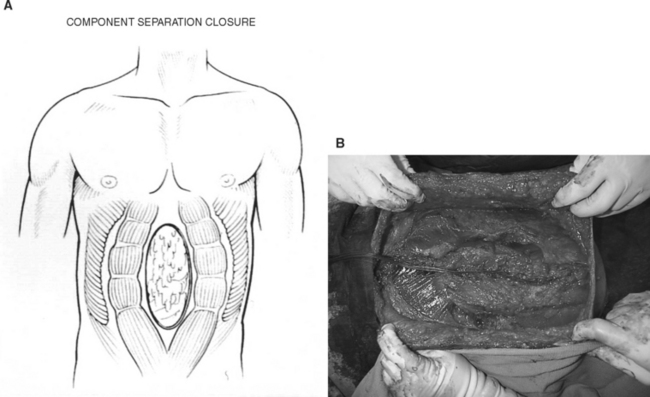
The component separation technique reconstructs the fascial defect with advancement flaps by transecting the external oblique just lateral to its insertion into the rectus sheath and separating it from the internal oblique. The rectus muscle can then be advanced medially and sutured in the midline to close the defect (Figure 22).34
This technique can approximate a 10-cm defect without undo tension. Using the modified component separation technique,35,36 several more centimeters of mobility can be obtained by separating the rectus muscle from the posterior rectus sheath.
The recurrence rate with this method alone is 22%–32%.37,38 Therefore, complementing or replacing this procedure with the use of Alloderm to bridge the fascial gap is an option.39
CONCLUSION
Damage control techniques and preventive measures to avoid the development of ACS are now the standards of care in the management of traumatic shock (Figure 23). Using a vacuum pack for temporary abdominal closure during the early stages of resuscitation protects the bowel and preserves the fascia.
1 Kron L, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984;199:28-30.
2 Richards WO, Scovill W, Shin B, Reed W. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183-187.
3 Kopelman T, Harris C, Miller RS, Arrillaga A. Abdominal compartment syndrome in patients with isolated extraperitoneal injuries. J Trauma. 2000;49:744-749.
4 Biffl WL, Moore EE, Burch JM, et al. Secondary abdominal compartment syndrome is a highly lethal event. Am J Surg. 2001;182:645-648.
5 Balough Z, McKinly BA, Cocanour CS, et al. Secondary abdominal compartment syndrome: an elusive complication of traumatic shock resuscitation. Am J Surg. 2002;184:538-544.
6 Balough Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, Kozar RA. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848-861.
7 Ertel W, Oberholzer A, Platz A, Stocker R, Trentz O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747-1753.
8 Saggi BH, Sugerman HJ, Ivatury RR, Bloomfield GL. Abdominal compartment syndrome. J Trauma. 1998;45:597-609.
9 McNelis J, Marini CP, Jurkiewicz A, Fields S, Caplin D, Stein D. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch Surg. 2002;137:133-136.
10 Morris JAJr, Eddy VA, Blinman TA, Rutherford EJ, Sharp KWVA. The staged celiotomy for trauma issues in unpacking and reconstruction. Ann Surg. 1993;217:576-586.
11 Eddy VA, Nunn C, Morris JAJr. Abdominal compartment syndrome: the Nashville experience. Surg Clin North Am. 1997;77:801-881.
12 Cheatham ML, Safcsak K, Block EFJ, Nelson LD. Predictors of mortality in patients with open abdomens. Crit Care Med. 1999;27(1 Suppl):170A.
13 Stone HH, Strom PR, Strom RJ, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532-535.
14 Rotondo MF, Zonies DH. Damage control sequence and the underlying logic. Surg Clin North Am. 1997;77:761-777.
15 Shapiro MB, Jenkins DH, Schwab W, Rotondo MF. Damage control collective review. J Trauma. 2000;49:969-978.
16 Rotondo MF, Schwab CW, McGonigal MD, et al. “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 2003;35:375-382.
17 Asensio JA, McDuffie L, Petrone P, et al. Reliable variables in the exsanguinated patient which indicate damage control and predict outcome. Am J Surg. 2001;182:743-751.
18 Asensio JA, Petrone P, Roldan G, et al. Has evolution in awareness of guidelines of institution of damage control improved outcome in the management of the posttraumatic open abdomen? Arch Surg. 2004;139:209-214.
19 Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure a 7-year experience with 112 patients. J Trauma. 2000;48:201-207.
20 Miller PR, Thompson JT, Faler BJ, Meredith JW, Chang MC. Late fascial closure in lieu of ventral hernia the next step in open abdomen management. J Trauma. 2002;53:843-849.
21 Miller RS, Morris JAJr, Diaz JJ, et al. Complications after 344 damage-control open celiotomies. J Trauma. 2005;59:1365-1371.
22 Mayberry JC, Burgess EA, Goldman RK, et al. Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: the plain truth. J Trauma. 2004;57:157-163.
23 Buechter KH, Leonvicz D, Hastings PR, Fonts C. Enterocutaneous fistulas following laparotomy for trauma. Am Surg. 1991;57:354-358.
24 Guy JS, Miller RS, Morris JA, Diaz J, May A. Early one-stage closure in patients with abdominal compartment syndrome: fascial replacement with human acellular dermis and bipedicle flaps. Am Surg. 2003;69:1025-1029.
25 Scott BG, Feanny MA, Hirshberg A. Early definitive closure of the open abdomen: a quiet revolution. Scand J Surg. 2005;94:9-14.
26 Holton LHIII, Kim D, Silverman RP, Rodriguez ED, Singh N, Goldberg NH. Human acellular dermal matrix for repair of abdominal wall defects: review of clinical experience and experimental data. J Long-Term Effects Med Implants. 2005;15:547-558.
27 Kolker AR, Brown DJ, Redstone JS, Scarpinato VM, Wallack MK. Multilayer reconstruction of abdominal wall defects with acellular dermal allograft (alloderm) and component separation. Ann Plast Surg. 2005;55:36-42.
28 Diaz JJJr, Guy J, Berkes MB, Guillamondegui O, Miller RS. Acellular dermal allograft for ventral hernia repair in the compromised surgical field. Am Surg. 2006;72(12):1181-1187.
29 Kim H, Bruen K, Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg. 2006;192(6):705-709.
30 Patton JHJr, Berry S, Kralovich KA. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg. 2007;193(3):360-363.
31 Cothren CC, Moore EE, Ciesla DJ, Johnson JL, Moore JB, Haenel JB, Burch JM. Postinjury abdominal compartment syndrome does not preclude early enteral feeding after definitive closure. Am Surg. 2004;188:653-658.
32 Blackburn GL, Jensen GL, Martindale RC. Nutrition support for the patient with an open abdomen after major abdominal trauma. Nutrition. 2003;19:563-566.
33 Tsuei BJ, Magnuson B, Swintosky M, Flynn J, Boulanger BR, Ochoa JB, Kearney PA. Enteral nutrition in patients with an open peritoneal cavity. Nutr Clin Pract. 2003;3:253-258.
34 Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519-526.
35 Fabian TC, Croce MA, Pritchard FE, et al. Planned ventral hernia staged management for acute abdominal wall defects. Ann Surg. 1994;219:643-653.
36 Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G. Staged management of giant abdominal wall defects acute and long-term results. Ann Surg. 2003;238:349-357.
37 Lowe JB3rd, Lowe JB, Baty JD, Garza JR. Risks associated with “components separation” for closure of complex abdominal wall defects. Plast Reconstr Surg. 2003;111:1276-1283.
38 de Vries Reilingh TS, van Goor H, Rosman C, Bemelmans MH, de Jong D, van Nieuwenhoven EJ, van Engeland MI, Bleichrodt RP. “Components separation technique” for the repair of large abdominal wall hernias. J Am Coll Surg. 2003;196:32-37.
39 Espinosa-de-los-Menteros A, de la Torre JI, Marrero I, Andrades P, Davis MR, Vasconez LO. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann Plast Surg. 2007;58(3):264-267.