CHAPTER 3 Abdomen Trauma
BLUNT TRAUMA
Computed Tomography Technique
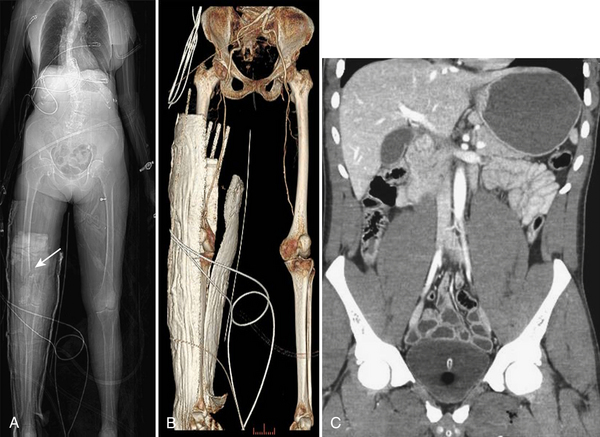
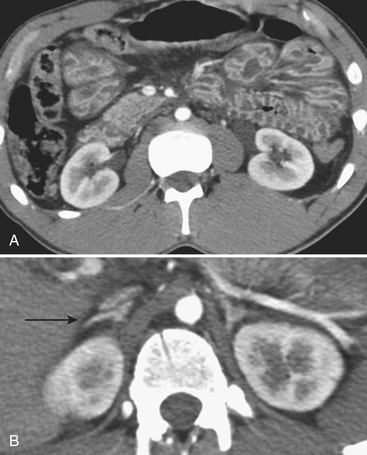
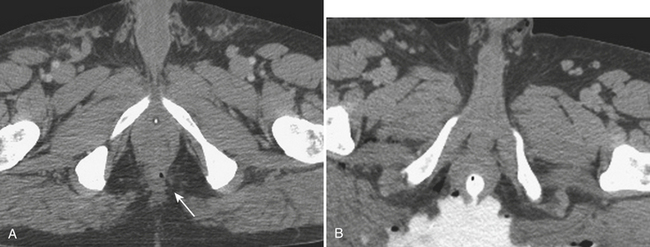
Regardless of the scanning protocol employed, modern 16- and 64-row detector scanners share several definite advantages over earlier-generation scanners. The most important of these is their markedly improved temporal resolution. With the development of these multidetector-row scanners, thin images (1 to 2 mm) can be easily acquired while still keeping scan time at 8 seconds or less per body part. In order to facilitate review at the interpretation workstations, it is advisable to reconstruct a separate set of thicker axial images by “fusing” the thin sections. For example, images acquired with 0.625- or 1.25-mm thickness can be reconstructed at 3.75- or 5-mm thickness. In addition, sagittal and coronal reformations are now generated almost routinely, taking advantage of the rapid scan times that nearly eliminate motion artifact. These sagittal and coronal reformations are ideal for adequate evaluation of the diaphragm, long vascular territories, and thoracic and lumbosacral spine, and reduce the need for lumbosacral and thoracic spine radiographs in the vast majority of patients. All series are sent to the Picture Archival Computer System (PACS) and are available at the time of interpretation and for further postprocessing (if necessary). Other benefits include the ability to combine routine protocols with CT angiograms of multiple body parts while still using a single bolus of contrast. This is possible due to the increased scanner table length (2 m) available with many of the 64 MDCT scanners. Using the scout images of the whole body, multiple complex CT examinations (including CT angiograms of the neck or extremities) are planned and combined in succession into one scan using a single contrast injection (Fig. 3-2).
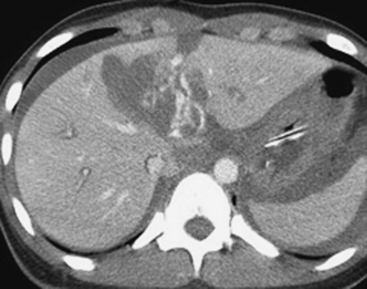
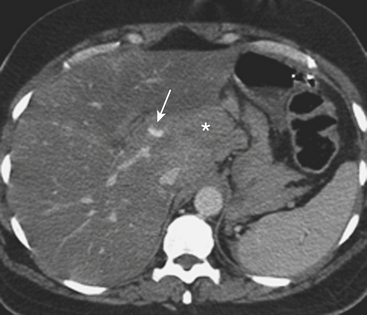
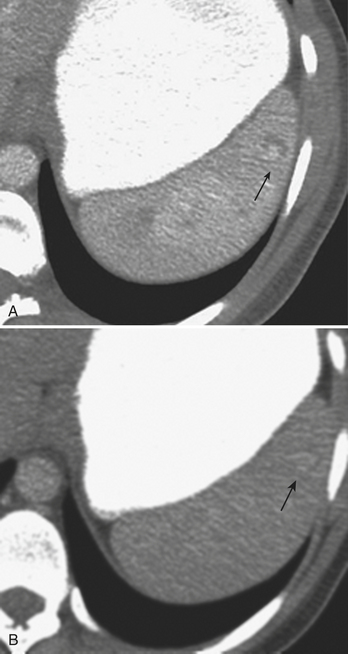
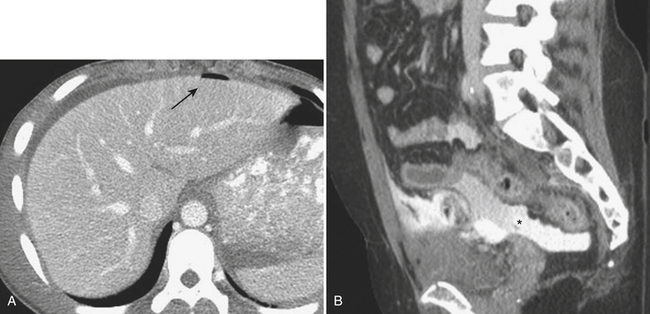
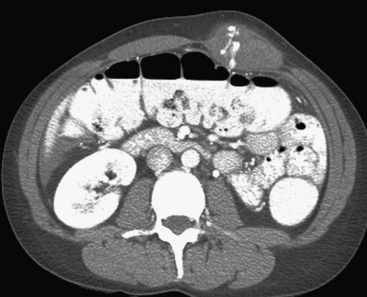
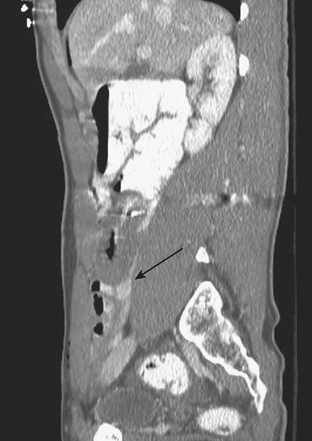
In addition to portal venous phase imaging, the acquisition of delayed images has become an increasingly important part of the trauma CT evaluation. Delayed images can be useful in evaluating vascular injuries as well as injuries to both the solid organs and the bowel and mesentery. Delayed CT acquisitions allow for improved characterization of solid organ injuries by helping to differentiate contained injuries (such as arterial pseudoaneurysms and arteriovenous fistulas) from uncontrolled active extravasation of contrast-enhanced blood. On delayed images, areas of active extravasation persist as hyperattenuating foci (relative to the aorta) and change configuration as blood (with contrast) diffuses into a potential space (Fig. 3-3), whereas pseudoaneurysms show an attenuation coefficient that remains similar to the aorta, with no change in overall size or shape. Delayed images also improve detection and characterization of bladder and renal injuries, as discussed later in this chapter. Finally, delayed scans can help in the characterization of findings seen on portal venous phase images that could potentially represent foci of extravasation and could be related to the acute injury (Fig. 3-4). However, the routine use of delayed images is unnecessary and should be discouraged, since the majority of trauma CT scans performed in emergency rooms today show no evidence of abdominal injury and the additional radiation dose is unnecessary. As an alternative, delayed images can be acquired selectively and used only when solid organ or bowel injry is detected or suspected on the initial CT acquisition. Additionally, since the sole purpose of this delayed scan is to characterize an injury seen or suspected on the initial scan, it is possible to employ a reduced radiation dose technique, typically 100 milliAmpere second (mAs) (or similar dose reduction with automated dose modulation).
HEPATIC TRAUMA
Ultrasonography
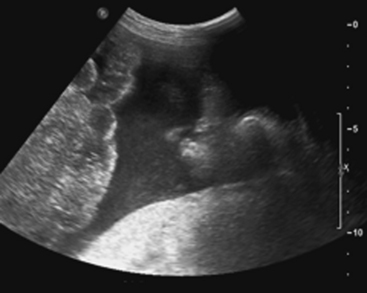
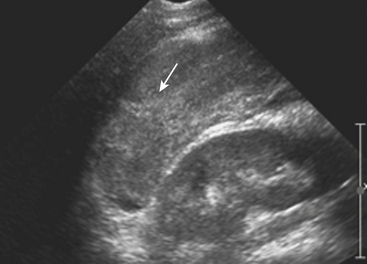
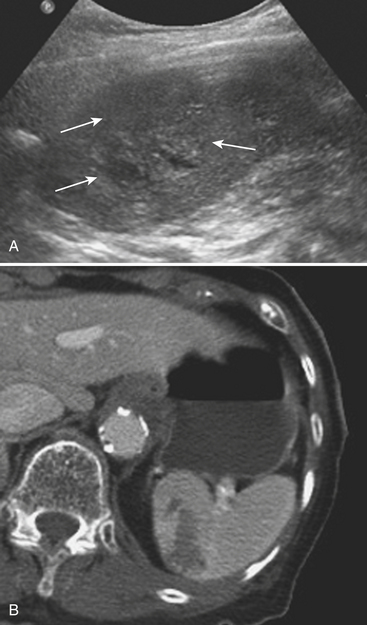
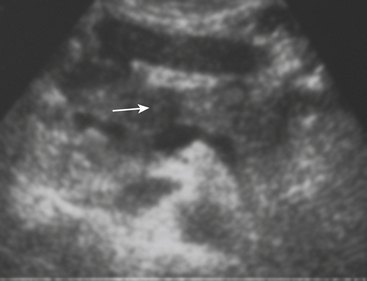
Sonographic evaluation for hepatic injuries is mostly limited to screening the trauma patient for indirect signs of injury, such as free fluid adjacent to the liver (as part of the FAST scan). When fluid is detected along the margin of the liver, it can appear complex and can contain echogenic clot due to its hemorrhagic nature. Although a careful inspection of the liver can demonstrate lacerations and contusions as focal areas of parenchymal distortion, various factors limit the use of US beyond the search for free peritoneal fluid. These include technical limitations such as difficult access to appropriate sonographic windows and the variability in operator experience and availability. However, advances in US technology and the development of sonographic contrast agents has led to increased use of this modality for direct evaluation of the solid parenchymal organs, including the liver, especially in European countries. On noncontrast US examinations, hepatic parenchymal injuries can produce three different morphological patterns. The most common pattern is that of a focal area of increased echogenicity with respect to the background liver, which is thought to correspond to the focal lacerations or hematomas seen on CT. The other two are a more diffuse area of increased echogenicity and focal areas of decreased echogenicity. Liver lacerations can be difficult to detect on initial exams, often appearing more prominent in the days following the initial injury (Fig. 3-5). The advent of sonographic contrast agents has increased the ability of ultrasound to detect acute hepatic injuries. Generally, the contrast agent is given in a bolus and the area of interest is scanned continuously for 4 to 6 minutes. On contrast-enhanced US, liver injuries are best seen during the portal venous phase of imaging. Liver lacerations can also appear as focal linear or branching hypoechoic areas, often oriented perpendicular to the liver surface. Contusions may appear as geographic areas of decreased echogenicity, often with ill-defined borders. Similar to active contrast extravasation on CT, active bleeding can be detected by the presence of micro-bubbles (contrast material) extending into a hematoma. In general, despite recent advances in technology, US is still considered an adjunctive test.
Computed Tomography
CT is the dominant imaging modality in emergency rooms in the United States and most other Western countries. Improvements in the rate of CT detection of liver injuries, as well as in the proper characterization of most injuries, are some of the reasons that support the trend toward conservative management of such injuries. As previously mentioned, liver injuries are optimally seen on CT performed during the portal venous phase of contrast enhancement. Once identified, it is important to document the type and location of such injury. In addition, it is especially important to note the presence of active extravasation of contrast-enhanced blood and the potential for injury to central hepatic vessels such as the hepatic veins and inferior vena cava. Hepatic injuries are typically characterized as either lacerations or hematomas (subcapsular or parenchymal). While many radiologists rely exclusively on morphological descriptors in their report, it is useful to understand the liver injury scale developed by the American Association for the Surgery of Trauma (AAST) (Table 3-1). This grading scale takes into account features such as the size of subcapsular or parenchymal hematomas and lacerations, as well as evidence of active extravasation and major vascular injuries of the liver; these findings are all readily identified on well-performed CT examinations. The value of this scale lies more in the ability to communicate properly with trauma surgeons about the extent of the injury than in the ability to predict individual patient prognosis or the type of therapy necessary.
| Grade | Description |
|---|---|
| I |
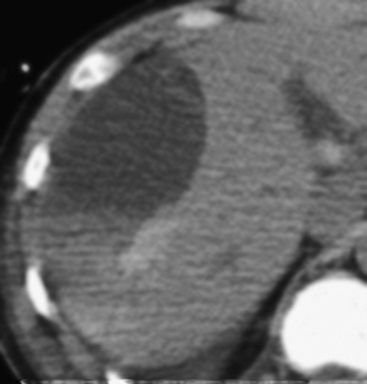
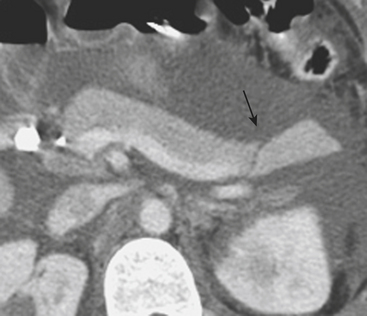
A subcapsular hematoma is typically hypodense to the enhancing liver parenchyma and appears elliptical, conforming to the confines of the liver capsule (Fig. 3-6). Such collections are usually easily distinguished from perihepatic fluid. Intraparenchymal hematoma appears as an ill-defined hypoattenuating region within the liver. If seen on noncontrast CT, hematomas are typically hyperdense to the background liver parenchyma. Liver lacerations appear as hypoattenuating linear, often branching, and complex regions within the parenchyma of the liver (Fig. 3-7). Extension to the hepatic surface is very common. Even small lacerations can be associated with perihepatic blood. It is important to identify lacerations that extend to the periportal region, since these patients are at an increased risk for the development of delayed bile leaks due to injury of the biliary ductal system.
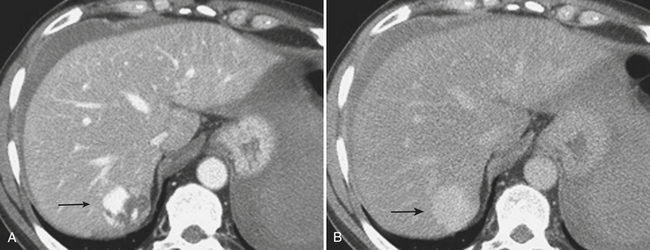
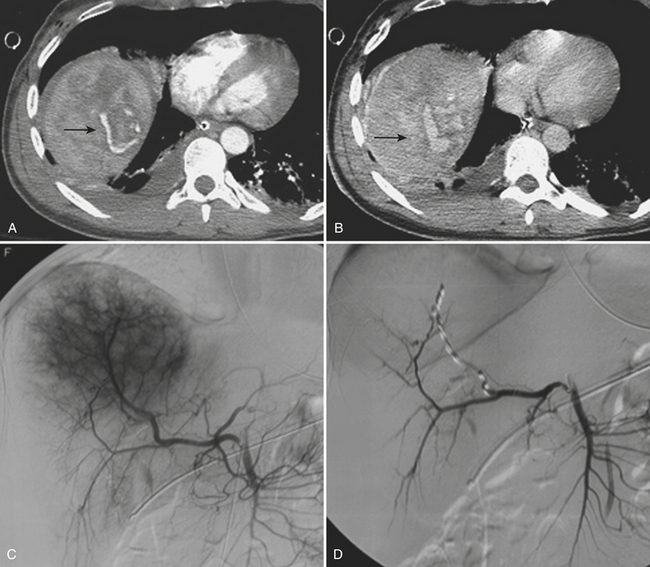
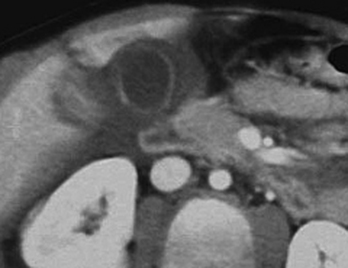
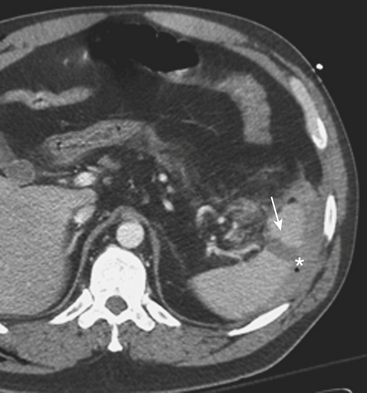
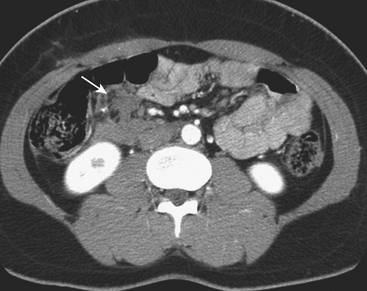
CT can also readily identify hepatic vascular injuries. Active extravasation of intravenous contrast, when seen during routine portal venous phase images, suggests ongoing hemorrhage from a hepatic arterial or portal venous source. Active extravasation may be confined to the hepatic parenchyma or may be seen as hyperattenuating collections of contrast-enhanced blood accumulating in the perihepatic spaces. On delayed CT images, the focus of active extravasation typically increases in size as the material continues to diffuse throughout the area of expanding hematoma (Fig. 3-8). In the past, active extravasation was considered an indication of the need for prompt surgical management. Currently, the demonstration of a sizable focus of active extravasation is more likely to trigger a response from the vascular interventional team for catheter angiography and coil embolization (see Fig. 3-8). Even patients with high-grade injuries can be managed conservatively using such techniques. Injuries to major hepatic vessels may also be directly depicted with CT. For example, direct evidence of portal venous injury may be seen as abrupt termination of a branch of an intrahepatic portal vein. Parenchymal injuries may extend centrally to involve the hepatic veins and inferior vena cava, seen on CT as abrupt termination of the hepatic veins, which may just begin to enhance during routine portal venous phase images. Such major venous injuries are more likely to require surgical management, since they can cause continued bleeding and hemodynamic instability, and are not readily treated by interventional radiology techniques (Fig. 3-9). Major venous injuries are also commonly associated with hepatic arterial trauma. Complications of hepatic vascular injuries include traumatic fistulas between various hepatic structures, including arterioportal, fistulas between hepatic arteries and biliary ducts, and, rarely, between a hepatic artery and adjacent bowel. Hepatic pseudoaneurysms can also occur as a result of hepatic injury and are extremely important to detect and document since they are at risk for delayed rupture, a potentially lethal complication. Hepatic artery pseudoaneurysms were considered rare, but are now detected more frequently due to improvements in the spatial resolution of CT and the ability to scan at the peak of contrast enhancement throughout the scan routinely (Fig. 3-10). Pseudoaneurysms appear as hyperattenuating foci on the early phase images and demonstrate washout on delayed phase images. If delayed rupture and hemorrhage are suspected based on clinical or laboratory parameters, CT is also the best method to detect subacute hemorrhage. On follow-up CT, delayed hemorrhage presents as an increase in the size of a previous hematoma. The more acute hemorrhage appears as focal hyperattenuating material in a previously documented hematoma or along the margin of the liver, the so-called “sentinel clot” sign. In addition to pseudoaneurysm formation and delayed hemorrhage, other complications of hepatic trauma result from associated bile duct injury and include the development of bilomas and abscess collections, persistent bile leaks with bile peritonitis, and bile duct strictures.
GALLBLADDER AND BILE DUCT TRAUMA
Computed Tomography
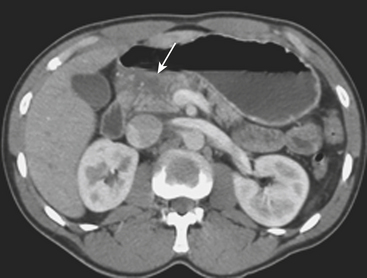
Gallbladder injuries are most often diagnosed at the time of the initial trauma CT scan. Contusions appear as diffuse gallbladder wall thickening. The presence of pericholecystic fluid is not specific, but may be an associated finding. High-attenuation fluid within the gallbladder lumen suggests hemorrhage and is a good indicator of acute injury. However, differentiation between high-attenuation sludge and blood may be difficult. Lacerations of the gallbladder wall are seen as focal disruption of the normal mural enhancement of the gallbladder wall. Dense contrast material in the gallbladder lumen or in the gallbladder fossa suggests active bleeding from injury to the cystic artery. If the gallbladder is avulsed from its pedicle, it may be displaced from the gallbladder fossa (Fig. 3-11). Injury of the extrahepatic bile ducts can be difficult to diagnose on CT, since perihepatic fluid is often caused by injury to other organs in the abdomen. Intrahepatic biliary ductal injury may be suggested on follow-up CT by the development or persistence of low-attenuation perihepatic fluid collections, usually with an obvious associated hepatic injury.
Hepatobiliary Scintigraphy
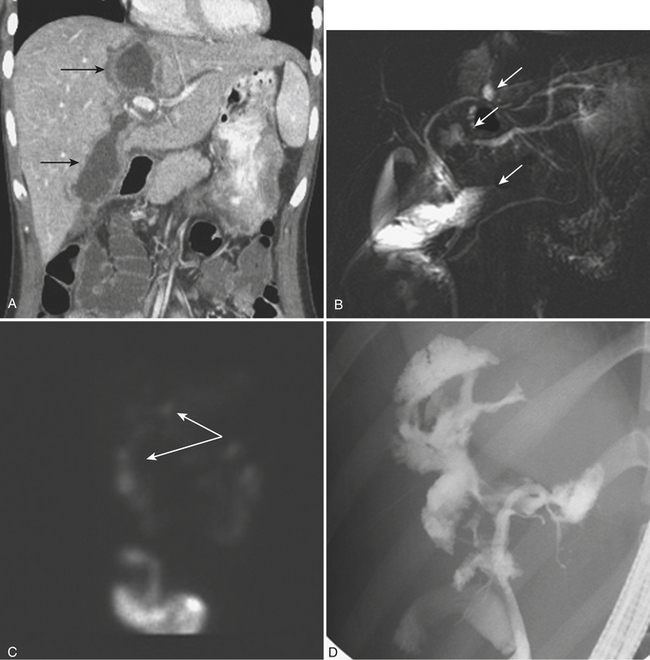
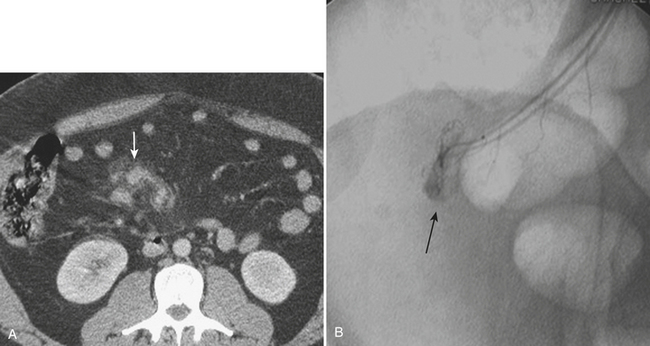
Once the patient with complex liver trauma has survived the acute phase of hepatic trauma, when bleeding and possible exsanguination are the main concerns, the possibility of developing bile leaks with complicating abscess and sepsis must be considered and treated. Persistent perihepatic fluid collections and increasing low-attenuation intraperitoneal fluid are common indicators of bile leaks that require direct therapy. Biliary scintigraphy is a simple and useful method for detecting and characterizing bile duct injuries. Hepatobiliary radiopharmaceutical agents are taken up by hepatocytes and excreted into the bile ducts. Sequential imaging over 1 to 2 hours identifies extraluminal collections that develop as the radiotracer is excreted into the biliary system and drains into the small bowel lumen. In some cases, images delayed 4 hours are necessary when there is no evidence of injury on the initial image acquisition. On hepatobiliary scintigraphy, accumulation of the radiopharmaceutical agent outside the bile ducts is indicative of a bile leak, which can be either contained (Fig. 3-12) or free if it extends into the peritoneal cavity. Small bilomas can be treated conservatively and followed, whereas larger collections may require percutaneous drainage, especially if there is superimposed infection. Early detection of bile leaks allows proper treatment by either percutaneous drainage or by ERCP with sphincterotomy and stent placement (see Fig. 3-12). A possible delayed complication of bile duct injury is the development of a bile duct stricture with obstruction and infection. MRCP is an ideal method for following hepatobiliary injuries for possible development of strictures.
SPLENIC TRAUMA
Splenic injuries are characterized as either hematomas or lacerations. As it has for the liver, the AAST has developed a scale for grading splenic trauma that is still commonly used for describing specific patterns of injury (Table 3-2). The prognostic implications of this scale are limited, since even complex injuries can heal without specific therapy. It is important to describe the type of injury, the location (parenchymal versus subcapsular), the size of the hematoma or laceration, and all associated complications. Severe injuries can affect the hilar vascular structures, leading to total or subtotal organ devascularization. Injuries to the splenic artery or branch vessels can cause active bleeding, which can be easily demonstrated with current MDCT examinations. Finally, splenic injuries can lead to the development of pseudoaneurysms, which are extremely important to note, since these patients have an increased risk of delayed morbidity and mortality due to pseudoaneurysm rupture.
| Grade | Description |
|---|---|
| I |
Ultrasonography
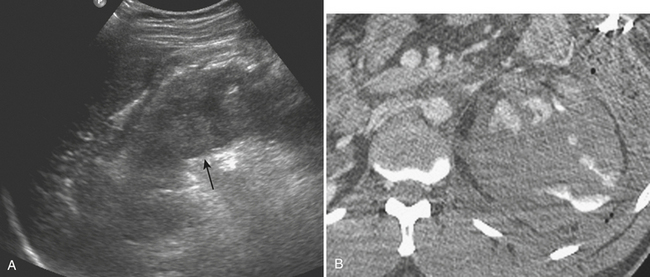
Splenic injuries can be detected by US evaluation of the abdomen and may be suspected based on the results of the initial FAST scan. However, the parenchymal injury can be difficult to detect. Instead, indirect evidence of injury is often identified, including hemoperitoneum and focal echogenic clot adjacent to the spleen. Splenic hematomas appear as heterogeneous and hypoechoic compared with the background spleen (Fig. 3-13). The borders are ill-defined and there is no associated mass effect or vessel displacement. Lacerations appear as linear or branching areas of decreased echogenicity compared with the normal spleen, often extending to the splenic surface. Although not commonly used in many countries, sonographic contrast agents have been shown to improve the ability to detect splenic injuries. The spleen can be readily imaged by contrast-enhanced US since it retains contrast for up to 5 to 7 minutes following intravenous injection. If the vascular pedicle is injured, there may be total or subtotal loss of enhancement in the spleen. Active contrast extravasation can be identified as a hyperechoic collection that develops in the early phase after contrast injection.
Computed Tomography
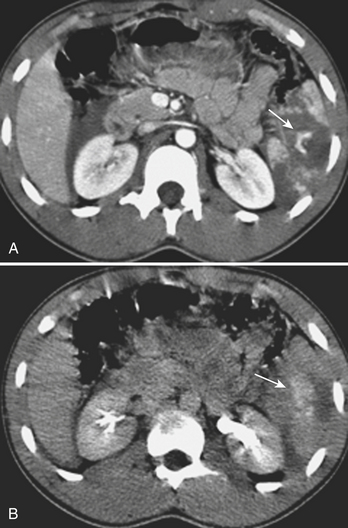
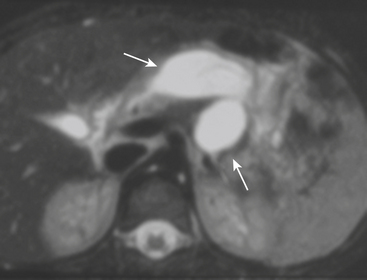
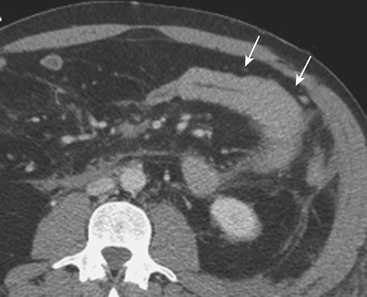
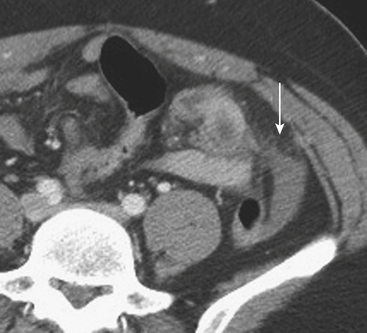
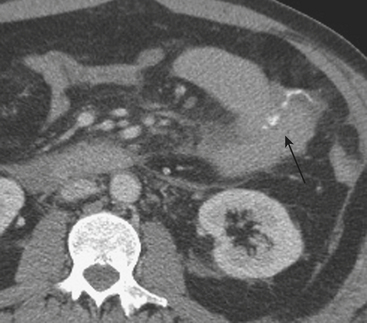
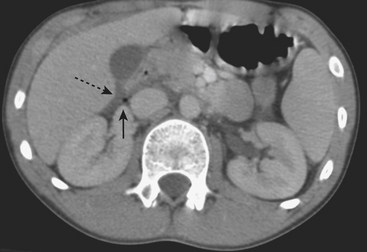
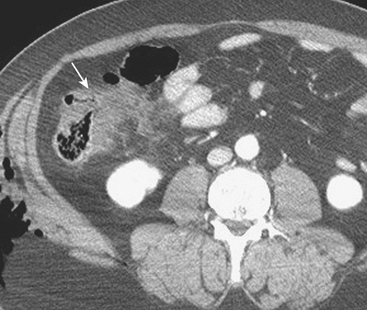
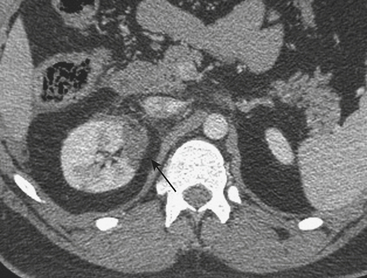
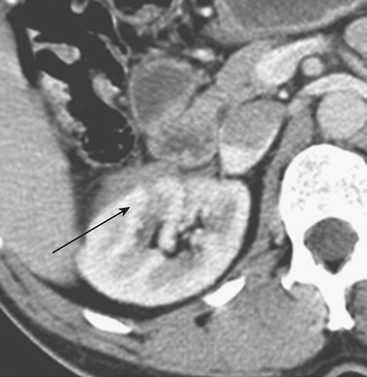
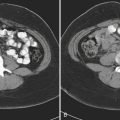
MDCT is the main imaging modality used to detect, characterize, and follow splenic trauma. Most splenic injuries are optimally detected on portal venous phase images of the abdomen following intravenous contrast injection. Splenic hematomas appear as focal areas of decreased attenuation compared with the background-enhancing splenic tissue (Fig. 3-14). Hematomas can be intraparenchymal or subcapsular in location. Lacerations appear as linear, irregular, and often branching areas of decreased attenuation (Fig. 3-15). Higher-grade injuries tend to be larger and involve more of the total volume of the spleen (Fig. 3-16). Injuries of the vascular pedicle lead to focal wedge-shaped areas of decreased enhancement, while severe injuries of the vascular pedicle may produce a markedly decreased or absent enhancement of most or all of the spleen on CT. In addition to describing the type of injury, it is important to note the presence of either active contrast extravasation or pseudoaneurysm formation. Active contrast extravasation in the spleen is characterized on CT by contrast density outside the expected lumen of the vessel, similar or higher in attenuation as compared with the adjacent vessels (Fig. 3-17). The shape is often irregular, with poorly defined margins due to the diffusion of the contrast material into the site of injury. Typically, contrast extravasation is identified within the splenic parenchyma or in a hematoma adjacent to the site of laceration in the perisplenic spaces. In contrast, splenic pseudoaneurysms are contained extraluminal collections that often have a more round or ovoid appearance (Fig. 3-18). These injuries are usually confined to the splenic parenchyma. In some cases, it is difficult to distinguish between active extravasation and pseudoaneurysms. If there is any question, delayed images are often useful in making this differentiation. On delayed CT images, active contrast extravasation shows a change in shape and usually an increase in size as the material continues to diffuse into the site of injury (see Fig. 3-17). On the other hand, pseudoaneurysms do not change in size or shape at different points in time, and the attenuation of the pseudoaneurysm tends to follow that of adjacent arteries such as the splenic artery or aorta (see Fig. 3-18). Other focal vascular injuries include the development of arteriovenous fistulas. Such injuries can be difficult to distinguish by CT alone, and are better characterized with catheter angiography. Once active contrast extravasation or a focal vascular injury such as a pseudoaneurysm is detected, the need for catheter angiography and possible endovascular therapy should be carefully assessed. Although practices vary among institutions, patients with proved active extravasation are more likely to undergo splenectomy, whereas contained vascular injuries such as pseudoaneurysms are more amenable to endovascular therapy with coil embolization.
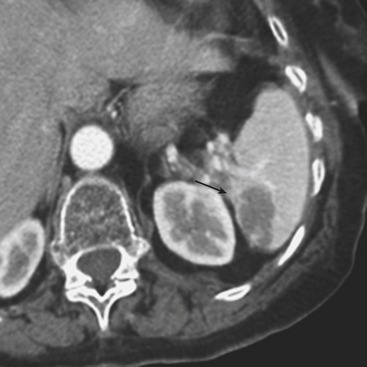
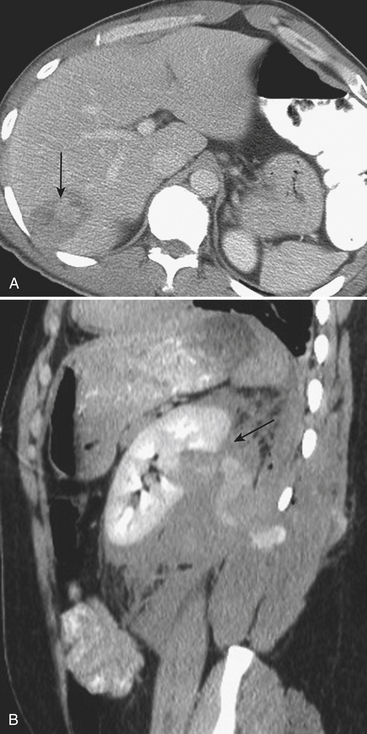
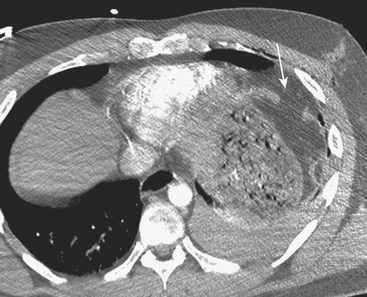
Figure 3-14 Splenic hematoma following motor vehicle collision. Axial CT during the portal venous phase demonstrates a wedge-shaped hypodense region in the spleen (arrow), consistent with a subcapsular hematoma and characterized as a grade II injury according to the AAST trauma scale (see Table 3-2).
PANCREATIC INJURY
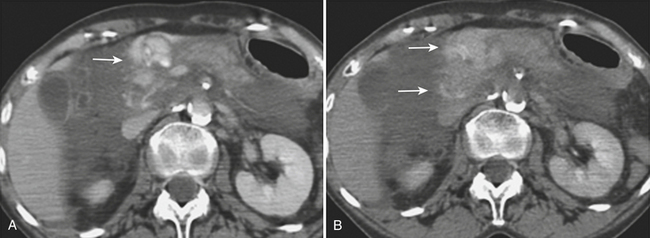
The imaging appearance of pancreatic trauma usually mimics the type of injury present. The role of ultrasonography is limited, but gland lacerations and transection can be seen as hypoechoic linear defects within the pancreatic parenchyma (Fig. 3-19). CT diagnosis of pancreatic injury is often difficult, with a reported sensitivity of 65% to 75% (although the sensitivity with newer MDCT scanners may be higher). Pancreatic injuries are typically detected on portal venous phase CT images of the abdomen. It is important to closely inspect the pancreas on thin-section images with liberal use of multiplanar reconstruction when available, since the many clefts of the pancreas can mimic or hide subtle injuries.
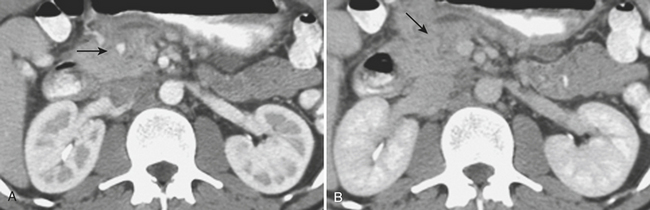
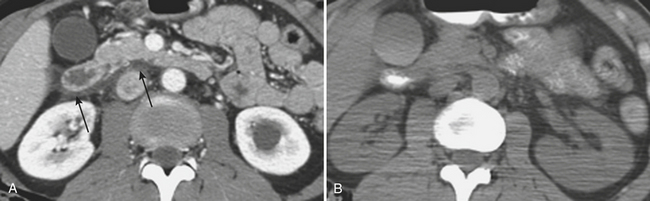
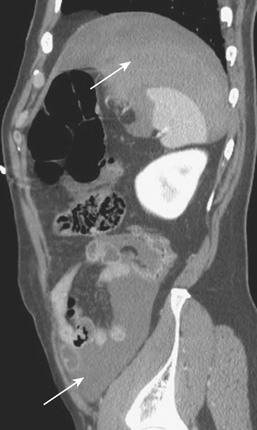
On contrast-enhanced CT, crush injuries (contusions) may show focal or diffuse enlargement and edema within the pancreas, characterized by areas of low attenuation extending through the planes of tissue within the gland (Fig. 3-20). Lacerations are detected as focal low-attenuation lines, most often perpendicular to the plane of the gland and duct (Fig. 3-21) and which may extend completely through the pancreas (termed pancreatic transection; Fig. 3-22). Additional indirect signs of injury include peripancreatic fluid/stranding as well as retroperitoneal hematoma in the peripancreatic region. A crucial part of the imaging characterization of pancreatic injury is to determine the depth of a laceration and any possible involvement of the main pancreatic duct. With modern MDCT technology,the pancreatic duct can be seen directly in the majority of patients. The depth of a laceration is also a useful predictor of main duct involvement: involvement of more than 50% of the anteroposterior thickness of the gland is often associated with duct transection (Fig. 3-23). While not commonly used, at least one CT grading system that parallels the surgical classification of Moore has been suggested: grade A, pancreatitis or superficial laceration (less than 50% pancreatic thickness); grade B1, deep laceration (greater than 50% pancreatic thickness) of the pancreatic tail; grade B2, transection of the pancreatic tail; grade C1, deep laceration of the pancreatic head; and grade C2, transection of the pancreatic head. Delayed complications, such as arterial pseudoaneurysms, abscesses, and pseudocysts, are all readily imaged by CT. Pseudoaneurysms typically appear as focal collections of contrast that enhance similarly to the aorta on all phases of imaging (Fig. 3-24). Pseudocysts and abscesses appear as focal pancreatic fluid collections of varying size. Percutaneous sampling under imaging guidance is very useful to confirm the diagnosis of superimposed infection.
One potential pitfall to be avoided is the misinterpretation of isolated low-attenuation fluid around the pancreas, without direct evidence of parenchymal trauma, as evidence of pancreatic injury (Fig. 3-25). In the trauma population, this can be the result of accumulation of fluid in the retroperitoneum from rapid or excessive administration of fluids for resuscitation. If there is a question, a repeat CT 24 to 48 hours later is advisable. Fluid related exclusively to exogenously administered replacements will decrease or resolve, whereas true pancreatic injuries lead to growing fluid collections and hematomas. Also, pancreatic lacerations may be very subtle on initial CT scans. Thus, if the patient develops abdominal pain after admission, a repeat CT with special attention to the pancreas is indicated.
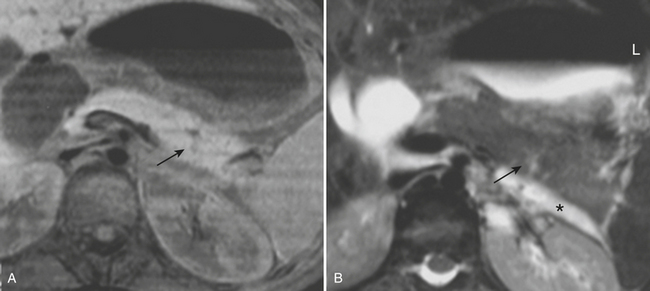
Once injury to the pancreas is identified, MR and MRCP can help in further assessing the status of the main pancreatic duct, especially for follow-up of pancreatic injuries in these typically young patients in whom unnecessary radiation should be avoided if possible (Fig. 3-26). Common MR pulse sequences acquired include fat-suppressed T1- and T2-weighted sequences and MRCP, performed by using heavily T2-weighted breath-hold or non-breath-hold sequences. Fast spin-echo (two-dimensional or three-dimensional) and rapid acquisition with relaxation enhancement (RARE) sequences performed in the coronal and axial planes are usually sufficient. In addition to evaluating the pancreatic duct, MR can be used to assess for pancreatic fluid collections that may have developed due to the pancreatic ductal injury (Fig. 3-27). Hemorrhagic components are also easily detected with MR. Although MRCP is useful for evaluating the pancreatic duct, ERCP is important because of its potential to definitively confirm communication of an apparently interrupted duct with surrounding fluid collections (see Fig. 3-23). In addition, ERCP provides a means for possible endoscopic therapy of pancreatic duct leaks and fluid collections.
BOWEL AND MESENTERIC INJURY
Injuries to the bowel and mesentery are uncommon but potentially devastating, since they are difficult to diagnose both clinically and with imaging. Most blunt injuries are secondary to motor vehicle collisions, although other mechanisms, including assaults and sports-related trauma, produce such injury. Injury of the small bowel is much more common than injury of the colon or stomach in the setting of blunt trauma (Box 3-1).
CT diagnosis of injuries to the bowel and mesentery is not always straightforward. Unlike most solid organ injuries, which are often obvious, the radiologist needs to carefully inspect the images for direct and indirect signs, each having varying ranges of sensitivity and specificity. Numerous CT signs have been reported to occur in the setting of bowel and mesenteric trauma (Box 3-2).
Bowel wall hematomas and contusions, when visible by CT, typically present with focal thickening of the bowel wall. The area of thickening may appear hyperattenuating relative to the normal bowel wall due to the presence of acute blood (Fig. 3-28). Depending on the severity of the injury, the hematoma may be eccentric or concentric in appearance. More severe injuries, which include lacerations of the bowel wall, are only rarely directly seen on CT as focal wall interruptions or frank discontinuity. Instead, other secondary signs of bowel laceration may be present to suggest such an injury. Free intraperitoneal air is one of the more common findings in patients with focal bowel wall lacerations (Fig. 3-29). However, the overall sensitivity of this finding varies between 20% and 75%. Free air may be found locally, adjacent to the site of perforation, or remotely in the upper abdomen near the surface of the liver or along the undersurface of the peritoneum. The presence of free air is not 100% specific for bowel injury, and other benign iatrogenic and traumatic causes (such as bladder rupture and air introduced at the time of Foley catheter placement) must be considered (Box 3-3).
Free intraperitoneal fluid is another sign associated with bowel and mesenteric trauma. In fact, it has been reported as the most common individual finding. This fluid can be seen adjacent to the site of the injury within the leaves of the mesentery, or diffusely throughout the abdomen and pelvis. While often present in combination with other CT findings, the significance of isolated free fluid on trauma CT scans is controversial, but it appears to be less commonly associated with bowel and mesenteric injuries than was once thought (see discussion on free fluid later in this chapter). However, isolated small puddles of free fluid trapped within the mesentery, seen on CT as small triangles outlining the mesenteric leaves (Fig. 3-30), should prompt a very careful review of the bowel loops for direct evidence of injury.
In addition to extraluminal air and fluid, oral contrast may also escape into the peritoneal cavity when a bowel laceration is present (Fig. 3-31). Unfortunately, this is an uncommon finding, with reported sensitivities as low as 6%, and is highly unlikely to be present as an isolated finding. This is the main reason why the mandatory use of oral contrast in blunt trauma has been questioned, and there is now a growing trend toward non-oral contrast CT for this indication. It should be noted that focal lacerations of the duodenum may produce free air and free fluid that is isolated to the retroperitoneum (Fig. 3-32). Close inspection of the duodenal wall should be made when such findings are present.
Bowel injuries may also produce focal changes in the appearance of the bowel wall itself that can be readily detected with MDCT. Partial thickness injuries may allow air to escape into the bowel wall, producing focal pneumatosis. Other findings include focal wall thickening and focal abnormal wall enhancement following intravenous contrast administration (Fig. 3-33). Enhancement may be increased or decreased, as occurs with severe devascularization from degloving injuries. Focal abnormal wall enhancement almost always indicates that an injury requiring surgical intervention has occurred. Colonic injuries may also manifest as focal wall thickening, usually with surrounding mesenteric hematoma (Fig. 3-34).
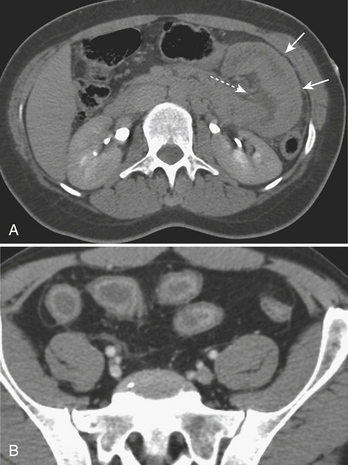
(B, Reprinted with permission from Stuhlfaut JW, Lucey BC, Varghese J Blunt abdominal trauma: Utility of 5-minute delayed CT with a reduced radiation dose. Radiology 238:473–479, 2006.)
Blunt trauma can result in significant injuries that are isolated to the mesentery, such as lacerations and vascular injuries. Mesenteric lacerations cannot be directly seen; however, they often produce indirect CT signs, such as mesenteric “stranding” (focal ill-defined increase in attenuation) or the formation of a frank mesenteric hematoma due to small vessel injury (Fig. 3-35). When larger vessels are injured, active contrast extravasation may be present (Fig. 3-36). Additionally, mesenteric lacerations can lead to the development of internal hernias. Close clinical follow-up of mesenteric injury is mandatory, because even small hematomas associated with small vessel injury can produce vascular compromise of the associated segment of bowel. The resulting bowel ischemia may be seen as a focal hypoenhancing segment by CT; however, delayed presentation of such injuries, such as bowel obstruction secondary to an ischemic stricture, is possible.
FREE PERITONEAL FLUID
Previous studies have shown that isolated free fluid is present in approximately 3% of all trauma CT scans. In the past, trauma surgeons often elected to surgically explore this subset of patients to fully evaluate the bowel for the presence of injury. However, these patients are now increasingly being managed nonoperatively. One study found that only 27% of patients with isolated free fluid require therapeutic laparotomy. Often, such patients are managed expectantly unless other clinical criteria are present to suggest acute bowel injury. The appearance and location of free fluid are often helpful in selecting patients for nonoperative management. Isolated free pelvic fluid in females is often of no clinical significance; there is also growing evidence to suggest that small amounts of low-attenuation fluid in male patients may be a benign finding, not associated with significant bowel or mesenteric injury (Fig. 3-37). However, large amounts of free fluid in males or females (especially if high in attenuation), focal mesenteric fluid, and free fluid in more than one space or location may be indicative of an underlying injury and may warrant surgery (Fig. 3-38), close clinical observation, or CT follow-up.
Finally, in addition to focal bowel injury, the initial trauma CT may show a diffuse abnormality of the bowel that can be seen in patients with hypovolemic shock (so-called shock bowel). Typically, the small intestine is diffusely thickened and there is increased enhancement of the small bowel mucosa (Fig. 3-39). The etiology of the intense mucosal enhancement is not completely understood, although it is thought to be due to increased vascular permeability of the bowel mucosa due to hypoperfusion. Occasionally, the bowel may appear dilated. This appearance is fairly classic and is usually reversible once the patient is appropriately resuscitated. In addition to small bowel abnormalities, profound hypovolemia is also associated with flattening of the inferior vena cava, decrease in caliber of the abdominal aorta, pancreatic swelling, and increased enhancement of the adrenal glands (typically greater than adjacent vascular structures), which maintain normal size and shape.
RENAL AND URETERAL TRAUMA
Renal Injury
The types of injuries that can affect the kidneys include renal contusions, hematomas, lacerations, fractures, shattered kidney, and renal vascular pedicle injury. Again, the AAST has developed a grading system for renal injury that can serve as a guideline for characterizing injuries at the time of CT interpretation (Table 3-3). Overall, most renal injuries fall into one of two categories: contusion and laceration. These injuries may appear severe at CT, due to the associated lack of enhancement of the traumatized segment, but are often stable and require no further intervention or follow-up. Contusions represent parenchymal injuries that produce interstitial edema and hemorrhage. The contused kidney is often swollen and hypofunctioning, either focally at the site of injury or diffusely with more severe injury. Lacerations represent focal tears in the parenchyma. If a laceration is present, the patient is at risk of developing a perirenal urinoma and hematoma. Only about 5% of traumatic lesions are classified as renal fractures, shattered kidneys, or vascular pedicle injury. These injuries may be unstable and usually require further intervention, either by catheter angiography or by surgical repair.
| Grade | Description |
|---|---|
| I |
Ultrasonography
Sonography is particularly limited for evaluation of the kidneys following blunt trauma. Acute hematomas are typically hyperechoic and difficult to differentiate from the echogenic renal sinus or perirenal fat (Fig. 3-40). Although early experience suggests that detection of parenchymal injuries is enhanced with the use of ultrasound contrast agents, their use in practice is limited, as is the case for most trauma applications. When seen, renal lacerations appear as linear hypoechoic defects within the parenchyma and may extend to the renal capsule. Injury to the vascular pedicle may be identified by total lack of enhancement of the kidney following contrast injection.
Computed Tomography
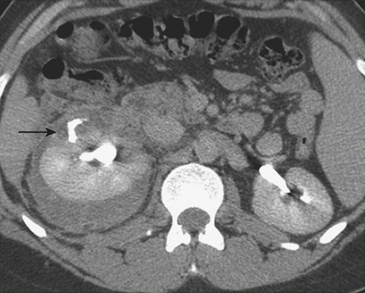
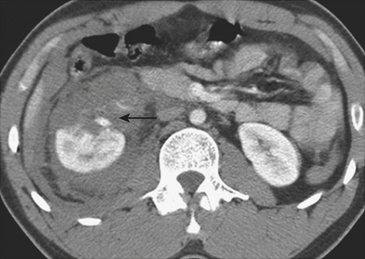
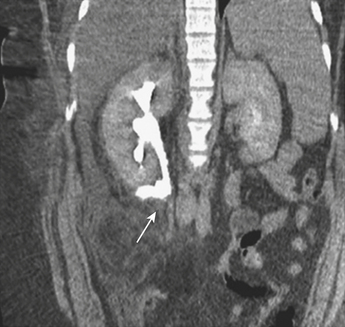
On CT, renal contusions appear hypoattenuating relative to the surrounding enhanced parenchyma and are optimally seen on the nephrographic phase of contrast enhancement, which typically occurs 90 to 120 seconds following initiation of intravenous contrast infusion. However, most injuries are also well seen in the portal venous phase of hepatic contrast enhancement, the timing used for most abdominal trauma CT scans. A contused kidney is often hypofunctioning, and CT demonstrates a delay in enhancement relative to the normal-functioning contralateral kidney or the ipsilateral noncontused areas (Fig. 3-41). On later phases, the contused kidneys may show a persistent nephrogram with delayed excretion of contrast into the collecting system. Renal laceration represents a tear in the parenchyma and manifests as a focal area of linear low attenuation on CT. The laceration typically extends to the surface and is often associated with a perirenal hematoma or fluid collection (Fig. 3-42). Once a laceration is identified, delayed images are extremely important for demonstrating a potential leak of urine containing dense contrast material into the perinephric space (Fig. 3-43). In addition, some lacerations are associated with active extravasation of contrast-enhanced blood (Fig. 3-44). Unless there is associated major vascular injury, most lacerations will heal without any specific therapy.
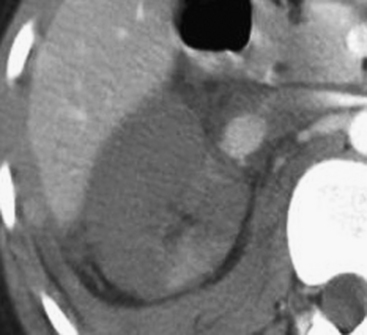
Finally, injury of the vascular pedicle typically represents either an acute traumatic dissection or transection of the renal artery or vein (Fig. 3-45). When such an injury occurs, the kidney will show an absence or near absence of contrast enhancement on the portal venous phase CT exam and there may be associated perirenal hematoma. This injury is severe, and patients may present in hypovolemic shock due to profound blood loss. This injury may be treated by surgical revascularization if detected early (typically within the first 6 hours following the initial trauma); otherwise, nephrectomy is indicated as the kidney is unsalvageable if the diagnosis is delayed or if the patient presents too long after the time of initial injury.
Ureteral Injury
More commonly, the ureter is evaluated with the routine trauma CT. When a focal injury of the collecting system or ureter is suspected, delayed CT images are mandatory. These are typically obtained 5 to 7 minutes following contrast injection to avoid unnecessary delay in patient care while the patient is undergoing diagnostic imaging evaluation. Early portal venous phase images may show abnormal fluid or stranding adjacent to the ureter; delayed images show extravasation of urine with contrast at the site of injury (Fig. 3-46). Multiplanar reformations are useful in highlighting the site of injury and may aid the urologist in treatment planning.
ADRENAL TRAUMA
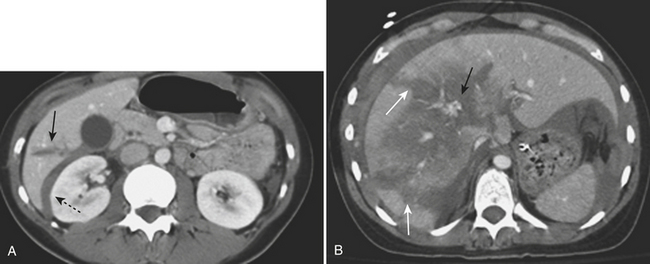
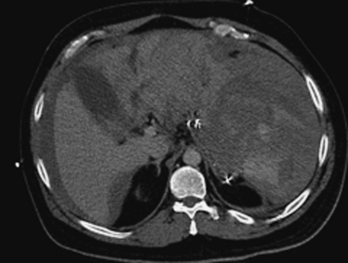
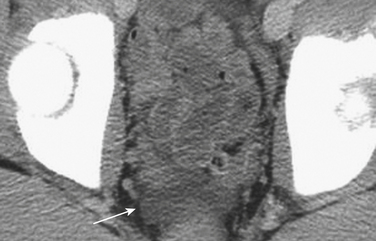
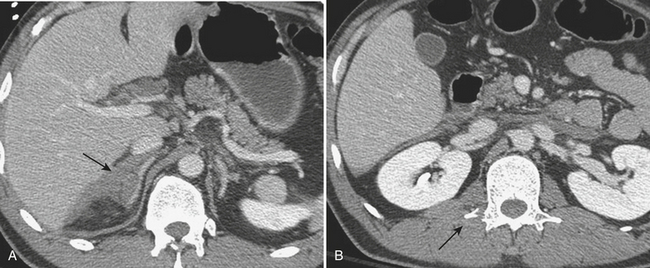
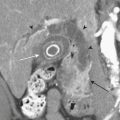
Injury of the adrenal gland is uncommon. It occurs in approximately 1% of all patients sustaining abdominal trauma. Isolated adrenal injury is even rarer, occurring in less than 5% of all patients with adrenal trauma. Instead, most patients with adrenal injuries also have injury to one or more other solid organs in the abdomen, most commonly the liver, spleen, or kidney. Ipsilateral rib fractures are also common. Adrenal injuries typically occur on the right side and are much less commonly seen in isolation in the left adrenal gland. Possible explanations for this discrepancy that have been offered include the position of the right adrenal gland in relatively tight quarters between the spine and the liver and the right adrenal gland being subject to higher venous pressure than the left when increased abdominal pressure occurs at the time of injury. The right adrenal gland might see relatively higher venous pressures transmitted from the vena cava, while venous pressures are filtered by the left renal vein before reaching the left adrenal gland. Regardless, injury to the adrenal gland can be readily identified on routine trauma CT imaging. Typically, adrenal injuries appear as round or ovoid nodules replacing the normal adrenal gland. Not uncommonly, there is a fracture of an ipsilateral transverse process (Fig. 3-47). These hematomas can be associated with surrounding periadrenal fat stranding on CT. It is important to distinguish adrenal hematomas from incidental adrenal nodules, which are commonly seen in the trauma population (and in the general population). It is helpful to measure the attenuation of the lesion. Most adrenal hematomas have attenuation coefficients higher than 50 Hounsfield units (HU). Adrenal injuries typically have a higher HU than other adrenal lesions such as adenomas, which often have attenuation measurements less than 10 HU. If available, delayed images (10 to 15 minutes after contrast injection) can help characterize the lesion by determining the washout characteristics. If there is any question, follow-up imaging can be obtained to assess for interval change in the suspected adrenal injury. On follow-up CT, adrenal hematomas typically regress or calcify. In addition, pseudocysts can develop as sequelae of adrenal injury. Rarely, patients with bilateral adrenal injury can develop clinical manifestations of adrenal insufficiency.
PELVIC TRAUMA
Although the pelvis communicates with the peritoneal and extraperitoneal compartments of the abdominal cavity and is imaged concomitantly with the abdomen at the time of admission CT, the unique anatomic disposition of the pelvic ring and the types of injuries encountered deserve a separate discussion in this chapter. In most settings, a portable radiograph of the pelvis is obtained upon arrival of a multiple-trauma patient to the trauma bay. If a displaced (and possibly unstable) fracture of the pelvic ring is demonstrated, the pelvic cavity should be investigated carefully for associated injuries to the vascular structures, rectum, bladder, or urethra. Strong forces are necessary to disrupt the osseous pelvic ring; the radiographic evaluation of trauma to the bony pelvis is discussed in detail in chapter 4 on trauma of the extremities.
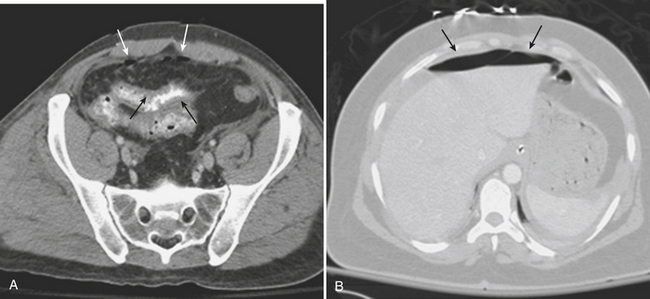
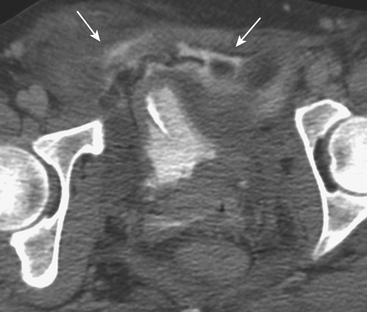
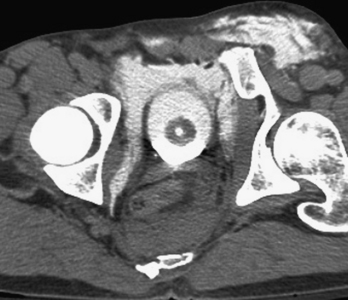
The possibility of vascular injury and major (sometimes life-threatening) bleeding should be considered in every patient with a disruption of the pelvic ring. CT has been shown to be valuable in evaluating for vascular injury in patients with pelvic trauma. Large hematomas and foci of active extravasation are the main findings that may prompt a consult to the interventional radiology service for possible endovascular therapy via embolization. Multiphasic CT imaging provides a temporal assessment of change in the size of the hematoma, and provides an indirect means of estimating the rate of bleeding (Fig. 3-48).
Bladder Trauma
CT Cystography
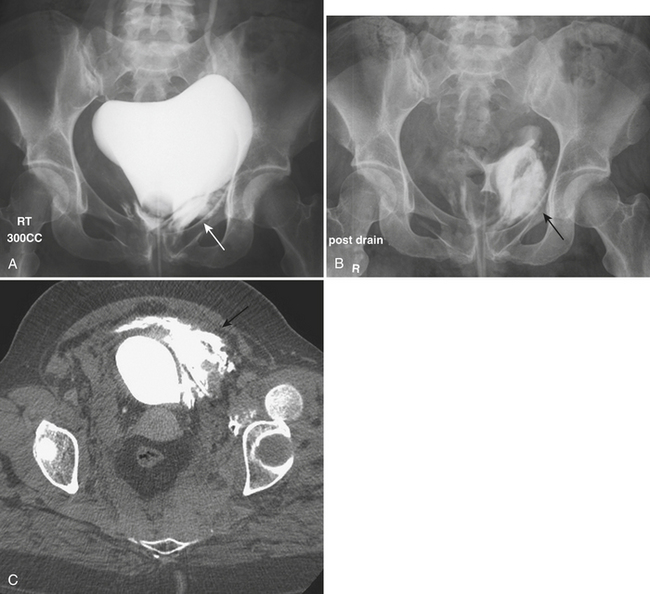
Most patients with bladder injuries have suffered multiple trauma and require abdominal or pelvic CT scans as part of their evaluation. The CT scan of the pelvis provides information on the status of the pelvic organs and osseous pelvis. Occasionally, bladder rupture is shown on the initial pelvic CT images with contrast-filled urine accumulating in the perivesical space or peritoneal cavity (Fig. 3-49). However, bladder integrity is not confirmed until full distention of the bladder with homogeneously opacified fluid is achieved. A CT cystogram is performed after the abdominopelvic CT is completed. With a Foley catheter secured in the bladder, diluted contrast is instilled to achieve full distention. CT images limited to the pelvis are then obtained. Although CT cystography lacks the temporal, dynamic information provided by fluoroscopy, the superior contrast resolution compensates for this limitation, often showing small accumulations of extravesical contrast. In the majority of major trauma centers, CT cystography has replaced conventional cystography as the most widely used method to assess bladder integrity.
Types of Bladder Rupture
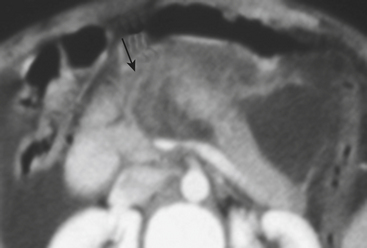
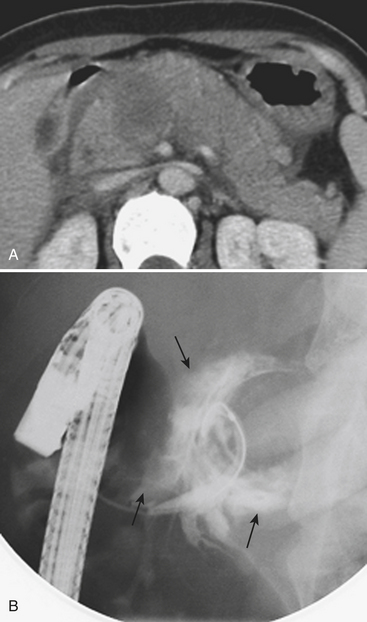
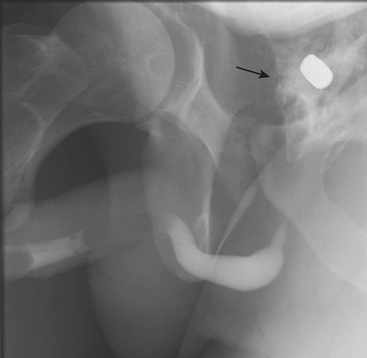
In blunt trauma, extraperitoneal bladder ruptures are almost invariably associated with pelvic fractures. Rupture may occur either from a direct perforation by a bony fragment (as with fractures of the anterior pubic arch) or from a burst injury or sudden shearing force from the pelvic ring at the time of the impact. The classic finding on cystography or CT cystography is contrast extravasation around the base of the bladder confined to the peri- and prevesical space (of Retzius); flame- or starburst-shape areas of contrast extravasation are characteristic (see Figs. 3-49 and 3-50). An associated pelvic hematoma may give the bladder a teardrop shape. With a more complex injury, the contrast material extends to the thigh, scrotum (or labia), penis, or perineum, or into the anterior abdominal wall (Fig. 3-51). Extravasation will reach the scrotum when the superior fascia of the urogenital diaphragm or the urogenital diaphragm itself has been disrupted. If the inferior fascia of the urogenital diaphragm is violated, the contrast material will reach the thigh and penis (contained by Colles’ fascia).
A typical intraperitoneal rupture results from a horizontal tear occurring in the dome of the bladder. The dome is the weakest and least supported area and the only portion of the adult bladder covered by peritoneum. The mechanism of injury is usually a direct blow to a fully distended urinary bladder. Initial CT images often demonstrate low-attenuation fluid within the peritoneal cavity, and gas if a Foley catheter has been introduced (Fig. 3-52). On cystography, contrast accumulates in the peritoneal cavity, outlines loops of bowel, and fills the paracolic gutters, pouch of Douglas, and other peritoneal spaces, including the subphrenic spaces. In combined intraperitoneal and extraperitoneal ruptures, cystography reveals contrast outlining the abdominal viscera and perivesical space. Combined ruptures are common after penetrating injuries from a high-velocity bullet or knife traversing the bladder.
Urethral Injury
Diagnosis Retrograde Urethrogram
Urethrography serves to assess integrity and to localize and characterize tears as complete or incomplete. In posterior urethral injuries, contrast material accumulates outside the urethra in the retropubic extraperitoneal space (Fig. 3-53). In partial rupture, there is at least some continuity, which allows partial filling of the bladder, in addition to the extravasated contrast. Complete tears are shown as an interruption of the urethra. Involvement of the urogenital diaphragm is assumed when contrast accumulates in the perineum. Partial tears of the posterior urethra usually heal uneventfully, whereas complete tears may heal with formation of a stricture. Other long-term symptoms associated with severe urethral injuries include impotence and, rarely, incontinence. These sequelae are more likely a reflection of the severity of the initial trauma, rather than caused by the urethral injury itself. In anterior urethral injuries, contrast may fill the corpora cavernosa or corpus spongiosum or it may reflux into the draining veins.
Rectal Injury
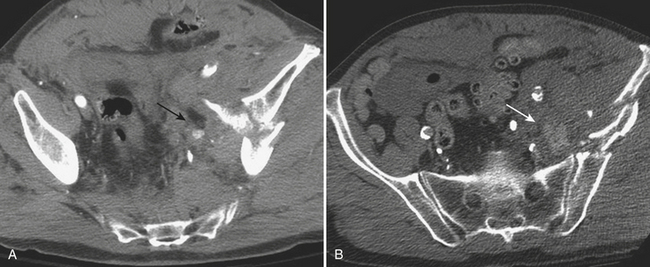
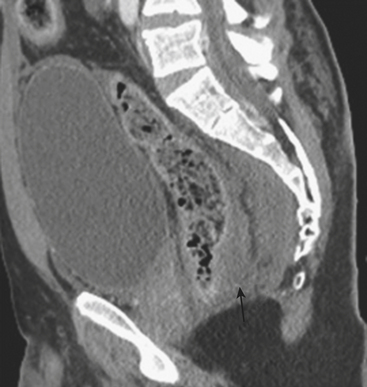
The rectum is rarely injured as a result of blunt trauma. When injured, there is often a history of direct perineal force at the time of the traumatic event, and patients often have associated pelvic fractures. Rectal injury can be difficult to detect clinically, although some patients present with bright red blood per rectum. Physical exam may detect the presence of blood or bone fragments in the rectal vault, indicating a high likelihood of injury. Often, patients with suspected injury are evaluated directly by rigid sigmoidoscope. However, rectal injuries are increasingly diagnosed at the time of diagnostic CT imaging. CT imaging may show focal rectal wall thickening (Fig. 3-54) or localized free air in the perirectal fat. In addition, hematoma may be present in the perirectal fat as a result of such injury. Water-soluble contrast administered as an enema may be necessary to demonstrate the site of perforation in questionable cases (Fig. 3-55).
PENETRATING ABDOMINAL TRAUMA
Computed Tomography
The diagnostic criteria used for interpretation of blunt trauma CT scans cannot be applied to penetrating trauma patients. Presence of free intraperitoneal air or free peritoneal fluid is a sign of peritoneal violation but is not definitive evidence of bowel injury, since air can be introduced into the peritoneal cavity by a bullet or knife wound and free fluid can be the result of bleeding from the peritoneal lining itself (Fig. 3-56). The only unequivocal sign of hollow viscus injury is the presence of extraluminal collections of oral or rectal contrast material (Fig. 3-57). Other CT findings considered highly indicative of bowel injury include the presence of focal bowel wall thickening or discontinuity and a bowel wall hematoma. Mesenteric injuries are confirmed by finding active extravasation of contrast material, as shown by the portal venous and delayed phase scans, or a focal mesenteric hematoma. Injuries to solid organs resulting from penetrating injuries are similar in appearance to those from blunt trauma (Fig. 3-58). Injury to the diaphragm is suspected when the trajectory of the missile or sharp object appears to extend toward or to the diaphragm. More specific signs, however, include finding herniated abdominal content into the chest through the diaphragmatic rent (Fig. 3-59), the CT “collar” sign (focal constriction of herniated abdominal fat or viscera at the site of diaphragmatic defect), and the finding of injured organs on either side of the diaphragm when only one injury was inflicted (thoracoabdominal injury). CT findings of potential diaphragm injury include a penetrating injury tract that extends to the diaphragm, thickening of the diaphragm, and an isolated focal defect in the normal continuity of the diaphragm without adjacent hemorrhage. If a question concerning the presence of peritoneal penetration persists during the period of clinical observation, laparoscopy should be performed as a definitive test.
Becker C.D., Spring P., Glattli A., et al. Blunt splenic trauma in adults: Can CT findings be used to determine the need for surgery? Radiographics. 2005;25:87-104.
Brasel K.J., Olson C.J., Stafford R.E., Johnson T.J. Incidence and significance of free fluid on abdominal computed tomographic scan in blunt trauma. J Trauma. 1998;44:889-892.
Breen D.J., Janzen D.L., Zwirewich C.V., et al. Blunt bowel and mesenteric injury: Diagnostic performance of CT signs. J Comput Assist Tomogr. 1997;21:706-712.
Brofman N., Atri M., Epid D., et al. Evaluation of bowel and mesenteric blunt trauma with multidetector CT. Radiographics. 2006;26:1119-1131.
Clancy T.V., Ragozzino M.W., Ranshaw D., et al. Oral contrast is not necessary in the evaluation of blunt abdominal trauma by computed tomography. Am J Surg. 1993;166:680-683.
Farahmand N., Sirlin C.B., Brown M.A., et al. Hypotensive patients with blunt abdominal trauma: Performance of screening US. Radiology. 2005;235:436-443.
Fleming K.W., Lucey B.C., Soto J.A., et al. Posttraumatic bile leaks: Role of diagnostic imaging and impact on patient outcome. Emerg Radiol. 2006;12:103-107.
Gralla J., Spycher F., Pignolet C., et al. Evaluation of a 16-MDCT scanner in an emergency department: Initial clinical experience and workflow analysis. AJR Am J Roentgenol. 2005;185:232-238.
Gupta A., Stuhlfaut J.W., Fleming K.W., et al. Blunt trauma of the pancreas and biliary tract: A multimodality imaging approach to diagnosis. Radiographics. 2004;24:1381-1395.
Hanks P.W., Brody J.M. Blunt injury to the mesentery and small bowel: CT evaluation. Radiol Clin North Am. 2003;41:1171-1182.
Killeen K.L., Shanmuganathan K., Poletti P.A., et al. Helical computed tomography of bowel and mesenteric injuries. J Trauma. 2001;51:26-36.
Kuan J.K., Wright J.L., Nathens A.B., et al. American Association for the Surgery of Trauma. American Association for the Surgery of Trauma Organ Injury Scale for kidney injuries predicts nephrectomy, dialysis, and death in patients with blunt injury and nephrectomy for penetrating injuries. J Trauma. 2006;60:351-356.
Levine C.D., Gonzales R.N., Wachsberg R.H. CT findings in bowel and mesenteric injury. J Comput Assist Tomogr. 1997;21:974-979.
Lingawi S.S., Buckley A.R. Focused abdominal US in patients with trauma. Radiology. 2000;217:426-429.
MacLean A.A., Durso A., Cohn S.M., et al. A clinically relevant liver injury grading system by CT, preliminary report. Emerg Radiol. 2005;12:34-37.
McGahan J.P., Wang L., Richards J.R. From the RSNA Refresher Courses: Focused abdominal US for trauma. Radiographics. 2001;21(Suppl):S191-S199.
Moore E.E., Cogbill T.H., Jurkovich J.G., et al. Organ injury scaling III: Chest wall, abdominal vascular, ureter, bladder, and urethra. J Trauma. 1992;33:337-339.
Moore E.E., Cogbill T.H., Malangoni M.A. Organ injury scaling II: Pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30:1427-1429.
Perry J.F.Jr. A five-year survey of 152 acute abdominal injuries. J Trauma. 1965;5:53-56.
Pinto A., Scaglione M., Pinto F., et al. Adrenal injuries: Spectrum of CT findings. Emerg Radiol. 2003;10:30-33.
Rana A.I., Kenney P.J., Lockhart M.E., et al. Adrenal gland hematomas in trauma patients. Radiology. 2004;230:669-675.
Richards J.R., McGahan J.P., Pali M.J., et al. Sonographic detection of blunt hepatic trauma: Hemoperitoneum and parenchymal patterns of injury. Trauma. 1999;47:1092-1097.
Rizzo M.J., Federle M.P., Griffiths B.G. Bowel and mesenteric injury following blunt abdominal trauma; Evaluation with CT. Radiology. 1989;173:143-148.
Rodriguez C., Barone J.E., Wilbanks T.O. Isolated free fluid on computed tomographic scan in blunt abdominal trauma: A systematic review of incidence and management. J Trauma. 2002;53:79-85.
Shanmuganathan K., Mirvis S.E., Boyd-Kranis R., et al. Nonsurgical management of blunt splenic injury: Use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 2000;217:75-82.
Sharma O.P., Oswanski M.F., Singer D. The role of computed tomography in diagnosis of blunt intestinal and mesenteric trauma. J Emerg Med. 2004;27:55-67.
Sinelnikov A.O., Abujudeh H.H., Chan D., et al. CT manifestations of adrenal trauma: Experience with 73 cases. Emerg Radiol. 2007;13:313-318.
Soto et al., Soto J.A., Lucey B.C., Stuhlfaut J.W., Varghese J.C. Use of 3D imaging in CT of the acute trauma patient: Impact of a PACS-based software package. Emerg Radiol. 2005;11:173-176.
Stafford R.E., McGonigal M.D., Weigelt J.A., et al. Oral contrast solution and computerized tomography for blunt abdominal trauma: A randomized study. Arch Surg. 1999;34:622-627.
Stuhlfaut J.S., Anderson S.W., Soto J.A. Blunt abdominal trauma: Current imaging techniques and CT findings in patients with solid organ, bowel, and mesenteric injury. Semin Ultrasound CT MR. 2007;28:115-129.
Stuhlfaut J.W., Lucey B.C., Varghese J.C., et al. Blunt abdominal trauma: Utility of 5-minute delayed CT with a reduced radiation dose. Radiology. 2006;238:473-479.
Stuhlfaut J.W., Soto J.A., Lucey B.C., et al. Blunt abdominal trauma: Performance of CT without oral contrast material. Radiology. 2004;233:689-694.
Tsang B.D., Panacek E.A., Brant W.E., et al. Effect of oral contrast administration for abdominal computed tomography in the evaluation of acute blunt trauma. Ann Emerg Med. 1997;30:7-13.
Vaccaro J.P., Brody J.M. CT cystography in the evaluation of major bladder trauma. Radiographics. 2000;20:1373-1381.
Valentino M., Serra C., Pavlica P., et al. Contrast enhanced ultrasound for blunt abdominal trauma. Semin Ultrasound CT MR. 2007;28:130-140.
Vasanawala S.S., Desser T., Jeffrey R.B. Value of delayed imaging in MDCT of the abdomen and pelvis. AJR Am J Roentgenol. 2006;187:154-163.
Wittenberg A., Minotti A.J. CT diagnosis of traumatic gallbladder injury. AJR Am J Roentgenol. 2005;185:1573-1574.
Yoon W., Jeong Y.Y., Kim J.K., et al. CT in blunt liver trauma. Radiographics. 2005;25:87-104.