19 Abdomen
Computed Tomography Technology and Terminology
Three distinct generations of CT technology are in current use. In order of historical development and scanner speed, these are conventional CT, spiral (or helical) CT, and multislice (or multidetector) CT. Rapid scanning is a desirable feature in abdominal CT for two reasons. First, unlike the brain, the abdomen moves with respiration, and imaging during a single breath-hold removes the risk of respiratory misregistration (i.e., structures missed between slices because successive breath-holds are not identical). Second, many applications in abdominal imaging are affected by the time images are acquired relative to the bolus of intravenous contrast medium, and accurate diagnosis may require imaging precisely timed to one or more phases of enhancement. Godfrey Hounsfield invented CT in 1972, with initial clinical installations in 1974. The first CT scanner developed by Hounsfield in his laboratory at Electrical and Musical Industries required several hours to acquire the data for a single slice, and took several days to reconstruct the corresponding image. Data acquisition and image reconstruction became progressively faster during the 1970s and 1980s, although the speed of the scanners remained limited by the need for “stop-start,” slice-by-slice acquisition. That is, in conventional CT, an axial slice is generated by rotating an x-ray tube and detector array in a 360° circle around the patient. After a 360° rotation, the rotating gantry reverses direction to prevent disruption of the tethered cables that transfer the data from the detector array to the computer. Such sequential slice acquisition limits the speed of conventional CT, prevents volumetric data acquisition, results in slice misregistration, and limits temporal resolution such that multiphase volumetric scanning is not possible. The development of spiral (or helical) CT in the late 1980s represented a technologic breakthrough. In spiral CT, data is carried from the rotating gantry to the computer by slip rings, which allow continuous gantry rotation and data transfer. Scanning can be performed while the patient is moved slowly but continuously through the gantry. The ability to continuously scan allows for “non-stop” volumetric data acquisition. Data is gathered on a three-dimensional volume in a spiral fashion. Images are reconstructed from the data volume. Physical and clinical performance studies of spiral CT were first reported at the 1989 Radiological Society of North America meeting.1 The first commercially available spiral CT scanner was released in 1990.2
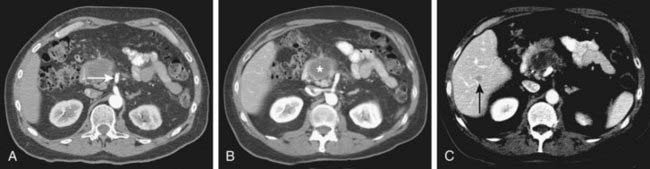
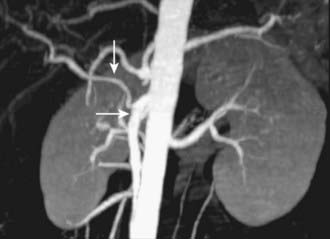
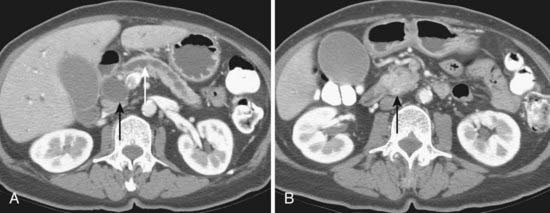
Spiral CT was an important technologic advance, but many limitations and compromises remained. The rotational speed of the gantry was generally one rotation per second, and clinical results suggested that a pitch (ratio of longitudinal distance moved by the tabletop during one gantry rotation to beam collimation) greater than 2 was undesirable. As a result, either large volumes could be covered, or thin sections acquired, but not both. The Elscint CT Twin scanner employed a dual array of two side-by-side detector rows, and represented an early version of multislice technology.3 However, although this scanner was an improvement over single-detector spiral CT in terms of coverage,4 substantial limitations remained. It was not until 1998 that a scanner with four detector rows became commercially available. Modern multislice CT using four or more detector rows essentially abolishes the remaining obstacles to rapid isotropic volumetric imaging by using multiple side-by-side detectors simultaneously. During recent years, top-of-the-line scanners have used 64 detector rows, although machines with 256 rows or flat-panel detectors are being investigated and will likely become more widely available in the next few years. Such multislice CT systems can collect multiple slices of data in approximately 300 milliseconds and reconstruct a 512 × 512 matrix image from millions of data points in less than a second. An entire body cavity (brain, chest, or abdomen) can be scanned in 5 to 10 seconds using the most advanced multislice CT systems. Faster imaging with modern scanners is due not only to multiple detector rows, but also to increased gantry rotational speed. Many commercial scanners are now capable of a complete gantry rotation in 0.3 seconds or less. Multislice CT has been a major advance in several areas of abdominal imaging, particularly those requiring imaging in multiple phases of enhancement after intravenous contrast medium administration (Fig. 19-1) and rapid near-isotropic (i.e., equal spatial resolution in all three planes) volumetric acquisition, such as CT angiography (Fig. 19-2). The ability to acquire scans of the abdomen during multiple phases of enhancement is particularly helpful when both arteriographic and parenchymal phases of enhancement are required for complete evaluation. For example, the arteriographic phase is critical for the assessment of arterial encasement in pancreatic cancer, but parenchymal enhancement phases are required for demonstration of the primary tumor and detection of hepatic metastases.
One of the unfortunate consequences of the increased ability to obtain multiphase imaging has been a corresponding increase in confusion with respect to the proper terminology for these studies.5 It has reasonably been suggested that the term phase should not be applied to noncontrast images.6 It also seems reasonable to use the term arteriographic phase for early arterial images in which contrast is primarily within the arteries, and the term parenchymal arterial phase for the subsequent period of early tissue enhancement when hypervascular masses are most prominent. Later periods of image acquisition can be referred to as portal venous or delayed, as appropriate. Until a universal terminology is established, it is probably best to avoid unclear terms such as dual or triple phase studies, and instead describe the specific phases acquired.
Cholangiocarcinoma
Pathologic Conditions
Cholangiocarcinomas are adenocarcinomas of the biliary ductal epithelium, and account for more than 90% of bile duct malignancies. Based on location, cholangiocarcinomas are divided into intrahepatic, proximal extrahepatic (i.e., perihilar), and distal extrahepatic. Perihilar cholangiocarcinomas, originating at the confluence of the right and left hepatic ducts, are the most common, and are also known as Klatskin tumors.7 Distal extrahepatic cholangiocarcinomas, originating from the common duct between the upper border of the pancreas and the ampulla of Vater, are the second most common. Intrahepatic cholangiocarcinomas are the least common. Cholangiocarcinomas are infiltrative tumors, and typically invade and occlude adjacent structures such as the bile ducts, portal veins, and hepatic arteries. Cholangiocarcinomas are often locally advanced before they present with clinical features of biliary obstruction such as painless jaundice, pruritus, dark urine, and pale stools. Regional extension occurs into the liver and the lymph nodes of the celiac and pancreaticoduodenal chains. Distant metastases may appear in the peritoneum, thoracic lymph nodes, and surgical or drain incisions. Intrahepatic cholangiocarcinomas may involve hepatoduodenal ligament, para-aortic, retro pancreatic, or common hepatic artery nodes. In addition, perihilar and left intrahepatic cholangiocarcinomas may spread along the left gastric nodes adjacent to the lesser curvature of the stomach.8
Imaging of Cholangiocarcinoma
The traditional standard of reference for the diagnosis and staging of cholangiocarcinoma has been the combination of conventional cholangiography and angiography.9 Modern cross-sectional imaging has largely replaced the need for these invasive tests, both with respect to diagnosis and staging. The diagnosis of cholangiocarcinoma should be suspected when US, CT, or MRI demonstrate dilated intrahepatic ducts with nondilated extrahepatic ducts in a patient with painless jaundice. Because of the infiltrative nature of cholangiocarcinoma, the primary tumor may or may not be seen, and visualization of a mass is not required to suggest the diagnosis. Potential differential diagnoses in this setting include postsurgical stricture (either iatrogenic bile duct injury or biliary enteric anastomotic stricture) or malignant masquerade (other hepatic masses, such as metastases, very rarely result in biliary dilatation). Postsurgical strictures are usually evident from the clinical history. Malignant masquerade is a rare nonneoplastic fibrous proliferation that occurs at the hilum of the liver and clinically and radiologically mimics perihilar cholangiocarcinoma.10 In a series of 94 patients with suspected obstructive malignancy at the hilum, 8 were found to have malignant masquerade at pathologic analysis.10 The 8 patients ranged in age from 37 to 66 years, and all presented with obstructive jaundice. One later developed cholangiographic changes of sclerosing cholangitis, and it may be that malignant masquerade represents a localized, masslike form of sclerosing cholangitis.11
Conventional Cholangiography
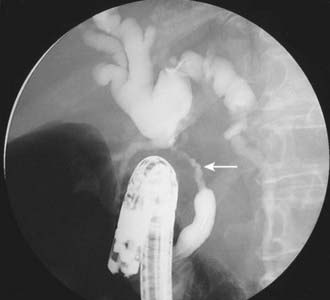
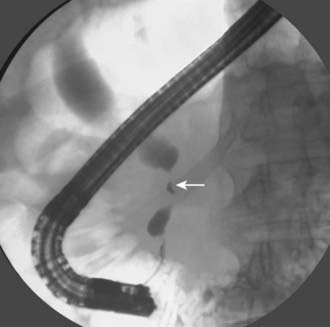
Conventional cholangiography can be performed by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC). ERCP has the advantage of excluding ampullary pathologic conditions by direct endoscopic evaluation and allowing for simultaneous tissue sampling. Palliative endobiliary stenting can also be performed at the same time. ERCP is usually favored more than PTC, but the latter may be appropriate when attempts at ERCP have been unsuccessful. PTC may allow access to proximal lesions with obstruction of both right and left hepatic ducts. Tissue may be obtained for cytologic analysis and drainage performed. The typical appearance of a cholangiocarcinoma at conventional cholangiography is of a tight, irregular stricture of variable length associated with marked dilatation of the proximal biliary tract (Fig. 19-3).
Ultrasound
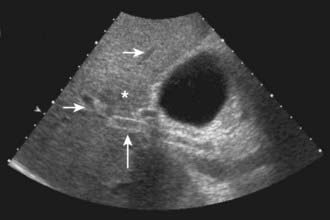
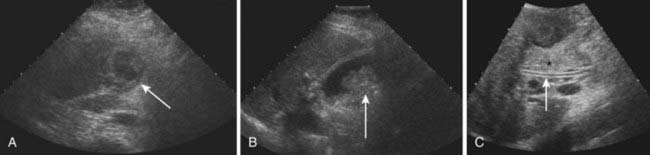
US is frequently the initial investigation requested in patients with suspected biliary obstruction. Klatskin tumors classically manifest at US as segmental dilatation and nonunion of the right and left hepatic ducts in the porta hepatis (Fig. 19-4). The papillary and nodular ductal variants of cholangiocarcinoma are relatively easy to see at US. Papillary tumors form polypoid intraluminal masses, whereas nodular cholangiocarcinoma manifests as a discrete smooth mass with associated mural thickening.12 Smaller or infiltrative tumors may be missed, although experienced operators can demonstrate high sensitivity. In a retrospective study of 49 patients with pathologically proved cholangiocarcinoma, US demonstrated the tumor in 47 (96%) cases; a mass was demonstrated in 44 and focal or diffuse thickening of the bile duct wall in 3.13 In a study of 39 patients with Klatskin tumors undergoing preoperative US,14 ductal masses were detected in 34 patients (87%). Masses were isoechoic in 22 (65%), hypoechoic in 7 (21%), and hyperechoic in 5 (15%). Morphologic tumor patterns included nodular mural thickening (n = 19; 56%), irregular infiltration (n = 9; 26%), and intraductal polypoid masses (n = 6; 18%). Vascular involvement is an important determinant of irresectability, and portal vein involvement can be assessed with an accuracy of up to 91% by Doppler US.15 In a study of 22 patients with hilar cholangiocarcinoma, vascular involvement was evaluated by Doppler US and arteriography, using operative findings as the standard of reference. Vascular patency or involvement was correctly determined by Doppler US in 19 patients (86%), compared with 18 by arteriography (82%).16 EUS enables both bile duct visualization and nodal evaluation, and EUS-guided fine-needle aspiration biopsy may be diagnostic when other diagnostic tests are inconclusive.17
Computed Tomography
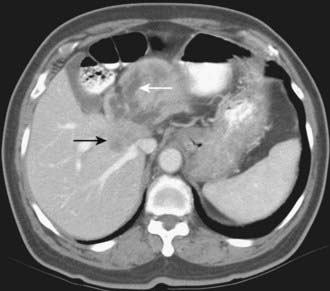
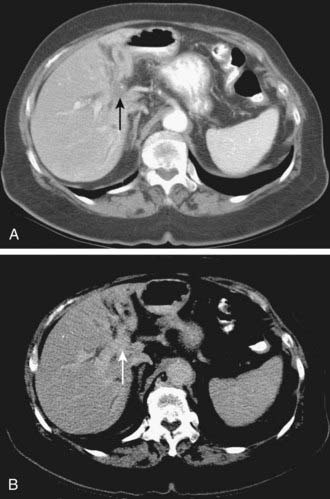
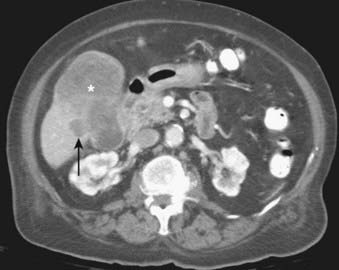
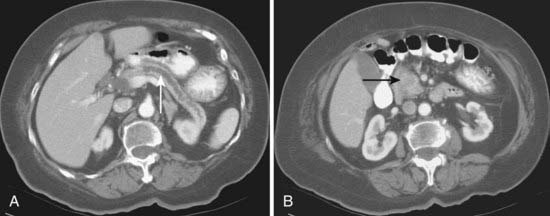
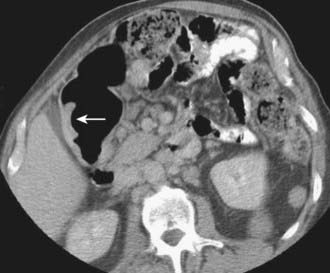
Depending on referral practices, CT may be the first investigation requested in patients with painless jaundice. The CT findings in Klatskin tumor resemble those seen at US, namely segmental dilatation and nonunion of the right and left hepatic ducts in the porta hepatis (Fig. 19-5). The reported frequency with which a primary tumor mass is seen varies from 40% to 91%.18–21 Delayed enhancement is a relatively recently described finding that has been reported in 36% to 74% of cases (Fig. 19-6).20,21 In one study, 3 of 47 cholangiocarcinomas were seen only in delayed images.21 The optimal time for acquisition of delayed images is 10 to 20 minutes after contrast media injection.20 Lobar atrophy is a nonspecific but useful ancillary feature of cholangiocarcinoma that may be better appreciated at CT than US. Lobar atrophy usually indicates both biliary and portal venous obstruction in that lobe. Another advantage of CT is the ability to detect metastases, such as nodal or peritoneal deposits. Adenopathy in cholangiocarcinoma arising secondary to primary sclerosing cholangitis should be interpreted with caution, because nodal enlargement in this context may be reactive or metastatic. Occasionally, CT may suggest the diagnosis of peripheral intrahepatic cholangiocarcinoma when a patient with vague or nonspecific symptoms is found to have a hypodense hepatic mass with peripheral enhancement, biliary dilatation, and delayed enhancement.22
Magnetic Resonance Imaging
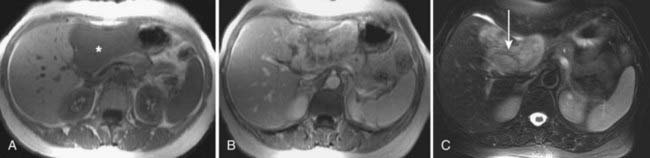
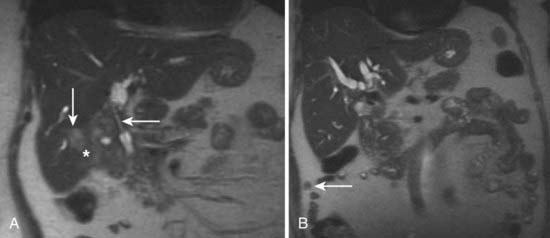
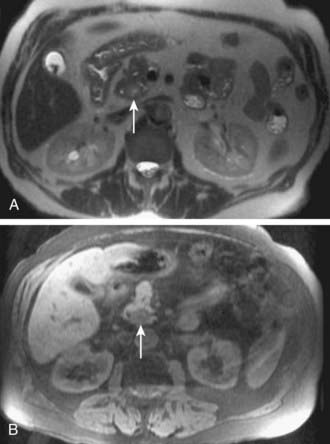
MRI findings in cholangiocarcinoma resemble those seen at CT: isolated intrahepatic biliary dilatation with or without a visible mass (Fig. 19-7). Although MRI is frequently reserved for patients with contraindications to contrast-enhanced CT, such as elevated creatinine or contrast allergy, the modality has several attractive imaging characteristics that may assist in tumor evaluation. MRI cholangiography provides similar information to conventional cholangiography, and may obviate the need for invasive biliary imaging.23 MRI angiography can be used to assess vascular involvement and may replace catheter angiography for vascular evaluation. In a study of 27 preoperative patients with Klatskin tumors, evaluation of vascular involvement by MRI and US were compared with intraoperative findings.24 Encasement, thrombosis, occlusion, and nonvisualization were considered to be evidence of vascular involvement. Of 16 involved veins, 12 were detected at MRI compared with 13 of 16 at US. There was one false-positive diagnosis of inferior vena cava involvement at both MRI and US. These results suggest that MRI and US provide comparable results for assessment of hepatic vein involvement by tumor. Central hypointensity on T2-weighted images appears to be a characteristic finding in intrahepatic cholangiocarcinoma (Fig. 19-8), and reflects severe fibrosis.25
Positron Emission Tomography
Early results suggest PET scanning may have a role to play in the evaluation of cholangiocarcinoma,26 because it may detect cholangiocarcinoma tumors as small as 1 cm in diameter and can simultaneously detect distant metastases. In a study of 54 patients, 26 with cholangiocarcinoma and 28 with no disease or benign disease, true-positive PET scans were obtained in 24 of 26 cholangiocarcinoma patients with only two false-positive results (sensitivity of 92% and specificity of 93%). Regional or hepatoduodenal lymph node metastases were detected with PET in only 2 of 15 cases, whereas distant metastases (peritoneal carcinomatosis, pulmonary metastases) were diagnosed in 7 of 10 cases.
Gallbladder Carcinoma
Imaging of Gallbladder Carcinoma
GBC is usually advanced at the time of diagnosis, and early-stage tumors are rarely encountered.27 As a result, imaging findings are usually relatively obvious, and can be well assessed by US, CT, or MRI. Radiologic findings include focal or diffuse thickening of the gallbladder wall (49%), a solid mass replacing the gallbladder in the gallbladder fossa (37%), or an intraluminal mass in the gallbladder (14%). Associated findings include invasion of adjacent structures such as the liver (67%), coexistent gallstones (64%), biliary duct dilatation (38%), coexistent porcelain gallbladder (4%), and distant metastases outside the liver (3%).28 Although gallbladder cancer may manifest as an intraluminal mass at imaging, it should be remembered that gallbladder polyps may be found in up to 2.5% of asymptomatic patients29 and that most small, noncalculous gallbladder filling defects are benign. In a study of 143 gallbladder polyps less than 1.5 cm in diameter removed by laparoscopic cholecystectomy, the final diagnoses were cholesterol polyp (74%), adenoma (11%), adenomyomatosis (7%), adenocarcinoma (4%), and hyperplastic or inflammatory polyp (4%).30 Historical reports have suggested the risk of GBC in patients with a porcelain gallbladder is between 12% and 61%,31 and such numbers are frequently quoted in clinical practice. Two recent series of 10,741 and 25,900 cholecystectomy specimens from the University of California, Los Angeles, and Massachusetts General Hospital, respectively, have suggested the true risk is 0% to 7%.31,32
Ultrasound
Sonographic findings of GBC include variable degrees of gallbladder wall thickening and irregularity (Fig. 19-9). Concomitant gallstones are frequently seen. Gallbladder wall thickening can also be seen with chronic cholecystitis and adenomyomatosis, but the wall thickening of these inflammatory and hyperplastic conditions is usually more diffuse than the relatively focal changes typical of GBC. GBC may also manifest as one or more intraluminal masses that are typically of homogeneous echotexture without acoustic shadowing. The polyps are usually sessile and only rarely have a stalk. With more advanced disease, US may demonstrate a necrotic mass, direct hepatic invasion, biliary dilatation, or hepatic metastases. US is of limited utility in the evaluation of nodal, peritoneal, or more distant metastases. Occasionally tumefactive sludge may mimic an intraluminal mass, but mobility on changes in patient position and absence of flow on Doppler insonation help in the correct identification of sludge.33 EUS has been used in some centers to study GBC, and early results suggest this modality may be helpful in assessing the depth of wall invasion.34 Loss of the normal multiple-layer pattern of the gallbladder wall has been reported as the most specific finding at EUS in the diagnosis of GBC.35
Computed Tomography
CT is helpful in both the diagnosis and staging of GBC, because it is able to detect gallbladder masses, wall thickening, and hepatic invasion (Fig. 19-10). Spiral CT has an accuracy of 83% to 86% in the local staging of GBC.36 The reported sensitivity for the detection of nodal metastases was more limited, ranging from 36% to 47%. CT has reasonably high positive predictive values for the detection of hepatic invasion of less than 2 cm (77%), hepatic invasion of more than 2 cm (100%), involvement of the common duct (90%), or invasion of the pancreas (100%).37 Xanthogranulomatous cholecystitis is a rare inflammatory process that cannot be reliably distinguished from GBC on CT scans because of multiple overlapping features such as gallbladder wall thickening and involvement of the surrounding tissues, including portal lymph nodes, fat, and the liver.
Magnetic Resonance Imaging
MRI findings in GBC resemble those seen at CT (Fig. 19-11). MRI and magnetic resonance cholangiopancreatography (MRCP) can provide information relevant to preoperative staging of GBC. GBC manifests at MRI as focal gallbladder wall thickening with an eccentric mass in 76% of cases. The most common types of regional spread demonstrated are direct hepatic invasion (91%), lymphadenopathy (76%), and biliary tract invasion (62%). Sensitivity for direct hepatic invasion is reported as 100%, and sensitivity for lymph node metastasis as 92%.38 In general, MRI findings are analogous to CT scanning. The tumor is usually poorly marginated and bright on T2-weighted images.
Pancreatic Cancer
Anatomy
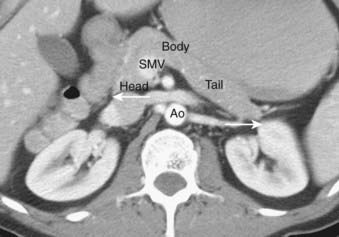
The pancreas is an exocrine and endocrine gland, situated transversely in the retro peritoneum, at the level of the L1 and L2 vertebrae. The head lies within the duodenal sweep, and the body and tail extend to the gastric border of the spleen. Although several systems for describing the anatomic subdivisions of the pancreas have been proposed, the tumor-node-metastasis classification is straightforward and divides the pancreas into head, body, and tail.39 According to this classification, the head lies to the right of the superior mesenteric vein, the body lies between the left border of the aorta and the superior mesenteric vein, and the tail lies to the right of the left border of the aorta (Fig. 19-12). The pancreatic head lies posterior to the stomach and transverse colon, and anterior to the inferior vena cava. The common bile duct courses through the pancreatic head to the ampulla of Vater. The pancreatic body lies anterior to the superior mesenteric vessels. The pancreatic tail extends laterally to abut the spleen and splenic flexure of the colon, and lies anterior to the left kidney. The blood supply of the pancreas is derived from the pancreaticoduodenal branches of the gastroduodenal and superior mesenteric arteries and from direct branches of the splenic artery. Pancreatic venous drainage is through the portal system to the liver. The pancreas has an extensive system of lymphatic drainage, primarily to superior and inferior pancreaticoduodenal, porta hepatis, and suprapancreatic nodes, but also to para-aortic nodes.
Pathologic Conditions
Most pancreatic cancers are adenocarcinomas arising from the pancreatic ductal epithelium. Pancreatic adenocarcinoma is often far advanced by the time symptoms occur. Approximately 75% of all pancreatic carcinomas occur within the head or body of the pancreas. Pancreatic cancer typically spreads first to regional lymph nodes, then to the liver, and finally to more distant sites such as the lungs. Regional nodes of the pancreas include supraperipancreatic nodes above the head and body, infrapancreatic nodes below the head and body, periceliac nodes, anterior and posterior pancreaticoduodenal nodes, pyloric nodes, peribiliary nodes, and proximal mesenteric nodes. Nodal involvement is seen in approximately 73% of patients undergoing surgical resection, and lowers the 5-year survival from 26% to 5%.40 Unfortunately, both CT and PET are of very limited accuracy in the evaluation of regional adenopathy in pancreatic cancer.41,42 Carcinoma of the head of the pancreas typically compresses the common bile duct and pancreatic duct, and causes biliary and pancreatic ductal dilatation. Peritoneal involvement is more common with carcinoma of the body and tail than with carcinoma of the head, presumably because such tumors are less likely to cause obstructive jaundice and therefore present in a more advanced stage.
Imaging of Pancreatic Carcinoma
Imaging modalities for pancreatic carcinoma include transabdominal US, EUS, CT, MRI, PET, and conventional cholangiography.43 Apart from distant metastases, involvement of the major vessels is the most important parameter for determining resectability in patients with pancreatic adenocarcinoma. Two common problems arise in imaging pancreatic carcinoma across all these modalities. First, pancreatic carcinoma is often infiltrative and the tumor may be poorly defined and difficult to appreciate. Even quite large tumors can be of similar appearance to the adjacent pancreatic parenchyma, and difficult to visualize. Such tumors may be diagnosed predominantly through the indirect findings of pancreatic or biliary dilatation (Fig. 19-13). Second, pancreatic carcinoma may be hard to distinguish from focal chronic pancreatitis.
Ultrasound
Transabdominal US is of limited utility in the diagnosis of pancreatic carcinoma, because of suboptimal acoustic contrast and variable obscuration of the pancreas by overlying bowel. US may be helpful in the initial workup of patients, because it can distinguish obstructive from nonobstructive jaundice and thus guide further investigations. EUS is an evolving modality that shows great promise in the diagnosis and staging of pancreatic carcinoma. EUS remains the most sensitive and specific method to identify pancreatic masses. Early results suggest EUS is more accurate than CT for staging pancreatic malignancy, including the evaluation of vascular invasion and local resectability. In a study of 151 patients with pancreatic carcinoma, the overall accuracy for T and N staging was 85% and 72% for EUS compared with only 30% and 55% for CT, respectively. The ability to accurately predict vascular invasion was 93% for EUS compared with 62% for CT. EUS was 93% accurate for predicting local resectability versus 60% for CT.44 In a study of 21 patients with pancreatic carcinoma, EUS demonstrated an accuracy of 81% in the assessment of vascular involvement compared with 38% for selective venous angiography.45 EUS can also be combined with targeted fine-needle aspiration biopsy, and has shown a sensitivity of 93% and a specificity of 100% in establishing the correct diagnosis when employed in patients with pancreatic masses in whom prior biopsies have been nondiagnostic.46
Computed Tomography
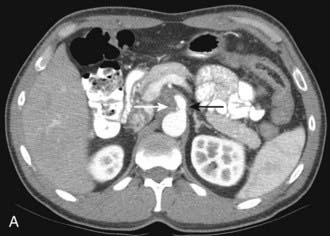
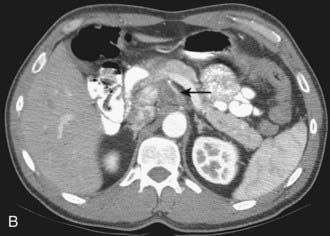
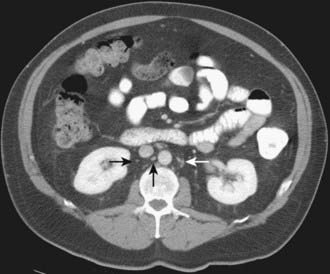
CT is the most frequently used modality for the evaluation of pancreatic carcinoma. CT-guided biopsy can be performed to provide a tissue diagnosis. The primary finding on contrast-enhanced CT is a focal hypodense mass (Fig. 19-14). The sensitivity of spiral CT for the detection of pancreatic carcinoma is between 89% and 97%.47 The overall accuracy of spiral CT in the determination of resectability has been reported as 77%.47 In a study of 80 evaluable major vessels in 25 patients, tumor contact with more than 50% of the vessel circumference demonstrated a sensitivity of 84% and specificity of 98% in the diagnosis of vascular invasion (Fig. 19-15).48 The development of spiral CT has allowed imaging of pancreatic carcinoma in several different phases of enhancement, and each may provide useful information. Arteriographic images are helpful in the evaluation of arterial encasement by pancreatic carcinoma.49 With respect to tumor detection, some studies have suggested tumor-to-background contrast is greater in the late arterial phase (scan delay of 40 to 70 seconds, sometimes called the pancreatic phase) than in the standard portal venous phase,50,51 although other studies have suggested late arterial phase images do not improve pancreatic tumor detection when compared with standard portal venous phase images.52,53 Accordingly, baseline studies for pancreatic carcinoma can reasonably be protocoled to include three phases: early arterial (arterial encasement), late arterial (tumor detection), and portal venous (hepatic evaluation).
Conventional Cholangiography
After CT, ERCP is perhaps the second most common investigation performed in patients with suspected pancreatic carcinoma (Fig. 19-16). Brush cytologic examination and forceps biopsy can be used to provide a tissue diagnosis. Endobiliary stents can also be inserted at the time of ERCP, although this may not always be appropriate in patients who are surgical candidates; two separate studies of periampullary tumors and proximal cholangiocarcinoma have shown that preoperative endobiliary stenting is associated with increased bacterial contamination of the biliary system, wound and intra-abdominal complications, and postoperative infections.54,55 The principal disadvantage of conventional cholangiography, such as ERCP or PTC, is that these invasive procedures have a major complication rate on the order of 3%. Such complications include sepsis, bleeding, bile leak, and death.56,57 EUS may be the most accurate test for the diagnosis of pancreatic cancer. Studies comparing EUS with CT have shown that EUS has a higher sensitivity and specificity for this diagnosis of pancreatic cancer, particularly for tumors less than 3 cm in diameter.58 In addition, EUS is highly accurate for detecting local invasion and nodal metastases from pancreatic cancer, although results are similar when compared with dual-phase multislice CT. CT does provide additional information about hepatic metastases.59 The side-viewing duodenoscope that delivers the US probe also permits the detection of ampullary and duodenal carcinomas.
Magnetic Resonance Imaging
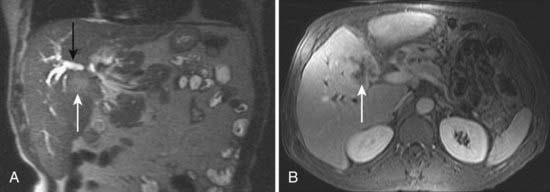
MRI is mainly used as a problem-solving modality in patients with suspected pancreatic carcinoma, typically in patients with an equivocal CT scan or with contraindications to iodinated contrast such as allergy or elevated creatinine. However, the ability of MRI to simultaneously image the pancreatic parenchyma and vasculature with gadolinium enhancement and the pancreaticobiliary system with MRCP provides an attractive combined assessment that may be superior to more traditional methods (Fig. 19-17).60–62 For example, in a study of 124 patients with suspected pancreatic carcinoma in whom the standard of reference was biopsy or 1 year of clinical follow-up, the sensitivity and specificity of MRCP for the diagnosis of pancreatic cancer was 84% and 97%, respectively, compared with a sensitivity and specificity of 70% and 94% for ERCP.58 Another study concluded that mangafodipir trisodium-enhanced MRI was as accurate as contrast-enhanced spiral CT for the detection and staging of pancreatic cancer but offers improved detection of small pancreatic metastases and of liver metastases.61 In a study of 33 patients with suspected pancreatic carcinoma, MRI was significantly better in the assessment of resectability than spiral CT.63
Positron Emission Tomography
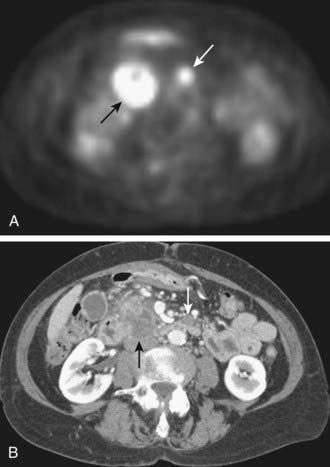
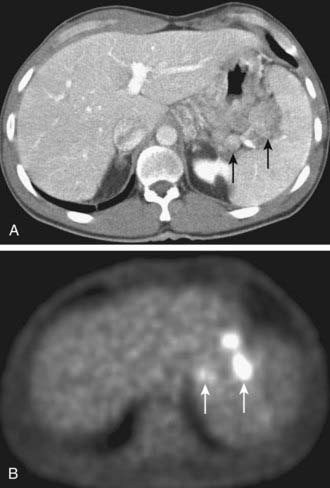
Initial studies suggest PET scanning may be helpful in the diagnosis of pancreatic carcinoma, detection of distant metastases, and evaluation of recurrent disease. In a study comparing PET and CT for tumor diagnosis in 73 patients with suspected pancreatic malignancy, PET had higher sensitivity (93% versus 80%) and specificity (93% versus 74%).63 In another study of 65 patients with suspected pancreatic carcinoma, PET again demonstrated higher sensitivity and specificity than CT in the correct diagnosis of pancreatic carcinoma (92% and 85% versus 65% and 61%).64 PET may also be helpful in the detection of small metastatic deposits that would preclude any attempt at surgical treatment (Fig. 19-18).
Retroperitoneal Lymphadenopathy
Pathologic Conditions
Virtually any malignancy can spread to the retroperitoneal nodes, particularly when widely disseminated. Tumors that commonly involve the retroperitoneal nodes include malignancies of the gastrointestinal tract, pancreas, kidney, and genitourinary system. The latter includes tumors of the testes, ovaries, and prostate. It is also common for lymphoma to involve the retroperitoneal nodes. In men presenting with a retroperitoneal nodal mass, in whom biopsy demonstrates a germ cell histologic condition, careful evaluation of the testes is important because the retroperitoneal adenopathy may represent a primary extragonadal germ cell tumor, or metastatic disease from a testicular primary. Testicular US in a patient with retroperitoneal germ cell tumor may demonstrate a typical primary testicular cancer (e.g., ovoid hypoechoic mass), or a dense echogenic focus. Such lesions are usually primary germ cell malignancies, often seminomas.65 Occasionally, no tumor is found within such a hypoechoic mass, and these are regarded as regressed primary tumors.66 Echogenic foci are thought to represent “scars” or “burnt-out” tumors.67
Imaging of Retroperitoneal Adenopathy
Computed Tomography
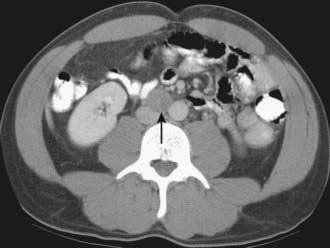
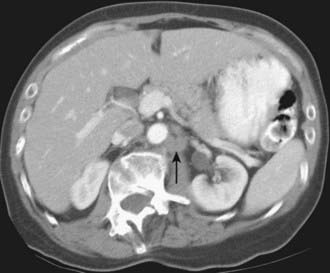
Normal retroperitoneal lymph nodes are either invisible or appear on CT as small round or oval densities less than 1 cm in diameter (Fig. 19-19). Abnormal nodes are also seen as soft-tissue densities, but of greater size and number. Adenopathy can occasionally be confluent or calcified. The accuracy of CT evaluation of retroperitoneal nodes is partially disease-specific, and this is illustrated by describing nodal assessment in testicular, endometrial, ovarian, and renal cancers. Right-side testicular cancer typically spreads to interaortocaval or paracaval nodes at the level of the entry of the right renal vein into the inferior vena cava (Fig. 19-20). Left-sided testicular cancers typically spread to left para-aortic nodes at the level of the entry of the left renal vein into the inferior vena cava (Fig. 19-21). These nodal sites should be carefully scrutinized in patients with testicular cancer. A study of 70 patients with newly diagnosed clinical stage 1 testicular nonseminomatous germ cell cancer undergoing preoperative CT showed that a short-axis size threshold of 1 cm had a sensitivity of 37% and a specificity of 100% in the diagnosis of nodal metastases. Progressively lower-size thresholds demonstrated greater sensitivity but lower specificity, so that a 4-mm threshold had a sensitivity of 93% and a specificity of 58%. The same study also showed that nodes lying anterior to the center of the aorta were more likely to be metastatic than nodes of similar size lying more posteriorly.68 The incidence of para-aortic nodal metastases in patients with clinical stages IA, IB, II, and III endometrial carcinoma is 2.5%, 8.5%, 15.7%, and 33.3%, respectively.69 Deep myometrial invasion, cervical involvement, and lymphovascular space invasion also significantly increase the likelihood of nodal metastases. In a study of 56 women with endometrial carcinoma that had preoperative CT scans and lymph node sampling, the sensitivity and specificity of CT for detecting nodal metastases were 57% and 92%, respectively.70 This study used a short-axis diameter of at least 1.5 cm to define adenopathy. Ovarian cancer can spread to pelvic and retroperitoneal nodes. In a study of 71 patients undergoing preoperative CT or MRI, the incidence of nodal metastases at surgery was 28% (20 of 71).71 CT showed a sensitivity of 50% and a specificity of 95% in the diagnosis of nodal metastases using a 1-cm short-axis diameter threshold. The evaluation of retroperitoneal adenopathy in renal cell carcinoma illustrates the importance of clinical context, because nodal enlargement in this setting is often reactive rather than metastatic. In a study of 163 patients undergoing preoperative CT and using a short-axis threshold of 1 cm to define adenopathy,72 lymphadenopathy was found in 43 patients, only 18 of whom had nodal metastases at surgery (positive predictive value of 43%). No adenopathy was detected in 120 patients, of whom 5 had metastases at surgery (negative predictive value of 96%).
Magnetic Resonance Imaging
MRI is rarely employed as the primary imaging modality for assessment of retroperitoneal adenopathy, because CT, even without intravenous contrast, is usually adequate. In general, the same size criteria are used at MRI as are used at CT, although a recent study suggests different size criteria may be appropriate.73 In a group of testicular cancer patients without metastases undergoing surveillance imaging, the 95th percentile for maximum short-axis diameter of retroperitoneal nodes at a variety of locations ranged from 3 to 5 mm.
Positron Emission Tomography
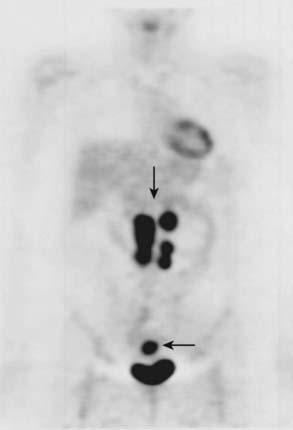
PET (Fig. 19-22) allows detection of small malignant nodes not identified or not meeting size criteria for malignancy by CT, or when lack of retroperitoneal fat or postsurgical change makes it difficult to identify retroperitoneal nodes with CT.74 PET scanning is also gaining importance in diagnosing and staging of cervical cancers. In a study comparing PET and CT in 101 consecutive patients with carcinoma of the cervix treated by chemotherapy and radiotherapy as clinically indicated and followed for a median of 15.4 months, baseline CT and PET showed retroperitoneal adenopathy in 7 and 21 patients, respectively. The 2-year progression-free survival, based solely on retroperitoneal adenopathy, was 64% in patients with negative results in both modalities, 14% in patients with positive results in both modalities, and 18% in patients with negative results on CT but positive results on PET. Multivariate analysis demonstrated that the most significant prognostic factor for progression-free survival was the absence of positive retroperitoneal nodes on PET. These results suggest that PET detects abnormal lymph nodes more often than CT, and that PET findings are a better predictor of outcome.75
Lymphangiography
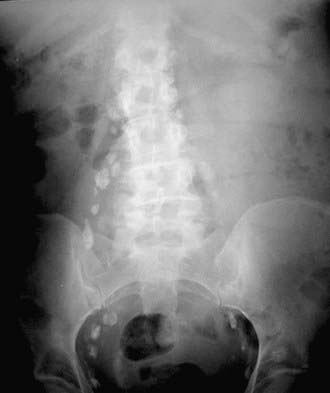
Lymphangiography (Fig. 19-23) has largely fallen into disuse because it is an invasive and technically challenging procedure, because few practicing radiologists have the necessary training to perform or interpret lymphangiograms, and because the available data do not suggest any incremental clinical benefit. Even when lymphangiography was available, it was largely confined to a few specialist centers and reserved for evaluation of testicular and cervical cancer. A recent meta-analysis reviewed the performance of lymphangiography, CT, and MRI in nodal assessment in cervical cancer. The three modalities had similar accuracy, and the authors concluded that as CT and MRI are less invasive than lymphangiography and also assess local tumor extent, they should be considered the preferred adjuncts to clinical evaluation of invasive cervical cancer.76 In a study comparing CT and lymphangiography in 50 patients with intra-abdominal metastatic testicular cancer, CT was more effective than lymphangiography. CT showed metastatic disease in 21 patients compared with 7 for lymphangiography. In the detection of relapse, lymphangiography showed nodal enlargement in 4 of 8 cases compared with 8 of 8 for CT.77
Gastric Cancer
Anatomy
The stomach lies in the left upper quadrant and is divided into fundus, body, and antrum. The gastroesophageal junction (cardia) and gastroduodenal junction are protected by the lower esophageal sphincter and pyloric sphincter, respectively. The shorter right border of the stomach forms the lesser curvature and the longer left border forms the greater curvature. The stomach wall is made up of five layers: mucosal, submucosal, muscular, subserosal, and serosal. The stomach is in contact with the esophagus, duodenum, diaphragm, liver, spleen, anterior abdominal wall, transverse colon, mesocolon, left adrenal gland, left kidney, pancreas, greater omentum, and small bowel loops, and any of these structures may be involved by extension of gastric carcinoma. The arterial blood supply to the stomach is from the celiac trunk, through the left and right gastric, short gastric, and left and right gastroepiploic arteries. Venous drainage parallels the arterial supply, but enters the portal vein to the liver. Lymphatic drainage generally follows the blood supply to the stomach, and local nodes are located at the cardia, along the greater and lesser curvatures and near the pylorus. Regional nodes lie along the left gastric, common hepatic, splenic, and celiac arteries. Involvement of hepatoduodenal, portal, mesenteric, and retroperitoneal nodes is classified as distant metastases.78
Imaging of Gastric Carcinoma
Gastroscopy is the standard of reference for the diagnosis of gastric carcinoma, with a reported accuracy of more than 95%. EUS may be helpful to supplement endoscopy. CT and MRI are useful for detection of metastases in advanced disease. The double-contrast upper-gastrointestinal examination with air and barium has been largely replaced by gastroscopy, but may still be useful in selected cases. Direct endoscopy is used for areas of the stomach that require particular scrutiny. Even in Japan, where double-contrast upper-gastrointestinal examinations are still frequently performed as a first-line investigation, approximately 26% (7 of 27) of gastric cancers may be completely occult radiographically.79
Endoscopic Ultrasound
EUS provides high spatial resolution images of the gastric wall, allowing visualization of all five layers, and also allows for evaluation of perigastric nodes. In a study of 119 patients with gastric cancer undergoing preoperative EUS, the modality demonstrated an accuracy of 70% and 65% in the prediction of depth of tumor invasion and nodal involvement, respectively.80 In another study, the overall accuracy, sensitivity, and specificity of EUS in the diagnosis of lymph nodal metastases was reported as 79%, 79%, and 80%, respectively.81
Computed Tomography
CT is of limited value in the primary diagnosis of gastric cancer (Fig. 19-24). In the preoperative staging of gastric carcinoma the criteria to be assessed with CT includes extension of the tumor along the wall and adjacent areas, lymph node metastases, and distant metastases.82 The use of water as a negative contrast agent to produce gastric distension by water may assist tumor visualization and assessment. In a study of 71 patients with gastric cancer undergoing thin-section spiral CT, the sensitivity for early stage tumors was only 26% (12 of 46), although all 29 advanced tumors were seen.83 The overall T staging was correct in only 66% (27 of 41). CT is more commonly used for detection of metastatic disease. In a study of 56 patients with node-positive gastric cancer undergoing spiral CT, the sensitivity, specificity, and accuracy in the detection of liver metastases were 89%, 98%, and 96%, respectively.84 However, peritoneal dissemination was not detected in 15 of 56 patients (27%), and stage IV disease was not correctly diagnosed in 18 of 40 patients (45%).
Magnetic Resonance Imaging
MRI is rarely used to evaluate gastric carcinoma, and only a few studies have been reported. Although the high tissue contrast and multiplanar capability of MRI are potentially attractive features for imaging gastric pathologic conditions, no convincing advantage has been shown for MRI versus CT. In a study of 30 patients with gastric carcinoma undergoing preoperative MRI and CT, MRI and CT were of similar accuracy in T staging (73% and 67%, respectively). Accuracy for nodal staging was also similar for MRI and CT (55% and 59%, respectively).85
Positron Emission Tomography
The role of PET (Fig. 19-25) in the imaging evaluation of gastric cancer continues to evolve. PET has essentially no role for the detection of gastric cancer, because endoscopy is the key test for diagnosis. PET is also particularly limited in the assessment of adenopathy associated with gastric cancer, with a reported sensitivity and specificity of 23% and 100%, respectively.86 Low sensitivity is likely due to a combination of confounding peristalsis and physiologic uptake. PET may have greater value in the prediction of prognosis and the early evaluation of therapeutic response. In a study of 65 patients with gastric cancer, those with a standardized uptake value (SUV) of less than 4 (n = 31) had a significantly better prognosis than those with a SUV more than 4.86 In another study of 35 patients with stage T3 or T4 gastric cancer, those with a metabolic response (n = 13; defined as at least a 35% reduction in SUV) 14 days after starting chemotherapy had a significantly better outcome than those who did not have a metabolic response.87 PET is also helpful in detecting recurrent disease. PET had an overall accuracy of 70% for the diagnosis of recurrent disease in a Belgian study of 33 patients with suspected recurrence after curative surgery using pathologic findings or follow-up as the standard of reference.88 Interestingly, a negative PET scan in and of itself was associated with a better prognosis in this study. The mean survival after a negative PET scan was 18.5 months compared with 6.9 months in those with a positive scan.
1 Kalender WA. Technical foundations of spiral CT. Semin Ultrasound CT MR. 1994;15:81-89.
2 Liang Y, Kruger RA. Dual-slice spiral versus single-slice helical scanning: Comparison of the physical performance of two computed tomography scanners. Med Phys. 1996;23:205-220.
3 Coakley FV, Cohen MD, Waters DJ, et al. The detection of pulmonary metastases with pathological correlation in a canine model: Effect of breathing on the accuracy of spiral CT. AJR Am J Roentgenol. 1997;169:1615-1618.
4 Catalano O. Proper terminology for multiple-phase helical CT of the liver. Letter. AJR Am J Roentgenol. 2001;176:547-548.
5 Dodd GD. AJR Proper terminology for multiple-phase helical CT of the liver. Reply. AJR Am J Roentgenol. 2001;176:547-548.
6 Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241-247.
7 Tsuji T, Hiraoka T, Kanemitsu K, et al. Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 2001;129:401-407.
8 Yeo CJ, Pitt HA, Cameron JL. Cholangiocarcinoma. Surg Clin North Am. 1990;70:1429-1447.
9 Hadjis NS, Collier NA, Blumgart LH. Malignant masquerade at the hilum of the liver. Br J Surg. 1985;72:659-661.
10 Goematis B, Giannopoulos A, Papachristou DN, et al. Sclerosing cholangitis of the bifurcation of the common hepatic duct. Mt Sinai J Med. 1982;49:38-40.
11 Bloom CM, Langer B, Wilson SR. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics. 1999;19:1199-1218.
12 Robledo R, Muro A, Prieto ML. Extrahepatic bile duct carcinoma: US characteristics and accuracy in demonstration of tumors. Radiology. 1996;198:869-873.
13 Hann LE, Greatrex KV, Bach AM. Cholangiocarcinoma at the hepatic hilus: Sonographic findings. AJR Am J Roentgenol. 1997;168:985-989.
14 Smits NJ, Reeders JW. Imaging and staging of biliopancreatic malignancy: role of ultrasound. Ann Oncol. 1999;10(Suppl 4):20-24.
15 Looser C, Stain SC, Baer HU, et al. Staging of hilar cholangiocarcinoma by ultrasound and duplex sonography: a comparison with angiography and operative findings. Br J Radiol. 1992;65:871-877.
16 Sans N, Fajadet P, Galy-Fourcade D, et al. Is capsular retraction a specific CT sign of malignant liver tumor? Eur Radiol. 1999;9:1543-1545.
17 Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51.
18 Feydy A, Vilgrain V, Denys A, et al. Helical CT assessment in hilar cholangiocarcinoma: Correlation with surgical and pathologic findings. AJR Am J Roentgenol. 1999;172:73-77.
19 Keogan MT, Seabourn JT, Paulson EK, et al. Contrast-enhanced CT of intrahepatic and hilar cholangiocarcinoma: Delay time for optimal imaging. AJR Am J Roentgenol. 2006;169:1493-1499.
20 Lacomis JM, Baron RL, Oliver JH3rd, et al. Cholangiocarcinoma: delayed CT contrast enhancement patterns. Radiology. 1997;203:98-104.
21 Valls C, Guma A, Puig I, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging. 2000;25:490-496.
22 Schwartz LH, Coakley FV, Sun Y, et al. Neoplastic pancreaticobiliary duct obstruction: Evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol. 1998;170:1491-1495.
23 Hann LE, Schwartz LH, Panicek DM, et al. Tumor involvement in hepatic veins: comparison of MR imaging and US for preoperative assessment. Radiology. 1998;206:651-656.
24 Maetani Y, Itoh K, Watanabe C, et al. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. AJR Am J Roentgenol. 2001;176:1499-1507.
25 Torok N, Gores GJ. Cholangiocarcinoma. Semin Gastrointest Dis. 2001;12:125-132.
26 Kluge R, Schmidt F, Caca K, et al. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029-1035.
27 Rooholamini SA, Tehrani NS, Razavi MK, et al. Imaging of gallbladder carcinoma. Radiographics. 1994;14:291-306.
28 Moriguchi H, Tazawa J, Hayashi Y, et al. Natural history of polypoid lesions in the gall bladder. Gut. 1996;39:860-862.
29 Huang CS, Lien HH, Jeng JW, et al. Role of laparoscopic cholecystectomy in the management of polypoid lesions of the gallbladder. Surg Laparosc Endosc Percutan Tech. 2001;11:242-247.
30 Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699-703.
31 Towfigh S, McFadden DW, Cortina GR, et al. Porcelain gallbladder is not associated with gallbladder carcinoma. Am Surg. 2001;67:7-10.
32 Ueno N, Tomiyama T, Tano S, et al. Diagnosis of gallbladder carcinoma with color Doppler ultrasonography. Am J Gastroenterol. 1996;91:1647-1649.
33 Fujita N, Noda Y, Kobayashi G, et al. Diagnosis of the depth of invasion of gallbladder carcinoma by EUS. Gastrointestinal Endosc. 1999;50:659-663.
34 Mizuguchi M, Kudo S, Fukahori T, et al. Endoscopic ultrasonography for demonstrating loss of multiple-layer pattern of the thickened gallbladder wall in the preoperative diagnosis of gallbladder cancer. Eur Radiol. 1997;7:1323-1327.
35 Yoshimitsu K, Honda H, Shinozaki K, et al. Helical CT of the local spread of carcinoma of the gallbladder: Evaluation according to the TNM system in patients who underwent surgical resection. AJR Am J Roentgenol. 2002;179:423-428.
36 Ohtani T, Shirai Y, Tsukada K, et al. Spread of gallbladder carcinoma: CT evaluation with pathologic correlation. Abdom Imaging. 1996;21:195-201.
37 Schwartz LH, Black J, Fong Y, et al. Gallbladder carcinoma: Findings at MR imaging with MR cholangiopancreatography. J Comput Assist Tomogr. 2002;26:405-410.
38 Illustrated guide to the TNM/pTNM classification of malignant tumors. ed 3. 1992; Springer-Verlag, New York.
39 Gebhardt C, Meyer W, Reichel M, et al. Prognostic factors in the operative treatment of ductal pancreatic carcinoma. Langenbecks Arch Surg. 2000;385:14-20.
40 Zeman RK, Cooper C, Zeiberg AS, et al. TNM staging of pancreatic carcinoma using helical CT. AJR Am J Roentgenol. 1997;169:459-464.
41 Valinas R, Barrier A, Montravers F, et al. [18 F-fluorodeoxyglucose positron emission tomography for characterization and initial staging of pancreatic tumors]. Gastroenterol Clin Biol. 2002;26:888-992.
42 Del Frate C, Zanardi R, Mortele K, et al. Advances in imaging for pancreatic disease. Curr Gastroenterol Rep. 2002;4:140-148.
43 Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: A large single-center experience. Gastrointest Endosc. 1999;50:786-791.
44 Ahmad NA, Kochman ML, Lewis JD, et al. Endosonography is superior to angiography in the preoperative assessment of vascular involvement among patients with pancreatic carcinoma. J Clin Gastroenterol. 2001;32:54-58.
45 Wiersema MJ. Accuracy of endoscopic ultrasound in diagnosing and staging pancreatic carcinoma. Pancreatology. 2001;1:625-632.
46 Valls C, Andía E, Sanchez A, et al. Dual-phase helical CT of pancreatic adenocarcinoma assessment of resectability before surgery. AJR Am J Roentgenol. 2002;178:821-826.
47 Lu DSK, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: Criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439-1443.
48 Raptopoulos V, Steer ML, Sheiman RG, et al. The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: correlation with findings at surgery. AJR Am J Roentgenol. 1997;168:971-977.
49 Lu DS, Vedantham S, Krasny RM, et al. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697-701.
50 O’Malley ME, Boland GW, Saez M, et al. Pancreatic-phase versus portal vein-phase helical CT of the pancreas: optimal temporal window for evaluation of pancreatic adenocarcinoma. AJR Am J Roentgenol. 1999;172:605-608.
51 Graf O, Boland GW, Warshaw AL, et al. Arterial versus portal venous helical CT for revealing pancreatic adenocarcinoma: conspicuity of tumor and critical vascular anatomy. AJR Am J Roentgenol. 1997;169:119-123.
52 Keogan MT, McDermott VG, Paulson EK, et al. Pancreatic malignancy: effect of dual-phase helical CT in tumor detection and vascular opacification. Radiology. 1997;205:513-518.
53 Hochwald SN, Harrison LE, Blumgart LH, et al. A preoperative biliary stent is associated with increased complications after pancreaticoduodenectomy. Arch Surg. 1998;133:149-154.
54 Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261-266.
55 Bilbao MK, Dotter CT, Lee TG, et al. Complications of retrograde cholangiography (ERCP): a study of 10,000 cases. Gastroenterology. 1976;70:314-320.
56 Harbin WP, Meuller P, Ferrucci JT. Transhepatic cholangiography: complications and use pattern of the fine-needle technique. Radiology. 1980;135:15-22.
57 Adamek HE, Albert J. Pancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356:190-193.
58 Mertz HR, Sechopoulos P, Delbeke D, et al. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367-371.
59 Midwinter MJ, Beveridge CJ, Wilsdon JB, et al. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg. 1999;86:189-193.
60 Schima W, Függer R, Schober E, et al. Diagnosis and staging of pancreatic cancer: Comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol. 2002;179:717-724.
61 Sheridan MB, Ward J, Guthrie JA, et al. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: A comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol. 1999;173:583-590.
62 Stollfuss JC, Glatting G, Friess H, et al. 2-(fluorine-18)-fluoro-2-deoxy-D-glucose PET in detection of pancreatic cancer: Value of quantitative image interpretation. Radiology. 1995;195:339-344.
63 Delbeke D, Rose DM, Chapman WC, et al. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999;40:1784-1791.
64 Glazer HS, Lee JKT, Melson GL, et al. Sonographic detection of occult testicular neoplasms. AJR Am J Roentgenol. 1991;138:673-675.
65 Gross GW, Rohner TJ, Lombard JS, et al. Metastatic seminoma with regression of testicular primary: Ultrasonographic detection. J Urol. 1986;136:1086-1088.
66 Imaging of retroperitoneal adenopathy. In: Hricak H, Hamm B, Kim B, editors. Imaging of the scrotum: textbook and atlas. New York: Raven Press; 1995:49-93.
67 Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: Assessment of size and distribution criteria. AJR Am J Roentgenol. 1997;169:521-525.
68 Hirahatake K, Hareyama H, Sakuragi N, et al. A clinical and pathologic study on para-aortic lymph node metastasis in endometrial carcinoma. J Surg Oncol. 1997;65:82-87.
69 Connor JP, Andrews JI, Anderson B, et al. Computed tomography in endometrial carcinoma. Obstet Gynecol. 2000;95:692-696.
70 Forstner R, Hricak H, Occhipinti KA, et al. Ovarian cancer: staging with CT and MR imaging. Radiology. 1995;197:619-626.
71 Studer UE, Scherz S, Scheidegger J, et al. Enlargement of regional lymph nodes in renal cell carcinoma is often not due to metastases. J Urol. 1990;144:243-245.
72 Grubnic S, Vinnicombe SJ, Norman AR, et al. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin Radiol. 2002;57:193-200.
73 Vesselle HJ, Miraldi FD. FDG PET of the retroperitoneum: normal anatomy, variants, pathologic conditions, and strategies to avoid diagnostic pitfalls. Radiographics. 1998;18:805-823.
74 Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745-3749.
75 Scheidler J, Hricak H, Yu KK, et al. Radiological evaluation of lymph node metastases in patients with cervical cancer, a meta-analysis. JAMA. 1997;278:1096-1101.
76 Williams MP, Husband JE. Computed tomography scanning and post-lymphangiogram radiography in the follow-up of patients with metastatic testicular cancer. Clin Radiol. 1989;40:47-50.
77 Fleming ID, Cooper JS, Henson DE, et al, editors. Manual for staging of cancer, American Joint Committee on Cancer, ed 5, Philadelphia: J.B. Lippincott, 1997.
78 Shindoh N, Nakagawa T, Ozaki Y, et al. Overlooked gastric carcinoma: pitfalls in upper gastrointestinal radiology. Radiology. 2000;217:409-414.
79 Wang JY, Hsieh JS, Huang YS, et al. Endoscopic ultrasonography for preoperative locoregional staging and assessment of resectability in gastric cancer. Clin Imaging. 1998;22:355-359.
80 Chen CH, Yang CC, Yeh YH. Preoperative staging of gastric cancer by endoscopic ultrasound: the prognostic usefulness of ascites detected by endoscopic ultrasound. J Clin Gastroenterol. 2002;35:321-327.
81 Angelelli G, Ianora AA, Scardapane A, et al. Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol. 2001;20:109-121.
82 Fukuya T, Honda H, Kaneko K, et al. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997;21:73-81.
83 Adachi Y, Sakino I, Matsumata T, et al. Preoperative assessment of advanced gastric carcinoma using computed tomography. Am J Gastroenterol. 1997;92:872-875.
84 Sohn KM, Lee JM, Lee SY, et al. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol. 2000;174:1551-1557.
85 Kim AY, Han JK, Seong CK. MRI in staging advanced gastric cancer: Is it useful compared with spiral CT? J Comput Assist Tomogr. 2000;24:389-394.
86 Mochiki E, Kuwano H, Katoh H, et al. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247-253.
87 Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604-4610.
88 Stahl A, Ott K, Weber WA, et al. Correlation of FDG uptake in gastric carcinomas with endoscopic and histopathological findings. J Nucl Med. 2001;42:78P-79P.