External and Intravascular Warming/Cooling Devices
An external surface or hydrogel pad temperature management device may be used to increase or decrease the body temperature. An intravascular warming and cooling device may be inserted to increase and decrease the body temperature. External surface cooling devices or intravascular cooling catheters are also used as a therapeutic treatment modality to reduce the body temperature after acute injury (such as cardiac arrest) to decrease cellular metabolism.19
PREREQUISITE NURSING KNOWLEDGE
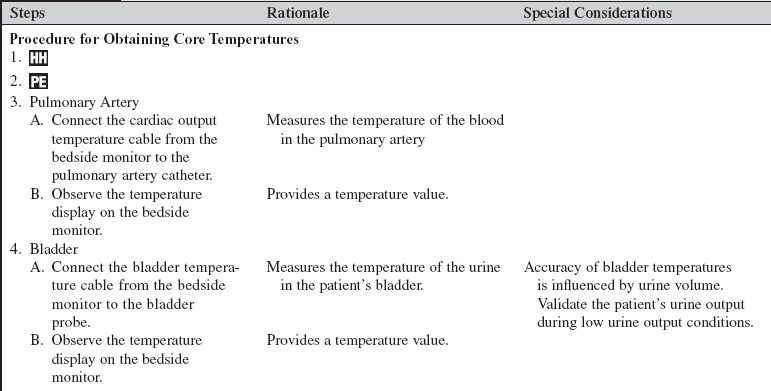
• The hypothalamus is the primary thermoregulatory center for the body; it maintains normothermia through internal regulation of heat production or heat loss. Sensory thermoreceptors are located in the skin and subcutaneous tissue. Superficial or shell-zone temperature information is transmitted by thermoreceptors to the posterior hypothalamus through the spinal cord. Thermoreceptors in the brain, heart, and other deep organs transmit the core-zone temperature. Effective temperature regulation depends on the ability of the posterior hypothalamus to receive and integrate the signals received from the core and shell zones.9,12,22
• Knowledge of terms associated with temperature is needed (Table 94-1).
Table 94-1
Terms Associated with Temperature
| Term | Definition |
| Normothermia/Euthermia | Optimal range of body temperature associated with health. |
| Hypothermia | Subnormal core body temperature equal to or below 35°C.21 |
| Induced hypothermia | Intentional reduction of body temperature to slow metabolic processes. This may be accomplished by surface means (transfer of heat from the skin to the cooling device) or central means (circulatory heat exchange in a cardiopulmonary bypass machine or cooling catheter) |
| Fever | Response to a pyrogen; the hypothalamus either resets its range higher, maintaining thermoregulation, or a change occurs in the sensitivity of the hypothalamus neuron activity to warmth and coldness.4 |
| Hyperthermia | Dysfunction of thermoregulation caused by an injury to the hypothalamus or by a person’s heat loss mechanisms being overwhelmed by high environmental heat. |
• The hypothalamus regulates temperature in the range of approximately 36.4° to 37.3°C (97.5° to 99.4°F). By initiating physiologic responses to changes above or below this range, the hypothalamus coordinates heat loss or gain. Vasoconstriction and vasodilation control the distribution and flow of blood to the organs, viscera, and skin surface; thus, the amount of heat loss to the environment is influenced by vasomotor activity. In response to heat loss, shivering and vasoconstriction occur, muscles tense and the extremities are drawn closer to the body, and the person conserves heat. In response to heat gain, sweating and vasodilation occur, muscles relax, and heat is lost through evaporative cooling and to the environment.12
• Heat flows from a higher temperature to a lower temperature until the gradient between the two temperatures diminishes. Mechanisms of heat loss include conduction, convection, radiation, and evaporation.5,9,12,22
 Conduction occurs when heat is lost by direct transfer from one surface to a second adjacent surface of a lower temperature.5,9,12,22
Conduction occurs when heat is lost by direct transfer from one surface to a second adjacent surface of a lower temperature.5,9,12,22
 Convection occurs when heat is lost by transfer from a surface to the surrounding air.5,9,12,22
Convection occurs when heat is lost by transfer from a surface to the surrounding air.5,9,12,22
 Radiation occurs when heat (thermal energy) is transferred through air or space between separated surfaces without direct contact.9,12,22
Radiation occurs when heat (thermal energy) is transferred through air or space between separated surfaces without direct contact.9,12,22
 Evaporation occurs when heat loss accompanies water loss from the skin to the surrounding air.9,12,22
Evaporation occurs when heat loss accompanies water loss from the skin to the surrounding air.9,12,22
• Alteration in thermoregulation can result from a primary central nervous system injury or disease (e.g., subarachnoid hemorrhage, traumatic brain injury, spinal cord injury, or neoplasm) and metabolic conditions (e.g., diabetes mellitus; toxic levels of ethanol alcohol or other drugs, such as barbiturates and phenothiazines).
• Body temperature is the measurement of the presence or absence of heat. Body heat is generated, conserved, redistributed, or dissipated during all physiologic processes. Factors such as age, circadian rhythm, and hormones influence body temperature.
• Body temperature may be measured with a variety of thermometers and at several body sites. Electronic or digital thermometers are used to obtain rectal, oral, and axillary temperatures. Thermistors within catheters or probes measure rectal, nasopharyngeal, esophageal, bladder, brain, and pulmonary artery temperatures. Infrared thermometers measure tympanic membrane and temporal artery temperatures. Choose the method of temperature monitoring that best meets the patient’s clinical condition. The most accurate temperature monitoring methods are intravascular (e.g., pulmonary artery catheter), esophageal, and bladder, followed by rectal, oral, and tympanic membrane methods.9,13,21
• Variations in temperatures normally occur in the body (Table 94-2).
Table 94-2
Normal Variations in Body Temperature Based on a Rectal Temperature of 37°C
| Type of Temperature Measurement | Degrees Lower Than Rectal Temperature |
| Oral | 0.3° to 0.5°C8 |
| Esophageal | 0.2°C8 |
| Pulmonary artery | 0.2° to 0.3°C8 |
| Tympanic membrane | 0.05° to 0.25°C8 |
| Bladder | 0.1° to 0.2°C8 |
| Axillary | 0.6° to 0.8°C 8 |
| Brain | 0.3° to 2°C higher than rectal17 |
| Temporal artery | >0.5° to <1.0°C different than pulmonary artery catheter9,12 |
• Site choice for temperature monitoring is based on the clinical data needed; the patient’s condition, safety, and comfort; environmental factors (e.g., room temperature); the indication for a catheter or a probe (e.g., pulmonary artery catheter); and the availability of equipment.
• An esophageal temperature probe can be inserted down the esophageal tract. Accurate placement of the temperature probe is necessary to obtain results similar to monitoring the temperature from the pulmonary artery.
• A consistent temperature site must be monitored during the application of warming or cooling therapy.
• Shivering is an involuntary shaking of the body generated to maintain thermal homeostasis. Shivering causes rhythmic tremors that result in skeletal muscle contraction and is a normal physiologic mechanism to generate heat production.4,11
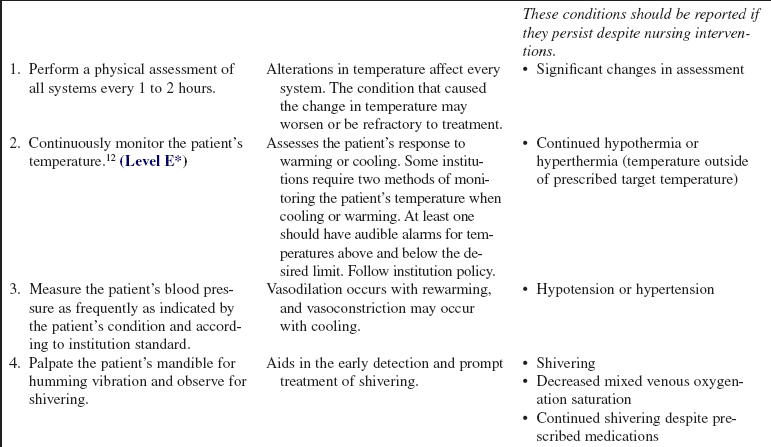
• Early detection of shivering can be accomplished by palpating the mandible and feeling a humming vibration. Electrocardiographic (ECG) artifact from skeletal muscle is seen on the bedside monitor. If not detected early, shivering can progress from visible twitching of the head or neck to visible twitching of the pectorals or trunk, and then to generalized shaking of the entire body and teeth chattering.
• Shivering may be visible on the Bispectral Index Monitor (BIS) in the form of an increase in EMG activity (see Procedure 86).
• Shivering increases the metabolic rate, carbon dioxide (CO2) production, oxygen consumption (by 40% to 100%),22 and myocardial work. If cardiopulmonary compensation does not occur to meet these demands, anaerobic metabolism occurs, resulting in acidosis.11,17,22
• Shivering is counterproductive to strategies intended to lower temperature.
• At a body temperature below 35°C, the basal metabolic rate can no longer supply sufficient body heat and an exogenous source of heat is needed.
• Table 94-3 outlines techniques to increase heat gain.
Table 94-3
Techniques to Increase Heat Gain
| Mechanism of Heat Transfer | Techniques to Increase Heat Gain |
| Radiation | Warming lights, warm environment, room temperature, blankets |
| Conduction | Warm blankets, circulating water blanket, continuous arteriovenous rewarming, cardiopulmonary bypass |
| Convection | Thermal fans, circulating air blanket |
| Evaporation | Head and body covers; warm, humidified oxygen |
• Hypothermia may be categorized as mild (32° to 35°C), moderate (28° to 31.9°C), severe (<28°C), or profound (<16.9°C).3 In severe to profound hypothermia, attempts at defibrillation are usually unsuccessful until the core temperature is above 28°C. The American Heart Association recommends only one attempt at defibrillation and then active rewarming should occur before reattempts at defibrillation.1
• Hypothermia may be caused by an increase in heat loss, a decrease in heat production, an alteration in thermoregulation, and a variety of clinical conditions.
• An increase in heat loss may occur from the following:
 Accidental (e.g., cold water drowning)
Accidental (e.g., cold water drowning)
 Induced vasodilation caused by high levels of ethanol alcohol, barbiturates, phenothiazines, or general anesthesia
Induced vasodilation caused by high levels of ethanol alcohol, barbiturates, phenothiazines, or general anesthesia
 Central nervous system dysfunction (e.g., spinal cord injury)
Central nervous system dysfunction (e.g., spinal cord injury)
 Dermal dysfunction (e.g., burns)
Dermal dysfunction (e.g., burns)
 Iatrogenic conditions (e.g., administration of cold intravenous fluids, hemodialysis, cardiopulmonary bypass)
Iatrogenic conditions (e.g., administration of cold intravenous fluids, hemodialysis, cardiopulmonary bypass)
• A decrease in heat production is associated with the following:
 Endocrine conditions (e.g., hypothyroidism)
Endocrine conditions (e.g., hypothyroidism)
 Neuromuscular insufficiency (e.g., resulting from a pharmacologic paralysis caused by a neuromuscular blocking agent or anesthetic agents)
Neuromuscular insufficiency (e.g., resulting from a pharmacologic paralysis caused by a neuromuscular blocking agent or anesthetic agents)
• Clinical conditions associated with hypothermia are sepsis, hepatic coma, prolonged cardiac arrest, and systemic inflammatory response syndrome (SIRS).
• Severe hypothermia may mimic death; resuscitative efforts should be initiated despite the absence of vital signs.
• Rewarming for cardiac arrest survivors who have undergone therapeutic hypothermia should not occur faster than 0.25° to 0.50°C per hour.20 Rapid rewarming can cause rewarming acidosis, electrolyte shifts, shivering, hypovolemic shock, temperature afterdrop, and temperature overshoot.9,16
• Afterdrop is a decrease in core temperature after rewarming is discontinued.
• Overshoot occurs when the thermoregulator mechanisms rebound or overcompensate.
• Termination of active external rewarming at 36° to 36.5°C may prevent temperature overshoot.
• Rewarming acidosis results from the increase in CO2 production associated with the temperature increase and from the return of accumulated acids in the peripheral circulation to the heart.
• Rewarming shock occurs when hypothermic vasoconstriction masks hypovolemia. If the patient’s circulating volume is insufficient during rewarming vasodilation, sudden decreases in blood pressure, systemic vascular resistance (SVR), and preload occur. In cases of severe to profound hypothermia, peripheral rewarming with external devices should be used with extreme caution. Core methods of rewarming should be considered.
• Hyperthermia occurs when the thermoregulator system of the body absorbs or produces more heat than it is able to release.
• Malignant hyperthermia is a rare, hereditary condition of the skeletal muscle that occurs on exposure to a triggering agent or agents.2,15 The triggering agents most commonly associated with malignant hyperthermia are anesthetic agents, particularly inhalation anesthetics and succinylcholine. Malignant hyperthermia involves instability of the muscle cell membrane, which causes a sudden increase in myoplasmic calcium and skeletal muscle contractures.
• The earliest indication of malignant hyperthermia is an increase in end-tidal carbon dioxide (Petco2) of 5 mm Hg more than the patient’s baseline. If the Petco2 is not being monitored, the earliest sign is tachycardia, which occurs within 30 minutes of anesthesia induction. Tachycardia is followed by ventricular ectopy, which may progress to ventricular tachycardia and ventricular fibrillation. Muscle rigidity usually begins in the extremities, chest, or jaws.2,15
• A cooling device is used to treat malignant hyperthermia after administration of the triggering agent is stopped and a muscle relaxant (e.g., dantrolene sodium) is given. The muscle relaxant blocks the release of calcium from the sarcoplasmic reticulum without affecting calcium uptake.15
• Heat stroke occurs when the outdoor temperature and humidity are excessive and heat is transferred to the body. Increased humidity prevents the body from cooling by evaporation. Other signs of heat stroke include hypotension, tachycardia, tachypnea, mental status changes from confusion to coma, and possibly seizures. The skin is hot and dry, and sweating may occur. The rectal temperature is greater than 40.0° to 41.1°C (104°F to 106°F). Initial interventions include support of airway, breathing, and circulation. Rapid cooling of the patient is the main treatment priority, with a goal of reducing the temperature to 38.3° to 38.9°C (101° to 102°F) within 1 hour.
• Fever occurs in response to a pyrogen and is defined as a temperature more than 38°C.21 During fever, the hypothalamus retains its function, and shivering and diaphoresis occur to gain or lose body heat. Fever may be an adaptive response and may be considered beneficial in the absence of neurologic disease processes. However, a febrile state increases the heart rate and metabolic rate and may be detrimental to a critically ill patient. The decision to reduce a fever needs to be based on the patient’s physical and hemodynamic stability during the fever.7,21
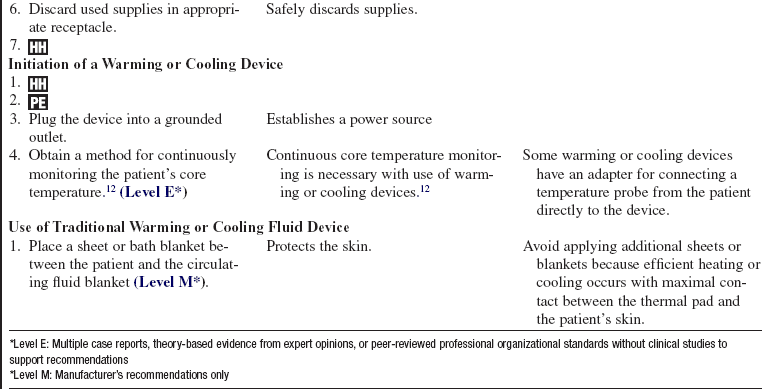
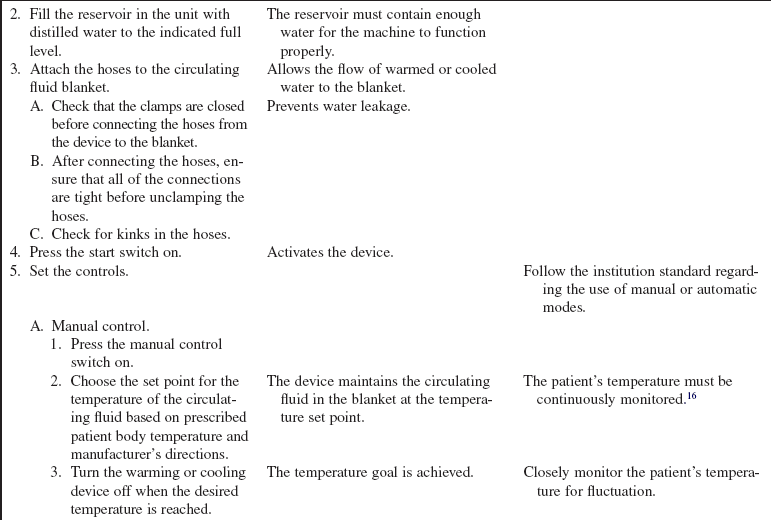
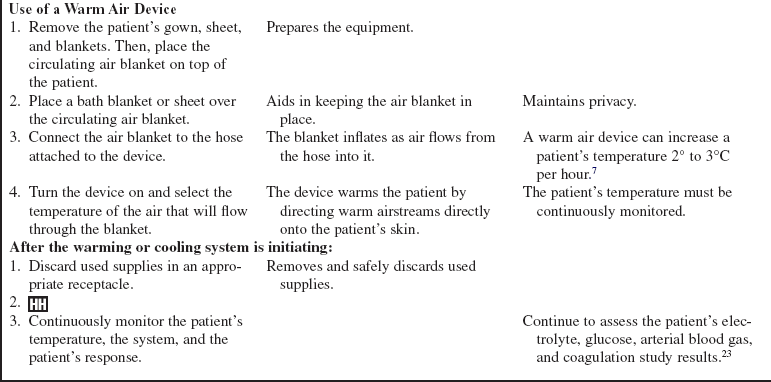
• Some external warming or cooling devices transfer warmth or coolness to the patient via conduction. Warmed or cooled fluids circulate through coils or channels in a thermal blanket or pad that is commonly placed under the patient.
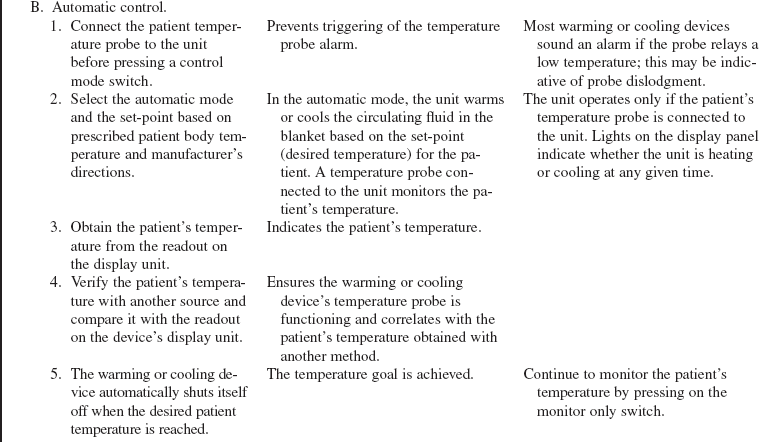
• Additional warming and cooling systems are available. Hydrogel pads or external wraps can be placed on the patient’s skin in the trunk and upper leg regions. These external systems are controlled through a feedback loop system with a core temperature (e.g., a bladder probe, an esophageal probe) that is attached to a central console and automatically regulates temperature according to programmed temperature target points. The feedback of patient temperature is compared to the set target temperature and the circulating water temperature is adjusted to ensure the target temperature is maintained.
• Other external devices transfer warmth to the patient via convection. A device used for warming blows warm air through microperforations on the underside of a blanket that is placed over the patient. The air is directed through the blanket onto the patient’s skin.3,12
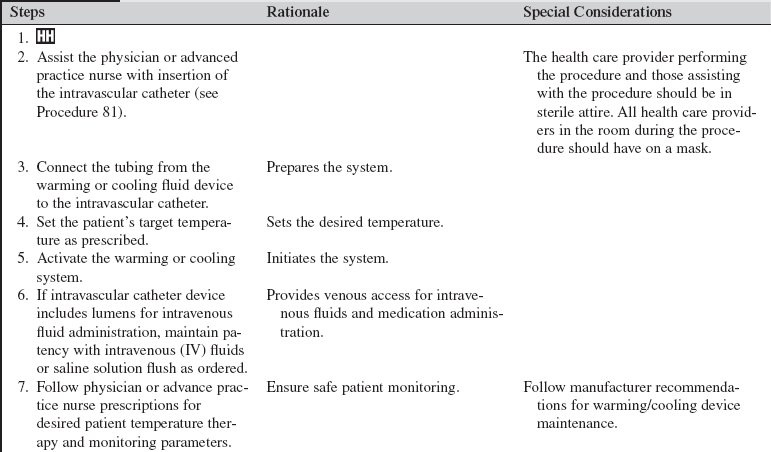
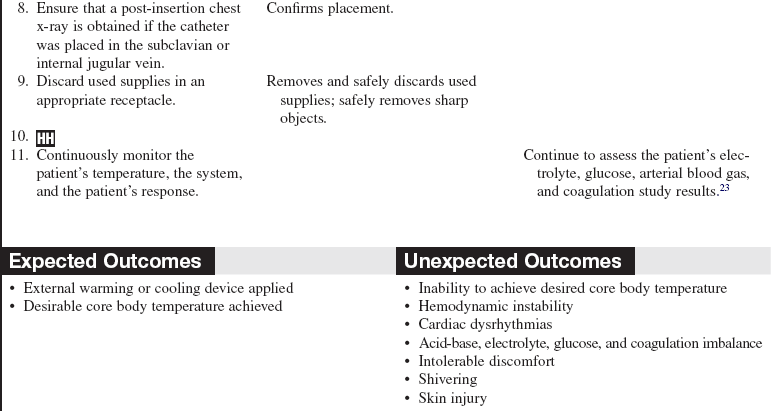
• Intravascular cooling and warming devices currently in use include central venous catheters with temperature-controlled saline solution balloons or distal metallic heat transfer elements that cool the blood as it flows by the catheter. The saline solution is not in direct contact with the systemic circulation. These devices may be inserted in the subclavian, internal jugular, or femoral vein.24 They are attached to a console with an automatic temperature control device that adjusts the pressure, temperature, and flow rate of the circulating saline solution based on the patient’s continuously monitored temperature (e.g., rectal, bladder, esophageal) and the set-points established by the healthcare provider).22
• Specific information about controls, alarms, troubleshooting, and safety features is available from each manufacturer and must be understood by the nurse before using the equipment.
EQUIPMENT
• Temperature probe, cable, and module to monitor the patient’s temperature (varies based on the type of site and thermometer selected and available)
• Pads or blankets needed by the equipment which is going to be used
Additional equipment to have available as needed includes the following:
PATIENT AND FAMILY EDUCATION
• Explain the reason for the use of a warming or cooling device and standard of care, including monitoring of the temperature, expected length of therapy, comfort measures, and parameters for discontinuation of the device.  Rationale: Explanation encourages the patient and family to ask questions and verbalize concerns about the procedure.
Rationale: Explanation encourages the patient and family to ask questions and verbalize concerns about the procedure.
• Assess the patient’s and family’s understanding of the warming or cooling therapy.  Rationale: Clarification and reinforcement of information are needed during times of stress and anxiety.
Rationale: Clarification and reinforcement of information are needed during times of stress and anxiety.
• Encourage the patient to notify the nurse of any discomfort. If the patient is unable to verbalize discomfort, look for signs and symptoms of discomfort such as grimacing, changes in heart rate, diaphoresis, etc.  Rationale: Identification of discomfort facilitates early intervention and promotes comfort.
Rationale: Identification of discomfort facilitates early intervention and promotes comfort.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess risk factors, medical history, the cause of the patient’s underlying condition, and the type and the length of temperature exposure.  Rationale: Assessment assists in anticipating, recognizing, and responding to the patient’s responses and potential side effects to therapy.
Rationale: Assessment assists in anticipating, recognizing, and responding to the patient’s responses and potential side effects to therapy.
• Assess the patient’s medication therapy.  Rationale: Medications such as vasopressors and vasodilators may affect heat transfer, increase the potential for skin injury, and contribute to an adverse hemodynamic response.
Rationale: Medications such as vasopressors and vasodilators may affect heat transfer, increase the potential for skin injury, and contribute to an adverse hemodynamic response.
• Obtain a core temperature (e.g., pulmonary artery, esophageal, bladder).  Rationale: Assessment determines baseline temperature and determines when a warming or cooling device is needed.
Rationale: Assessment determines baseline temperature and determines when a warming or cooling device is needed.
• Obtain vital signs and hemodynamic values (if using pulmonary artery monitoring).  Rationale: Assessment determines baseline cardiovascular data. Initially, cold temperatures activate the sympathetic nervous system, resulting in tachycardia, vasoconstriction, and shivering. Rewarming may result in vasodilation and hypotension. Heart failure may occur with malignant hyperthermia and heat stroke.
Rationale: Assessment determines baseline cardiovascular data. Initially, cold temperatures activate the sympathetic nervous system, resulting in tachycardia, vasoconstriction, and shivering. Rewarming may result in vasodilation and hypotension. Heart failure may occur with malignant hyperthermia and heat stroke.
• Monitor the patient’s cardiac rhythm.  Rationale: Monitoring determines the baseline cardiac rhythm. Hypothermia has a negative chronotropic effect on myocardial pacemaker tissue, which may lead to bradycardia. Hypothermia may cause repolarization abnormalities and is susceptible to atrial and ventricular fibrillation.3 Tachycardia and ventricular dysrhythmias may occur if the patient is hyperthermic.3,6,22
Rationale: Monitoring determines the baseline cardiac rhythm. Hypothermia has a negative chronotropic effect on myocardial pacemaker tissue, which may lead to bradycardia. Hypothermia may cause repolarization abnormalities and is susceptible to atrial and ventricular fibrillation.3 Tachycardia and ventricular dysrhythmias may occur if the patient is hyperthermic.3,6,22
• Assess the patient’s electrolyte, glucose, arterial blood gas, and coagulation study results.  Rationale: Alterations in temperature balance may result in acid-base imbalance, coagulopathy, electrolyte imbalance, glycemic imbalance, and hypoxemia.3,6,22,23 Close monitoring of metabolic parameters with careful consideration of replacement during cooling and warming therapy is necessary.22,23
Rationale: Alterations in temperature balance may result in acid-base imbalance, coagulopathy, electrolyte imbalance, glycemic imbalance, and hypoxemia.3,6,22,23 Close monitoring of metabolic parameters with careful consideration of replacement during cooling and warming therapy is necessary.22,23
• Assess the patient’s level of consciousness and neurologic function.  Rationale: Assessment determines baseline neurologic status. A change in mental status, level of consciousness, or impaired neurologic function may occur because of an undesirable high or low temperature or from the condition causing the alteration in temperature. Fatigue, muscle incoordination, poor judgment, weakness, hallucinations, lethargy, and stupor may occur with hypothermia. Seizures may occur with hyperthermia.
Rationale: Assessment determines baseline neurologic status. A change in mental status, level of consciousness, or impaired neurologic function may occur because of an undesirable high or low temperature or from the condition causing the alteration in temperature. Fatigue, muscle incoordination, poor judgment, weakness, hallucinations, lethargy, and stupor may occur with hypothermia. Seizures may occur with hyperthermia.
• Assess the patient’s ventilatory function.  Rationale: Hypoventilation, suppression of cough, and mucociliary reflexes associated with hypothermia may lead to hypoxemia, atelectasis, and pneumonia. Hypothermia shifts the oxygenation dissociation curve to the left, and less oxygen is released from oxyhemoglobin to the tissues. Because of peripheral vasoconstriction, digit-based pulse oximetry is often unreliable. Hyperthermia shifts the oxygenation dissociation curve to the right, and oxygen is readily released from oxyhemoglobin.3,6,22
Rationale: Hypoventilation, suppression of cough, and mucociliary reflexes associated with hypothermia may lead to hypoxemia, atelectasis, and pneumonia. Hypothermia shifts the oxygenation dissociation curve to the left, and less oxygen is released from oxyhemoglobin to the tissues. Because of peripheral vasoconstriction, digit-based pulse oximetry is often unreliable. Hyperthermia shifts the oxygenation dissociation curve to the right, and oxygen is readily released from oxyhemoglobin.3,6,22
• Assess the patient’s bowel sounds, abdomen, and gastrointestinal function.  Rationale: Assessment determines baseline status. Patients with hypothermia may develop an ileus because of decreased intestinal motility. Vomiting and diarrhea may occur with hyperthermia.
Rationale: Assessment determines baseline status. Patients with hypothermia may develop an ileus because of decreased intestinal motility. Vomiting and diarrhea may occur with hyperthermia.
• Assess the patient’s skin integrity.  Rationale: Assessment provides baseline data. An externally applied warming or cooling device can cause or exacerbate skin injury. Preexisting conditions such as diabetes and peripheral vascular disease increase the patient’s risk for skin injury.
Rationale: Assessment provides baseline data. An externally applied warming or cooling device can cause or exacerbate skin injury. Preexisting conditions such as diabetes and peripheral vascular disease increase the patient’s risk for skin injury.
Patient Preparation
• Ensure that the patient and family understand preprocedural education. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• If a warm air device will be used, remove the patient’s gown and top sheet.  Rationale: The warm air device works via convection and should be in direct contact with the patient’s skin for optimal results.
Rationale: The warm air device works via convection and should be in direct contact with the patient’s skin for optimal results.
• If the patient is unintentionally hypothermic, cover the patient’s head with a blanket or towel or an aluminum cap.  Rationale: This action minimizes additional heat loss.
Rationale: This action minimizes additional heat loss.
• Ensure that informed consent has been obtained for insertion of intravascular catheters.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient; however, in emergency circumstances, time may not allow for the consent form to be signed.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient; however, in emergency circumstances, time may not allow for the consent form to be signed.
• Perform a pre-procedure verification and time out with placement of intravascular catheters, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
References
![]() 1. American Heart Association. 2005 Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulaton. 2005; 112(Suppl 24):1–203. [IV].
1. American Heart Association. 2005 Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulaton. 2005; 112(Suppl 24):1–203. [IV].
2. Anderson-Pompa K, Foster, A, Parker, L, et al. Genetics and susceptibility to malignant hyperthermia. Crit Care Nurse. 2008; 28:32–36.
3. Aslam, AF, Aslam, AK, Vasavada, BC, et al, Hypothermia. evaluation, electrocardiographic manifestations and management. Am J Med 2006; 119:297–301.
4. Biddle, C. The neurobiology of the human febrile response. AANA J. 2006; 74:145–150.
![]() 5. Creechan, T, Vollman, K, Kravutske, ME. Cooling by convection vs cooling by conduction for treatment of fever in critically ill adults. Am J Crit Care. 2001; 10:52–59.
5. Creechan, T, Vollman, K, Kravutske, ME. Cooling by convection vs cooling by conduction for treatment of fever in critically ill adults. Am J Crit Care. 2001; 10:52–59.
![]() 6. Gentilello, L, Moujaes, S, Treatment of hypothermia in trauma victims. thermodynamic considerations. J Intensive Care Med 1995; 10:5–14.
6. Gentilello, L, Moujaes, S, Treatment of hypothermia in trauma victims. thermodynamic considerations. J Intensive Care Med 1995; 10:5–14.
![]() 7. Goheen, MSL, Ducharme, MB, Kenny, GP, et al. Efficacy of forced-air and inhalation rewarming by using a human model for severe hypothermia. J Appl Physiol. 1997; 83:1635–1640.
7. Goheen, MSL, Ducharme, MB, Kenny, GP, et al. Efficacy of forced-air and inhalation rewarming by using a human model for severe hypothermia. J Appl Physiol. 1997; 83:1635–1640.
![]() 8. Henker, R, Shaver, J. Understanding the febrile state according to an individual adaptation framework. AACN Clin Issues Crit Care Nurs. 1994; 2:186–193.
8. Henker, R, Shaver, J. Understanding the febrile state according to an individual adaptation framework. AACN Clin Issues Crit Care Nurs. 1994; 2:186–193.
![]() 9. Holtzclaw, B. Monitoring body temperature. AACN Clin Issues Crit Care Nurs. 1993; 4:44–55.
9. Holtzclaw, B. Monitoring body temperature. AACN Clin Issues Crit Care Nurs. 1993; 4:44–55.
10. Hooper, VD, Andrews, JO, Accuracy of noninvasive core temperature measurement in acutely ill adults. the state of the science. Biol Res Nurs 2006; 8:24–34.
![]() 11. Iampietro, P, Vaughn, JA, Goldman, RF, et al. Heat production from shivering. J Appl Physiol. 1960; 15:632–634.
11. Iampietro, P, Vaughn, JA, Goldman, RF, et al. Heat production from shivering. J Appl Physiol. 1960; 15:632–634.
12. Keresztes, PA, Brick, K. Therapeutic hypothermia after cardiac arrest. Dimens Crit Care Nurs. 2006; 25:71–76.
13. Lawson, L, Bridges, EJ, Ballou, I, et al. Accuracy and precision of noninvasive temperature measurement in adult intensive care patients. Am J Crit Care. 2007; 16:485–496.
![]() 14. Lefrant, JY, Muller, L, de La Coussaye JE, et al, Temperature measurement in intensive care patients. comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med 2003; 29:414–418.
14. Lefrant, JY, Muller, L, de La Coussaye JE, et al, Temperature measurement in intensive care patients. comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med 2003; 29:414–418.
15. Litman, RS, Flood, CD, Kaplan, RF, et al, Postoperative malignant hyperthermia. an analysis of cases from the North American Malignant Hyperthermia Registry. Anesthesiology 2008; 109:825–829.
![]() 16. Loke, AY, Chan, HC, Chan, TM. Comparing the effectiveness of two types of cooling blankets for febrile patients. Nurs Crit Care. 2005; 10:247–254.
16. Loke, AY, Chan, HC, Chan, TM. Comparing the effectiveness of two types of cooling blankets for febrile patients. Nurs Crit Care. 2005; 10:247–254.
17. Mahmood, MA, Zweifler, RM. Progress in shivering control. J Neurol Sci. 2007; 261:47–54.
![]() 18. McIlvoy, L, Comparison of brain temperature to core temperature. a review of the literature. J Neurosci Nurs 2004; 36:23–31.
18. McIlvoy, L, Comparison of brain temperature to core temperature. a review of the literature. J Neurosci Nurs 2004; 36:23–31.
19. Moore, K, Hypothermia in trauma. J Trauma Nurs 2008; 15 :62–64.
20. Neumar, RW, Nolan, JP, Adrie, C, et al, Post cardiac arrest syndrome. epidemiology, pathophysiology, treatment, and prognosticationa consensus statement from the International Liaison Committee on Resuscitation. Circulation 2008; 118:2452–2483.
21. O’Grady, NP, Barie, PS, Bartlett, JG, et al, Guidelines for evaluation of new fever in critically ill adults patients. 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 2008; 36:1330–1349.
![]() 22. Polderman, KH, Application of therapeutic hypothermia in the ICU. opportunities and pitfalls of a promising treatment modality: part 2: practical aspects and side effects. Intensive Care Med 2004; 30:757–769.
22. Polderman, KH, Application of therapeutic hypothermia in the ICU. opportunities and pitfalls of a promising treatment modality: part 2: practical aspects and side effects. Intensive Care Med 2004; 30:757–769.
23. Polderman, K. H, Ingeborg, H., Therapeutic hypothermia and controlled normothermia in the intensive care unit. Practical considerations, side effects, and cooling methods. Critical Care Medicine, 2009; 37:1101–1119.
24. Simosa, HF, Petersen, DJ, Agarwal, SK, et al. Increased risk of deep venous thrombosis with endovascular cooling in patients with traumatic brain injury. Am Surg. 2007; 73:461–464.
25. Zoll Medical Corporation (2009), Intravascular Temperature Management. Products/Catheter Family, 2009. http://www.alsius.com/products/catheters.html [Text available from].