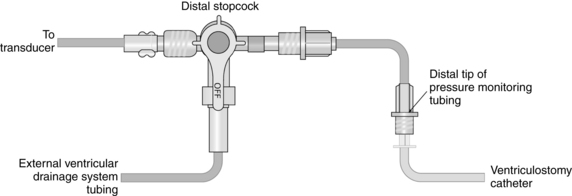
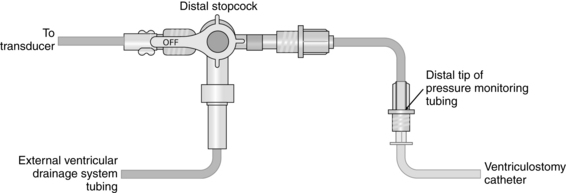
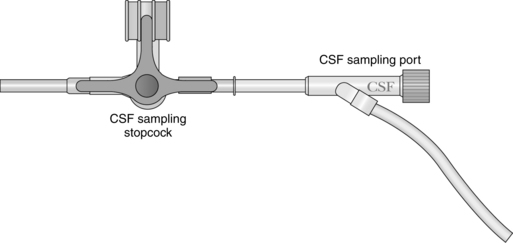
Intraventricular Catheter with External Transducer for Cerebrospinal Fluid Drainage and Intracranial Pressure Monitoring
PREREQUISITE NURSING KNOWLEDGE
• Knowledge of neuroanatomy and physiology is needed.
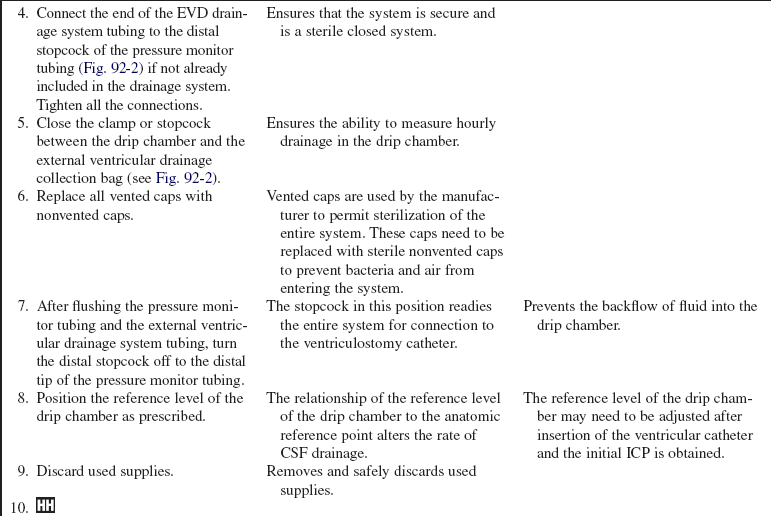
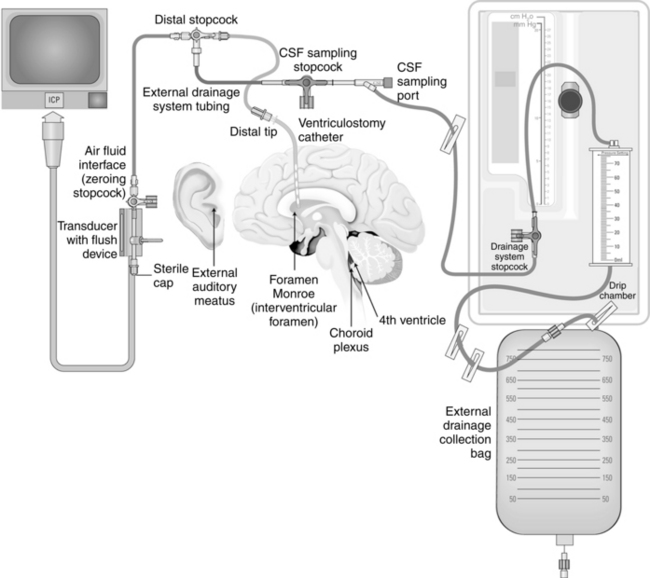
• Understanding is needed regarding the assembly and maintenance of the intraventricular catheter with an external transducer and drainage system, care of the insertion site, and drainage techniques.
• Principles of aseptic technique should be understood. Of all the intracranial pressure monitoring devices, external ventricular drains (EVDs) have the greatest risk of infection.3,6
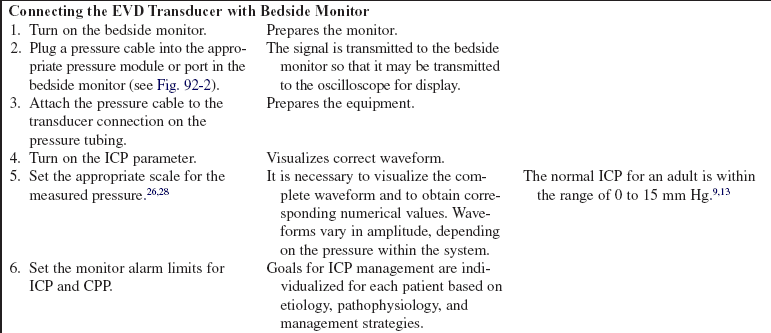
• The normal range for intracranial pressure (ICP) is 0 to 15 mm Hg.3,25,28 This measurement reflects the pressure exerted by the intracranial contents within the skull, including brain, blood, and cerebrospinal fluid.25
• Cerebral perfusion pressure (CPP) is a derived mathematic calculation that indirectly reflects the adequacy of cerebral blood flow. The CPP is calculated by subtracting the ICP from the mean arterial pressure (MAP); thus, CPP = MAP – ICP. 3,27 The normal CPP range for adults is approximately 60 to 100 mm Hg21 or a mean of 80 mm Hg.19,28 The optimal CPP for a given patient and clinical condition is not entirely known. ICP and CPP should be managed concomitantly. According to the Brain Trauma Foundation Guidelines, an acceptable CPP for an adult with a severe traumatic brain injury (Glasgow Coma Scale [GCS] score of equal to or less than 8) lies between 50 and 70 mm Hg.6 Patients with aneurysmal subarachnoid hemorrhage vasospasm may need higher CPPs to maintain adequate perfusion through vasospastic cerebral blood vessels.2 Patients with other neurologic injuries require individualized CPP parameters reflective of the neuropathology and brain perfusion needs.
• Elevations in ICP result when one or more intracranial components—blood, cerebrospinal fluid (CSF), or brain tissue—increase without an accompanying decrease in one or two of the other intracranial components. This is known as the Monro-Kellie doctrine or hypothesis.3,19
• Clinical conditions that frequently result in increased intracranial pressure include traumatic brain injury, subarachnoid hemorrhage,2 intraparenchymal hemorrhage,8 brain tumor, meningitis, and hydrocephalus.3,26 An EVD may be indicated in the management of intracranial pressure in each of these conditions.21
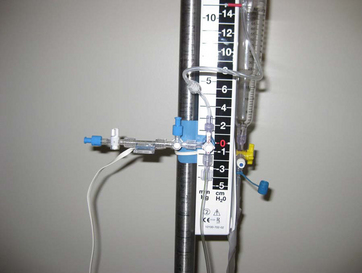
• Fiberoptic catheters and the microsensors that are placed during surgery in the surgical site or through a bolt in the skull are also used to monitor the ICP. They may be placed in the epidural, subdural, subarachnoid, ventricular, and intraparenchymal spaces.27,28 These catheters are sentinels for increased ICP but are not designed for treatment of increased ICP with CSF drainage.27,28 In contrast, when the ventricular catheter is inserted and transduced at the level of the foramen of Monro, approximately at the level of the external auditory canal, it produces a value and a waveform that reflects the ICP. The EVD is considered the most accurate ICP monitor.3,6
• CSF is formed within the lateral ventricles of the cerebral hemispheres by the choroid plexus. From the lateral ventricles, fluid drains into the foramen of Monro, the intraventricular foramina, into the third ventricle adjacent to the thalamus. Although most of the CSF is made in the choroid plexus of the lateral ventricles, the third ventricle contributes some CSF, which then passes through the aqueduct of Sylvius into the fourth ventricle at the pons and medulla. The choroid plexus in the roof of the fourth ventricle contributes an additional small amount of CSF. The fluid then enters into the subarachnoid space, with the major portion of the fluid moving through the foramen of Magendie, where it is dispersed around the spinal cord and through the foramen of Luschka, where it flows around the brain. CSF is absorbed by the arachnoid villi, also known as arachnoid granulations, where it drains into the venous system to be returned to the heart.5,7
• CSF is a clear colorless liquid of low specific gravity with no red blood cells and only 0 to 5 white blood cells (WBCs). Approximately 150 mL of CSF circulates within the CSF pathways in the brain and spinal subarachnoid space. CSF is secreted at the rate of 0.35 mL/min or approximately 20 mL/hr.5
• ICP waveform morphology reflects transmission of arterial and venous pressure through the CSF and brain parenchyma. The normal ICP waveform has three or four peaks, with P1 being of greater amplitude than P2, and P2 of greater amplitude than P3. P1 is thought to reflect arterial pressure; P2, P3, and P4 (when present) have been described as choroid plexus or venous in origin (see Fig. 88-2).3,27 The amplitude of P2 may exceed P1 with increased ICP or decreased intracranial compliance (see Fig. 88-3).
• ICP waveform trends include a, b, and c waves. The a waves, also referred to as plateau waves, are associated with ICP values of 50 to 100 mm Hg and last 5 to 20 minutes. The a waves are associated with abrupt neurologic deterioration and herniation (Fig. 88-4). The b waves (Fig. 88-5), with ICP values of 20 to 50 mm Hg, last 30 seconds to 2 minutes and may become a waves. The c waves (Fig. 88-6) may coincide with ICPs as high as 20 mm Hg but are short lasting and without clinical significance (see Fig. 88-6).3
• Some external ventricular drainage systems may also provide simultaneous drainage and trending of the intracranial pressure.
• Management of acute brain injury is aimed at decreasing secondary brain injury from increased intracranial pressure, decreased cerebral perfusion pressure, impaired autoregulation, hypotension, hypoxemia, cerebral ischemia, hypercarbia, hyperthermia, hypoglycemia, hyperglycemia, or abnormalities in cerebral blood flow. Interventions should include a decrease in environmental stimuli, elevation of the head of the bed, alignment of the head and neck in a straight position to promote venous drainage, the avoidance of constrictive devices about the neck that might impede arterial flow to the brain and venous drainage from the brain, and attaining and maintaining normothermia without shivering.3,25
• In addition to CSF drainage, management of increased ICP frequently requires the use of certain pharmacologic agents to lessen intracranial pressure, including sedation and analgesia, osmotic diuretics, hypertonic saline, neuromuscular blockade, and barbiturates. In the case of barbiturate coma, continuous electroencephalographic (EEG) monitoring for burst suppression is necessary to achieve the desired decrease in cerebral oxygen consumption and electrical stimuli.13 Additional strategies include decompressive craniectomy and hemispherectomy.3,12,27,32
• Underdrainage of CSF may result in sustained increased intracranial pressure and herniation.21,24,27,28
• Overdrainage of CSF may result in headache, subdural hematoma, pneumocephalus, and herniation.21,24,27,28
EQUIPMENT
• Cranial access tray with drill
• Pressure monitor tubing kit, including pressure tubing, transducer, a three-way stopcock, or a flushless transducer with stopcock
• External drainage system, including tubing, collection chamber, and drainage bag
• Preservative-free normal saline solution
• Pressure monitoring cable and module
• Local anesthetic (lidocaine 1% or 2% without epinephrine)
• Laboratory forms and specimen labels (for CSF specimens)
• Sterile CSF specimen tubes (for collection of CSF)
• Caps, masks, sterile drapes, gloves, and gowns
• Cautery as required by institutional standard (for bedside insertion)
PATIENT AND FAMILY EDUCATION
• This procedure may be performed at the bedside or in an operating room. The patient may need to be sedated, paralyzed, and intubated.  Rationale: Patient cooperation during cranial access is of utmost importance. In the presence of an intact neurologic examination, the patient may not need intubation. However, the patient and family should be aware that the patient may need to be intubated to maintain a patent airway, ensure adequate oxygenation, and maintain a normal ICP and an adequate CPP.
Rationale: Patient cooperation during cranial access is of utmost importance. In the presence of an intact neurologic examination, the patient may not need intubation. However, the patient and family should be aware that the patient may need to be intubated to maintain a patent airway, ensure adequate oxygenation, and maintain a normal ICP and an adequate CPP.
• Assess the patient and the family for understanding of ICP pressure monitoring.  Rationale: Knowledge and information may lessen anxiety.
Rationale: Knowledge and information may lessen anxiety.
• Explain the waveforms on the bedside monitor and how this pressure is continually observed for signs of increased ICP. In the case of increased ICP, the drain is opened to drain CSF continuously or intermittently to alleviate the pressure.  Rationale: This explanation presents to the patient and family a more realistic expectation of the events to come.
Rationale: This explanation presents to the patient and family a more realistic expectation of the events to come.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Obtain a baseline assessment to include level of consciousness, mental status, motor and sensation, cranial nerves, and vital signs.  Rationale: This assessment provides baseline data.
Rationale: This assessment provides baseline data.
• Obtain the patient’s medical and surgical history to include use of aspirin, anticoagulants, prior craniotomies, the presence of aneurysm clips, embolic materials, permanent balloon occlusions, detachable coils, or a ventriculoperitoneal shunt. Obtain laboratory results to assess coagulation status as needed.  Rationale: The information obtained determines and guides future treatment based on the neurologic examination results and evidence from radiology and angiography.
Rationale: The information obtained determines and guides future treatment based on the neurologic examination results and evidence from radiology and angiography.
• Assess for allergies.  Rationale: Insertion of an external ventricular catheter requires the use of an antiseptic to cleanse the site, local anesthetic, and possibly systemic analgesic and sedation. External ventricular catheters may be impregnated with antibiotics (e.g., clindamycin, rifampin, and minocycline), or systemic antibiotics may be given periprocedurally or prophylactically.6,21,26
Rationale: Insertion of an external ventricular catheter requires the use of an antiseptic to cleanse the site, local anesthetic, and possibly systemic analgesic and sedation. External ventricular catheters may be impregnated with antibiotics (e.g., clindamycin, rifampin, and minocycline), or systemic antibiotics may be given periprocedurally or prophylactically.6,21,26
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and family understand preprocedural teaching. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient. However, in emergency circumstances, time may not allow for the consent form to be signed.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient. However, in emergency circumstances, time may not allow for the consent form to be signed.
• Initiate intravenous (IV) access or assess the patency of the IV.  Rationale: Readily available IV access is necessary if the patient needs to be sedated or paralyzed or needs other medications.
Rationale: Readily available IV access is necessary if the patient needs to be sedated or paralyzed or needs other medications.
• Perform a pre-procedure verification and time out, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
References
![]() 1. Arabi, Y, Memish, ZA, Balkhy, HH, et al, Ventriculostomy-associated infections. incidence and risk factors. Am J Infect Control 2005; 33:137–143.
1. Arabi, Y, Memish, ZA, Balkhy, HH, et al, Ventriculostomy-associated infections. incidence and risk factors. Am J Infect Control 2005; 33:137–143.
2. Bederson, JB, Connolly, ES, Batjer, HH, et al, Guidelines for the management of aneurysmal subarachnoid hemorrhage. a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009; 40:994–1025.
3. Bershad, EM, Humphreis, WE, Suraz, JI. Intracranial hypertension. Semin Neurol. 2008; 28:690–702.
![]() 4. Bisnaire, D, Robinson, L. Accuracy of levelling intraventricular collection drainage systems. J Neurosci Nurs. 1997; 29:261–268.
4. Bisnaire, D, Robinson, L. Accuracy of levelling intraventricular collection drainage systems. J Neurosci Nurs. 1997; 29:261–268.
![]() 5. Blumenfeld, H, Ventricles and cerebrospinal fluid in neuroanatomy through clinical cases,. Sinauer Associates, Sunderland, MA, 2002:128–133.
5. Blumenfeld, H, Ventricles and cerebrospinal fluid in neuroanatomy through clinical cases,. Sinauer Associates, Sunderland, MA, 2002:128–133.
6. Bratton, SL, Chesnut, RM, Ghajar, J, et al, Guidelines for the management of severe traumatic brain injury. a join project of the Brain Trauma Foundation, American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), AANS/CNS Joint Section on Neurotrauma and Critical Care. J Neurotrauma. 2007; 24(Suppl 1):S1–S106.
7. Brodbelt, A, Stoodley, M, CSF pathways. a review. Br J Neurosurg 2007; 21:510–520.
8. Broderick, J, Connolly, S, Feldman, E, et al, Guidelines for the early management of spontaneous intracerebral hemorrhage in adults. guidelines from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke 2007; 38:2001–2023.
![]() 9. Czosnyka, M, Pickard, JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004; 75:813–821.
9. Czosnyka, M, Pickard, JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004; 75:813–821.
![]() 10. Czosnyka, M, Czosnyka, ZA, Richards, HK, et al. Hydrodynamic properties of extraventricular drainage systems. Neurosurgery. 2003; 52:619–623.
10. Czosnyka, M, Czosnyka, ZA, Richards, HK, et al. Hydrodynamic properties of extraventricular drainage systems. Neurosurgery. 2003; 52:619–623.
11. Dasic, D, Hanna, SJ, Bojanic, S, et al, External ventricular drain infection. the effect of a strict protocol on infection rates and a review of the literature. Br J Neurosurg 2006; 20:296–300.
![]() 12. Dennis, LJ, Mayer, SA. Diagnosis and management of increased intracranial pressure. Neurol India. 2001; 49(Suppl 1):S37–S50.
12. Dennis, LJ, Mayer, SA. Diagnosis and management of increased intracranial pressure. Neurol India. 2001; 49(Suppl 1):S37–S50.
![]() 13. Dunn, LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002; 73(Suppl I):i23–i27.
13. Dunn, LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002; 73(Suppl I):i23–i27.
14. Fountas, KN, Kapsalaki, EZ, Machinis, T, et al. Review of the literature regarding the relationship of rebleeding and external ventricular drainage in patients with subarachnoid hemorrhage of aneurysmal origin. Neurosurg Rev. 2006; 29:14–18.
15. Hebl, JR. The importance and implications of aseptic techniques during regional anesth. Reg Anesth Pain Med. 2006; 31:311–323.
![]() 16. Hetherington, NJ, Dooley, MJ. Potential for patient harm from intrathecal administration of preserved solutions. Med J Aust. 2000; 173:141–143.
16. Hetherington, NJ, Dooley, MJ. Potential for patient harm from intrathecal administration of preserved solutions. Med J Aust. 2000; 173:141–143.
![]() 17. Kinirons, B, Mimoz, O, Lafendi, L, et al. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children. Anesthesiology. 2001; 94:239–244.
17. Kinirons, B, Mimoz, O, Lafendi, L, et al. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children. Anesthesiology. 2001; 94:239–244.
![]() 18. Kirkness, CJ, Mitchell, PH, Burr, RL, et al, Intracranial pressure waveform analysis. clinical and research implications. J Neurosci Nurs 2000; 32:271–277.
18. Kirkness, CJ, Mitchell, PH, Burr, RL, et al, Intracranial pressure waveform analysis. clinical and research implications. J Neurosci Nurs 2000; 32:271–277.
![]() 19. Kirkness, CJ, March, K. Intracranial pressure management. In: Bader MK, Littlejohns LR, eds. AANN core curriculum for neuroscience nursing. St Louis: Saunders; 2004:249–267.
19. Kirkness, CJ, March, K. Intracranial pressure management. In: Bader MK, Littlejohns LR, eds. AANN core curriculum for neuroscience nursing. St Louis: Saunders; 2004:249–267.
20. Komotar, R, Zacharia, BE, Mocco, J, et al, Controversies in the surgical treatment of ruptured intracranial aneurysms. the first annual J. Lawrence Pool Memorial Research Symposium-Controversies in the management of cerebral aneurysms. Neurosurgery 2008; 62:396–407.
21. Leeper, B, Lovasik, D, Cerebrospinal drainage systems. external ventricular and lumbar drains. Littlejohns LR, Bader MK, editors. AACN-AANN protocols for practice: monitoring technologies in critically ill patients. 2009. Jones and Bartlett: Sudbury, MA:71–82.
![]() 22. Littlejohns, LR, Trimble, B, Ask the experts. our policy on external ventricular drainage systems includes the procedure for priming the system. Does it really have to be primed. Crit Care Nurse 2005; 25:57–59.
22. Littlejohns, LR, Trimble, B, Ask the experts. our policy on external ventricular drainage systems includes the procedure for priming the system. Does it really have to be primed. Crit Care Nurse 2005; 25:57–59.
![]() 23. Lozier, AP, Sciacca, RR, Romagnoli, MFet al. Ventriculostomy-related infections. a critical review of the literature. Neurosurgery. 2002; 51:170–182.
23. Lozier, AP, Sciacca, RR, Romagnoli, MFet al. Ventriculostomy-related infections. a critical review of the literature. Neurosurgery. 2002; 51:170–182.
24. Maniker, A, Vaynman, AY, Karimi, RJ, et al, Hemorrhagic complications of external ventricular drainage. Neurosurgery . 2006; 59(ONS Suppl 4 ):ONS419–424.
![]() 25. March, K. Intracranial pressure monitoring and assessing intracranial compliance in brain injury. Crit Care Nurs Clin North Am. 2000; 12:429–436.
25. March, K. Intracranial pressure monitoring and assessing intracranial compliance in brain injury. Crit Care Nurs Clin North Am. 2000; 12:429–436.
![]() 26. March, K, Intracranial pressure monitoring. why monitor. AACN Clin Issues 2005; 16:456–475.
26. March, K, Intracranial pressure monitoring. why monitor. AACN Clin Issues 2005; 16:456–475.
27. March, K, Madden, L. Intracranial pressure management. In: Littlejohns LR, Bader MK, eds. AACN-AANN protocols for practice: monitoring technologies in critically ill patients. Sudbury, MA: Jones and Bartlett; 2009:35–69.
![]() 28. March, K, Wellwood, J, Arbour, R. Technology. In: Bader MK, Littlejohns LR, eds. AANN core curriculum for neuroscience nursing. St Louis: Saunders; 2004:199–204.
28. March, K, Wellwood, J, Arbour, R. Technology. In: Bader MK, Littlejohns LR, eds. AANN core curriculum for neuroscience nursing. St Louis: Saunders; 2004:199–204.
![]() 29. Ng, I, Lim, J, Wong, HB, Effects of head posture on cerebral hemodynamics. its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery 2004; 54:593–598.
29. Ng, I, Lim, J, Wong, HB, Effects of head posture on cerebral hemodynamics. its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery 2004; 54:593–598.
![]() 30. O’Grady, NP, Alexander, M, Dellinger, Pet al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30:476–489.
30. O’Grady, NP, Alexander, M, Dellinger, Pet al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30:476–489.
31. Reynolds, F. Neurologic infections after neuraxial anesthesia. Anesthesiol Clin. 2008; 26:23–52.
![]() 32. Vincent, JL, Berre, J. Primer on medical management of severe brain injury. Crit Care Med. 2005; 33:1392–1399.
32. Vincent, JL, Berre, J. Primer on medical management of severe brain injury. Crit Care Med. 2005; 33:1392–1399.