History
Comments
Current Medications
Current Symptoms
Physical Examination
Comments
Laboratory Data
Electrocardiogram
Findings
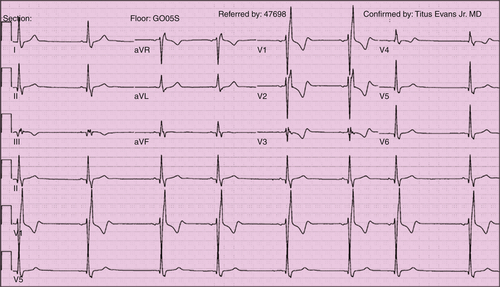
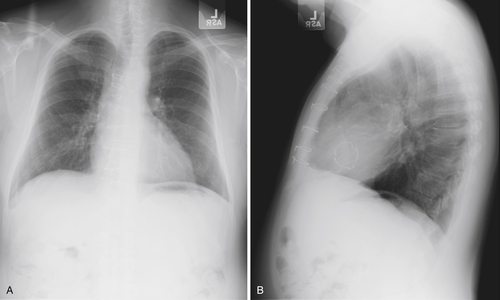
FIGURE 9-1
Chest Radiograph
Findings
Exercise Testing
Echocardiogram
Findings
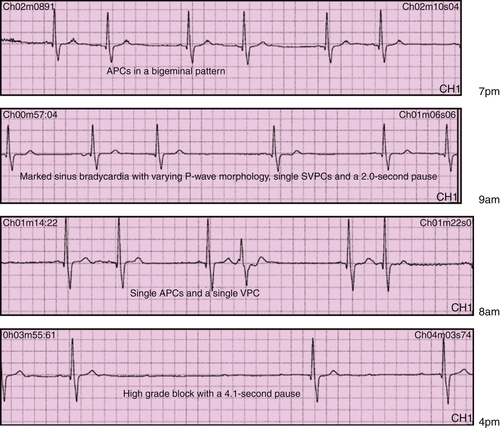
Physiologic Tracings
Findings
Focused Clinical Questions and Discussion Points
Discussion
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
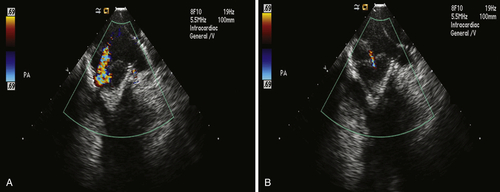
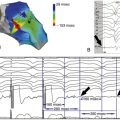
FIGURE 9-4
Outcome
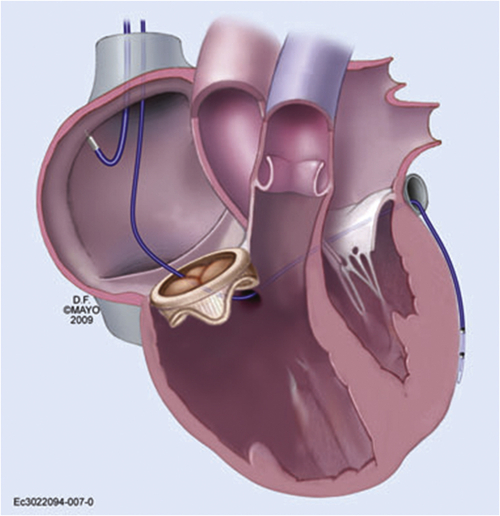
FIGURE 9-5
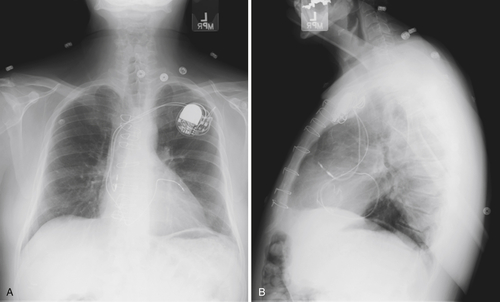
FIGURE 9-6
Selected References
1. Bleeker G.B., Schalij M.J., Nihoyannopoulos P. et al. Left ventricular dyssynchrony predicts right ventricular remodeling after cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2264–2269.
2. Eleid M.F., Blauwet L.A., Cha Y.-M. et al. Bioprosthetic tricuspid valve regurgitation associated with pacemaker or defibrillator lead implantation. J Am Coll Cardiol. 2012;59:813–818.
3. McLeod C.J., Attenhofer Jost C.H., Warnes C.A. et al. Epicardial versus endocardial permanent pacing in congenital heart disease. J Interv Card Electrophysiol. 2010;28:235–243.