Infectious Disease Emergencies
Edited by Peter Cameron
9.1 Approach to undifferentiated fever in adults
Jonathan Knott
Introduction
Fever is a common presenting symptom to the emergency department (ED); about 5% of patients give fever as the reason for their visit. Most patients with fever have symptoms and signs that indicate the site or region of infection. A prospective study of patients aged 16 years or older who presented to an ED with fever≥37.9°C found that 85% had localizing symptoms and signs that suggested or identified a source of fever and 15% had unexplained fever after the history and examination [1].
Fever with no localizing symptoms or signs at presentation is often seen in the first day or two of the illness. Many patients with such a problem will ultimately prove to have self-limiting viral infections, but others will have non-viral infections requiring treatment. Among this latter group are illnesses that may be serious and even rapidly fatal.
Over one-third of patients who have fever for more than a few days with no localizing symptoms and signs are likely to have a bacterial infection [1,2].
If no cause is found in an adult with fever present for over 3 days, there is a good chance the patient will have a bacterial infection that needs treatment. Over half of these infections are likely to be in the respiratory or urinary tracts [1].
The most important task in the ED for febrile patients without localizing features is not to miss early bacterial meningitis, bacteraemia, such as meningococcaemia and early staphylococcal and streptococcal toxic shock syndromes.
Approach
The management of febrile patients varies according to the severity, duration and tempo of the illness, the type of patient and the epidemiological setting. Although the steps in management of a febrile patient in the ED, listed below, may be set out in a sequential manner, in reality the mental processes involved occur simultaneously by the bedside.
Step 1: identify the seriously ill patient who requires urgent intervention
The first step in managing febrile patients is to identify those in need of immediate resuscitation, urgent investigations and empirical therapy. The presence of any of the following features justifies immediate intervention: shock, coma/stupor, cyanosis, profound dyspnoea, continuous seizures and severe dehydration.
Step 2: identify those with localized infections or easily diagnosable diseases
Having excluded those who need urgent intervention, the doctor has more time to attempt a diagnosis. The history and physical examination are usually sufficient to localize the source of community-acquired fever in most cases, especially if the illness has been present for several days.
History
A precise history remains the key to diagnosis of a febrile illness. An inability to give a history and to think clearly is a sign of potential sepsis.
Illness
An abrupt onset of fever, particularly when accompanied by chills or rigors and generalized aches, is highly suggestive of an infective illness.
Localizing symptoms, their evolution and relative severity, help to identify the site of infection; localized pain is particularly valuable in this way.
The severity and the course of the illness can be assessed by the patient’s ability to work, to be up and about, to eat and sleep and the amount of analgesics taken.
Previous state of health
Underlying diseases predispose patients to infection at certain sites or caused by certain specific organisms. Knowledge of any defects in the immune system is similarly helpful. For example, asplenic patients are more prone to overwhelming pneumococcal septicaemia and renal transplant patients to Listeria meningitis.
A past history of infectious diseases, particularly if properly documented, may be useful in excluding infections such as measles and hepatitis.
Predisposing events
Recent operations, accidents and injuries and medications taken may be the direct cause of the illness (e.g. drug fever or rash from co-trimoxazole, ampicillin) or may affect the resistance of the patient, predisposing to certain infections. Concurrent menstruation raises the possibility of toxic shock syndrome.
Epidemiology
Information on occupation, exposure to animals, hobbies, risk factors for blood-borne viruses and travel overseas or to rural areas may suggest certain specific infections, e.g. leptospirosis, acute HIV infection, hepatitis C, malaria, etc.
Contact with similar diseases and known infectious diseases
This information is useful in the diagnosis of problems such as meningococcal infection, viral exanthema, respiratory infection, diarrhoea, and zoonoses.
Examination
Physical examination in the febrile patient serves two purposes: to assess the severity of the illness and to find a site of infection.
Bedside assessment of severity and ‘toxicity’ based on intuitive judgement is frequently wrong and many patients with severe bacterial infections do not appear obviously ill or toxic.
Physical examination may yield a diagnosis in a febrile patient who has not complained of any localizing symptoms. A checklist of special areas to be examined is useful.
 Heart: murmurs and pericardial rubs.
Heart: murmurs and pericardial rubs.
 Lungs: subtle crackles may be heard in pneumonic patients without respiratory symptoms.
Lungs: subtle crackles may be heard in pneumonic patients without respiratory symptoms.
 Marked muscle tenderness is a frequent sign of sepsis.
Marked muscle tenderness is a frequent sign of sepsis.
 Neck stiffness may be a clue to meningitis in a confused patient who cannot give a history.
Neck stiffness may be a clue to meningitis in a confused patient who cannot give a history.
 Any area that is covered, e.g. under plasters or bandages, for evidence of sepsis.
Any area that is covered, e.g. under plasters or bandages, for evidence of sepsis.
There are two caveats when assessing local symptoms and signs:
Step 3: look for the ‘at-risk’ patient
If no diagnosis is forthcoming after the first two steps, the next task is to identify the ‘at-risk’ patient who may not appear overtly ill but who, nonetheless, requires medical intervention. This applies particularly to those with treatable diseases that can progress rapidly, such as bacterial meningitis, bacteraemia and toxic shock syndromes.
Four sets of pointers are helpful in identifying these ‘at-risk’ patients: the type of patient (host characteristics), exposure history, the nature of the non-specific symptoms and how rapidly the illness evolves.
Clinical pointers: type of patient
Clinical manifestations of infections are often subtle or non-specific in young children, the elderly and the immunocompromised. The threshold for intervention in these patients should be lowered. The issue of fever in children is not addressed in this chapter.
Elderly patients
Elderly patients with infections often do not mount much of a febrile response and fever may be absent in 20–30% of these patients [3].
Infectious diseases in the elderly, as in the very young, often present with non-specific or atypical symptoms and signs and may progress rapidly [4].
In adult patients with unexplained fever, up to one-third may have bacteraemia or focal bacterial infection. This proportion is even higher in those over the age of 50 [1]. In the elderly, a fever>38°C indicates a possible serious infection [5] and is associated with increasing risk of death [6].
The urinary tract is the most frequent site of infection and source of bacteraemia; symptoms of urinary tract infection are frequently absent in the elderly. The respiratory tract is the next most common site of infection; fever and malaise may be the only clues of pneumonia in the elderly. Urinalysis and chest X-ray will identify about half of occult infections [1].
An unexplained fever in a person over the age of 50 should be regarded as being caused by a bacterial infection until proved otherwise and is generally an indication for admission to hospital.
Alcoholic patients
Alcoholic patients present with multiple problems, many of which cause fever. Most are caused by infections, the commonest of which is pneumonia. Multiple infections may occur at the same time [7].
Non-infectious causes of fever frequently coexist with infections and conditions such as subarachnoid haemorrhage, alcoholic withdrawal and alcoholic hepatitis and require admission.
The initial history and physical examination in the alcoholic may be unreliable and diagnosis may be difficult.
Alcoholic patients with fever for which no obvious cause is found should be admitted to hospital for investigations and observation.
Injecting drug users
The risk of injecting drug users acquiring serious or unusual infections is high through repeated self-injection with non-sterile illicit substances, the use of contaminated needles and syringes and poor attention to skin cleansing prior to injections [8].
Many intravenous drug users presenting with fever have a serious infection. Some have obvious focal infections, such as cellulitis and pneumonia. Others present simply with fever and the presence of bacteraemia and endocarditis must be suspected.
Clinical assessment cannot differentiate trivial from potentially serious conditions in these patients [8]. A history of chills, rigors and sweats strongly suggest the presence of a transient or ongoing bacteraemia. Back pain may be a subtle symptom of endocarditis or vertebral osteomyelitis.
It is difficult to distinguish the patient with endocarditis from other drug users with fever due to another cause. Hospitalization of febrile injecting drug users would be prudent if 24-hour follow up is not possible. Intravenous drug use in the previous 5 days is a predictor of occult major infection and is an indication for admission to hospital [9].
Patients with diabetes mellitus
Diabetic patients are more prone to developing certain bacterial infections [1]. A diabetic patient with an unexplained fever is more likely to have an occult bacterial infection than a non-diabetic patient. In general, an insulin-dependent diabetic patient, especially if aged over 50, with fever and no obvious source of infection, should be investigated and preferably admitted.
Febrile neutropaenic patients
Febrile neutropaenic patients (absolute neutrophil count<500/μL or<1000/μL and falling rapidly) must be hospitalized regardless of their clinical appearance. Infections may become fulminant within hours in these patients and the clinical manifestations of their infective illnesses are frequently modified by the underlying disease, therapy received and coexisting problems.
Splenectomized patients
Splenectomized patients with fever must be very carefully assessed because of their increased risk of overwhelming bacterial infection. If the fever cannot be readily explained, admission for intravenous antibiotics is usually indicated.
Other immunocompromised patients
Fever in transplant patients (renal, hepatic or cardiac) and those with HIV infection is not an absolute indication for admission, but the threshold of intervention should be considerably lowered and they are best assessed by their usual treating doctors.
Patients recently discharged from hospital may have hospital-acquired infections or infections caused by multiresistant organisms. Recent operations or procedures may be a clue to the site of infection.
Clinical pointers: exposure history
Overseas travellers or visitors
Returned travellers or overseas visitors may have diseases such as malaria and typhoid fever that need early diagnosis and treatment. Any fever in a traveller returned from a malaria-endemic area should be regarded as due to malaria until proved otherwise.
Influenza in febrile returned travellers is a concern to EDs worldwide. Outbreaks of avian influenza occur periodically in bird populations throughout Asia. Although the virus does not typically infect humans, direct bird-to-human transmission of H5N1 influenza has been documented. The virus is highly pathogenic and the mortality of the disease is high. Travellers acquiring influenza overseas may also introduce this infection. Most cases occur within 2–4 days after exposure, but incubation is as long as 8 days. Suspected influenza infection requires isolation and respiratory precautions. The peak season is generally during the winter months, but can vary, especially in the tropics [10].
Although rare, viral haemorrhagic fever in returned travellers represents a true medical emergency and a serious public health threat. Viral haemorrhagic fevers are caused by several distinct families of virus, including Ebola and Marburg, Lassa fever, the New World arenaviruses (Guanarito, Machupo, Junin, and Sabia) and Rift Valley fever and Crimean Congo haemorrhagic fever viruses. Most exist in Africa, the Middle East or South America. Although some types cause relatively mild illnesses, many can cause severe, life-threatening disease. Viral haemorrhagic fever should be considered in any febrile patient who has returned from an area in which viral haemorrhagic fever was endemic, especially if they have come into contact with blood or other body fluids from a person or animal infected with viral haemorrhagic fever or worked in a laboratory or animal facility handling viral haemorrhagic fever specimens. All these infections have incubation periods of up to 2–3 weeks, so it may be possible to exclude viral haemorrhagic fever on epidemiological grounds alone. Isolation measures should be instituted immediately in these persons [11].
Staff working in emergency departments should be aware of regional outbreaks of unusual pathogens. These are reported by State and National Departments of Health. Returning travellers who are unwell will commonly go directly to an emergency department and this may be a critical point to limit further spread.
Contact with animals
A contact history with animals, either at work or at home, is frequently the clue to a zoonosis, particularly if the illness is a perplexing fever of several days’ duration. The occurrence of multiple cases at work or at home should also make one suspect these infections early.
Contact with meningococcal and Haemophilus meningitis
Close contacts of patients with these infections have a high risk of acquiring the same infections. Early symptoms may be subtle and a high index of suspicion must be maintained.
Clinical pointers: non-specific clinical features (Table 9.1.1)
There are several non-specific clinical features whose presence should suggest the possibility of sepsis. These warrant careful scrutiny even when the patient does not appear toxic. They are by no means specific indicators of serious problems and there will be many false positives. However, ignoring them is frequently the cause of missed or delayed diagnosis of sepsis.
Table 9.1.1
Clinical pointers: non-specific clinical features (‘alarm bells’)
Severe pain in muscles, neck or back
Impairment of conscious state
Vomiting, especially in association with headache or abdominal pain
Severe headache in the presence of a normal CSF
Unexplained rash
Jaundice
Severe sore throat or dysphagia with a normal looking throat
Repeated rigors
Severe pain in muscles, neck or back
Severe muscle pain, even in the absence of overt fever, may be an early symptom of meningococcaemia, staphylococcal or streptococcal bacteraemia. It is also a feature of myositis and necrotizing fasciitis.
Impairment of conscious state
A change in conscious state may be the sole presenting manifestation of sepsis, especially in the elderly.
Vomiting
Unexplained vomiting, especially in association with headache or abdominal pain, should raise concern. Vomiting without diarrhoea should not be attributed to a gastrointestinal infection. It is a common symptom of CNS infections and occult sepsis.
Severe headache in the presence of a normal CSF
This is especially important in a person who seldom gets headaches. Severe headache in a febrile patient with normal CSF should not be diagnosed as a viral infection; many focal infections, e.g. pneumonia and bacterial enteritis, may also present in this manner. CSF may be normal in cerebral abscess and in the prodromal phase of bacterial meningitis.
Unexplained rash
An unexplained rash in a febrile patient should be regarded as meningococcaemia until proved otherwise, even in the absence of headache or CSF pleocytosis.
Jaundice
Jaundice in the febrile patient is associated with a greatly increased risk of death, admission to ICU and prolonged hospital stay [6]. Jaundice in a febrile patient is unlikely to be due to viral hepatitis, but occurs in serious bacterial infections, such as bacteraemia, cholangitis, pyogenic liver abscess and malaria.
Sore throat or dysphagia
Severe sore throat or dysphagia with a normal-looking throat is frequently the presenting symptom of Haemophilus influenzae epiglottitis in adults.
Repeated rigors
Although repeated rigors may occur in some viral infections, they should generally be regarded as indicators of sepsis, in particular abscesses, bacteraemia, endocarditis, cholangitis and pyelonephritis.
Clinical pointers: evolution of illness (Table 9.1.2)
How rapidly the illness evolves is often an indication of its severity. Previously healthy individuals do not seek medical attention unless they are worried. Notice should be taken of any person seeking help within 24 hours of the onset of illness or a person whose illness appears to have progressed rapidly within 24–48 hours (e.g. from being up and about to being bedridden). Similarly, the patient who presents to the ED on more than one occasion over a 24–48-hour period warrants a careful work-up.
Table 9.1.2
Clinical pointers: evolution of illness
Those presenting early (<24 hours)
Those presenting with rapidly evolving symptoms
Patients presenting to ED on>1 occasion over a 24–48-hour period
Step 4: a final caveat
A major concern in the management of undifferentiated fever in adults is missing the diagnosis of meningococcal bacteraemia when the patient does not appear ill on presentation.
There are a number of infections that must be treated rapidly to minimize morbidity and mortality (Table 9.1.3). With the exception of meningococcal bacteraemia, there are usually some clues in the history or physical examination.
Table 9.1.3
Infections requiring urgent treatment
| Disease | Clues |
| Meningococcaemia | Myalgia, rash. May be none |
| Falciparum malaria | Travel history, blood film |
| Bacterial meningitis | Headache, change in conscious state, CSF findings |
| Post-splenectomy sepsis | Past history, abdominal scar |
| Toxic shock syndromes | Presence of shock and usually a rash |
| Infections in the febrile neutropaenic | Past history, blood film |
| Infective endocarditis | Past history, murmur, petechiae |
| Necrotizing soft-tissue infections | Pain, tenderness, erythema and swelling in skin/muscle, toxicity |
| Space-occupying infection of head and neck | Localizing symptoms and signs |
| Focal intracranial infections | Headache, change in conscious state, neurological signs, CT findings |
Meningococcal infection is peculiar in its wide spectrum of severity and variable rate of progression in different individuals. It may be fulminant and cause death within 12 hours or it may assume a chronic form that goes on for weeks.
When the patient presents with fever and a petechial rash, meningococcaemia can easily be suspected if one remembers the golden rule of medicine that ‘fever plus a petechial rash is meningococcaemia (or staphylococcal bacteraemia) until proved otherwise’. However, only 40% of meningococcal diseases present with a petechial rash.
It is less well known that the early meningococcaemic rash may be macular, i.e. one that blanches with pressure. This is the basis of another golden rule in infectious disease: early meningococcal rash may resemble a non-specific viral rash.
Rarely, meningococcal disease presents with symptoms and signs of a localized infection other than meningitis, e.g. pneumonia, pericarditis or urethritis. These presentations should not pose any management problems.
The risk of missing the diagnosis increases markedly when the patient with meningococcal disease presents with fever and non-specific symptoms without a rash. Abrupt onset of fever and generalized aches may be due to influenza, but it could be due to meningococcaemia.
It is prudent to single out meningococcal disease and ask oneself: could this patient have meningococcaemia? If in doubt, the safest course is to take cultures, give antibiotics and admit.
Clinical investigations
Most febrile patients seen in the ED justify a fever work-up.
Full blood examination is of limited use. White cell count (>15×109/L), marked left shift, neutropaenia or thrombocytopaenia are pointers to a possible bacteraemia or occult bacterial infections, but they may also be seen in viral infections [12]. Similarly, non-specific markers of inflammation, such as C-reactive protein and erythrocyte sedimentation rate, have not been shown to be useful in predicting outcomes for febrile patients in the ED [13].
Urinalysis and urine culture should be done in febrile adults over the age of 50 unless the pathology clearly lies in another body system. However, if the history does not suggest urinary sepsis and the dipstick urinalysis is normal, then urine cultures are usually negative [14].
A chest X -ray is usually indicated unless a definite diagnosis has been made, e.g. chickenpox, tonsillitis.
Blood cultures should be done in anyone suspected of having bacteraemia, endocarditis or meningitis, in compromised patients with a fever, all febrile patients over the age of 50 and, possibly, in anyone with an unexplained high fever. It should be noted that only 5% of blood cultures in this setting will be positive and less than 2% will alter clinical management [15]. In general, a patient considered ‘sick enough’ to warrant blood cultures should be admitted to hospital or followed up within 24 hours.
Disposition
Patients who have any of the following features are in need of resuscitation, followed by work-up and admission: shock, coma/stupor, cyanosis, profound dyspnoea, continuous seizures and severe dehydration.
With few exceptions, the following groups of febrile adults should be investigated and admitted:
 patients with diabetes mellitus
patients with diabetes mellitus
 immunologically compromised patients
immunologically compromised patients
In general, there should be close liaison with the admitting unit and the issue of empirical therapy for septic patients should be discussed. For the dangerously ill, e.g. those with septic shock or bacterial meningitis, antibiotics should be commenced almost immediately.
There is an increasing tendency to start antibiotics in the ED as soon as possible to reduce the length of hospital stay. Time to antibiotic therapy is used as a key performance indicator for the ED, e.g. for febrile immunocompromised patients.
Patients who do not require intervention after the basic work-up in the ED are discharged home after a period of observation. Because of the time taken to interview the patient, perform investigations and wait for the results, the patient will usually have been observed for 1–2 hours and progression or lack of progression may be a help in deciding what to do. During observation one must be aware that the apparent improvement of the patient may be the result of pain relief or a fall in temperature due to antipyretics.
Arrangement must be made for the patient to be reviewed by their general practitioner or at the hospital. This is an essential component of the care of a febrile patient seen in an ED.
There is no easy way of detecting occult bacterial sepsis. The infectious process is a dynamic one and the doctor must maintain contact with the patient or family during the 24–72 hours following the initial visit.
Patients with fever>39°C must be seen within 24 hours. Review by a doctor within 6–12 hours may be necessary in those who have had a lumbar puncture and is advisable in those who have had blood cultures taken. A verified phone number should be clearly recorded in the medical history.
All febrile patients discharged from the ED should be encouraged to seek review if there is any adverse change to their condition. A patient re-presenting to the ED has provided an opportunity to ensure that they are being managed appropriately and to rectify any errors.
Fever due to most common viral infections will resolve by about 4 days. Many other infections will be diagnosed when new symptoms or signs appear.
If fever persists beyond 4–5 days without any localizing symptoms or signs, a less common infection or non-infective cause should be suspected and the patient should be thoroughly investigated. In this situation, the threshold of admission to hospital should be low.
The establishment of ED short-stay units allows fast-track treatment and observation, usually for 24–48 hours, for carefully selected febrile patients who are not suitable for immediate discharge home.
Future research directions
9.2 Meningitis
Andrew Singer
Introduction
Definition
Meningitis is an inflammation of the leptomeninges, the membranes that line the central nervous system, as well as the cerebrospinal fluid (CSF) in the subarachnoid space. It is usually the result of an infection, but can be due to an inflammatory response to a localized or systemic insult.
Classification
Meningitis is usually classified according to the aetiology or location as bacterial, aseptic (viral, tuberculous, fungal or chemical) or spinal (where the infection specifically affects the spinal meninges).
Aetiology
Bacterial
Bacterial meningitis is a serious cause of morbidity and mortality in all age groups. The causes vary according to age, as shown in Table 9.2.1. Neisseria meningitidis serogroups A and C tend to cause endemic cases of meningitis, especially in Aboriginal populations, whereas serogroup B is more commonly associated with epidemics [1]. There has been an increase in the incidence of penicillin-resistant Streptococcus pneumoniae, especially in children [2].
Table 9.2.1
| Viral | Bacterial | Other |
| Echovirus 6, 9,11, 30 | Neonates (<3 months old): | Mycobacterium tuberculosis |
| Coxsackie viruses A9, A16, B1, B5, B6 Enterovirus 71 H Herpes simplex 1 & 2 |
Group B streptococcus (Streptococcus agalactiae) Escherichia coli Listeria monocytogenes |
Cryptococcus neoformans (especially in immunocompromised) Aseptic |
| Cytomegalovirus Varicella zoster Epstein-Barr virus | Coagulase-negative Staphylococcus aureus Pseudomonas aeruginosa |
|
| Children (<6 years old): | ||
| Haemophilus influenzae type b | ||
| Neisseria meningitidis | ||
| Streptococcus pneumoniae | ||
| Adults | ||
| Neisseria meningitidis (especially in young adults) | ||
| Streptococcus pneumoniae | ||
| Listeria monocytogenes (especially in adults over 50) | ||
| Klebsiella pneumoniae | ||
| Staphylococcus aureus | ||
| Escherichia coli (in the immunocompromised) |
Aseptic
Aseptic meningitis may be either due to an immune response to a systemic infection (usually viral) or to a chemical insult.
Viral
Enteroviruses are the most common cause of meningitis, often in clusters of cases. Herpes viruses often cause meningitis as part of a more generalized infection of the brain (meningoencephalitis) or as part of an immune response to a systemic infection. A generalized viraemia may also cause aseptic meningitis, owing to an immune reaction without direct infection.
Fungal
Fungal causes of meningitis, especially that due to Cryptococcus neoformans, tend to occur in immunocompromised patients, such as those with HIV/AIDS or those on immunosuppressant medication or cancer chemotherapy. It can occur in immunocompetent individuals as well, particularly the elderly.
Tuberculous
Tuberculous meningitis is rare in industrialized countries, but can occur in all age groups. It tends to follow an insidious course, with a lack of classic signs and symptoms. Diagnosis is often difficult, owing to the low yield from CSF staining and the 4-week time frame required to culture the organism. Suspicion should be high in patients with immunocompromise or chronic illness. It tends to have a high mortality.
Spinal
Spinal meningitis is usually bacterial and due to direct spread from a localized infection in the spine.
Epidemiology
The epidemiology of meningitis is different for groups according to age, as well as immunocompetence:
 Neonates: Table 9.2.1 shows the main causes of bacterial meningitis in neonates. There is an overall incidence of 0.17–0.32 cases per 1000 live births. There is 26% mortality, which is even higher in premature infants [3].
Neonates: Table 9.2.1 shows the main causes of bacterial meningitis in neonates. There is an overall incidence of 0.17–0.32 cases per 1000 live births. There is 26% mortality, which is even higher in premature infants [3].
 Children: until the introduction of Haemophilus influenzae type b (Hib) immunization in the early 1990 s, this organism was the major cause of bacterial meningitis in children under 5 years (until 1990, the incidence of childhood Hib meningitis was 26.3 per 100 000 [152 per 100 000 in Aboriginal children]) [4]. Between 1990 and 1996 there was a 94% reduction in the incidence of Hib disease. N. meningitidis and S. pneumoniae remain common causes of both meningitis and generalized sepsis [5]. The introduction of immunization programmes for some strains of both of these bacteria will reduce the incidence of meningitis caused by these organisms in the future, although it is important to understand that not all strains are covered by vaccines.
Children: until the introduction of Haemophilus influenzae type b (Hib) immunization in the early 1990 s, this organism was the major cause of bacterial meningitis in children under 5 years (until 1990, the incidence of childhood Hib meningitis was 26.3 per 100 000 [152 per 100 000 in Aboriginal children]) [4]. Between 1990 and 1996 there was a 94% reduction in the incidence of Hib disease. N. meningitidis and S. pneumoniae remain common causes of both meningitis and generalized sepsis [5]. The introduction of immunization programmes for some strains of both of these bacteria will reduce the incidence of meningitis caused by these organisms in the future, although it is important to understand that not all strains are covered by vaccines.
 Adults: N. meningitidis and S. pneumoniae are common causes in all age groups, with N. meningitides predominating in adults under 24 years. Listeria monocytogenes is more common in adults over 50 years. The overall incidence in adults is 3.8 per 100 000 population [6]. More unusual organisms occur in patients following neurosurgery or chronic illness, such as alcoholism, hepatic cirrhosis, chronic renal failure and connective tissue disease [7] (GNRs, coagulase-negative Staphylococcus aureus, Mycobacterium tuberculosis, Klebsiella pneumoniae).
Adults: N. meningitidis and S. pneumoniae are common causes in all age groups, with N. meningitides predominating in adults under 24 years. Listeria monocytogenes is more common in adults over 50 years. The overall incidence in adults is 3.8 per 100 000 population [6]. More unusual organisms occur in patients following neurosurgery or chronic illness, such as alcoholism, hepatic cirrhosis, chronic renal failure and connective tissue disease [7] (GNRs, coagulase-negative Staphylococcus aureus, Mycobacterium tuberculosis, Klebsiella pneumoniae).
 Patients with HIV/AIDS: Cryptococcus neoformans is relatively common, with an incidence of 5 per million of population or 10% of HIV-infected patients. Tuberculosis, Listeria, Klebsiella and syphilis are also causes of meningitis in this group, as well as viral causes of meningoencephalitis [8].
Patients with HIV/AIDS: Cryptococcus neoformans is relatively common, with an incidence of 5 per million of population or 10% of HIV-infected patients. Tuberculosis, Listeria, Klebsiella and syphilis are also causes of meningitis in this group, as well as viral causes of meningoencephalitis [8].
Pathogenesis
Initially, there is colonization of the infectious agent, commonly in the nasopharynx in the case of the enteroviruses and bacteria, such as meningococcus and Hib. Other infections may spread from already established foci, such as otitis media or sinusitis (e.g. pneumococcus). There is either haematogenous or local spread to the meninges and subarachnoid space, with inflammation of this area and the production of a purulent exudate approximately 2 hours after invasion of the area. The inflammatory response is initiated by bacterial subcapsular components, such as lipoteichoic acid in S. pneumoniae, a lipo-oligosaccharide in H. influenzae and other Gram-negative endotoxins. These substances stimulate the release of cytokines, such as interleukin-1 and -6, tumour necrosis factor (TNF) and arachidonic acid metabolites, as well as the complement cascade. There is a subsequent increase in neutrophil and platelet activity, with increased permeability of the blood–brain barrier. This response is often worse after the initial destruction of bacteria by antibiotics. If left untreated, fibrosis of the meninges may occur. In viral and aseptic meningitis, there is a more limited inflammatory response, with mild-to-moderate infiltration of lymphocytes. In the more chronic causes, such as fungi or tuberculosis, the exudate is fibrinous, the main cells being a mixture of lymphocytes, monocytes/macrophages and plasma cells. The base of the brain is most commonly affected.
Presentation
History
There are some differences in the history with different causes of meningitis, which may allow an early differential diagnosis to be made. There are no pathognomonic single symptoms or signs for meningitis, so a high index of suspicion is necessary.
The combination of fever, headache, meningism and mental obtundation is found in approximately 85% of cases of bacterial meningitis [9]. It is also a common pattern in viral or aseptic meningitis, where obtundation is less of a feature. In fungal or tuberculous meningitis, these symptoms are much less common (less than 40% of cases of cryptococcal meningitis). Elderly patients or those who have had recent neurosurgery may present with subtle or mild symptoms and lack a fever [10].
The headache is usually severe and unrelenting. It may be either global or located in a specific area. The main symptoms of meningism are nuchal rigidity (neck stiffness) and photophobia. The nuchal rigidity is something more than merely pain on movement of the neck. It is clinically important when the patient complains of a painful restriction of movement in the sagittal plane (i.e. forwards and backwards only). Up to 35% of cases have associated nausea and vomiting.
As a general rule, the height of the fever is a poor indication of the possible cause, although the fever may often only be mild in tuberculous or fungal meningitis or in bacterial meningitis that has been partially treated by antibiotics. The spectrum of mental obtundation can range from mild confusion, to bizarre behaviour, delirium or coma. The severity of obtundation is a good indication of the severity of the illness.
Focal neurological signs occur in around 10–20% of cases of bacterial meningitis, but are also associated with cerebral mass lesions, such as toxoplasmosis or brain abscess. They are also a feature of tuberculous meningitis. Seizures are relatively uncommon (13–30%), but may occasionally be the only sign of meningitis if the patient has been partially treated with oral antibiotics.
There may also be associated systemic symptoms. Myalgias and arthralgias are often associated with viral causes, but may also be the sole presenting symptom in meningococcal meningitis. HIV/AIDS patients may show stigmata associated with that disease.
The course of the illness may also indicate the cause. Meningococcal or pneumococcal meningitis is often characterized by a rapid, fulminating course, often going from initial symptoms to death over an interval of hours. Viral causes tend to be a slower course over days. Fungal or tuberculous meningitis shows a more chronic course over days to weeks, with milder symptoms.
Risk factors for meningitis include the extremes of age, pre-existing sinusitis or otitis media, recent neurosurgery, CSF shunts, splenectomy, immunological compromise and chronic diseases such as alcoholism, cancer, connective tissue disorders, chronic renal failure and hepatic cirrhosis.
Examination
The physical examination will often reflect symptoms elicited in the history, with fever, physical evidence of meningism, stigmata of AIDS, etc.
As stated above, neck stiffness is only clinically significant when it occurs in the sagittal plane. There will be a restriction of both passive and active movement. Other tests to elicit meningism include Kernig’s sign and Brudzinski’s sign, although these are only present in 50% of adult cases of bacterial meningitis. Kernig’s sign is elicited by attempting to extend the knee of a leg that has been flexed at the hip with the patient lying supine and the other leg flat on the bed. The sign is positive if the knee cannot be fully extended due to spasm in the hamstrings. The test can be falsely positive in patients with shortening of the hamstrings or other problems involving the legs or lumbar spine. In Brudzinski’s sign, flexing the head causes the thighs and knees to also flex. It can also be tested in children by the inability to touch the nose with the flexed hips and knees in the sitting position. These are both late signs.
Focal neurological signs should be a cause for concern, as they can indicate a poor prognosis.
Papilloedema is rare and late, as is a bulging fontanelle in infants and should alert one to alternative diagnoses.
A rash, often starting as a macular or petechial rash on the limbs, is seen in sepsis due to N. meningitidis and S. pneumoniae. A petechial rash is a particularly serious sign and is an indication to start antibiotics immediately. A maculopapular rash is also a feature of viral causes.
Investigations
Lumbar puncture
A CSF sample via a lumbar puncture (LP) is an important source of information for making the diagnosis and determining the likely aetiology and treatment. As the procedure may be time-consuming, treatment should not be delayed if there will be more than a 20-minute delay before the lumbar puncture and there is a reasonable clinical suspicion that a bacterial cause is present. Blood cultures should be taken prior to the administration of antibiotics.
Indications
Precautions
The main features to note during lumbar puncture are the opening pressure and the physical appearance of the CSF. The sample should be sent for Gram staining, culture, sensitivities, polymerase chain reaction (PCR) analysis for bacteria and Herpes simplex virus, a cell count and protein and glucose levels. If fungal meningitis is suspected, an India-ink stain and cryptococcal antigen screen should be requested. If tuberculous meningitis is suspected, multiple 5 mL samples of CSF will be required to increase the likelihood of a positive result. If there has been prior administration of antibiotics, a bacterial antigen screen should also be requested.
Turbid CSF is indicative of a significant number of pus cells and is an indication for immediate administration of antibiotics. The patient should usually rest supine for a few hours after the procedure to prevent a worsening of the headache. This has been known to occur up to 24 hours following the procedure. The evidence for the benefits of enforced rest after lumbar puncture is equivocal.
The pattern of cell counts and glucose and protein levels is shown in Table 9.2.2. This can act as a guide only and the clinician needs to be guided by the complete clinical picture.
Table 9.2.2
Expected CSF values in meningitis
| Parameter | Normal range | Bacterial | Viral | Fungal or TB |
| Pressure (cm H20) | 5–20 | >30 | Normal or mildly raised | |
| Protein (g/L) | 0.18–0.45 | >1.0–5.0 | <1.0 | 0.1–0.5 |
| Glucose (mmol/L) | 2.5–3.5 | <2.2 | Normal | 1.6–2.5 |
| Glucose ratio – CSF/serum | 0.6 (0.8 in infants) | <0.4 (allow 2–4 hour equilibration) | 0.6 | <0.4 |
| White cell count/μL | <3, usually lymphocytes (if the tap is traumatic, allow 1 WBC for every 1000 RBC) | >500 (90% PMN) | <1000, predominantly monocytes (10% are>90% PMN, 30–40%>50% PMN) | 100–500 |
| Gram stain | No organisms | 60–90% positive | No organisms |
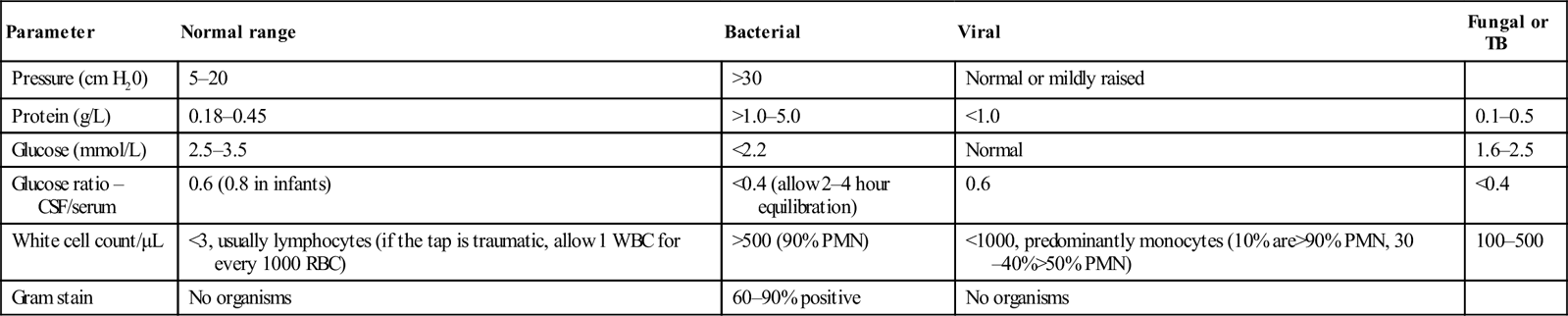
A leucocyte count (WCC) of more than 1000/μL with a predominantly neutrophilic pleocytosis is considered positive for bacterial meningitis. Ten per cent of cases, especially early in the course of the illness, may have a predominance of lymphocytes. As a general rule, bacterial meningitis is characterized by a raised CSF protein and a low CSF glucose level. The ratio of CSF to serum glucose levels is also lowered. The combination of CSF glucose<1.9 mmol/L, CSF to serum glucose ratio<0.23, CSF protein>2.2 g/L and either a total WCC>2000/μL or a neutrophil count of>1180/μL has been shown to have a 99% certainty of diagnosing bacterial meningitis [11]. Aseptic meningitis will often have cell counts near the normal range. This does not exclude infection with less common agents, such as herpes viruses or L. monocytogenes.
CT scan
CT scanning of the brain is indicated as a prelude to lumbar puncture in the presence of focal neurological signs, mental obtundation or abnormal posturing. It must be noted though, that a normal CT does not exclude the risk of cerebral herniation in bacterial meningitis [12] and, therefore, those with the above signs should have lumbar puncture delayed until they are conscious and stable.
Microbiology
Apart from microscopy and culture of CSF, there are a number of other methods that may allow the causative organism to be identified.
Skin lesion aspirate
In cases where a petechial rash is present, Gram staining or culture from some of the skin lesions may yield the causative organism. This has a reported sensitivity of 30–70%.
Throat swab
Throat swabs are useful in identifying a bacterial cause spread by nasopharyngeal carriage and should be performed in a case of suspected bacterial meningitis.
Polymerase chain reaction
This potentially allows identification of the causative organism and even the serotype for organisms, such as meningococcus. The test can be performed on CSF or EDTA blood samples and may remain positive for up to 72 hours after the commencement of antibiotics. In CSF, the reported sensitivity is 89% with a specificity of 100% and in blood a sensitivity of 81% with a specificity of 97% [13].
Serology
Tests to detect IgM to specific organisms are available for meningococcus and some viruses. For meningococcus, the test has a sensitivity and specificity of 97% and 95%, but is only reliable in adults and children over 4 years old and takes 5–7 days after onset of the illness to reach diagnostic levels.
Antigenic studies
Latex agglutination, immunoelectrophoresis or radioimmunoassay techniques can be used to screen for antigens from S. pneumoniae, Hib, group B streptococcus (S. agalactiae), Escherichia coli K1, N. meningitidis and C. neoformans. The tests can be performed on serum, CSF or urine. Serum or urine samples tend to allow greater sensitivities (around 96–99%) than CSF (82–99%). The test is no more sensitive in untreated cases than either a positive Gram-stain or the presence of CSF pleocytosis [14]. The main purpose of antigenic studies is in allowing rapid identification of the causative organism in cases confirmed by the CSF findings or in cases where partial treatment with antibiotics renders the CSF sterile on culture. In many laboratories, these tests have been superseded by PCR methods.
General investigations
Full blood count (FBC), urea and electrolyte counts (UEC), blood cultures, erythrocyte sedimentation rate (ESR) and a throat swab can assist in building an overall picture.
Blood cultures should be taken prior to parenteral antibiotics, especially in patients where lumbar puncture has been delayed. One study found that blood cultures grew the causative organism in 86% of proven cases of bacterial meningitis and that the combination of blood culture, CSF Gram staining and antigen testing identified the cause in 92% of cases [15].
Differential diagnosis
 Generalized viral infections, with meningism as a component.
Generalized viral infections, with meningism as a component.
 Brain abscess: this tends to produce focal signs due to local pressure at the site of the abscess.
Brain abscess: this tends to produce focal signs due to local pressure at the site of the abscess.
 Focal cerebral infections, such as those due to Toxoplasma gondii in HIV/AIDS patients.
Focal cerebral infections, such as those due to Toxoplasma gondii in HIV/AIDS patients.
 Severe pharyngitis with cervical lymphadenopathy causing neck stiffness.
Severe pharyngitis with cervical lymphadenopathy causing neck stiffness.
Management
Management depends on the likely causative agents, as well as the severity of the illness.
General
Patients should rest in bed, particularly following a lumbar puncture. A quiet, darkened room will be beneficial to those with headache or photophobia. Simple analgesics may be used to treat the headache, with or without codeine. Opiates may be required in severe headache.
Sedation may be necessary if the patient is very agitated or delirious. Suitable drugs are diazepam 5–10 mg IV or midazolam 2–10 mg IV or IM, with or without the addition of an antipsychotic, such as haloperidol 5–20 mg IV or IM, or chlorpromazine 12.5–50 mg IV or IM.
Seizures should be treated appropriately, initially with a benzodiazepine, then maintenance with phenytoin or phenobarbitone. Meningitis can occasionally be associated with status epilepticus, which should be treated in the standard way.
Patients with raised intracranial pressure may need pressure monitoring and measures to reduce the pressure, such as nursing the patient 30° head up and the administration of hyperosmotic agents, such as mannitol. Hyperventilation is controversial as it may reduce intracerebral pressure at the expense of reduced cerebral perfusion. Obstructive hydrocephalus requires appropriate neurosurgical treatment with CSF shunting.
If septic shock has intervened, it should be treated in the usual way, with IV fluids and inotropes.
Antimicrobials
The choice of antimicrobial agent will be determined by the likely causative organism and is therefore determined primarily by age and immune status. It is important that antibiotic therapy is not delayed by investigations such as lumbar puncture or CT and should be administered as soon as the diagnosis is made. Table 9.2.3 shows the recommended choice of antimicrobial for different situations and organisms. Table 9.2.4 shows the recommended dosage of each. As a general rule, the combination of a third-generation cephalosporin and benzylpenicillin will cover most organisms in all age groups. It is important to note that there is emerging resistance to penicillins in S. pneumoniae (currently 7.6% of isolates in Australia). If Gram-positive diplococci are found or S. pneumoniae is identified on antigen or PCR testing, vancomycin should be added to the therapy.
Table 9.2.3
Choice of antimicrobial in meningitis [16]
| Organism | First-line drug | Second-line drug | Duration |
| Pre-hospital | Benzylpenicillin | ||
| Neonates | Ampicillin PLUS cefotaxime PLUS vancomycin | ||
| Organism unknown | Cefotaxime or ceftriaxone PLUS benzylpenicillin | 7–10 days | |
| H. influenzae type b | Cefotaxime or ceftriaxone | Ampicillin or chloramphenicol | 7–10 days |
| N. meningitidis | Benzylpenicillin or cefotaxime or ceftriaxone | Ciprofloxacin or chloramphenicol | 5–7 days |
| S. pneumoniae | Benzylpenicillin | Cefotaxime or ceftriaxone or vancomycin | 10 days |
| L. monocytogenes | Benzylpenicillin | Trimethoprim+sulfamethoxazole | 3–6 weeks |
| C. neoformans | Amphotericin PLUS flucytosine | Fluconazole | 4–6 weeks |
| Herpes simplex | Acyclovir | 14 days |
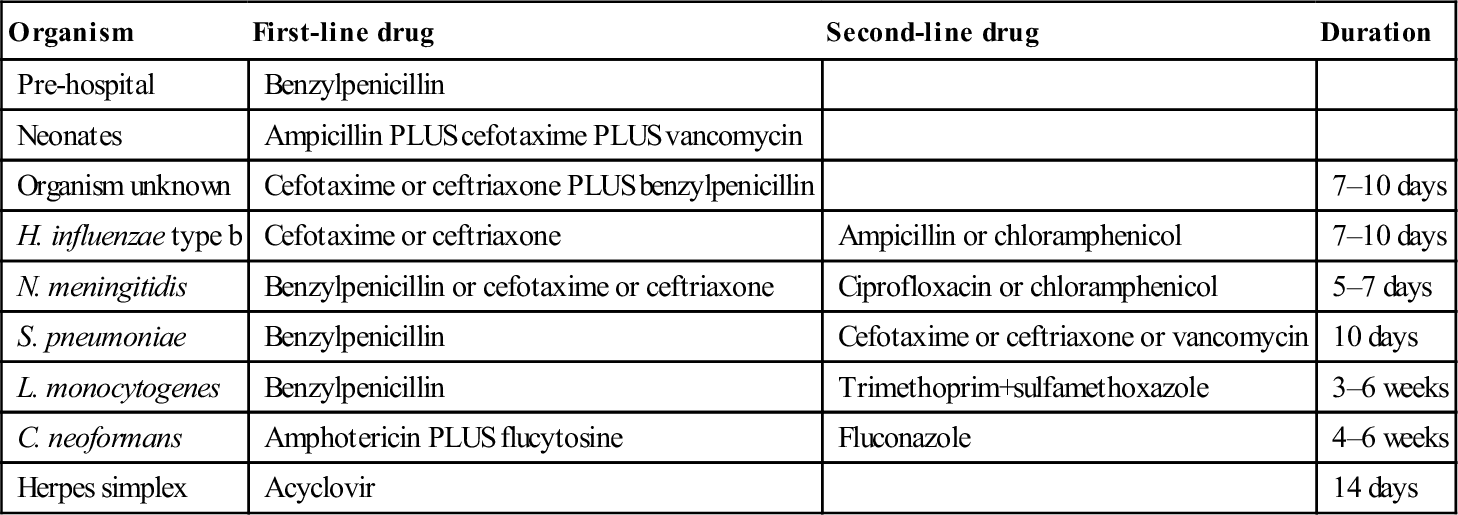
After eTG complete [internet]. Melbourne: Therapeutic Guidelines Limited; 2013 July with permission.
Table 9.2.4
Antibiotic doses in treating meningitis [17]
| Antibiotic | Adult dose | Child dose | Route | Frequency |
| Cefotaxime | 2 g | 50 mg/kg | IV | q 6 h |
| Ceftriaxone | 4 g | 100 mg/kg | IV | Daily |
| Benzylpenicillin | 2.4 g | 60 mg/kg | IV | q 4 h |
| Ampicillin | 50 mg/kg | IV | q 6 h | |
| Trimethoprim+sulphamethoxazole | 160+800 mg | 4+20 mg/kg | IV | q 6 h |
| Chloramphenicol | 1 g | 25 mg/kg | IV | q 6 h |
| Acyclovir | 10 mg/kg | 20 mg/kg in full-term neonates, 10 mg/kg otherwise | IV | q 8 h |
| Amphotericin B | 0.7–1 g/kg | 0.7–1 g/kg | IV | Daily |
| Flucytosine | 25 mg/kg | 25 mg/kg | IV or PO | q 6 h |
| Vancomycin | 1.5 g | 30 mg/kg (if under 12 years old) | IV | q 12 h |
| Ciprofloxacin | 400 mg | 10 mg/kg | IV | q 12 h |
| Moxifloxacin | 400 mg | 10 mg/kg | IV | q 12 h |
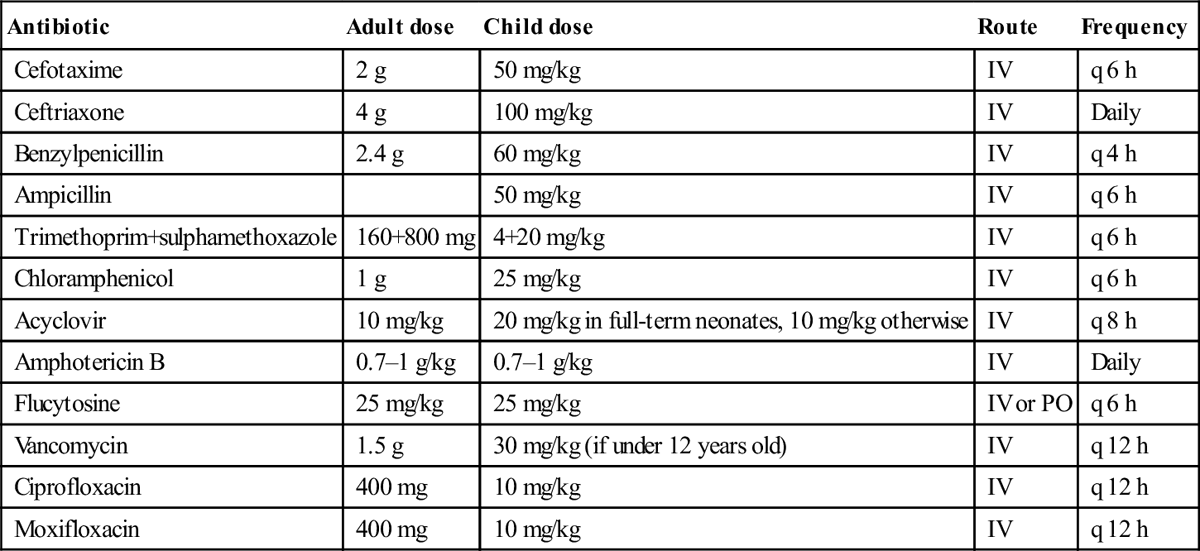
After eTG complete [internet]. Melbourne: Therapeutic Guidelines Limited; 2013 July with permission.
Steroids
Steroids have been shown to improve the prognosis of bacterial meningitis in both adults and children. There is a reduction in complications, such as sensorineural deafness and short-term neurological deficits (in high-income countries). The most benefit appears to be derived with infections from H. influenzae and S. pneumoniae. No clear mortality benefit has been established. Steroids are usually administered as dexamethasone 0.15 mg/kg IV q 6 h (up to 10 mg), started before or with the first dose of antibiotics and continued for 4 days. The main adverse effect is gastrointestinal bleeding, which may be reduced by limiting treatment to 2 days [17].
Disposition
All cases of bacterial meningitis require admission for IV antibiotics, as well as supportive therapy. They often require intensive therapy, especially if septic shock has supervened. Viral meningitis will usually require supportive therapy only, but this may require admission. Mild cases of viral or aseptic meningitis, with a clear diagnosis, can be safely sent home.
Prognosis
Over the last 20 years, the mortality of bacterial meningitis has ranged from 6 to 20% and is higher in the very young or the very old. Meningitis in immunocompromised individuals carries a high mortality of up to 50%. Bacterial meningitis in children can lead to a number of long-term sequelae, such as sensorineural hearing loss, learning difficulties, motor problems, speech delay, hyperactivity, blindness, obstructive hydrocephalus and recurrent seizures. These sequelae are less common in adults.
Prevention
Prophylaxis should be offered in cases of H. influenzae type b, or Meningococcus infection to:
 passengers adjacent to the index case on a trip of 8 hours’ or longer duration
passengers adjacent to the index case on a trip of 8 hours’ or longer duration
 healthcare workers who have given mouth-to-mouth resuscitation to an index case
healthcare workers who have given mouth-to-mouth resuscitation to an index case
■ ciprofloxacin 500 mg orally as a single dose – preferred for females on oral contraceptives
■ ceftriaxone 250 mg (125 mg in children<12 years IM in 1% lignocaine – preferred in pregnant women
■ rifampicin 600 mg orally 12-hourly for 2 days (5 mg/kg in neonates<1 month, 10 mg/kg in children).
■ rifampicin 600 mg orally daily for 4 days (10 mg/kg in neonates<1 month, 20 mg/kg in children)
■ ceftriaxone 1 g IM daily for 2 days (50 mg/kg in children)
Casual, neighbourhood or hospital contacts are not required to receive prophylaxis.
Meningococcal vaccine should be considered in populations where cases are clustered. The vaccine is currently only available for serogroup C.
9.3 Septic arthritis
Trevor Jackson and Varadarajulu Suresh
Introduction
Septic arthritis is defined as bacterial infection of the synovial space. The knee is the most commonly affected joint in adults and the hip joint in the paediatric age group [1].
Aetiology, pathogenesis and pathology
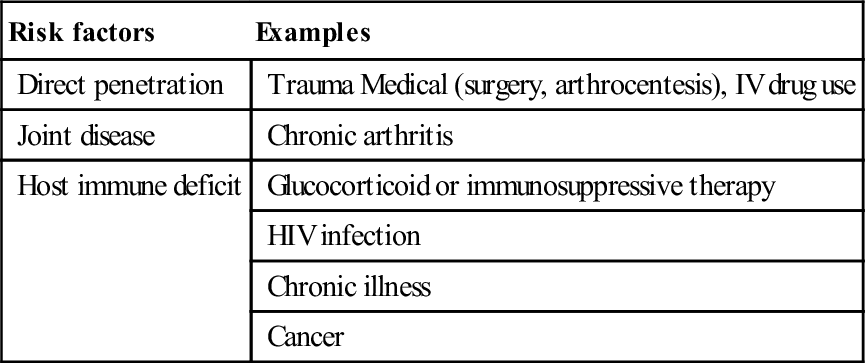
Septic arthritis can be caused by haematological spread or direct invasion. Bacteria are the usual pathogens by haematogenous seeding of the joint. Direct spread from adjacent infection or via trauma are less common. Once inside the joint, bacterial growth and invasion can occur uninhibited. Phagocytic and neutrophil responses to the bacteria lead to proteolytic enzyme release and cytokine production, resulting in synovial abscess formation and cartilage necrosis [2]. Co-morbidity or deficient host defences are risk factors for infection [3] and can be associated with more rapid and severe disease (Table 9.3.1).
Table 9.3.1
Risk factors for septic arthritis
| Risk factors | Examples |
| Direct penetration | Trauma Medical (surgery, arthrocentesis), IV drug use |
| Joint disease | Chronic arthritis |
| Host immune deficit | Glucocorticoid or immunosuppressive therapy |
| HIV infection | |
| Chronic illness | |
| Cancer |
The majority of cases are community acquired and occur in children and young adults [4]. Prosthetic joint surgery and invasive management of chronic arthritis are factors in the increased prevalence observed in older age groups.
Epidemiology
The incidence of proven and probable septic arthritis in Western Europe is 4–10 per 1 00 000 patients per year. This is more in lower socioeconomic groups in both Northern Europe and Australia.
The prevalence is 29 cases per 1 00 000 of the Aborigine population with a relative risk of 6.6 compared with the white Northern Territory Australian population.
The incidence of septic arthritis is increasing and is linked to an increase in orthopaedic-related infection, an ageing population, more invasive procedures being under taken and an enhanced use of immunosuppressive treatment [5].
Clinical features
History
This will usually reveal the recent onset of a painful, hot and swollen joint, most commonly the hip or knee, although any joint may be affected. Systemic features of fever or rigors should be sought, plus the presence of any risk factors.
Examination
Typical findings include a hot, tender joint with marked limitation of passive or active movement owing to pain. An effusion will be evident in most cases. A polyarticular presentation is more common in gonococcal infection or in the setting of chronic arthritis. In general, fever is low grade and few patients will appear ‘toxic’ and unwell. The elderly and immunosuppressed may present non-specifically with anorexia, vomiting, lethargy or fever.
Differential diagnosis
Non-septic arthritis or synovitis may be differentiated on clinical features and joint fluid analysis. Fractures will generally be evident on joint radiographs, but detection of osteomyelitis may require more advanced imaging techniques, such as nuclear or computed tomography (CT) scanning. Rheumatic fever and brucellosis are rare causes.
Clinical investigations
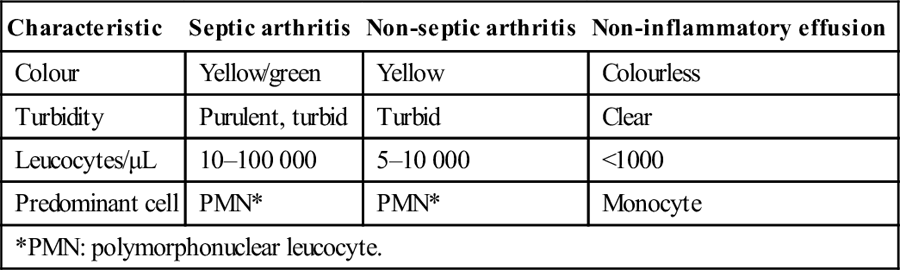
Synovial fluid examination and culture
Aspiration should be performed promptly with local anaesthetic and a large-bore needle for cell count, crystals, Gram stain and culture to confirm the diagnosis. Typical findings in septic arthritis and its differential diagnoses are shown in Table 9.3.2[6].
Table 9.3.2
Synovial fluid characteristics
| Characteristic | Septic arthritis | Non-septic arthritis | Non-inflammatory effusion |
| Colour | Yellow/green | Yellow | Colourless |
| Turbidity | Purulent, turbid | Turbid | Clear |
| Leucocytes/μL | 10–100 000 | 5–10 000 | <1000 |
| Predominant cell | PMN* | PMN* | Monocyte |
| *PMN: polymorphonuclear leucocyte. | |||
Most infections are acute and bacterial (Table 9.3.3) [6], although fungal and mycobacterial pathogens have been recognized in chronic infections.
Table 9.3.3
Bacterial causes of septic arthritis
| Age group | Typical bacteria |
| Children | Staphylococcus aureus |
| Group A streptococci (B in neonates) | |
| Haemophilus influenza | |
| Young adults | Neisseria gonorrhoeae Staphylococcus aureus |
| Older adults | Staphylococcus aureus |
| Gram-negative species* | |
| Group A streptococci |
Other laboratory investigations
Blood cultures should always be taken and may be positive in up to 50%. Inflammatory markers (erythrocyte sedimentation rate and C-reactive protein) are elevated, with typically a neutrophil-predominant leucocytosis. These are non-diagnostic, but aid in monitoring response to therapy.
Imaging studies
Plain radiographs should be performed in all cases: they may reveal effusions or local oedema and help to exclude alternative conditions. Ultrasound is very sensitive in detecting effusions and excellent for facilitating needle aspiration.
Fluoroscopy may also be used. Nuclear medical studies are very sensitive early, but not specific for sepsis. CT and magnetic resonance imaging (MRI) have a small role in difficult joints (e.g. hip and sacroiliac).
Criteria for diagnosis [7]
This depends on positive culture of synovial fluid from an affected joint, a positive Gram stain or blood culture in the context of an inflamed joint suspicious of sepsis, macroscopic pus aspirate and appropriate response to antibiotics.
Management
Joint drainage and empiric parenteral antibiotic therapy must take place without delay. Surgical drainage is usually employed in children, with needle drainage more commonly first line in adults. Newer arthroscopic techniques are increasingly being used [2,8–10]. Repeated drainage procedures will often be necessary to ensure complete resolution of the infection.
Antibiotic therapy is initiated after culture specimens have been obtained, with clinical presentation and Gram stain guiding the choice of agents. All regimens must include an antistaphylococcal agent with Gram-negative cover as indicated by the clinical setting.
Suggested initial empiric regimen [11]
Di(flu)cloxacillin: 2 g (25–50 mg/kg up to 2 g) intravenously, 6-hourly. If Gram-negative bacteria are suspected, add ceftriaxone 2 g (25–50 mg/kg up to 2 g) intravenously daily. If methicillin resistance is suspected, add vancomycin 1 g (25 mg/kg) intravenously 12-hourly.
Definitive therapy will be tailored to later laboratory identification of the organism and its sensitivities.
The duration and route of therapy remain controversial but, in uncomplicated acute cases, parenteral antibiotics will be required for at least 3 days in children and 2 weeks in adults, with a total treatment duration of 3–6 weeks [10,12]. Specific organisms, such as Neisseria spp., will respond more rapidly, whereas chronic infections and co-morbidity will necessitate aggressive and more prolonged therapy.
General care, with initial joint rest, appropriate analgesia and physical therapy, is important. All patients require admission until their joint sepsis is controlled. Thereafter, ongoing therapy may be monitored as an outpatient or via domiciliary hospital services.
Prognosis
This depends upon the organism, patient co-morbidity and the adequacy and rapidity of treatment. Gonococcal and paediatric infections have a generally good response, with low rates of ensuing joint morbidity. Polyarticular sepsis in rheumatoid arthritis has been associated with mortality rates of up to 15% and major morbidity in up to 50% of survivors [2,6,12].
Prevention
Safe sexual practice can reduce gonorrhoeal infections. Strict aseptic technique, good patient selection and prophylactic antibiotics help prevent cases associated with invasive joint procedures. The overall incidence of infection after arthroplasty ranges from 0.5 to 2% [2].
Table 9.3.4
Likely developments in future [14]
| Synovial markers to distinguish septic arthritis from other sources of non-traumatic joint pain may become available to the emergency physician |

After Carpenter CR, Schuur JD, Everett WW, Pines JM. Evidence-based diagnostics: adult septic arthritis. Acad Emerg Med 2011;18:781–96.
9.4 Urinary tract infections
Salomon Zalstein
Introduction
Urinary tract infections are the most common bacterial infections and the major cause of Gram-negative sepsis in hospitalized patients [1,2].
Definitions
Urinary tract infection
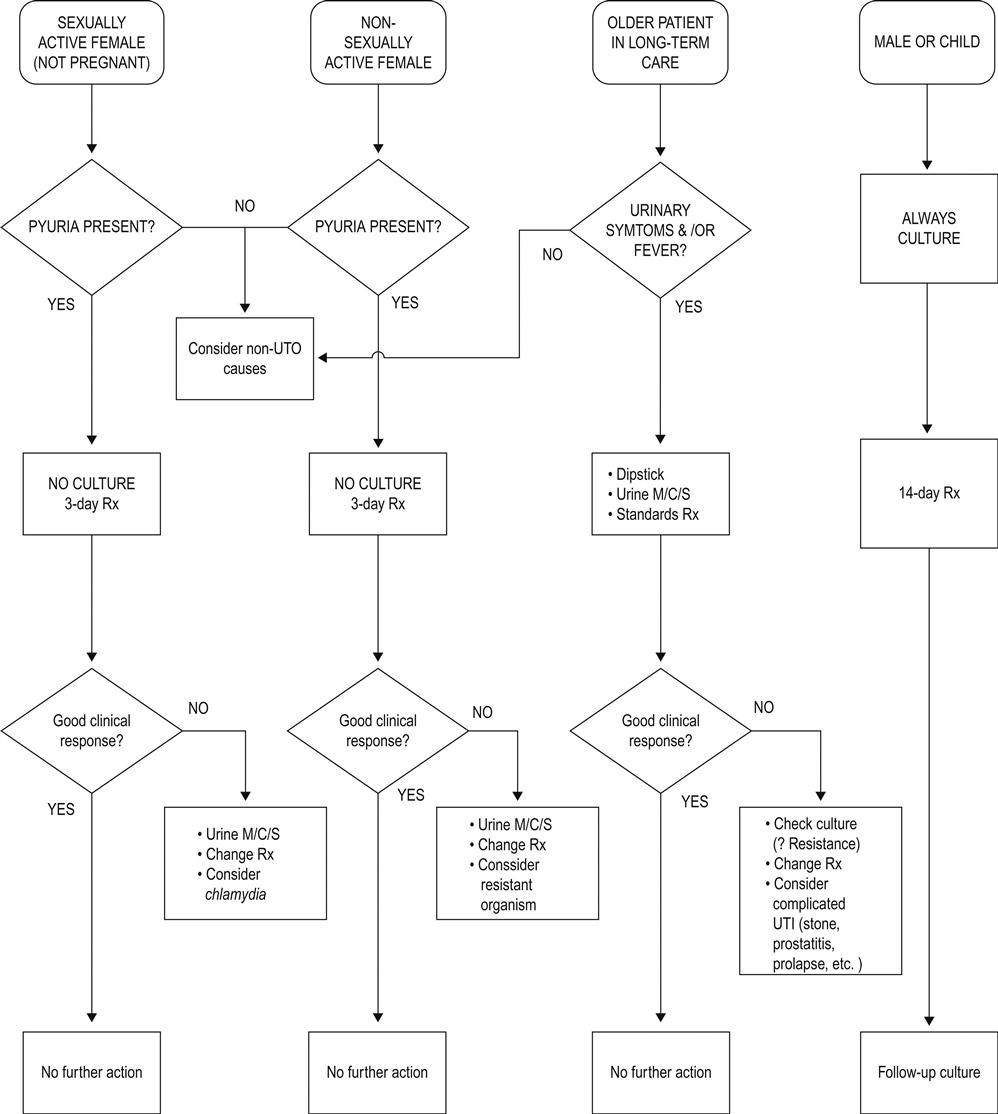
The term urinary tract infection (UTI) is non-specific and may refer to a variety of clinical conditions, including asymptomatic bacteriuria, urethritis, cystitis, female urethral syndrome and acute and chronic pyelonephritis. The most common clinical presentations are cystitis and acute pyelonephritis, although the clinical distinction between these diagnoses may not be as straightforward as the terms imply, with up to 50% of patients having unrecognized pyelonephritis [3].
UTI is considered in two main groups: simple (or uncomplicated) and complicated. Simple UTIs occur in an otherwise healthy person with a normal urinary tract, most commonly a young non-pregnant female. A complicated UTI is one associated with anatomical abnormality, urinary obstruction or incomplete bladder emptying due to any cause: instrumentation or catheterization, pregnancy or significant underlying disease, such as immunosuppression or diabetes mellitus.
Significant bacteriuria
Significant bacteriuria most commonly refers to more than 105 bacteria/mL of urine, reported as colony forming units per mL (cfu/mL). This usually represents infection as opposed to contamination (see Quantitative culture), although there are significant exceptions to this generalization (see Urethral syndrome).
Asymptomatic bacteriuria
Asymptomatic bacteriuria (ASB) refers to significant bacteriuria in the absence of symptoms of infection.
Epidemiology
UTIs are very common, particularly in women in whom age, degree of sexual activity and the form of contraception used are all factors that affect the incidence and prevalence of infection. While the overall rate of infection is difficult to estimate since UTI is not a reportable disease, in a USA health survey, the self-reported incidence of UTI is 12.1% among women and 3% among men. By age 32, 50% of women will report at least one UTI [4]. In non-pregnant women aged 18–40 years, the rate of infection has been stated to be between 0.5 and 0.7 per person per year, with much higher rates in pregnancy [5].
In males, the prevalence of bacteriuria beyond infancy is 0.1% or less. Between the ages of 21 and 50, infection rates may be as low as 0.6–0.8/1000 [6]. With increasing prostatic disease, the frequency of bacteriuria may rise to 3.5% in healthy men and to more than 15% in hospitalized men by age 70 [7]. Homosexual men are at increased risk of UTI.
In the presence of chronic disease and institutionalization in the elderly, the incidence of bacteriuria may be as high as 50%, although this is most commonly asymptomatic [8].
Aetiology
The aetiology of uncomplicated UTI has remained unchanged for decades, although increased antibiotic resistance in the bacteria responsible has been well documented. In community-acquired UTI, Escherichia coli accounts for 75–90% of cases, Staphylococcus saprophyticus accounts for 5–15% (especially in young, sexually active women), with enterococci and Gram-negative organisms, such as Klebsiella spp. and Proteus mirabilis, responsible for 5–10% [9,10]. Which bacteria are isolated is influenced by factors such as whether the infection is initial or recurrent; the presence of obstruction, instrumentation or anatomical abnormalities; and whether the patient is an inpatient or outpatient. In simple acute cystitis, the most common presentation of UTI, a single organism is usually isolated. On the other hand, in complicated UTI, E. coli is isolated in 20–50% of cases and non-E. coli organisms, such as Proteus and Klebsiella species, are more commonly seen. In the presence of structural abnormalities, it is more common to isolate multiple organisms and antibiotic resistance is frequently found [10].
Pathogenesis
In healthy individuals, the perineum, vagina, vaginal introitus and urethra and periurethral areas each have their respective flora and are normally colonized by bacteria different from those commonly associated with UTI, that is by non-pathogens. The periurethral area may become colonized by such UTI-causing (uropathogenic) bacteria, which then ascend via the urethra into the bladder and thence may ascend further to the kidney, causing pyelonephritis. The reservoir for these bacteria is the gastrointestinal tract [4]. There are host and bacterial mechanisms involved in determining whether a UTI will occur.
Host mechanisms
Anatomic considerations (men) and prostatic secretions
In males, the length of the urethra, its separation from the anus and the presence of prostatic secretions all contribute to the prevention of colonization and subsequent UTI.
Sexual activity, contraceptive practices, use of diaphragm/spermicides
Sexual activity is the most important risk factor for acute cystitis, with recent or frequent sexual activity increasing that risk. The use of a diaphragm with a spermicide (an inhibitor of normal vaginal flora) promotes vaginal colonization with uropathogenic bacteria and has also been shown to increase the risk of UTI [4].
Secretor/non-secretor status
Blood group antigens are secreted in the body fluids by some women. The urethral and periurethral mucosae in women who do not secrete these antigens (non-secretors) in their body fluids, have a higher affinity for bacterial adhesins (see below) than the mucosae of women who do. These non-secretors are more susceptible to recurrent infections [11].
Entry of bacteria into the bladder
Instrumentation of the bladder (see below) is a well-recognized mechanism by which bacteria are introduced into the bladder. Other factors have been considered but have not been conclusively demonstrated. These include frequency and timing of voiding, hormonal changes and personal hygiene habits [12].
Bladder defence mechanisms
The healthy bladder can normally clear itself of bacteria. There are three factors involved: voiding; urinary bacteriostatic substances, such as organic acids, high urea concentrations and immunoglobulins; and active resistance by the bladder mucosa to bacterial adherence.
Obstruction
This may be extrarenal (congenital anomalies, such as urethral valves, calculi, benign prostatic hypertrophy) or intrarenal (nephrocalcinosis, polycystic kidney disease, analgesic nephropathy). Complete obstruction of the urinary tract predisposes to infection by haematogenous spread. In the absence of such obstruction, haematogenous seeding of bacteria to the kidneys accounts for about 3% of infections. Partial obstruction does not have this effect.
Vesicoureteric reflux
Incompetence of the vesicoureteric valve is a congenital problem that is five times more common in boys than in girls, but tends not to be a significant factor in adults. It allows infected urine to ascend to the kidney and is the most common factor predisposing to chronic pyelonephritic scarring.
Instrumentation
Although any instrumentation of the urinary tract predisposes to infection, catheterization is the most common of these. A single catheterization will result in UTI in 1% of ambulatory patients but, in hospitalized patients, 10% of women and 5% of men will develop a UTI after one catheterization. Once in place, catheters produce infection in up to 10% of patients per day and nearly all catheterized patients will be bacteriuric by 1 month [13]. All chronically catheterized patients are bacteriuric.
Pregnancy
Changes to the urinary tract occur normally during pregnancy as a result of both anatomical alterations and hormonal effects: dilatation of the ureters and renal pelves, decreased peristalsis in the ureters and decreased bladder tone. These changes begin before the end of the second month. The prevalence of bacteriuria rises with age and parity. A large proportion of asymptomatic bacteriuric women develop symptomatic pyelonephritis later in that pregnancy, with significant increases in toxaemia and prematurity (see Asymptomatic bacteriuria).
Diabetes mellitus
The relationship between diabetes mellitus on the one hand and asymptomatic bacteriuria and UTI on the other has been debated in the past. Current evidence indicates that asymptomatic bacteriuria is more common in diabetic women than non-diabetic women. The evidence in men is less clear cut. Good evidence from prospective studies for increased incidence of symptomatic urinary tract infection in diabetics is lacking. What appears clear is that diabetes is a significant and independent risk factor for pyelonephritis, complicated UTI, urosepsis, hospitalization and other, often rare, complications (such as emphasematous pyelonephritis, papillary necrosis and candidal infections). The precise pathogenetic mechanism is unclear but involves many factors not necessarily related to glycaemic control [14–16].
Ageing
UTI is the most frequent bacterial infection in residents of long-term care facilities. Asymptomatic bacteriuria is highly prevalent in residents of long-term care facilities with up to 30% of men and 50% of women showing such bacteriuria. The likelihood of bacteriuria correlates with the degree of functional impairment. Several factors may be involved: chronic degenerative neurological diseases may impair bladder function as well as bladder and bowel continence, prostatic enlargement in men and oestrogen deficiency in women can both lead to incomplete bladder emptying, the use of devices, such as indwelling catheters or condom drainage, predisposes to bacteriuria [8].
Bacterial factors
A number of studies [17–19] have shown that the strains of E. coli (and a number of other Gram-negative bacteria) that cause UTI are not just the most prevalent in the bowel of the patient at the time of the infection, but have specific characteristics, termed virulence factors, that give them certain capabilities: increased intestinal carriage, persistence in the vagina and the ability to ascend and invade the normal urinary tract. Thus, there are clearly uropathogenic strains of these bacteria. In cases of complicated UTI (e.g. those associated with reflux, obstruction or foreign body), these virulence factors are not significantly involved.
Presentation
History
A careful history should be taken in any patient presenting with symptoms of apparent UTI, looking for risk factors for complicated or recurrent infection (such as previous UTIs and their treatment, the presence of known anatomical abnormalities and investigations or instrumentation, the possibility of pregnancy and history of diabetes mellitus), as well as seeking to identify those patients with urethritis and vaginitis. In men, the most common cause of recurrent lower tract UTI is prostatitis, so evidence of prostatitis, such as chills, dysuria and prostatic tenderness, should be sought.
Lower tract infections (cystitis) typically present with irritative micturition symptoms, such as dysuria and frequency, suprapubic discomfort and, sometimes, macroscopic haematuria. There is usually no fever. Women presenting with dysuria and frequency without vaginal discharge or irritation have a 90% probability of cystitis [20]. The classic symptom complex of loin pain, fever (>38°C), chills and urinary symptoms is usually associated with pyelonephritis. Severe pain should raise the suspicion of a ureteric calculus that, combined with infection, poses a greater risk of sepsis and of permanent injury to the kidney.
Patients with chronic indwelling catheters usually have no lower tract symptoms at all, but may develop loin pain and fever.
In elderly patients, particularly in long-term care facilities, the long-held view that symptoms of increased confusion and reduced mobility in the absence of fever, are due to urinary tract infection has been cast into doubt (see Treatment of specific groups: elderly patients) [8].
Examination
The clinical signs of lower UTI are few and non-specific, however, patients should be examined to exclude other causes for their symptoms, particularly vaginitis in women and prostatitis in men. The presence of renal angle tenderness, associated with fever, chills and dysuria suggests pyelonephritis.
Investigations
The key step in the diagnosis of UTI is examination of the urine, most commonly a midstream specimen (MSU). Catheterization is appropriate in patients with altered mental state or who cannot void because of neurological or urological reasons. Suprapubic aspiration is commonly used in paediatric practice but can be used in adults if other techniques have failed or are unable to be used.
The next step is to look for the presence of pyuria and, subsequently, the specimen may be sent for quantitative culture and antibiotic sensitivity testing. Testing for haematuria, proteinuria and nitrites may be of supportive value but is not diagnostic.
Reagent test strips
In considering the use of reagent strips in the diagnosis of UTI, it should be noted that variations in published sensitivity and specificity exist and are due to: (1) the use of different brands of reagent strips; (2) the use of different ‘gold standards’ against which comparison is made (e.g. counting chamber or cells/HPF counts, ‘cut-off’ criterion of the test used); (3) the nature of the study (blinded, unblinded); (4) the reader of the test (lab worker, doctor, nurse); and most importantly, (5) the clinical setting or target population (e.g. symptomatic emergency department patients rather than an asymptomatic population in a clinic or office environment) – in other words, the pre-test probability.
A reagent strip test for leucocyte esterase is now the most common screening test for pyuria (see below). Taken alone, this has a sensitivity of 48–86% and a specificity of 17–93% for detecting pyuria (as defined below). A positive predictive value (in symptomatic individuals) of 50% and a negative predictive value of 92% makes it a valuable test for screening the emergency department population. Most studies indicate that when the combination of leucocyte esterase and nitrite is considered, the sensitivity of the test is 68–88% and a negative test excludes the presence of infection [21]. Recent work by Sultana and others has shown that reagent strips significantly improve the clinician’s accuracy in diagnosing UTI in symptomatic emergency department patients [22]. The clinical probability of UTI must be considered when using such screening tests. In the patient with typical urinary tract symptoms, it may provide an adequate screen. It should, however, be used with great caution in the presence of fever of unknown cause in the elderly, the patient with an indwelling catheter or the patient with an impaired mental state, as pyuria and the implied bacteriuria may not be the cause of the problem.
Pyuria
Pyuria, indicates inflammation in the urinary tract and, as an indicator of infection, is second only to bacteriuria determined by quantitative culture (see below). The ‘gold standard’ definition of pyuria is based on early work involving the measurement of the rate of excretion of polymorphs in the urine. This work showed that excretion of 400 000 polymorphs per hour was always associated with infection and was also found to be represented by 10 polymorphs per mm3 in a single (unspun) midstream specimen of urine [23]. Thus, ‘significant pyuria’ was defined as 10 000 polymorphs per mL of urine. It was subsequently shown that more than 96% of symptomatic patients, defined as having significant bacteriuria, had significant pyuria and conversely less than 1% of asymptomatic people without bacteriuria have this degree of pyuria. Other definitions of pyuria, such as>5 leucocytes/high power field are based on examination of either the urinary sediment or of centrifuged urine and are inherently inaccurate because they cannot be standardized, but are nevertheless often used [24]. ‘Sterile’ pyuria indicates the presence of significant pyuria without the presence of bacterial growth in standard culture (Table 9.4.1).
Table 9.4.1
Common causes of sterile pyuria
Non-specific urethritis in males
Prostatitis
Renal tract neoplasm
Renal calculi
Catheterization
Renal TB
Previous antibiotic treatment
Nitrites
This reagent strip-based test is dependent on the bacterial reduction of urinary nitrate to nitrite, a function of coliform bacteria but not of Enterococcus spp. nor S. saprophyticus. The test has a low sensitivity (45–60%), better specificity (85–98%) but a high false-negative rate (about 45% in many studies). False-negative results are likely if the infecting organism is Gram positive or Pseudomonas, if the diet lacks nitrate or if there is diuresis or extreme frequency, as a period of bladder incubation is necessary to form nitrites.
Haematuria
Although a frequent accompaniment of UTI, this finding is non-specific as there are many other causes of haematuria.
Proteinuria
Most commonly with UTI protein excretion is less than 2 g/24 h. It is another common but non-specific finding.
Quantitative culture
Urine culture is not essential in the management of the pre-menopausal sexually active female with an uncomplicated UTI as the probability of UTI in these patients is 90% [20]. Culture should always be performed in patients with recurrent infection, possible pyelonephritis, potentially complicated UTI, males, the elderly or in cases where the cause of infection is not clinically evident. In symptomatic patients, a single specimen with a bacterial count in urine of>105 colony forming units (cfu)/mL has a 95% probability of representing infection [25]. However, it has been shown that 30–50% of women with symptoms of dysuria will have bacterial counts less than 105 cfu/mL [26]. Of these, about one-half have bacterial UTI with low numbers of bacteria. The rest may be considered in two groups; one group has urethritis due to Chlamydia trachomatis or Neisseria gonorrhoeae and the other has negative cultures and may have Ureaplasma urealyticum urethritis. In men, counts as low as 103 cfu/mL suggest infection [27].
In patients with indwelling urethral or suprapubic catheters, or those who intermittently self-catheterize and have symptoms or signs of UTI, a colony count of≥103 cfu/mL of more than one bacterial species in a single catheter or MSU specimen if the catheter has been removed within the previous 48 hours does indicate UTI [28].
Blood cultures
Blood cultures are normally not taken in afebrile patients with symptoms of cystitis. Current evidence indicates that blood cultures do not alter management and are therefore unnecessary in the majority of cases of uncomplicated pyelonephritis since the infecting organism is able to be isolated from a urine specimen [29,30]. Blood cultures should be taken in the following circumstances:
Imaging
Imaging is not required in cases of uncomplicated cystitis. In pyelonephritis, imaging should be performed if there is:
 pain suggestive of renal colic or obstruction
pain suggestive of renal colic or obstruction
 failure to defervesce within 72 hours
failure to defervesce within 72 hours
 rapid relapse on cessation of antibiotic treatment or within 2 weeks
rapid relapse on cessation of antibiotic treatment or within 2 weeks
These circumstances have been shown to be associated with stones or renal scarring. CT scanning is the preferred modality as this has greater sensitivity for demonstrating not only stones and obstruction, but rare gas-forming infections, haemorrhage and inflammatory masses.
Management
Ideally, treatment of UTI should rapidly relieve symptoms, prevent short-term complications, such as progression from cystitis to pyelonephritis and subsequent sepsis or long-term sequelae, such as renal scarring, and prevent recurrences by eliminating uropathogenic bacteria from vaginal and perineal reservoirs. Treatment should be cost-effective and have few or no side effects.
There is no evidence that non-specific treatments, such as pushing fluids or attempting to alter urinary pH, improve the outcome of normal antibiotic treatment.
Antibiotic treatment
Serum levels of antibiotics are largely irrelevant in the elimination of bacteriuria. Reduction in urinary bacterial numbers correlates with the sensitivity of the organism to the urinary concentration of the antibiotic. Inhibitory concentrations are usually achieved in the urine after oral doses of the commonly used antibiotics. On the other hand, blood levels are vitally important in the treatment of bacteraemic or septic patients or those with renal parenchymal infections. In considering antibiotic treatment, it is important to recognize the rapidly and constantly evolving antibiotic resistance patterns of common urinary pathogens. In order to ensure effective treatment, while participating in worldwide efforts to mitigate the rapid development of highly resistant forms, clinicians should always refer to the latest available guidelines. Empirically, the choice of antibiotic is based on the clinical presentation and the bacteria likely to be involved (Table 9.4.2).
Table 9.4.2
Choice of treatment depending on bacteria involved (see text)
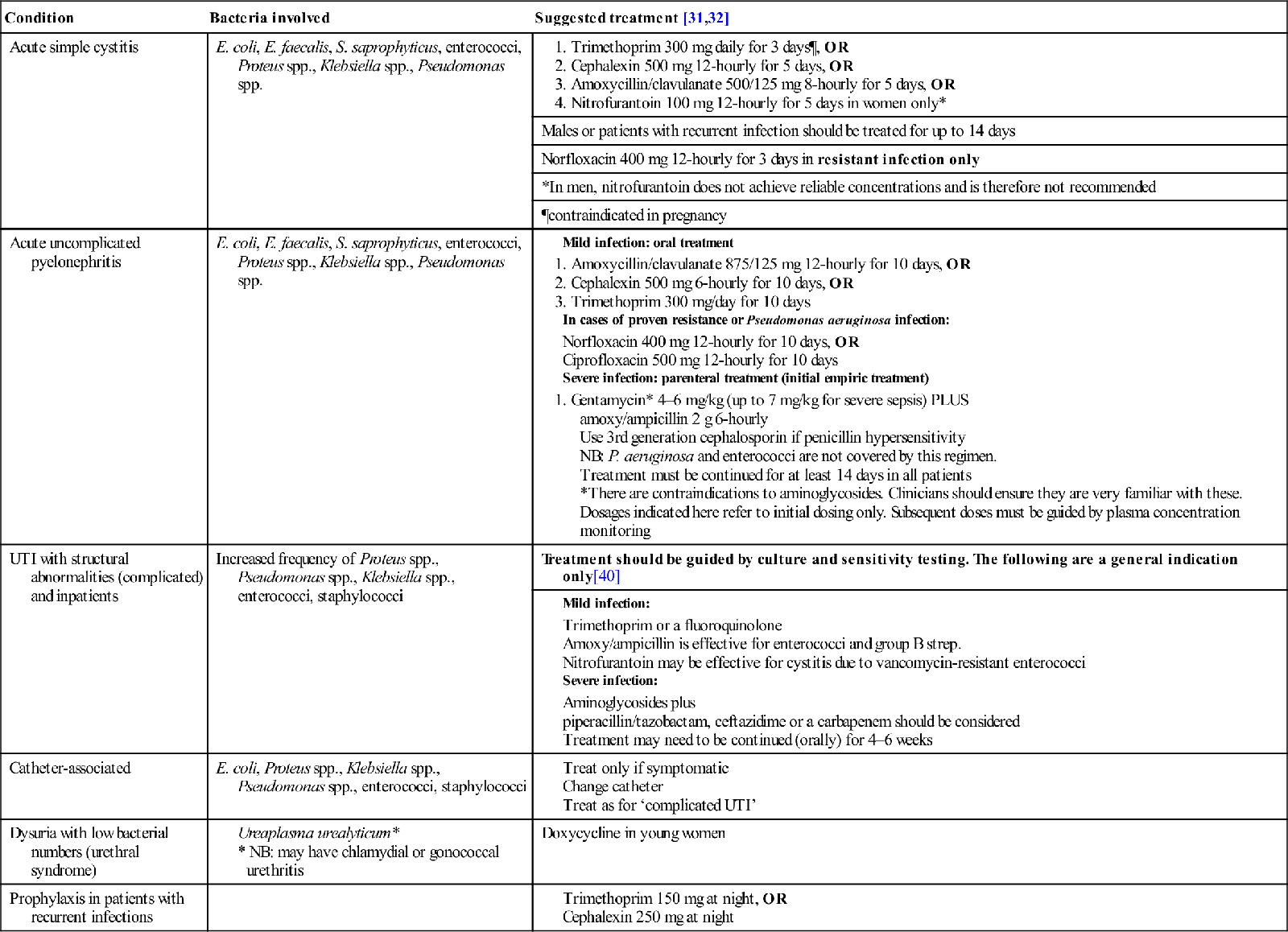
Management of specific groups (Fig. 9.4.1)
Frequency dysuria syndrome: presumed simple cystitis
A non-pregnant, non-diabetic woman first presenting from the community with typical lower urinary symptoms should have vulvovaginitis excluded and an MSU taken and examined or tested by dipstick for pyuria. If pyuria is confirmed, culture of the urine specimen is not necessary and treatment should be commenced empirically.
There is now good evidence that in this group of patients short-course treatment is effective in both treating the infection and eradicating uropathogenic strains of bacteria from reservoirs. Three-day treatment is superior to a single dose in eradicating the reservoirs of uropathogenic organisms, thereby reducing the incidence of recurrence. Longer courses have an increased incidence of side effects but not higher cure rates. The antibiotic of choice for 3-day treatment is trimethoprim [31,32]. The emergence of trimethoprim resistant uropathogens has been well documented in some communities. The subsequent overuse of fluoroquinolones as first-line agents has resulted in a rapid rate of development of resistance to these agents in parts of Europe and in North America [33,34]. In general, fluoroquinolones should not be used as first-line agents for simple cystitis [31]. Awareness of local antibiotic resistance patterns is thus an important factor in choice of the most appropriate antibiotic.
Amoxycillin/clavulanic acid, nitrofurantoin and cephalexin are suitable for 5-day therapy, but amoxycillin alone should not be used as there is a high incidence of resistant E. coli in community-acquired UTI. If there is no clinical response, MSU should be sent for culture and, in sexually active women, treatment for C. trachomatis commenced (doxycycline 100 mg bd). In non-sexually active women, further treatment is guided by the results of sensitivity testing. Short-course treatment is inappropriate in women who are at risk of upper urinary tract infection (despite lower tract symptoms), which includes those with a history of previous infections due to resistant organisms, with symptoms for more than 1 week or those with diabetes mellitus.
Males with UTI should be investigated for urinary tract abnormality, associated prostatic or epididymal infection, must have urine culture initially and should have at least 14 days of treatment with any of the agents used for treatment of young women with simple cystitis (see Table 9.4.2) [31]. In men over 50 years, there is a high probability of invasion of prostatic tissue and treatment may need to be continued for 4–6 weeks.
Recurrent UTI
Recurrent UTI is defined as a symptomatic UTI which follows resolution of a previous UTI. These may be re-infections (with the same organism or another) or relapses (regrowth of the same organism within 2 weeks of treatment). Re-infections are more common than relapses, but the two may be indistinguishable [35]. It is important to consider the risk factors specific to the age and gender of the patient, e.g. sexual activity and use of spermicides in the young pre-menopausal woman or the higher rate of asymptomatic bacteriuria in the older patient. A careful search for causes and reversible factors (e.g. of complicated UTI due to stone or obstruction, previously undiagnosed diabetes mellitus, prostatitis in males) should be made together with urine culture and sensitivity testing. Treatment is generally as for pyelonephritis, with an appropriate antibiotic guided by the results of sensitivity tests for at least 10–14 days. Female patients may benefit from post-intercourse or maintenance prophylaxis with, e.g. cephalexin 250 mg or trimethoprim 150 mg at night for several months (see Host factors). There has recently been burgeoning interest in the use of cranberry, either as juice or in tablet form, for UTI prophylaxis. Evidence is variable, but a recent Cochrane review of 24 studies concluded that cranberry cannot be recommended for UTI prevention [36].
Acute pyelonephritis
Patients presenting with the typical symptoms of pyelonephritis are at risk of bacteraemia or sepsis syndrome and therefore must rapidly have adequate concentrations of appropriate antibiotics delivered to both the blood and the urine. In order to meet this requirement, particularly in patients who are vomiting, parenteral (intravenous) treatment is usually required initially, but seldom for longer than 24–48 hours, by which time the patient is usually afebrile and not vomiting.
The choice of antibiotics is of necessity empirical at this stage. In cases of mild infection in patients who are not vomiting, 10-day treatment with one of the antimicrobials used for simple cystitis is appropriate, with ciprofloxacin or norfloxacin reserved for resistant organisms or proven Pseudomonas aeruginosa. For severe infections, parenteral ampicillin or amoxycillin (2 g 6-hourly) together with gentamicin (4–6 mg/kg and up to 7 mg/kg as initial dose for severe sepsis) are appropriate, with a third-generation cephalosporin as an alternative to gentamicin when use of aminoglycosides is contraindicated. In patients with hospital- acquired infections and suspected Gram-negative sepsis or infections with Pseudomonas aeruginosa, broader-spectrum agents, such as ceftazidime, piperacillin/tazobactam, ticarcillin/clavulanic acid and imipenem, perhaps in combination with aminoglycosides, may be required. Parenteral treatment is followed by oral therapy for 2 weeks.
The use of short-stay observation units is now a standard part of the practice of emergency medicine. The safety and efficacy of treatment of pyelonephritis in such units with intravenous antibiotics and fluid administration, followed by oral therapy is widely accepted [37]. ‘Hospital in the home (HITH)’ programmes are also now commonplace, allowing close supervision of these patients by hospital-based staff and once- or twice-daily intravenous antibiotic administration at home. The efficacy and safety of HITH is well established, but requires careful patient selection to exclude those at risk of complicated infections. Appropriate follow up is essential, although repeat urine cultures are not recommended in asymptomatic patients following simple pyelonephritis [38,39].
Pregnancy
UTI in pregnancy are associated with an increased incidence of premature delivery and low birthweight infants. This has also been demonstrated to occur with asymptomatic bacteriuria, although up to 40% of asymptomatic women develop acute pyelonephritis later in pregnancy. Therefore, screening for bacteriuria and treatment of pregnant women is essential and urine must be sent for culture and antibiotic sensitivity testing. Three-day courses of treatment are not widely recommended, although it may be reasonable to use them with close follow up in an effort to reduce antibiotic usage; however, 10-day treatment courses are the norm. Cephalexin, nitrofurantoin or amoxycillin/clavulanate are appropriate for use in pregnancy, sulphas and trimethoprim being contraindicated [31].
Complicated UTI
As there is a greater range of organisms causing infection in these circumstances and a higher probability of antibiotic resistance, urine culture is essential and initial empiric treatment must cover the broader spectrum of organisms potentially involved. If possible, antibiotic treatment should be delayed until results of urine culture and antibiotic sensitivities are known and, if empirical therapy is instituted, management should be reviewed as soon as such results are available [40]. Trimethoprim or a quinolone is appropriate for mild infections. More serious infections may need combinations of agents, such as aminoglycosides with amoxycillin or imipenem/cilastatin.
Catheter-associated UTI
Catheter-associated UTI (CA-UTI) are the most common nosocomial infections. In patients with short-term catheters who develop infection, the catheter must be removed or changed and treatment instituted as for complicated UTI. For those with chronic indwelling catheters (such as patients with spinal injuries), bacteriuria is universal and treatment is only indicated in the presence of symptoms such as fever, chills or loin pain. Patients with chronic spinal injuries may present with autonomic dysreflexia syndrome, the symptoms of which include sudden hypertension, muscle spasm and sweating, with or without fever. Antibiotic selection should again be based on culture or empirically as for complicated UTI. The most important strategy for prevention of CA-UTI is minimizing catheter use and duration whenever possible. Preventative strategies based on use of methanemine, cranberry or prophylactic antibiotics at time of catheterization or catheter change are not recommended [28,31].
Elderly patients
As previously stated, asymptomatic bacteriuria and UTI are very common in older patients and more so with increasing functional impairment. Symptomatic infection is a significant cause of morbidity and mortality as this age group also has a higher incidence of bacteraemia associated with pyelonephritis and septic shock commonly follows. Given the high rate of asymptomatic bacteriuria, the diagnosis of UTI in such individuals is difficult. The traditional view that non-specific symptoms, such as increased confusion (without fever), falling or deteriorating mobility are due to UTI has been called into question. Evidence indicates that UTI should only be considered in patients with fever or specific genitourinary symptoms or both. In patients with non-specific symptoms, non-infective causes should be sought and, in the case of fever alone, other potential sources of infection must be considered [8].
Antibiotic treatment of symptomatic UTI in the elderly patient is no different initially to that of younger patients; however, it should be borne in mind that a greater variety of organisms may be cultured in this age group and urine for culture should be obtained at the outset whenever possible.
Asymptomatic bacteriuria
Asymptomatic bacteriuria (ASB) is defined as the presence of significant bacteriuria (as previously defined) in a person without signs or symptoms of UTI. The presence of pyuria per se should not be taken to indicate bacteriuria – a quantitative culture is essential for the diagnosis. Current evidence indicates that many patient groups may be harmed, or at least not benefit from antibiotic treatment for asymptomatic bacteriuria. Antibiotics should therefore not be given to:
Conversely, two significant groups receive clear benefit from antibiotic treatment for ASB: pregnant women and patients about to undergo urological procedures in which mucosal bleeding is anticipated (e.g. TURP).
Pregnant women with ASB have a 20–30-fold increased risk of developing pyelonephritis during pregnancy with consequent premature delivery and low birthweight infants. Therefore, pregnant women should be actively screened for ASB in early pregnancy and treated as for uncomplicated cystitis (without nitrofurantoin), with repeat cultures to confirm bacterial clearance. Periodic re-testing is recommended.
Patients who undergo urological procedures with mucosal bleeding (e.g. TURP) have a 60% rate of bacteraemia, with sepsis in 6–10% of these. These patients should be screened for ASB prior to the procedure and antibiotic treatment commenced shortly prior to the procedure and continued until after the procedure or removal of the post-procedure catheter [41].
Disposition
Patients with simple UTI should have follow up to confirm clinical cure. Failure of symptomatic improvement in 48 hours may indicate antibiotic resistance, which requires urine culture to elucidate. Recurrence of symptoms within 1–2 weeks may indicate occult renal infection and necessitates urine culture and at least 7 days’ treatment.
Prognosis
In adults with normal urinary tracts, UTI does not cause long-term sequelae. In the presence of urinary-tract abnormalities, infection may be a factor in producing renal damage or altering its rate of onset. Imaging of adults as part of their follow up should detect this group of patients.
9.5 Skin and soft-tissue infections
Rabind Charles
Introduction
Infectious disease is one of the most common reasons for patients to present to the emergency department (ED) and skin and soft- tissue infections (SSTIs) make up an important subset of these. SSTIs are a diverse group of aetiologically and anatomically distinct infections. Bacteria cause the majority of SSTIs encountered in the ED. The pathogenesis of these infections usually involves direct inoculation of bacteria as a result of violation of the skin or its defences, although there may also be spread of infection from a distant source via the haematogenous or lymphatic systems. The severity of infections encountered may range from mild to life threatening. Most recommendations for the diagnosis and treatment of SSTIs are based on tradition or consensus, as there are few randomized clinical trials on the subject. Some of the challenges to the emergency physician include:
Aetiology
The majority of SSTIs are caused by aerobic Gram-positive bacteria, commonly Staphylococcus aureus and group A streptococcus. Deeper complicated infections, commonly seen in the immunocompromised host, are usually caused by Gram-negative, anaerobic or mixed organisms (Table 9.5.1).
Table 9.5.1
Causes of skin and soft-tissue infections
| Risk factor/setting | Expected pathogen |
| Simple cutaneous infection | Staphylococcus aureus. Also Staph. epidermidis, Staph. hominis, Streptococcus viridans |
| Perianal, genital, buttocks, ungual and cervical areas | Bacteroides fragilis, Escherichia coli, Klebsiella and Proteus |
| Immunocompromised host | Cryptococccus neoformans, Coccidioides, Aspergillus, Mycobacterium kansasii, M. tuberculosis and Yersinia enterocolitica |
| Human bite | Eikenella corrodens, Fusobacterium, Prevotella, streptococci |
| Dog bite | Pasteuralla multocida, Capnocytophaga canimorsus |
| Cat bite | P. multocida |
| Injection drug abuse | S. aureus, Clostridium spp., E. corrodens, S. pyogenes |
| Body piercing | S. aureus, S. pyogenes, P. aeruginosa, C. tetani |
| Hot tub/wading pool | Ps. aeruginosa |
| Fresh water injury | A. hydrophila |
| Salt water injury | V. vulnificus |
| Fish tank exposure | Mycobacterium marinum |
Examination
History
When taking a history, it is important to elicit the following:
Physical examination
Investigations
SSTIs are usually diagnosed from their clinical presentation. Laboratory and radiological investigations play a secondary and limited role in routine evaluation but may be useful in the ED management of immunocompromised patients or those with signs and symptoms of severe sepsis. In such situations, the following parameters should be considered [1]:
 Urea/creatinine: elevated levels suggest intravascular volume depletion or renal failure.
Urea/creatinine: elevated levels suggest intravascular volume depletion or renal failure.
 Creatine kinase: elevated levels may indicate myonecrosis caused by necrotizing fasciitis.
Creatine kinase: elevated levels may indicate myonecrosis caused by necrotizing fasciitis.
 Blood culture and drug susceptibility tests: the yield from these may be less than 10% and may be compounded by false-positive results [2]. In addition, emergency physicians do not have the luxury of time to await blood culture results before initiating the appropriate antibiotic treatment.
Blood culture and drug susceptibility tests: the yield from these may be less than 10% and may be compounded by false-positive results [2]. In addition, emergency physicians do not have the luxury of time to await blood culture results before initiating the appropriate antibiotic treatment.
Patients with a chronic, recurrent or unusual infection should have their immune status checked, including serology for HIV. Soft-tissue radiographs may demonstrate a foreign body or gas in deep tissues. Computed tomography (CT) or magnetic resonance imaging (MRI) may be needed to define the depth and extent of the infective process when entertaining the diagnoses of fasciitis or myonecrosis. Ultrasonography in the ED may be a useful adjunct in evaluating soft-tissue infections for the presence of subcutaneous abscesses.
Management
Analgesia
Oral or parenteral analgesia should be prescribed, as most patients with SSTIs will present with pain. Simple measures, such as immobilization, elevation, heat or moist warm packs, should not be overlooked as they may help to alleviate pain in cellulitis. Abscess pain is best resolved by timely incision and drainage.
It is important to have a high index of suspicion for necrotizing fasciitis in any patient who has cellulitis with an inordinate amount of pain, or exquisite muscle tenderness where there is no history of musculoskeletal trauma.
Antibiotic therapy
Antibiotics are recommended for patients with signs of systemic toxicity, high fever, tachycardia, who are flushed and who look unwell, who are immunocompromised, who have abscesses in high-risk areas (hands, perineal region or face) and where deep necrotizing infection is suspected [2].
It is important for the emergency physician to recognize patients with serious skin and soft-tissue infections and to initiate appropriate care. The choice of antibiotic is often empiric and thus must be guided by the patient’s history, where they have been recently institutionalized and knowledge of the typical range of pathogens associated with each type of infection and their resistance patterns. The antibiotic of choice is the one that has proven efficacy against the range of expected pathogens, is associated with minimal toxicity and is cost-effective. Where possible, narrow- spectrum antibiotics should be used in preference to broad-spectrum ones [3]. Published guidance on antibiotic therapy is often deliberately non-prescriptive, reflecting the wide variety between differing patient populations, resistance patterns, methicillin-resistant Staph. aureus (MRSA) risk and local governance policies. It is also prudent to remember that SSTI clinical trials often exclude the most severely ill patients and may be powered to demonstrate non-inferiority only. The Infectious Diseases Society of America recently released guidelines for treating MRSA in SSTIs [4]. Unlike inpatient or chronic care settings, emergency physicians more frequently have to initiate empiric antibiotics based on clinical judgement and prevailing antibiograms, due to absence of culture and susceptibility results.
In the last decade, new agents active against Gram-positive bacteria have emerged and those licensed for treating complicated SSTIs include linezolid, daptomycin and tigecycline.
Surgical intervention
Certain SSTIs are best treated surgically. Effective treatment of abscesses and carbuncles and large furuncles entails incision, drainage of pus and breaking up of loculations, followed by regular dressings. Necrotizing fasciitis requires early aggressive surgical debridement together with broad-spectrum antibiotics in order to achieve best morbidity and mortality outcomes [5].
Tetanus and other prophylaxis
All wounds should be considered to be tetanus prone and treated accordingly. The patient’s immunization status should be checked and, where appropriate, tetanus toxoid plus tetanus immunoglobulin should be administered. Deep and penetrating wounds and wounds that have significant tissue devitalization or where there is heavy contamination (e.g. soil, dust, manure and wood splinters) are best treated with prophylactic antibiotic cover. The antibiotic of choice is penicillin; patients who are allergic to penicillin should receive cephalexin. If there is a history of severe penicillin allergy, use erythromycin or vancomycin.
Rabies prophylaxis should be considered for all feral and wild animal bites and in geographical areas where there is a high prevalence of rabies.
In cases involving human bites, consideration should also be given to screening for blood-borne pathogens such as hepatitis B virus, hepatitis C virus, HIV and syphilis.
Disposition
SSTIs are among the most frequently encountered conditions in the emergency observation setting. Good candidates for the observation/short-stay unit include patients likely to respond to empirical therapy, with a low likelihood of infection with unusual and/or resistant organisms.
Patients who have systemic toxicity (fever, tachycardia, rigors, altered mentation, severe pain), involvement of vital structures (fingers, hand, face and neck, genitourinary, scrotal and anal regions), those unable to take oral medication, who have failed outpatient therapy or who are immunocompromised (HIV positive, cancer, diabetes mellitus, hepatic or renal failure) are highly likely to require admission. Other prognostic factors include low serum bicarbonate, elevated creatinine, elevated creatine kinase and marked left shift polymorphonuclear neutrophils. The emergency physician must also be alert to scenarios requiring not just inpatient care but also urgent subspecialty consultation, e.g. necrotizing fasciitis.
Superficial skin infections
Clinical presentation
Patients usually present with a complaint of localized pain, redness and swelling. They may have been self-treating or have had previous treatment with oral antibiotics without success. Frequently, an abscess is fluctuant and indurated with surrounding erythema. The patient may also have associated lymphadenitis, regional lymphadenopathy and cellulitis. If the patient is febrile or there is systemic involvement, their immune status needs to be examined.
The possibility of a foreign body associated with an abscess needs to be considered. A careful history needs to be taken to determine whether this is possible and radiography may be necessary. Ultrasound can be useful in identifying the presence of a foreign body. The patient should also be questioned in relation to use of immunosuppressive agents.
Impetigo
This is a localized purulent skin infection, usually caused by group A streptococcus (Strep. pyogenes) and is seen more in warm humid climates. Topical therapy with mucipirocin often suffices, but oral antibiotics (first-generation cephalosporin or erythromycin) may be needed in cases with extensive lesions or perioral lesions.
Folliculitis
A superficial infection characterized by reddened papules or pustules of the hair follicles. Most cases are caused by Staph. aureus. Pseudomonas aeruginosa may be the cause following swimming pool or hot tub (spa) exposure. Treatment may only require the use of an antibacterial soap or solution. Removal of the hair in limited infections usually results in rapid resolution.
Furuncle and carbuncle
A furuncle arises secondarily to an infected hair follicle, where an abscess forms in the subcutaneous tissue. Furuncles most commonly occur on the back, axilla or lower extremities. Staphylococcus species are the most common associated organism. When the infection extends to involve several adjacent follicles, resulting in a coalescent inflammatory mass, the lesion is termed a carbuncle. Small furuncles are best treated with moist heat. Larger furuncles and all carbuncles require incision and drainage. The most common site is the back of the neck and diabetics are particularly prone to this. Systemic antibiotics are usually unnecessary unless there is extensive surrounding cellulitis or fever or if the patient has diabetes or is immunocompromised, in which case di(flu)cloxacillin (500 mg q 6 h oral), cephalexin (500 mg q 6 h) or clindamycin (450 mg q 8 h oral) can be used.
Erysipelas
Erysipelas is a rapidly progressive, erythematous, indurated, painful, sharply demarcated superficial skin infection caused by Strep. pyogenes (other causes are non-group A streptococci, Haemophilus influenzae, Staph. aureus and Strep. pneumoniae). The classic description is of a butterfly facial distribution, but recent evidence suggests that erysipelas is commonly found on the lower limbs. There is a clear line of demarcation between involved and uninvolved skin. It is common in young children and the elderly. Systemic symptoms (fever, chills, rigors and diaphoresis) are common and 5% will have bacteraemia. Erysipelas may rapidly progress to cellulitis (i.e. involvement beyond the upper dermis), abscess formation and, occasionally, fasciitis. Treatment consists of the use of antibacterial soap and oral penicillin (di(flu)cloxacillin 500 mg q 6 h oral).
Herpetic whitlow
Herpetic whitlow is a superficial infection with herpes simplex virus and is an occupational hazard of jobs having contact with oral mucosa, e.g. dentistry and anaesthesiology. Incision and drainage is contraindicated and may in fact spread the viral infection.
Cellulitis
Cellulitis is an acute spreading infection of the skin involving the deeper dermis and subcutaneous fat. In patients with a normal immune system who are otherwise healthy, the infection is caused by bacteria that normally colonize skin, principally Staph. aureus and group A β-haemolytic streptococci. Predisposing factors include conditions leading to a disrupted cutaneous barrier and/or impaired local host defences, such as trauma and inflammatory dermatoses, e.g. eczema, oedema from venous insufficiency or lymphatic obstruction.
Despite the common occurrence of cellulitis, there is a paucity of published research on issues such as criteria for antibiotics and admission and severity assessment. The presence of an underlying abscess should be considered in patients who ‘fail’ initial antibiotic therapy: treatment failure may be caused by an undrained abscess rather than inadequate antimicrobial therapy and bedside soft-tissue ultrasonography is a useful tool that is increasingly available in EDs.
Treatment consists of elevation of the affected part and administration of an antistaphylococcal penicillin such as di(flu)cloxacillin (2 g q 6 h IV) or a first-generation cephalosporin, such as cephazolin (2 g q 12 h IV).
Therapy may need to be escalated in special settings, such as diabetes, or particular anatomical areas. Anaerobes or Gram-negative organisms have been identified in 95% of affected diabetic foot ulcers, with Staph. aureus found in approximately 33%. Broad-spectrum antibiotic treatment, e.g. metronidazole (400 mg q 12 h orally) plus cephazolin (2 g q 12 h IV), is recommended.
Infections that originate from wounds involving the feet may be due to Pseudomonas aeruginosa; this organism is also associated with osteomyelitis of the foot. Antibiotic treatment should consist of an antipseudomonal β-lactam, such as carbenacillin, or a third- generation cephalosporin, such as ceftriaxone, and an aminoglycoside.
Cellulitis is a well-known complication in women who have undergone axillary lymph node dissection and surgery for breast cancer. The major mechanism is thought to be an altered lymphatic and/or venous circulation related to the surgical procedure and to radiation therapy. Empiric antibiotic therapy is targeted at Staph. aureus and β-haemolytic streptococci and choices include cephalexin or cefazolin. If the patient has received recent chemotherapy and is neutropaenic, then the antibiotic regimen must be broadened to include coverage for aerobic Gram-negative bacilli, including Pseudomonas aeruginosa.
Facial cellulitis, including periorbital and orbital cellulitis is a serious infection occurring in adults and children [6]. The causal organisms include Staph. aureus, H. influenzae type b and Staph. pneumoniae. This type of cellulitis may arise from an infected sinus. Broad-spectrum antibiotic therapy is required, the agent of choice being dicloxacillin 1 g IV 6-hourly. Radiological evaluation, including CT scanning, may be necessary to identify underlying sinusitis.
Abscesses
Pilonidal abscess
Pilonidal abscesses occur in the superior gluteal fold and arise from the disruption of the epithelium, causing the formation of a pit lined with epithelial cells that may become plugged with hair and keratin, leading to an abscess. Treatment involves incision and drainage, usually in the operating theatre, although smaller abscesses can be drained in the ED under local anaesthetic. They are usually associated with mixed organisms, both aerobic and anaerobic.
Hidradenitis suppurativa
This is a chronic suppurative abscess of the upper apocrine sweat glands in the groin and axilla. It is much more common in females, in obesity and in patients who have poor hygiene or who shave the region. Organisms include Staph. aureus, Strep. viridans and Proteus spp. Treatment is incision and drainage, usually in the operating theatre. Definitive treatment may require removal of the apocrine sweat glands from the region.
Bartholin’s abscess
This occurs as a result of the obstruction of a Bartholin’s duct and is usually composed of mixed vaginal flora. Neisseria gonorrhoeae and Chlamydia trachomatis may also be involved. Treatment is incision, drainage and marsupialization of the cyst in the operating theatre.
Paronychia
This is a superficial abscess of the lateral aspect of the nail, commonly associated with patients whose hands are frequently wet. Common organisms involved are Staph. aureus, Candida and anaerobes. Some cases may require incision and drainage, with advice to keep the hands dry.
Perianal abscess
These are thought to originate in the anal crypts and extend into the ischiorectal space. Patients frequently complain of pain on defaecation and sitting. Perianal abscesses may be associated with inflammatory bowel disease and fistula formation. Treatment should be incision and drainage in the operating theatre under general anaesthesia. When the abscess is superficial and ‘pointing’, drainage in the ED is possible.
Infected sebaceous cyst
Sebaceous cysts become infected when the duct is obstructed. They can occur anywhere on the body, but tend to favour the head and neck region. Treatment is incision and drainage, recurrence is not uncommon.
Treatment
Incision and drainage of cutaneous abscesses is the key to treatment. Some patients require oral antibiotic therapy. Patients who are immunosuppressed or who have diabetes mellitus should be treated with appropriate antibiotic therapy based on a knowledge of the probable pathogen. Patients at risk of developing bacterial endocarditis require prophylactic antibiotics prior to incision and drainage. The treatment of superficial skin abscesses has in recent years been complicated by the emergence of MRSA. Proponents of the practice of ‘routine culture’ of abscess fluid say that surveillance of antimicrobial susceptibility allows therapeutic adjustment. Detractors point out that for simple abscesses, incision and drainage without antibiotics is usually sufficient and thus if antibiotics are not considered clinically useful it is unlikely that culture results will alter the management.
Deep soft-tissue infections
Necrotizing fasciitis
Necrotizing fasciitis is a rare, rapidly progressing, life-threatening infectious process involving primarily the superficial fascia (i.e. all the tissue between the skin and underlying muscles – the subcutaneous tissue). Patients usually present with the triad of exquisite pain – often out of proportion to initial physical findings – swelling and fever. Early diagnosis is sometimes thwarted by the paucity of cutaneous findings early in the course of the disease. The clinician should have a high index of suspicion based on the clinical presentation as well as the patient’s underlying co-morbidities (diabetes, chronic alcoholism, and immunosuppression). Laboratory values may be used in risk scoring, e.g. the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC), which has been validated prospectively and has a high sensitivity and positive predictive value of 92% in patients with scores of six points and above. Patients with scores of five points and below are considered at low risk of necrotizing fasciitis [7,8].
Numbness of the involved area is characteristic of advanced necrotizing fasciitis – this is a result of infarction of the cutaneous nerves. Eighty per cent of cases show clear origins for an accompanying skin lesion (insect bite, minor abrasion, furuncle, and IVDU injection site) but, in the remaining 20%, no skin lesion can be found [9,10].
Patients appear extremely toxic with a high fever, tachycardia and malaise. Pathognomonic features include extensive undermining of the skin and subcutaneous tissues, with separation of the tissue planes. The subcutaneous tissues may have a hard, wooden feel. Bullous lesions and skin ecchymoses may also be evident. Crepitation may be clinically evident and gas may be visualized on X-ray in some 80% of patients. The gas is typically layered along fascial planes. CT or MRI may aid in confirming the clinical suspicion.
Bacteria involved in this infection are usually mixed: Staph. aureus, haemolytic streptococci, Gram-negative rods and anaerobes. Sometimes only group A streptococci, either alone or in combination with Staph. aureus, are found. Aggressive therapy is essential, as mortality approaches 50%. Immediate surgical intervention extensively to open and debride the wound is required, as myonecrosis may be present [11]. Appropriate antimicrobial therapy should be commenced immediately: meropenam (1 g q 8 h IV) plus clindamycin (600 mg q 8 h IV) or lincomycin (600 mg q 8 h IV). Hyperbaric oxygen therapy should be considered.
Fournier’s gangrene is a form of necrotizing fasciitis which involves the scrotum, penis or vulva and is usually seen in diabetics. It usually originates from perianal or urinary tract infections (which extend into the periurethral glands) and can progress explosively. The management is early recognition and surgical debridement and intravenous antibiotics.
Gas gangrene
Gas gangrene is an acute life- and limb- threatening deep-tissue infection, also known as clostridial myonecrosis. Aetiological agents include Clostridium perfringens, Cl. histolyticum, Cl. septicum and Cl. novyi. Cl. perfringens is the most common cause in traumatic gas gangrene, whereas spontaneous gangrene is principally associated with Cl. septicum. This infection is characterized by the rapid development (often within hours) of intense pain in the region of a wound, followed by local swelling and a haemoserous exudate. A characteristic foul smell is also a good indication of the diagnosis. The area becomes tense and may develop a bluish and bronze or dusky discoloration. The presence of gas is typical, although it may be a late finding. It is frequently found on X-ray, where it has a feathered pattern as gas develops within the muscle itself. Aggressive treatment is required as the patient may present in an advanced stage with tachycardia, altered mental status, shock and haemolytic anaemia.
Classically, the gas gangrene occurs in extensive and or deep wounds with predisposing factors, including vascular ischaemia, diabetes and presence of foreign bodies. Gram stain frequently reveals relatively few white blood cells and large numbers of club-shaped Gram-positive rods.
Early surgical intervention is essential, including wide debridement of necrotic muscle and other tissues, administration of high-dose penicillin C (benzylpenicillin 2.4 g q 4 h IV), an aminoglycoside and hyperbaric oxygen therapy. Early hyperbaric oxygen therapy has been demonstrated to result in improved outcome [3,10–13].
One should note that the presence of gas certainly raises the suspicion of a deep-tissue infection, including gas gangrene, but that it may also be present because of previous wound manipulation, self-injection of air, localized gas abscess or other gas-producing organisms, including anaerobes, E. coli, streptococci and staphylococci.
Pyomyositis
Pyomyositis is the presence of pus within individual muscle groups and the usual culprit is Staph. aureus. A positive blood culture yield is found in only 5–30% of cases. Typical presenting symptoms included localized pain in a single muscle group, usually in an extremity, and fever. Ultrasonography or CT may be warranted to differentiate the condition from a suspected deep vein thrombosis.
Toxic complications of wound infections
A number of bacteria produce toxins that result in systemic symptoms.
Tetanus
Tetanus, albeit rare in developed countries, still occurs despite the fact that immunization is completely effective in preventing it. All wounds should be treated as tetanus prone. Tetanus may occur with trivial wounds that may not even be apparent. The incubation period is variable, ranging from 3 days to several weeks after inoculation; the disease is more severe at the extremes of age. Difficulty in swallowing and a fever with progression to stiffness and trismus is pathognomonic. Tetanus is also associated with autonomic nervous system dysfunction. Occasionally, localized tetanus may occur with muscle spasm in the area adjacent to the wound. This is sometimes associated with cranial nerve dysfunction. Treatment is largely supportive, often requiring deep sedation, paralysis and ventilation for prolonged periods. Antibiotic therapy with high-dose penicillin should also be given in addition to tetanus immunization and tetanus immunoglobulin (Table 9.5.2).
Table 9.5.2
| Time since vaccination | Type of wound | Tetanus toxoid | Tetanus immunoglobulin |
| History of 3 or more doses of tetanus toxoid | |||
| <5 years | All wounds | No | No |
| 5–10 years | Clean minor wounds | No | No |
| All other wounds | Yes | No | |
| >10 years | All wounds | Yes | No |
| Uncertain vaccination history or less than 3 doses of tetanus toxoid | |||
| Clean minor wounds | Yes | No | |
| All other wounds | Yes | Yes | |
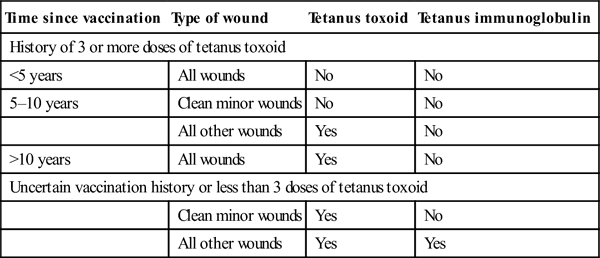
Reproduced with permission from Antibiotic Expert Group. Melbourne: Therapeutic guidelines Limited, 2013 eTG [14].
Toxic shock syndrome
Toxic shock syndrome (TSS) is a life-threatening multisystem disease caused by inflammatory immune responses to toxogenic strains of Staph. aureus. TSS has been classically associated with the use of tampons, although 10–40% of cases are non-menstrual related. Onset of menstrual TSS symptoms occurs within 3 days of the menstrual cycle and usually has no preceding clinically apparent infection. Non-menstrual cases occur after childbirth, abortions, in bone and skin infections including postoperative wound infections, burns, mastitis and varicella-related cellulitis. The wound itself may look insignificant. There is a rapid onset of fever, usually>38.9°C, hypotension and an initial diffuse and later desquamating erythematous rash. Multiorgan involvement may include muscular (myalgia), neurological (headache, and altered sensorium) and gastrointestinal (nausea, and diarrhoea) symptoms. Occasionally, Staph. aureus can be cultured locally, although blood cultures are rarely positive. Antibiotics do not affect the course of TSS but may lower the recurrence rate by 59–73% [15]. An antistaphylococcal agent should be given with an aminoglycoside. Patients are frequently haemodynamically compromised, requiring aggressive fluid resuscitation and inotropic support. Debridement of necrotic wounds, if present, and elimination of the source of infections – e.g. removal of the tampon – should be carried out urgently. A similar syndrome can develop due to infection with group A β-haemolytic streptococci. This is known as ‘wound’ or ‘surgical’ scarlet fever. Treatment is the same as for TSS.
Special infections
Human bites
Human bite wounds may occur as a result of an accidental injury, deliberate biting or closed fist injuries. The bacteriology reflects the normal oral flora of the biter: streptococci in 50–80% of wounds, staphylococci, Eikenella corrodens and anaerobic organisms. Therapy consists of irrigation and topical wound cleansing and prophylactic antibiotics should be initiated as early as possible in all patients, regardless of the appearance of the wound.
Clenched fist injuries over the metacarpophalangeal joint warrant hospitalization for formal washout and intravenous antibiotics. Appropriate antibiotic choices include amoxicillin-clavulanate (875+125 mg q 12 h oral), metronidazole (400 mg q 12 h oral) plus either ceftriaxone (1 g daily IV) or cefotaxime (1 g daily IV). In cases of β-lactam allergy, metronidazole plus doxycycline, ciprofloxacin or trimethoprim-sulfamethoxazole may be used.
Animal bites
Most bites are from dogs (80%) or cats, but bites from exotic pets and feral animals also occur. Pasteurella and bacteriodes species are the most common bacterial isolates and Capnocytophaga canimorsus can cause bacteraemia and fatal sepsis, especially in patients with underlying liver disease or asplenia. Infected bites presenting<12 hours after injury are more likely to be infected with Pasteurella spp., whereas those presenting>24 hours post-bite are more likely to be infected with staphylococci or anaerobes. Wounds should be cleansed with sterile normal saline and infected wounds should not be closed. Cat bite wounds have less crush injury and wound trauma than dog bites, but have a higher proportion of osteomyelitis and septic arthritis. The oral agent of choice for both dog and cat bites is amoxicillin–clavulanate, with doxycycline as an alternative. Intravenous options include second-generation cephalosporins, piperacillin–tazobactam and carbapenams. Cellulitis and abscesses usually respond to 5–10 days of therapy. Rabies prophylaxis should be considered for all feral and wild animal bites and in geographical areas where there is a high prevalence of rabies.
Water-related infections
Water-related infections may be caused by unusual organisms. Vibrio vulnificus, V. alginolyticus and other non-cholera vibrios are found in salt and brackish water and can result in serious and life-threatening infections, especially in patients with hepatic disease. Aggressive infection can progress rapidly over 2–4 hours. It is associated with saltwater exposure or the ingestion of raw shellfish. Infections can mimic gas gangrene, with rapid progression and tissue destruction; septicaemia may occur and can be fatal. If parenteral therapy is required, a third- generation cephalosporin can be combined with an aminoglycoside and/or doxycycline.
Exposure to fresh or brackish water (rivers, mud and caving) can result in infection with the Gram-negative bacillus Aeromonas hydrophila[16]. Aeromonas infections can result in superficial skin infections, myositis and septicaemia. Treatment consists of administration of cefotaxime 1 g IV 8-hourly or ceftriaxone 1 g IV daily. If oral therapy is possible, consider ciprofloxacin 500 mg orally 12-hourly.
Mycobacterium marinum, M. ulcerans, M. chelonei, M. gordanae. and M. fortuitum are found in fish tanks and can result in ‘fish fancier’s finger’. After 2–6 weeks of incubation, an ulcerating granuloma develops. Treatment options include clarithromycin, trimethoprim–sulfamethoxazole or a combination of ethambutol and rifampicin. Systemic infection is uncommon.
Saltwater fish handlers may develop infections due to Erysipelothrix rhusiopathiae; this causes erysipeloid, a type of cellulitis. It also causes infections in people handling fish, poultry, meat and hides. Coral cuts are often infected with Streptococcus pyogenes; other marine pathogens may be involved (including Vibrio species). Treatment should consist of phenoxymethylpenicillin 500 mg 6-hourly.
Mastitis
Infections of the breast can occur in both sexes and in all ages; however, breast infections are most common in nursing mothers and the prevalence of lactational mastitis in Australia is estimated at 20% [17]. Staph. aureus is the most common pathogen in infective mastitis.
Treatment consists of regular emptying of the breast. If breastfeeding needs to be stopped because of the severity of the infection or the risk to the neonate, a pump or manual expression methods should be employed (at least temporarily). If symptoms are not resolving within 12–24 hours of effective milk removal and analgesia, antibiotic treatment should be commenced to prevent abscess formation. Eleven per cent of patients who are not treated appropriately with antibiotics will develop an abscess. Options include di(flu)cloxacillin (500 mg q 6 h oral) or a first-generation cephalosporin, such as cephalexin (500 mg q 6 h oral) or erythromycin (250–500 mg q 6 h oral). Severe infections may require parenteral or more prolonged therapy. Local care to the region is also important, including warm compresses, breast support, analgesia and the application of a moisturizing cream to the nipple and areolar region. Patients who develop an abscess will require percutaneous aspiration or open drainage [18].
Decubitus ulcers
Decubitus ulcers are cutaneous ulcerations caused by prolonged pressure that results in ischaemic necrosis of the skin and underlying soft tissue. They are most commonly found in patients who are bedbound, particularly elderly nursing home patients and patients with sensory deficits, such as paraplegia and quadriplegia. Immobility, compounded by vascular insufficiency and neuropathy, results in ulcer formation and, unless treated aggressively, serious complications can follow [19]. Complications include cellulitis and deep soft-tissue necrosis, osteomyelitis, septic thrombophlebitis, bacteraemia and sepsis. Culture of the ulcer invariably reveals a mixed bacterial flora of both aerobes and anaerobes, which do not distinguish between colonization and tissue infection. The most common organisms found are staphylococci, streptococci, coliforms and a variety of anaerobes. Systemic antibiotics are required for patients with clinical signs of sepsis or osteomyelitis.
Varicose ulcers
Varicose ulcers are cutaneous ulcers caused by oedema and poor tissue drainage as a result of dysfunction of the venous system, including varicose veins. These are more common in the elderly and obese. They may be chronic, and healing is often difficult. Complications include cellulitis and, occasionally, bacteraemia. Culture of the ulcer variably reveals a mixed bacterial flora of both aerobes and anaerobes that cannot distinguish between colonization and tissue infections. The most common organisms found are staphylococci, streptococci, coliforms and a variety of anaerobes.
Treatment consists of debridement of necrotic tissue, pressure area and general nursing care, as well as treatment of infection, if present. Antibiotic treatment is only indicated where there is systemic evidence of infection or where there is a complicating infection, such as osteomyelitis or bacteraemia. Surgical debridement is frequently as important, if not more important, than antibiotic therapy, particularly where the bacterial infection is localized.
Diabetic foot infections
Foot infections are a common complication of diabetes and require both local (foot) treatment and systemic (metabolic) optimization, which is best undertaken by a multidisciplinary team including surgeons, podiatry services and the endocrinologist or physician.
The peripheral neuropathy associated with diabetes results in the loss of protective pain sensation and results in repetitive injuries, followed by the development of ulcers that become infected. Vascular insufficiency and impaired immune function contribute to the increased risk of acute and chronic infection. Infections in foot ulcers are often polymicrobial and both the number of bacterial groups and bacterial density are thought to affect healing [16]. Aerobes include Staph. aureus, coagulase-negative staphylococci and streptococci. Enterobacteriaceae and Corynebacterium are not uncommon. Anaerobes which have been isolated from up to 48% of patients include Bacteroides and Clostridium spp. The presence of anaerobes is associated with a high frequency of fever, foul-smelling lesions and the presence of an ulcer. Cultures obtained using curettage following debridement should be used in preference to wound swabs to identify causative organisms and sensitivities.
Local signs and symptoms predominate and include those secondary to infection, vasculopathy and neuropathy. Pain and tenderness are often minimal due to the neuropathy and pulses are frequently reduced or absent. Wound infections must be diagnosed clinically on the basis of local (and occasionally systemic) signs and symptoms of inflammation. Laboratory (including microbiological) investigations are of limited use for diagnosing infection, except in cases of osteomyelitis radiography and/or a bone scan may be warranted to exclude osteomyelitis.
A recent systemic review [20] reported that there is no strong evidence for any particular antimicrobial agent in the prevention of amputation, resolution of infection or ulcer healing. For mild to moderate infections with no evidence of osteomyelitis or septic arthritis, consider amoxicillin–clavulanate (875+125 mg q 12 h oral) for at least 5 days. Alternatives include ciprofloxacin 500 mg q 12 h with clindamycin 600 mg q 8 h. For severe limb- or life-threatening infections, intravenous piperacillin–tazobactam 4+0.5 g q 8 h or ticarcillin–clavulanate 3+0.1 g q 6 h or meropenem 500 mg q 8 h are all acceptable empiric therapy. Prolonged use of appropriate bactericidal antibiotics may be required, especially in the setting of osteomyelitis or septic arthritis.
Surgical-site/postoperative wound infection
Surgical-site infections are the most commonly occurring adverse events in patients who have undergone surgery, accounting for as much as 38% of nosocomial infections in postoperative patients. Surgical-site infections are usually diagnosed by the usual features of inflammation: wound pain, redness, swelling and purulent discharge. These external signs of inflammation may manifest late in morbidly obese patients or those with deep, multilayer wounds. Most bacterial wound infections present with fever only after 48 hours. Earlier symptoms may be seen in Strep. pyogenes and clostridial infections.
The mainstay of treatment for surgical site infections is early opening of the incision, coupled with evacuation of any infected material and sending off of wound cultures. This should be done after consultation with the surgeon involved, where possible. There has been a paucity of evidence regarding the use of antibiotics combined with drainage [21], but expert consensus generally advocates the use of empirical antibiotics for patients with temperature>38.5°C and/or pulse rate>100 in the presence of obvious wound infection [5].
Post-traumatic wound infection
The goals of wound care are to avoid infection and to achieve a functional and cosmetically acceptable scar. Adequate wound management requires a thorough history, with particular attention directed at factors adversely affecting healing. Factors, such as the extremes of age, diabetes, chronic renal failure, malnutrition, alcoholism, obesity and patients on immunosuppressive agents, cause an increased risk of infections and impaired wound healing. Wounds located in highly vascular areas, such as the scalp or face, are less likely to become infected than wounds in less vascular areas.
In order to reduce the incidence and severity of infections, wounds need to be thoroughly cleansed and irrigated. Devitalized tissue should be removed, injuries to associated structures need to be excluded and the wound closed appropriately. The method of closure depends on the location of the wound, the level of contamination and whether it is an ‘old’ wound (over 6 hours old). Wounds that should not be closed because of a high risk of infection, such as heavily contaminated wounds, should be treated by delayed primary closure 3–5 days after initial management. Where primary closure is possible the wound should be closed and a protective non-adherent dressing applied for a minimum of 24–48 hours, with both the wound and the dressing kept dry [22].
The use of prophylactic antibiotics is not recommended except where there is significant bacterial contamination, foreign bodies, the patient is immunosuppressed or the wound is the result of a bite (human or animal) or associated with an open fracture. Most wounds can be treated with amoxicillin–clavulanate (875+125 mg q 12 h oral) or metronidazole (400 mg q 12 h oral) plus di(flu)cloxacillin (500 mg q 6 h oral). Broad-spectrum antibiotics should be limited to heavily contaminated and bite wounds and immunosuppressed patients (see Table 9.5.2).
Intravenous drug users
Intravenous drug users frequently develop unusual infections because the needles and the drug paraphernalia used are contaminated. They also have alterations to their skin and flora and frequently have poor nutrition and immune function [23]. Many are hepatitis B, C and HIV positive. Intravenous drug users frequently have mixed organisms, particularly anaerobes, including Klebsiella, Enterobacter, Serratia and Proteus. They have mixed Gram-positive and Gram-negative infections. Some develop fungal infections, including candidaemia. Subacute bacterial endocarditis and endocarditis need to be considered in IV drug users. If endocarditis is not suspected, treatment should consist of flucloxacillin 2 g IV 6-hourly and gentamicin 5–7 mg/kg/day as a single daily dose.
9.6 Hepatitis
Helen E Stergiou and Biswadev Mitra
Introduction
Hepatitis is a non-specific clinicopathological term that encompasses all disorders characterized by hepatocellular injury and by histological evidence of a necroinflammatory response [1]. Prolific research has resulted in the identification of specific hepatotrophic viruses. An important distinction is that between acute and chronic viral hepatitis. Acute viral hepatitis refers to a process of self-limited liver injury of less than 6 months’ duration [1]. Chronic viral hepatitis is diagnosed on pathological criteria and is characterized by a duration of more than 6 months [1].
Clinical presentations of viral hepatitis
An appropriate clinical pattern of illness and specific laboratory confirmation are necessary for the diagnosis of acute viral hepatitis. Patients with acute viral hepatitis may present quite variably: they may be asymptomatic with only mildly deranged liver function tests (LFTs), they may be symptomatic with or without jaundice or they may present with fulminant disease (severe liver failure which develops within 8 weeks of symptom onset) [2].
Various clinical phases characterize acute viral hepatitis [1–4]. The incubation phase is the time between the original infection and the initial symptoms and is the time of viral replication and laboratory evidence of hepatitis. During the pre-icteric phase non-specific symptoms evolve, such as malaise, fatigue, anorexia, nausea, vomiting, myalgias, arthralgias, abdominal discomfort. If fever is present, it is generally low grade. Cough, coryza, pharyngitis and a distaste for alcohol and tobacco smoke may be evident. Rarely meningoencephalitis may occur.
The icteric phase features a variable degree of jaundice, dark urine (bilirubinuria), pale stools (absence of bile pigment in the stool), pruritus, hepatomegaly and splenomegaly. During the convalescent phase symptoms resolve, as do liver enzyme abnormalities. In patients presenting to the emergency department (ED) during the pre-icteric phase, the diagnosis may be challenging, given their non-specific symptomatology. If the patients present during the icteric phase, focused history taking, examination and the appropriate investigations should result in a definitive diagnosis.
Laboratory investigations
Blood test abnormalities are a prominent aspect of acute viral hepatitis. Serum transaminases are typically elevated at>500 μ/L and often>1000 μ/L [5]. Alanine aminotransferase (ALT) may be characteristically higher than aspartate aminotransferase (AST) [5]. Alkaline phosphatase may be normal or mildly elevated. Serum bilirubin is variably elevated and is usually divided between conjugated and unconjugated fractions. Albumin and the prothrombin time should be normal unless hepatic synthetic function is significantly impaired. Neutropaenia and lymphopaenia may be evident transiently. Severe acute hepatitis may cause hypoglycaemia. Further specific laboratory tests for viral hepatitis will be presented subsequently.
Management
In cases of acute viral hepatitis, the fundamental management is supportive care. Many of these patients can be managed on an outpatient basis. Patients require hospitalization when they have intractable vomiting with inadequate oral intake and when they demonstrate clinical features of liver failure. Bed rest is recommended during the symptomatic phase. A well-balanced diet is beneficial. It is recommended that alcohol be avoided during the acute phase, but there is no definitive evidence that alcohol consumption post-recovery causes either relapses or progression to chronic disease [2]. Given that the liver is involved in the metabolism of a plethora of drugs, all medications must be carefully prescribed to patients with acute hepatitis.
In managing fulminant hepatic failure, it is imperative that potential patients be identified as early as possible. In the emergency setting, intubation and the concomitant critical care are necessary for patients with progressive encephalopathy. Early referral to an appropriate intensive care unit (ICU) is mandatory.
Prevention and immunization
Prevention of viral hepatitis is possible via the introduction of public health programmes, improved sanitation and vaccination programmes. Post-exposure prophylaxis regimens are particularly relevant to healthcare workers.
Hepatitis A virus (HAV)
As the most common cause of viral hepatitis, HAV contributes significantly to the global burden of disease. Multiple genotypes exist and infection with one genotype confers immunity against others [6]. (See Table 9.6.1 for virology.)
Table 9.6.1
Characteristics of the main hepatitis viruses
| HAV | HBV | HCV | HDV | HEV | |
| Family | Picornavirus | Hepadnavirus | Flavivirus | Incomplete | Calicivirus |
| Nucleic acid | RNA | DNA | RNA | RNA | RNA |
| Diameter (nm) | 27 | 42 | 32 | 36 | 34 |
| Incubation period (weeks) | 2–6 | 6–24 | 2–26 | 6–9 | 2–10 |
| Spread | |||||
| Faeces | Yes | No | No | No | Yes |
| Blood | Uncommon | Yes | Yes | Yes | No |
| Sexual | Uncommon | Yes | Uncommon | Yes | ? |
| Vertical | No | Yes | Uncommon | Yes | No |
| Chronic infection | No | Yes | Yes | Yes | No |
| Vaccine | Available | Available | Nil | Nil | Nil |
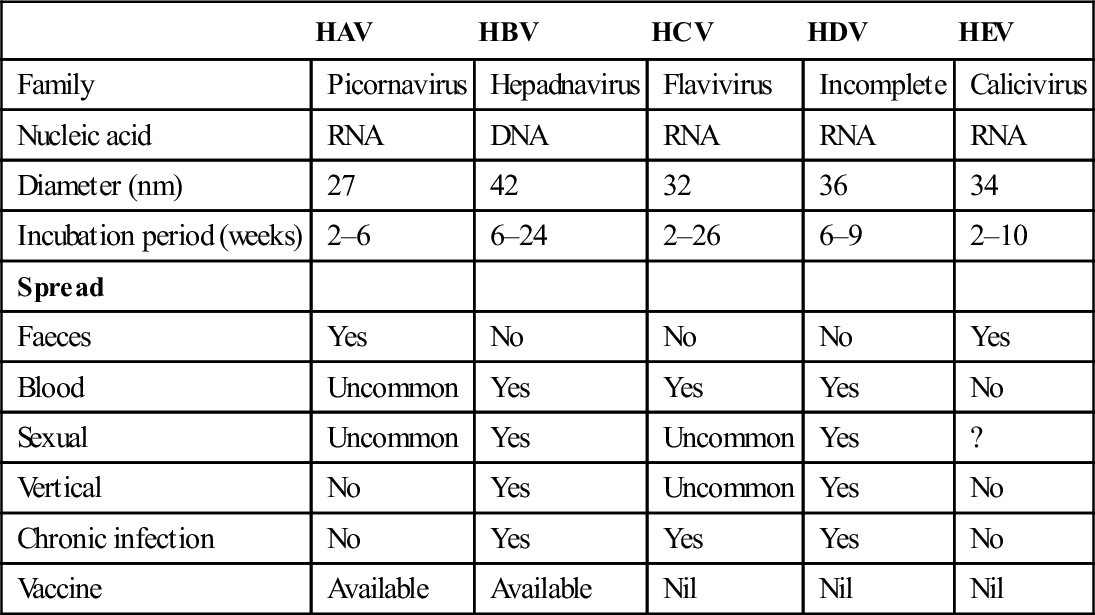
Epidemiology
HAV is highly endemic in developing countries and can often be traced to contaminated water or food.
Natural history
Virus is excreted in the stool of the infected person for 1–2 weeks prior to and for 1 week after the onset of symptoms. A non-specific prodrome may be followed by jaundice and tender hepatomegaly. The clinical severity of the illness increases with age, with more than 80% of children being asymptomatic [1]. HAV has been associated with extrahepatic features, such as cutaneous vasculitis, renal failure, pancreatitis, bradycardia and, rarely, convulsions, transverse myelitis and aplastic anaemia [7]. Relapsing hepatitis has been described in 20% of those with HAV infection [2]. Relapses are generally benign and may occur 4–15 weeks after the original illness. Complete recovery is the typical outcome. Fulminant hepatic failure occurs in less than 1% of cases. Chronic infection never ensues.
Laboratory investigations
Serum antibody is present from the onset of HAV disease in both IgM and IgG forms. After approximately 3–12 months, anti-HAV IgM disappears and anti-HAV IgG persists, thereby conferring lifelong immunity against re-infection.
Management
Supportive management is of primary importance. Bed rest is indicated until any jaundice settles. Potentially hepatotoxic medications must be ceased. Alcohol must not be consumed during acute episodes because of the direct nephrotoxic effects.
Prevention and immunization
General measures are imperative – safe water supplies, proper sewage disposal and careful hand washing. HAV vaccines can prevent HAV infection and, importantly, they have excellent safety profiles. Persons who have been exposed to HAV and who have not been previously vaccinated should receive the vaccine within 2 weeks of exposure. Travellers to endemic areas require inactivated hepatitis vaccine, which confers long-term immunity to more than 90% of persons.
Hepatitis B virus (HBV)
Of the viral causes of hepatitis few are of greater global importance than HBV. HBV infection is endemic in certain parts of the world – Southeast Asia, China and sub-Saharan Africa. It is estimated that there are 350 million carriers worldwide [8]. By 2017, it is estimated there will be a two- to threefold increase in the number of hepatitis B-induced liver cancer cases and a marked increase in the number of deaths attributable to hepatitis B under current treatment patterns in Australia [9]. (See Table 9.6.1 for virology.)
Epidemiology
Transmission occurs by percutaneous and mucosal exposure to infected blood products and bodily fluids, hence unprotected sexual contact with infected individuals, the use of contaminated paraphernalia during intravenous drug use and vertical transmission from mother to infant are commonly implicated.
Natural history
Many acute HBV infections are asymptomatic, particularly in younger patients. The non- specific symptoms of the acute episode may be preceded by a serum-sickness syndrome with fevers, urticaria and arthralgias [5].
Approximately 90% of patients completely recover from an acute episode of HBV infection. Fulminant hepatic failure may develop in 1% of patients and has a mortality rate of up to 80%.
Progression to chronic HBV infection occurs in 5–10% of cases, with 90% of these experiencing an asymptomatic carrier state and the remaining 10% proceeding to cirrhosis and hepatocelluar carcinoma. The risk of developing chronic disease is related to the age at which HBV is first contracted – there is a greater than 90% risk of developing chronic HBV in neonates and a less than 5% risk in immunocompetent adults [1,2]. Although chronic HBV infection is generally a lifelong condition, a small percentage of infected individuals will experience complete viral eradication. In chronic HBV infection, the incidence of cirrhosis is about 2–3% per year [10]. Variables associated with progression to cirrhosis are persistence of viral replication, older age, elevation of ALT levels and HBeAg positivity [11].
Laboratory investigations
HBsAg indicates acute hepatitis or a carrier state if it persists beyond 6 months. Anti-HBc IgM indicates acute HBV and high infectivity. Anti-HBc IgG indicates previous infection. HBeAg indicates ongoing viral replication, high infectivity or chronic hepatitis.
Management
Supportive care is the primary aim of management. Household contacts require adequate education. In cases of chronic HBV infection, the aims are to suppress HBV replication and to reduce liver injury. Interferon-alpha (IFN-α) has antiviral, antiproliferative and immunomodulatory effects and is an effective treatment option against HBV. Patients with normal serum ALT levels have a poor response to interferon- alpha because the lack of hepatic dysfunction is suggestive of low immune-mediated hepatic inflammation. The limiting factor in the use of IFN-α is the side-effect profile, which includes an influenza-like illness, gastrointestinal symptoms, psychological sequelae (particularly depression), bone marrow suppression, thyroid dysfunction and possible birth defects [5]. Lamivudine is an oral nucleoside analogue that potently inhibits HBV DNA synthesis. Human monoclonal antibodies may be directed against different epitopes of HBsAg, bind HBV particles and reduce serum viral titres and HBsAg levels. Further research is ongoing.
Prevention and immunization
The pre-exposure administration of HBV vaccine is fundamental to immunoprophylaxis. The vaccine is protective in over 90% of individuals [1]. Current recommendations include all infants at birth and individuals with high exposure risk, such as healthcare personnel, injecting drug users and high-risk sexual workers. Antibody titres may decrease with time, but the protective effects persist. The risk of HBV infection in the occupational setting is related primarily to the degree of contact with blood and to the HBeAg status of the donor. In needle-stick injuries, the risk of developing clinical hepatitis if the blood is both HBsAg- and HBeAg-positive has been estimated to be up to 30%. Post-exposure prophylaxis involves the administration of hepatitis B immunoglobulin in addition to the recombinant vaccine series.
Hepatitis C virus (HCV)
International studies estimate that up to 3% of the world’s population is infected with HCV [2,12]. (See Table 9.6.1 for virology.) The identification of six major genotypes of the HCV has important clinical implications in that such genomic sequence variation makes vaccine development extremely difficult.
Epidemiology
Parenteral exposure leads to HCV infection, the use of contaminated needles and syringes being a predominant factor. Sexual and perinatal transmission of HCV is negligible. Transfusion-related HCV transmission has essentially been eradicated via donor screening. Up to 10% of HCV cases do not have an identifiable source of infection.
Natural history
A pre-icteric phase featuring non-specific symptoms develops in 15–20% of patients. When the icteric phase develops it typically lasts for 1–2 weeks. Fulminant hepatic failure rarely results from acute HCV infection.
Following an acute episode, 75–85% of adults and 55% of children will enter a chronic phase [12]. There is a high proportion of subclinical chronic HCV infection, hence patients may not manifest any pathology until incidental blood tests or end-stage liver disease many years after the initial infection. Approximately 20–30% of chronic HCV patients develop cirrhosis, with subsequent hepatocellular carcinoma occurring in up to 20% of the latter group [1,5].
Laboratory investigations
A fluctuating titre of HCV RNA is detectable within days to weeks of the initial HCV infection. The rate at which HCV antibodies develop is variable. Notably, HCV antibodies are neither neutralizing nor protective. It may not be possible to distinguish between acute and chronic HCV infection, given that the same laboratory markers can be present in both conditions. Further specific laboratory tests for viral hepatitis are presented in Table 9.6.2.
Table 9.6.2
Laboratory tests in viral hepatitis
| Test | Interpretation of positive test | Clinical significance |
| Tests for HAV | ||
| Anti-HAV IgM | Recently acquired HAV | Acute hepatic illness |
| Anti-HAV IgG | Previous infection/vaccination | Immunity |
| Tests for HBV | ||
| HBsAg (surface Ag) | Current/chronic infection | Structural viral component |
| Anti-HBsAg (surface Ab) | Previous infection/vaccination | Immunity |
| Anti-HBclgM (core Ab) | Recently acquired HBV | Test for acute HBV |
| HBeAg | Marker of viral replication | High infectivity |
| Anti-HBeAg | No viral replication | Low infectivity |
| HBV DNA | Complete virus present | High infectivity |
| Tests for HCV | ||
| Anti-HCV | HCV exposure | Variable infectivity |
| HCV RNA | Virus present | |
| Tests for HDV | ||
| Anti-HDV IgG/IgM | HDV exposure | Acute or chronic HDV |
| Delta Ag | HDV present | Acute or chronic HDV |
| Tests for HEV | ||
| Anti-HEV IgM | Recently acquired HEV | Acute hepatic illness |
| Anti-HEV IgG | Previous exposure |
Modified from Talley N, Martin C. Clinical Gastroenterology: A Practical Problem-based Approach, 2nd edn. Edinburgh: Churchill Livingstone, 2006 with permission.
Management
Supportive management is fundamental in addressing HCV infection. Relevant education and counselling regarding high-risk behaviours and referrals to appropriate support networks are necessary. Avoidance of alcohol is advisable as some studies indicate that alcohol may promote the progression of HCV infection [13,14]. Standard therapy for HCV infection has consisted of a combination of pegylated interferon-α and ribavirin. However, the combination therapy leads to cure in only abut 50% of cases [15]. Recent advances include the use of HCV protease inhibitors, polymerase inhibitors, NS5A inhibitors and host factor inhibitors, such as cyclophilin antagonists [16,17].
Prevention and immunization
Currently, there is no effective vaccination available against HCV infection, nor is there any specific post-exposure prophylaxis regimen. Vaccination against HAV and HBV is advisable. HCV is not transmitted efficiently through occupational exposures to blood. The average incidence of anti-HCV seroconversion after accidental exposure from an HCV-positive source is<2% [18].
Hepatitis D virus (HDV)
As a defective virus, HDV requires the presence of HBV for virion assembly and for viral replication [1,2]. (See Table 9.6.1 for virology.)
Epidemiology
Only patients with acute or chronic HBV infection are susceptible to infection with HDV. An estimated 5% of HBV carriers are infected with HDV worldwide [1,2,5]. Parenteral exposure is the primary transmission mode. HDV can occur as a co-infection with acute HBV (acquired at the same time) or as a superinfection in chronic HBV carriers.
Natural history
In cases of HDV and HBV co-infection, acute HDV infection generally presents as a benign acute hepatitis with subsequent resolution in up to 80–95% of patients. Chronic HDV/HBV infection may occur in 5–10% of patients [2]. HDV superinfection results in progression to chronic HDV/HBV in 70–80% of cases [5]. Chronic HDV/HBV infection manifests as a chronic healthy carrier state or severe liver disease. HDV superinfection may result in fulminant hepatitis in 2–20% of cases [5]. Chronic HDV infection leads to more severe liver disease than HBV monoinfection and is associated with accelerated fibrosis progression, earlier hepatic decompensation and an increased risk for the development of hepatocellular carcinoma.
Laboratory investigations
HBsAg must be detected to diagnose acute HDV/HBV co-infection. Anti-HDV IgM is transiently present in acute infections. Anti-HDV IgG appears late in acute infections.
Management
There is no specific cure for HDV infection other than suppressing HBV replication. IFN-α treatment has proven antiviral activity against HDV in humans and has been linked to improved long-term outcomes. Studies conducted in the past 2 years on the use of PEG-IFN-α show that a sustained virologic response to therapy, measured in terms of undetectable serum HDV RNA levels, can be achieved in about one-quarter of patients with hepatitis D [19].
Prevention and immunization
Currently, there is no vaccine for preventing HDV infection. HBV immunization has been shown to provide protection against the development of HDV.
Hepatitis E virus (HEV)
See Table 9.6.1 for virology.
Epidemiology
HEV is endemic in developing countries, such as Southeast and Central Asia and the Indian subcontinent. The primary transmission mode is the faecal–oral route, with contaminated drinking water and food supplies being primary sources of infection. Young adults are often predominantly affected.
Natural history
The clinical course is similar to that of acute HAV infection. Full recovery from the acute HEV infection is the norm. There have not been any recorded cases of chronic HEV infection.
Overall mortality from acute HEV infection is about 5%. For reasons which remain unclear, fulminant hepatic failure with a subsequent high mortality rate occurs in 25% of women with HEV infection during the third trimester of pregnancy. Liver transplant recipients may be at a greater risk for HEV infection, which can lead to chronic hepatitis.
Laboratory investigations
Anti-HEV IgM occurs between 1 week and 6 months after the illness onset. Anti-HEV IgG is evident during the convalescent phase or post-exposure.
Management
Supportive management is the key.
Prevention and immunization
Disease control depends on good personal hygiene and improved environmental sanitation. There is no effective vaccine.
Hepatitis G virus (HGV)
Exposure to blood products is a recognized route of acquisition of HGV infection in humans. Chronic viraemia results and reported prevalences of HGV infection range from 1% to 3% in most populations, incidences that are higher than those of either HBV or HCV in these populations [20]. A causal relationship between the prevalence of HGV and hepatitis, however, has not been proven. Although HGV RNA may persist in serum of patients acutely infected with HGV for as long as 16 years, in about 90% of these patients persistence is not accompanied by evidence of hepatocellular injury [21]. Currently, there should be no need to test for HGV in the emergency setting.
Non-hepatotrophic viruses
Several non-ABCDE viruses cause viral hepatitis. The cytomegalovirus (CMV) and Epstein–Barr virus (EBV) commonly contribute to abnormal LFTs and icteric hepatitis may also occasionally be noted. In immunocompromised patients, herpes simplex may lead to a hepatitic picture. Progression to chronic hepatitis has not been demonstrated with any of these viruses.
Non-viral hepatitis
Of the causes of non-viral hepatitis, the following are important in the emergency setting: alcoholic hepatitis, non-alcoholic steatohepatitis (NASH), drug-induced hepatitis and autoimmune hepatitis.
Alcoholic hepatitis
Alcoholic hepatitis is an important clinical syndrome which is variably characterized by anorexia, nausea, jaundice, hepatomegaly and features of portal hypertension, such as ascites and encephalopathy. Cirrhosis and death are possible sequelae if the patients do not cease their alcohol consumption.
Non-alcoholic steatohepatitis
Defects in the processing of fatty acids through the liver may cause steatosis-induced inflammation (steatohepatitis). Ten to 50% of patients with NASH are at risk of developing cirrhosis [14].
Drug-induced hepatitis
Toxic exposure to certain medications, vitamins, herbal remedies and food supplements may result in a drug-induced hepatitis. Drug-induced hepatitis may occur as an expected consequence of a drug’s toxicity profile or as an idiosyncratic reaction to a standard dose. Hepatotoxic agents result in variable clinicopathological patterns of liver injury via toxic and immune mechanisms [22]. Commonly, the formation of reactive hepatotoxic metabolites is the primary underlying mechanism [23]. Extensive lists of hepatotoxic drugs can be found in the literature. Acute liver injury may be necroinflammatory (e.g. paracetamol), cholestatic (e.g. chlorpromazine) or of a mixed type. Table 9.6.3 lists drugs which may induce hepatitis and which are encountered in the emergency setting [22,23].
Table 9.6.3
Hepatitis-inducing drugs [13,14]
| Drug | Pathology |
| Allopurinol | Hepatic granulomas |
| Cloxacillin | Lobular hepatitis |
| Chlorpromazine | Cholestatic hepatitis |
| Dantrolene | Cytolytic hepatitis |
| Erythromycin | Cholestasis with hepatitis |
| Flucloxacillin | Cholestatic hepatitis |
| Halothane | Hepatocellular injury |
| Isoniazid | Cytolytic hepatitis |
| Non-steroidals | Primarily cholestasis |
| Paracetamol | Cytolytic hepatitis |
| Phenothiazines | Cholestatic hepatitis |
| Phenytoin | Non-caseating granulomas |
| Sulphonamides | Cytolytic hepatitis |
After Thomas D, Astemborski J, Rai R. The natural history of hepatitis C virus infection. Journal of the American Medical Association 2000;284:45; Friedman L, Keeffe E, Schiff E. Handbook of Liver Disease, 2nd edn. Edinburgh: Churchill Livingstone, 2004.
Autoimmune hepatitis
Autoimmune hepatitis is a self-perpetuating hepatocellular inflammation of unknown cause which is associated with hypergammaglobulinaemia and serum antibodies [14]. Fatigue, anorexia and jaundice may progress to liver failure. Corticosteroids are the basis of treatment.